Abstract
The correlation between water physicochemical parameters and eukaryotic benthic composition was examined in Río Tinto. Principal component analysis showed a high inverse relationship between pH and most of the heavy metals analyzed as well as Dunaliella sp., while Chlamydomonas sp. abundance was positively related. Zn, Cu, and Ni clustered together and showed a strong inverse correlation with the diversity coefficient and most of the species analyzed. These eukaryotic communities seem to be more influenced by the presence of heavy metals than by the pH.
Natural extreme acidic rivers are scarce worldwide (18). Extreme acidic environments, characterized by a pH of <3, are often the consequence of anthropogenic influences (e.g., mining activity or acid rain) (14). Thus, most ecological studies of acidic waters have been focused on environments affected by human activity. In addition, most of the information available about acidophilic communities in aquatic environments is focused on bacterial communities, although microbial eukaryotes are also present and could also play a critical role in these places. There have been few reports on eukaryotes, and most of them are related to acid mine drainages instead of naturally acidic locations (2, 3, 16). Moreover, these studies are often carried out in lakes, probably because of the difficulty in obtaining integrated representative samples in rivers.
In this regard, the Río Tinto (southwestern Spain), a 92-km-long river, is one of the most extensive examples of a naturally extreme acidic environment. The river springs up in the core of the Iberian Pyritic Belt, one of the largest bodies of iron and copper sulfide deposits in the world (5). Ferric iron and sulfuric acid are the most common components found in this acidic environment, establishing a buffer system at pH values of approximately 2.3. Ferric iron is produced by the metabolism of iron-oxidizing microorganisms, which are very active in the aerobic part of the river; sulfuric acid originates from sulfides by chemical oxidation or the activity of sulfur-oxidizing microorganisms, depending on the sulfide mineral substrate (15). The result is a strongly acidic solution of ferric iron which brings into solution other heavy metals, increasing their concentrations in relation to neighboring rivers with higher pH (12).
It is usually assumed that high metal concentrations in acidic habitats limit eukaryotic growth and diversity due to their toxicity. It has been also proposed that metal hydroxide deposition could change the physicochemical conditions of surfaces, resulting in a reduction of epiphytic growth on rocks (8). However, what makes Río Tinto a unique acidic extreme environment is that eukaryotic organisms are the principal contributors of biomass in the river, over 65% of the total biomass, as well as the unexpected degree of eukaryotic diversity found in its waters (1, 21, 28). Members of the Bacillariophyta, Chlorophyta, and Euglenophyta phyla as well as ciliates, cercomonads, amoebae, stramenopiles, fungi, and yeast have been detected.
The main purpose of the current investigation was to study the seasonal dynamics of the epiphytic eukaryotic community as well as to evaluate the influences of different physicochemical characteristics of water in the biodiversity structure and population abundance. Although chemistry and microbiology should be linked, there have been few reports in which both have been described in detail for acidic environments.
MATERIALS AND METHODS
Sample collection and in situ measurements.
Twelve sites along the Río Tinto (between 0 and 50 km from its source) were selected for in situ measurements, water sampling, and epilithon collection (Fig. 1). A general description of Río Tinto geological records and hydrochemistry conditions has been previously reported (12, 13). Samples were taken for all 12 sites in January, June, and September during 2001 and 2002. Measurements of water conductivity, temperature, redox potential, pH, and oxygen concentration were carried out as described previously (12). Epilithon samples were collected as described in previous studies (30). Identification of algae and heterotrophic protists was carried out by direct microscopic observation using different phenotypic features based on previous studies of the eukaryotic communities in this river (1, 20, 21). Biological diversity was estimated through Simpson's coefficient, D (29).
FIG. 1.
Locations of the 12 investigated sampling points in the Tinto River (southwest Spain).
Chemical analysis.
