Abstract
Oligonucleotide primers were designed for the 18S rRNA genes of members of the Neocallimastigales and used in a nested PCR protocol to amplify 787-bp fragments of DNA from landfill site samples. The specificities of the primers were confirmed by phylogenetic analysis of the environmental clone sequences, and this method can therefore now be used to investigate the ecology of the obligately anaerobic fungi. To our knowledge, this is the first demonstration of the occurrence of members of the Neocallimastigales outside the mammalian gut, and their distribution across the landfill samples examined here suggests that they are actively involved in cellulose degradation.
The discovery of obligate anaerobiosis in the fungi in 1975 with the isolation and cultivation of Neocallimastix frontalis from a sheep (9) provided rumen microbiologists with another microbial group to add to the bacteria and protozoa upon which herbivore nutrition is absolutely dependent. In fact, the anaerobic gut fungi have attracted more widespread interest as the most active cellulose degraders in the biological world (13), although their isolation, cultivation, and maintenance are not straightforward. This may partly explain the complete absence of information on their occurrence outside the herbivore gut, where they colonize and degrade plant fiber. Anaerobic fungi are chytridiomycetes classified in the order Neocallimastigales and, having permanently lost a mitochondrial genome while retaining the hydrogenosome that is derived from the mitochondrion (3), serve as useful markers in eukaryote evolution. Relatively few representatives are available for comparison, however, and obtaining strains from anaerobic ecosystems other than the rumen is an obvious priority. There is a high degree of conservation in 18S rRNA gene sequences across the Neocallimastigales, for which morphological criteria have been used as the principal means of classifying the six genera and their species that constitute the order. There has, however, been more recent progress on the use of internally transcribed spacer region sequences as a reliable means of identifying anaerobic fungi to the genus level (2, 10).
There is no shortage of environments other than the herbivore gut where cellulose is degraded under anaerobic conditions, and landfill sites in particular could contain active populations. The aim of the study reported here was to design oligonucleotide PCR primers that could specifically amplify the 18S rRNA gene of members of the Neocallimastigales and to use the data to demonstrate their occurrence in those landfill sites in which cellulose degradation is proceeding under anaerobic conditions. Apart from the difficulties of isolating and cultivating anaerobic fungi, the paucity of information on their occurrence outside the rumen could also be due to competition with large populations of cellulolytic anaerobic bacteria, such as those that predominate in landfill sites (11). In the rumen, anaerobic fungi are heavily outnumbered by bacteria (7) but have a high specific activity against cellulose and therefore make a very significant contribution to its degradation (1). This could also apply to landfill sites, and detection sensitivity can be improved by employing a nested PCR protocol analogous to that used for monitoring populations of sulfate-reducing bacteria in landfill sites (5).
Two sites were used as the source of samples for DNA extraction. The Drigg site is the United Kingdom's principal facility for the disposal of solid low-level radioactive waste. Historically, untreated waste was tumble tipped into a series of trenches cut into the local geostrata, with a natural low permeability layer forming the base (6). The waste came in a variety of forms, including rubble, spoil, redundant equipment, scrap, and process waste, and typically contained significant amounts of metallic and cellulosic materials. A series of open “gas vents” have been driven into the waste and consist of a hollow tube with a perforated sidewall to facilitate gas migration and allow sampling of leachate and monitoring of water levels. Samples of a sediment/leachate mix were recovered from the bottoms of 71 gas vent pipes from across the site. The second site (Brogborough) comprised test cells initiated by the United Kingdom Department of Energy to demonstrate several easily applied techniques for accelerating landfill gas production. Each 20-m-deep cell accommodated 15,000 tons of waste, at a density of 1 metric ton m−3 (4). Prior to final closure of the site, a leachate sample (1 liter) was obtained from each of the six test cells. The leachate samples were then concentrated by centrifugation (14,000 × g for 30 min) before subsequent DNA extraction from the pelleted material. Bovine rumen fluid was used as a control sample known to contain anaerobic fungi. Total genomic DNA was isolated from all samples by use of a Bio101 FastDNA spin kit for soil (Anachem, United Kingdom). In some cases, the extracted DNA was not sufficiently pure for further applications and required processing via the Wizard DNA cleanup system (Promega).
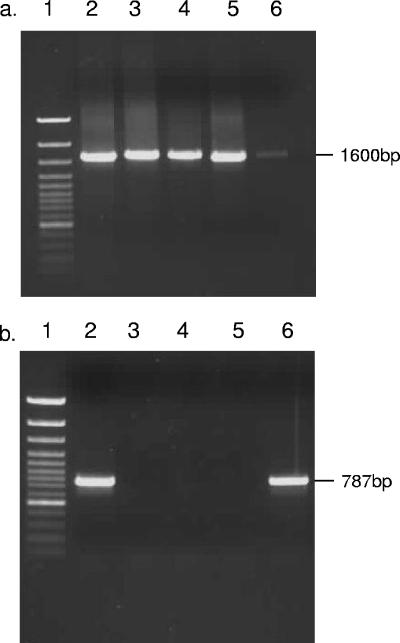
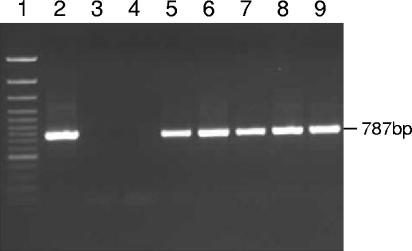
The initial PCR (annealing temperature, 50°C) was carried out using primer NS1-eukaryotic (5′-CCAGTAGTCATATGCTTGTC-3′) (12) and reverse primer Univ-1390-universal (5′-GACGGGCGGTGTGTACAA-3′) (14). The second round of amplification was carried out using an internal primer set (annealing temperature, 60°C) consisting of Neocal-forward (5′-GCACTTCATTGTGTGTACTG-3′) and Neocal-reverse (5′-GGATGAAACTCGTTGACTTC-3′), designed in this study to be specific for members of the Neocallimastigales. Specificity was established using the “search for short, nearly exact matches” function at the National Centre for Biotechnology Information (NCBI) website. Amplification products of the expected size (787 bp) were obtained from DNA extracts of Neocallimastix frontalis and rumen fluid but not from DNA extracts of three nontarget fungal species (Fig. 1). Neocallimastigales DNA was detected in 34 of the 71 samples retrieved from the gas vents at Drigg, by application of this nested PCR procedure (see Fig. 2). Drigg is an unusual landfill site in that biological activity is very low due to the absence of putrescible waste, and the limited amounts of carbon dioxide and methane detectable (6) almost certainly have their origin in cellulose degradation. This, together with the absence of any inputs that could contain anaerobic gut fungi, suggests that