Water samples were filtered through 0.45-μm Millipore membranes. The total concentrations of eight recoverable metals (Zn, Cu, Fe, Co, Ni, As, Cd, and Cr) were measured for each water sample using X-ray fluorescence reflection and inductively coupled plasma-mass spectrometry. These metals were used to calculate the toxicity index (TI). This index was used as a measure of relative heavy metal presence in each sampling station following the equation previously proposed (11).
Distances between sampling sites based on biodiversity and water physicochemical parameters.
Two different measures of distance have been defined. In order to compare biodiversity, we calculated first the relative frequency of each species at each site with the equation fiα = niα/Nα, where the subindex i represents the species and the superindex α is for the sampled site. The similarity dαβ between two sites is defined in the following equation:
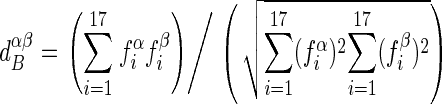 |
which represents the cosine of the angle formed by the two vectors characterizing the biodiversity of each site (9, 19).
The comparison of the physical chemical states of different sites can be carried out with the following equation:
 |
where the sum is performed over all measures taken (including pH, conductivity, and metals), Mei represents each of those variables, and Memin represents their minimal concentrations (7). The two distances defined above yield two independent matrices from which phenograms establishing the degree of similarity among sites were derived. We used the unweighted pair group method with arithmetic mean (UPGMA, or “average linkage”) (31, 32).
All physicochemical and microbiological parameters for each sampling site and season were organized in a single matrix. Because of heterogeneous variances in physicochemical and microbial parameters, Kruskal-Wallis nonparametric tests were used to analyze the differences between stations and seasons. An estimate of possible relation between physicochemical and biological parameters was developed by correlation analysis and principal components analysis (PCA), performed with the Statistica v.6.0 program.
RESULTS
The average pH was ca. 2, remaining rather constant during the last two surveys of each year (June and September) (see Fig. S1 in the supplemental material). A significant increase was detected during the winter in most of the sampling sites analyzed (P < 0.05) (see Fig. S1a). Three stations stand out due to their low pH values, RI (pH ca. 1.0), UMA (pH ca. 1.2), and ANG (pH ca. 1.6). The average water temperature was ca. 25°C during summer and autumn, decreasing to 15°C in winter. Only one sampling site, ANG, with an average temperature of ca. 23°C, showed no seasonality (see Fig. S1b). In general, no statistically significant differences in conductivity were found among seasons (P > 0.05) (see Fig. S1c), although dissolved oxygen displayed seasonal variations (see Fig. S1d), with enhanced concentrations in January and comparatively lower concentrations in June and September. Most of the sampling sites did exhibit significant differences in redox values among seasons (P < 0.05) (see Fig. S1e).
In general, heavy metals presented the highest values during the low water flow period, summer and autumn, decreasing significantly in winter (see Fig. S2 in the supplemental material). Additionally, the concentration of metallic ions showed a relative decrease from the source to the mouth of the river. Significant variations among the different sampling sites were also found, reaching up to 3 orders of magnitude in some metals (i.e., the Fe amount between RI and LPC) (see Fig S2a).
The data obtained for the TI are summarized in Fig. 2a. The TI showed significant differences among sampling stations (P < 0.05). Three locations near the origin, RI, UMA, and ANG, had the highest TI values (ca. 5.5). Surprisingly, the lowest average TI value (2.53 ± 1.28 [mean ± standard error]) was reached at FE, located at the very origin of the river, followed closely by LPC (TI, 2.9 ± 0.23) located ca. 50 km downstream and usually considered the less extreme of the sites sampled in this study. The TI showed evidence of seasonal variations, with enhanced values in September and significantly lower values in June and January, for most of the sampling sites analyzed.
FIG. 2.
(a) TI values for water collected from each sampling site. (b) Means and standard errors of benthic eukaryotic cell densities at each sampling site and the time point as determined by direct microscopic enumeration. (c) The diversity index at each sampling site.
We have microscopically identified 18 individual species belonging to different genera of Bacillariophyta (Pinnularia sp.), Euglenophyta (Euglena mutabilis), Rhodophyta (Cyanidium sp.), Chlorophyta (Chlamydomonas sp., Chlorella sp., and Dunaliella sp.), and Streptophyta (Zygnemopsis sp. and Klebsormidium sp.), 2 species of amoeba (Vahlkampfia sp. and Naegleria sp.), 1 species of heliozoan (Actinophrys sp.), 4 species of flagellates (Bodo sp., Cercomonas sp., Ochroomonas sp., and Labirynthula sp.), 2 species of ciliates (Oxytrichia sp. and Colpidium sp.), and 1 species of rotifer (Rotaria sp.).
Direct microscopic counts were used to estimate total biomass abundances and diversity at each site over the course of this study (Fig. 2). There were significant differences in total cell numbers among the sampling locations and among sampling dates (P < 0.05) (Fig. 2b). Only three locations, RI, ANG, and CEM, did not show significant differences throughout the year (P > 0.05). Average abundance of cells in summer was ca. 3.7 × 106 cells cm−2, with a range of 8 × 103 in Iz-Iz to 13 × 106 cells cm−2 in CEM. In winter, the cell abundance was much lower than in summer. The lowest abundance appeared at the Iz-Iz station for all the seasons sampled. The results showed an average diversity index (D) of 0.36 ± 0.1, varying from 0.02 to 0.78. Only four stations (ANG, 3.2, CEM, and LPC) showed no statistically significant differences among seasons (P > 0.05) (Fig. 2c). For the remaining sampling sites, in general, the diversity index was lower in winter. The lowest diversity index appeared at 3.2 and BRR.
To determine whether community composition could be correlated with the measured environmental parameters, we completed a statistical study of their correlations (see Table S3 in the supplemental material). The statistical analysis was performed for each season independently as well as for the average values (January, June, and September). Since no statistically significant differences were found among them (P > 0.05), only the results for the average values are presented in this study. As expected, metals, TI, and conductivity were positively correlated (R range, 0.74 to 0.98) and were negatively correlated with pH (R range, −0.62 to −0.95). Correlation coefficients relating heavy metals among themselves and to the TI were also consistently high (R range, 0.68 to 0.95), always showing positive correlations. In general, none of the individual physical parameters, heavy metals, or TI values demonstrated significant relationships with individual eukaryotic populations, except for Chlamydomonas sp. with oxygen (R, 0.64) and euglenas with iron (R, −0.61). By contrast, a consistently significant and strong relationship (R > 0.70; P < 0.001) among Dunaliella sp., pH, conductivity, and most heavy metals was detected. Also, some eukaryotic groups were positively correlated with each other. The highest correlation values were obtained among euglenas, diatoms, Cyanidium sp., and filamentous algae (R, 0.58 to 0.91).
UPGMA similarity clusterings of the different sampling sites based on physicochemical and biological data are represented in Fig. 3. The analysis based on physicochemical data (Fig. 3a) shows three main separate clusters. One cluster (RI, UMA, ANG, and Iz-Iz) grouped the sampling sites that showed lower pH and higher levels of heavy metals, indicating a strong similarity among them. There was also relative homogeneity among sampling sites located in the origin (AG, 3.2, and FE) and among most of those located in the lower part of the river (EST, CEM, BRR, LPC, and NU). Similarly, clustering analysis from eukaryotic communities based upon the presence or absence of all 18 observed eukaryotic species as well as their abundances also demonstrated a strong similarity among the most extreme sampling sites (Fig. 3b, UMA, Iz-Iz, and RI). However, in this case the clustering of the sampling sites was slightly different: 3.2-BRR-FE-EST grouped together while CEM-NUR-AG demonstrated, based on biological data, a higher similarity among them. LPC and ANG grouped independently.
FIG. 3.
UPGMA similarity cluster analysis of the different sampling locations analyzed in the Tinto River based on physicochemical water parameters (a) and biological data from epiphytic eukaryotic communities (b).
A PCA of the whole data set was performed in order to elucidate the main relationships between biological and physicochemical variables. Figure 4 shows the distribution of the variables in the space formed by the first two components of the analysis, which explained 60.16% of the variance. Four principal components (PC) with an eigenvalue of >1 were extracted. PC1 explained 40.95% of the observed variance and included the variables pH, conductivity, Fe, Co, As, Cd, Cr, and TI as well as Dunaliella sp. number (R > 0.65). PC2 explained 19.24% of the observed variance and contained Cu, Ni, and Chorella sp., diatoms, euglenoids, filamentous algae, Cyanidium sp., and total biomass numbers. PC3 (Ta, oxygen, Zn, and diversity coefficient) and PC4 (redox and Chlamydomonas number) were of less importance, explaining only 9.9 and 7.1% of the variance. PC1 can be described as an abiotic factor, as it contained most of the physicochemical parameters, while PC2 had a strong biotic component, including most of the species studied.
FIG. 4.
Two-dimensional plot of the PCA performed for the whole data set, including physicochemical and biological data. Ta, temperature; Cond, conductivity; D, diversity index; Ti, toxicity index; Cya, Cyanidium; Diato, diatoms; Filam, filamentous algae; Hetero, heterotrophic flagellates; Eugl, Euglena; Duna, Dunaliella; Chlo, Chlorella; Chlamy, Chlamydomonas.
PCA showed a high inverse correlation between pH and most of the heavy metals analyzed as well as Dunaliella sp., while Chlamydomonas sp. presence was directly related to pH. Three heavy metals (Zn, Cu, and Ni) remained separate from the rest and showed a strong inverse correlation with the diversity coefficient, D, and most of the species analyzed except for Chlamydomonas sp., Dunaliella sp., and Chlorella sp. Biomass and the diversity coefficient exhibited a high positive correlation with most of the species as well as a high inverse correlation with the TI.
DISCUSSION
The two main objectives of this work were to report the seasonal changes in the benthonic eukaryotic community of an extreme acidic environment, Río Tinto, as well as to investigate the possible impact of the most important water physicochemical parameters on the eukaryotic community assemblage. Although other studies have been performed on acidic environments, to our knowledge this is the first report in which the water physicochemical parameters of an acidic river have been analyzed regarding the eukaryotic community.
Upper Río Tinto water, from the origin to the UMA sampling station, was found to be the most extreme part of the river. The average pH was ca. 1.66, and water was highly mineralized and characterized by a high conductivity (ca. 40 mS cm−1), which remained constant throughout the year (see Fig. S1 in the supplemental material). In this part of the river, three stations should be pointed out for their extreme physicochemical characteristics, RI, UMA, and ANG. RI showed the lowest pH values (ca. 1.1), followed by UMA (ca. 1.3) and ANG (ca. 1.5). They also presented the highest concentrations of Fe and other heavy metals, such as Co, As, Cd, Cr, and Ni (see Fig. S2 in the supplemental material). On the contrary, our last sampling point, LPC, showed higher pH values (ca. 2.2) and lower concentrations of heavy metals, pointing out the existence of a possible toxicity gradient in the longitudinal direction of the river. This means that, in general, the environmental parameters observed during sampling become less extreme in their values as the mouth of the river is approached.
Comparison of levels of physical characteristics and heavy metal ions in winter, summer, and late summer denoted a clear bimodality in their annual distribution. Generally, pH and oxygen showed higher values in winter, while heavy metals reached their peaks in summer and late summer. This fact is coincident with the bimodality in the annual water availability reported in previous work in the Río Tinto area (12). The climograms of this area showed a clear bimodality in the pluviosity and water availability, consisting of a humid and temperate season alternating with a warm and dry season. The high phreatic level maintains the river flow during the summer, although a high rate of evaporation induces higher amounts of heavy metals due to concentration processes.
This seasonal bimodality greatly influences the eukaryotic community biomass. In winter the eukaryotic biomass was lower than in summer (Fig. 2b). The increase in the eukaryote population in the summer and late summer is mainly due to the occurrence of two species of filamentous green algae (Klebsormidium sp. and Zygnemopsis sp.) as well as to the presence of higher amounts of Euglena mutabilis. In general, green algae are responsible for nearly the total eukaryotic biomass increase during summer, which agrees with other studies in extremely acidic environments (25, 34). This fact is closely related to the significant increase in temperatures and daylight as well as to the decrease in water flow, all of which facilitate cell deposition and biofilm formation.
Regarding diversity, there is a strong indication that sites with lower pH, such as Iz-Iz, RI, or UMA, showed lower species diversity (Fig. 2c). This finding agrees with other studies conducted in acidic lakes in which increases in acidity produce a reduction in species richness (24, 26). In the same manner, data from sampling stations located along the river showed that the highest diversity index appeared at the stations located closer to the mouth of the river, where the physicochemical water conditions were less extreme. Only two exceptions were found, 3.2 and BRR, where the Simpson's coefficient was close to zero and the physicochemical water conditions were not particularly extreme in comparison with others. In both sampling sites Euglena mutabilis, one of the best-adapted organisms to acidic environments, is the main dominant species present in the benthos (3). This low diversity could be due to an abiotic factor, since both sampling stations showed the deepest water column (ca. 6 m in 3.2 and ca. 0.5 m in BRR). Due to the high amount of Fe in the water, light can only reach the first few centimeters of the water column, reducing drastically the amount of sediment capable of being colonized by other algal species in these two places.
Besides these results, some discrepancies have also been found. For example, although Iz-Iz and ANG have similar TI values, they differ in cell number by 2 to 3 orders of magnitude (Fig. 2a and b); in the same manner, some sampling stations showed the same results in relation to TI and diversity index (i.e., RI showed a high TI as well as a high diversity index) (Fig. 2a and c). These results could be due, at least in part, to the cell distribution within the river. In the Río Tinto, the eukaryotic community is mainly distributed in diverse biofilms located all over the riverbed. Microscopic observations revealed a variety of prokaryotic morphotypes, algae, protozoa, or fungi, and the whole community is usually embedded in a mucilaginous coating that most probably changes the inner physicochemical conditions in the biofilms, protecting the microbial community from the external water conditions.
The correlation analysis carried out disclosed the relationship between environmental and biological parameters in Río Tinto (see Table S3 in the supplemental material). From all the species included in the analysis, only Dunaliella sp. showed statistically significant correlations with pH (negative correlation) and with conductivity and most of the heavy metals (positive correlations). This is not surprising, since the genus Dunaliella includes several species adapted to the most extreme environments, including pH ranges between 0 and 3 (27).
The principal component analysis also supports a direct relationship between Dunaliella sp., pH, and heavy metal concentrations (Fig. 4). In this analysis, Dunaliella sp. clustered together with most of the heavy metals, while pH appeared in the opposite quadrant, indicating an inverse relationship between them. It is well documented that pH has a considerable effect on the availability and, as a consequence, the toxicity of heavy metals (22). Acidic environments tend to contain unusually high concentrations of heavy metals because their solubility increases markedly as the pH decreases (22).
The same analysis showed also an inverse relationship between the remaining groups of eukaryotes and the TI. The biomass and the diversity coefficient also gave similar results. Based on the data in the current study, it appears that these eukaryotic communities are more influenced by the presence of heavy metals than by the pH. Furthermore, although the metals included in the TI are the eight most toxic heavy metals known to influence aquatic biota (6), the close correlation among three of them (Zn, Cu, and Ni) and the TI lends support to the conclusion that these metals play a substantial role in controlling the epiphytic eukaryotic community structure in the river. In this regard, the lowest diversity coefficient found in the river corresponded to BRR, the sampling site that showed some of the highest concentrations of Zn and Cu.
Both analyses, correlation and PCA, showed significant associations among species. Thus, Euglena and diatoms or filamentous algae were positively correlated, while Chlamydomona and Dunaliella showed a negative correlation (Fig. 4; see also Table S3 in the supplemental material). The association between species could be of double benefit for the algae, because a microenvironment is created that has low concentrations of heavy metals (23) and an increased carbon dioxide availability for photosynthesis (33). The algae can take up carbon dioxide that is excreted by the heterotrophs, preventing the loss of carbon and other nutrients from the biofilm. Under such conditions, algae provide organic substrates for heterotrophic organisms in the biofilm (17).
UPGMA analysis of similarity among sampling sites based on physicochemical characteristics shows three well-defined areas that support the idea of the existence of a gradient of pH and heavy metals along the river (Fig. 3a). The sampling sites were grouped in three different clusters that comprised three stations located in the origin (AG, 3.2, and FE) and five located in the middle and last part of the river (EST, CEM, BRR, LPC, and NUR), and the last cluster grouped the four most extreme sampling sites located in the origin (RI, UMA, ANG, and Iz-Iz). The analysis with UPGMA cladograms has the advantage of including in one single output all the information on the variables used. This method is more global than variable-to-variable correlations (as shown in Table S3 of the supplemental material) or even PCA, which is usually restricted to the two main components in its representation. It is interesting that, as the degree of detail regarding individual variables is lost as we use more global methods, the classification of sampling sites and their corresponding diversity is still consistent with our expectations. More precise and reliable site classification would benefit from the inclusion of additional environmental parameters as well as from the evaluation of species that, though present in low amounts, may play an important role in sustaining the biodiversity at each point.
Since the comparison between the physicochemical results and the clustering obtained using biological data yielded different results (Fig. 3b), we cannot conclude that similar physicochemical sites showed similar eukaryotic assemblages. This is true for most of the sampling sites except for the four most extreme ones, which grouped together in both analyses, showing high similarities in physicochemical and biological compositions. In this regard, diversity has been linked to a number of ecosystem processes (10). To what extent biotic factors, such as predation or competition, or abiotic factors, such as light, temperature, or habitat size, control diversity in each particular system is still an open question. Our results indicate that, although pH influences the eukaryotic community (Fig. 4), heavy metals also play a key role in diversity and biomass for most of the species analyzed. In the same regard, it cannot be discarded that differences in biotic compositions may be due to the intrinsic dynamics of the populations, in which case they would not be directly reducible to environmental changes (4).
In conclusion, the correlation analysis showed strong positive interactions between pairs of species (e.g., filamentous algae and euglenas) and between species and heavy metals (e.g., Dunaliella sp. and most of the heavy metals analyzed) not previously observed. The clustering study showed high similarities among locations with comparable metal contain in the river in terms of water chemistry and eukaryotic species composition, indirectly supporting the presence of a toxicity gradient from the origin of the river to the estuary. Finally, the PCA revealed interesting results, such as the relevance of three heavy metals (Zn, Cu, and Ni) out of eight analyzed and the toxicity index, as well as deleterious influences of these metals on the diversity and biomass.
In summary, Río Tinto has unique characteristics that distinguish it from other acidic freshwater environments, such as extension, extreme conditions, and highly unexpected eukaryotic diversity. Continued research on these microbial communities and their relationships with environmental conditions is essential to understand their physiology as well as their ecological role under such unique natural extreme conditions.
Supplementary Material
Acknowledgments
Work by A.A. and S.C.M. was supported by the Spanish Ministry of Education and Science through the Program Ramón y Cajal. This work has been supported by grants to the Centro de Astrobiología at the Instituto National de Técnica Aeroespacial and grant CGL2005-05470/BOS.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Amaral, L. A., F. Gómez, E. Zettler, B. G. Keenan, R. Amils, and M. L. Sogin. 2002. Eukaryotic diversity in Spain's river of fire. Nature 417:137. [DOI] [PubMed] [Google Scholar]
- 2.Baffico, G. D., M. M. Díaz, T. Wnzel, M. Koschorreck, M. Schimmele, T. R. Neau, and F. Pedrozo. 2004. Community structure and photosynthetic activity of epilithon from a highly acidic (pH<2) mountain stream in Patagonia, Argentina. Extremophiles 8:403-475. [DOI] [PubMed] [Google Scholar]
- 3.Baker, B. J., M. A., Lutz, S. C. Dawson, P. L. Bond, and J. F. Banfield. 2004. Metabolically active eukaryotic communities in extreme acidic mine drainage. Appl. Environ. Microbiol. 70:6264-6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becks, L., F. M., Hilker, H. Malchow, K. Jürgens, and H. Arndt. 2005. Experimental demonstration of chaos in a microbial food web. Nature 435:1226-1229. [DOI] [PubMed] [Google Scholar]
- 5.Boulter, C. A. 1996. Did both extensional tectonics and magmas act as major driver of convection cell during the formation of the Iberian Pyrite Belt massive sulfide deposits? J. Geol. Soc. Lond. 153:181-184. [Google Scholar]
- 6.Boudou, A., B. Inza., S. Lemaire-Gony, R. Maury-Brachet, M. Odin, and F. Ribeyre. 1998. Metal bioaccumulation in freshwater systems: experimental study of the actions and interactions between abiotic and contamination factors, p. 451-482. In G. Schüürmann and B. Markert (ed.), Ecotoxicology. John Wiley & Sons, New York, N.Y.
- 7.Briones, C., S. C. Manrubia, E. Lázaro, A. Lazcano, and R. Amils. 2005. Reconstructing evolutionary relationships from functional data: a consistent classification of organisms based on translation inhibition response. Mol. Phylog. Evol. 34:371-381. [DOI] [PubMed] [Google Scholar]
- 8.Courtney, L. A., and W. H. Clements. 2002. Assessing the influence of water and substratum quality on benthic macroinvertebrate communities in a metal-polluted stream: an experimental approach. Freshwater Biol. 47:1766-1778. [Google Scholar]
- 9.Derrida, B., S. C. Manrubia, and D. H. Zanette. 2002. On the genealogy of a population of biparental individuals. J. Theor. Biol. 203:303-315. [DOI] [PubMed] [Google Scholar]
- 10.Estrada, M., P. Henrikesen, J. M. Gasol, E. O. Casamayor, and C. Pedrós-Alió. 2004. Diversity of planktonic photoautotrophic microorganisms along a salinity gradient as depicted by microscopy, flow cytometry, pigment analysis and DNA-based methods. FEMS Microbiol. Ecol. 49:281-293. [DOI] [PubMed] [Google Scholar]
- 11.Feris, K. P., P. W. Ramsey, C. Frazar, M. Rilling, J. N. Moore, J. E. Gannon, and W. E. Holben. 2004. Seasonal dynamics of shallow-hyporheic-zone microbial community structure along a heavy metal contamination gradient. Appl. Environ. Microbiol. 70:2323-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernández-Remolar, D. C., N. Rodríguez, F. Gómez, and R. Amils. 2003. Geological record of an acidic environment driven by the iron hydrochemistry: the Rio Tinto system. J. Geophys. Res. 108:5080-5095. [Google Scholar]
- 13.Fernández-Remolar, D. C., J. Gómez-Elvira, F. Gómez, E. Sebastián, J. Martín, J. A. Manfredi, J. Torres, C. González-Kesler, and R. Amils. 2004. The Tinto river, an extreme acidic environment under control of iron, as an analog of the Terra Meridiani hematite site of Mars. Planet. Space Sci. 52:239-248. [Google Scholar]
- 14.Geller, W., H. Klapper, and M. Schultze. 1998. Natural and anthropogenic sulphuric acidification of lakes, p. 3-14. In W. Geller, H. Klapper, and W. Salomon (ed.). Acidic mining lakes: acid mining drainage, limnology and reclamation. Springer, New York, N.Y.
- 15.González-Toril E., E. Llobet-Brossa, E. O. Casamayor, R. Amann, and R. Amils. 2003. Microbial ecology of an extreme acidic environment, the Tinto River. Appl. Environ. Microbiol. 69:4853-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross, W. 2000. Ecophysiology of algae living in highly acidic environments. Hydrobiologia 433:31-37. [Google Scholar]
- 17.Haack, T. K., and G. A. McFeters. 1982. Microbial dynamics of an epilithic community in a high alpine stream. Appl. Environ. Microbiol. 43:702-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutchinson, G. E. 1957. A treatise on limnology, p. 113. Wiley, New York, N.Y.
- 19.Kimura, M. 1983. The neutral theory of molecular evolution, p. 156. Cambridge University Press, Cambridge, England.
- 20.López-Archilla, A. I., and R. Amils. 1999. A comparative ecological study of two acidic rivers in SW Spain. Microb. Ecol. 38:146-156. [DOI] [PubMed] [Google Scholar]
- 21.López-Archilla, A. I., I. Marín, and R. Amils. 2001. Microbial community composition and ecology of an acidic aquatic environment: the Tinto river, Spain. Microb. Ecol. 41:20-35. [DOI] [PubMed] [Google Scholar]
- 22.Mason, A. Z., and K. D. Jenkins. 1995. Metal speciation and biovailability in aquatic ecosystems, p. 236. John Wiley & Sons Ltd., Chichester, England.
- 23.Nakatsu, C., and T. Hutchinson. 1988. Extreme metal and acid tolerance of Euglena mutabilis and an associated yeast from Smoking Hills, NW Territories, and their apparent mutualism. Microb. Ecol. 16:213-231. [DOI] [PubMed] [Google Scholar]
- 24.Niederlehner, B. R., and J. Cairns. 1990. Effects of increasing acidity on aquatic protozoan communities. Water Air Soil Pollut. 52:183-196. [Google Scholar]
- 25.Nixdorf, B., U. Mischke, and D. Lessmann. 1998. Chrysophytes and Chlamydomonads: pioneer colonists in extremely acidic mining lakes (pH<3) in Lusitania (Germany). Hydrobiologia 369/370:315-327.
- 26.Packroff, G. 2000. Protozooplankton in acidic mining lakes with special respect to ciliates. Hydrobiologia 433:157-166. [Google Scholar]
- 27.Pick, U. 1998. Dunaliella acidophila a most extreme acidophilic alga, p. 467-472. In J. Seckbach (ed.), Enigmatic microorganisms and life in extreme environments. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 28.Sabater, S., T. Buchaca, J. Cambra, J. Catalán, H. Guasch, N. Ivorra, I. Muñoz, E. Navarro, M. Real, and A. Romaní. 2003. Structure and function of benthic algal communities in an extremely acid river. J. Phycol. 39:481-489. [Google Scholar]
- 29.Simpson, E. H. 1949. Calculation of diversity coefficients in ecology. Nature 163:688. [Google Scholar]
- 30.Snoeijs, P., and F. Snoeijs. 1993. A simple sampling device for taking quantitative microalgal samples from stone surfaces. Arch. Hydrobiol. 129:121-126. [Google Scholar]
- 31.Sokal, R., and P. Sneath. 1973. Numerical taxonomy. Freeman, San Francisco, Calif.
- 32.Swofford, D. L., and G. J. Olsen. 1990. Phylogenetic reconstruction, p. 411-501. In D. M. Hillis and C. Moritz (ed.), Molecular systematics. Sinauer Associates, Sunderland, Mass.
- 33.Turner, M., M. Jackson, D. Finslay, R. Graham, E. DeBruyn, and E. Vandermeer. 1987. Early responses of periphyton to experimental lake acidification. Can. J. Fish. Aquat. Sci. 44:135-149. [Google Scholar]
- 34.Walton, K. C., and D. B. Johnson. 1992. Microbiological and chemical characteristics of an acidic stream draining a disused copper mine. Environ. Pollut. 76:169-175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






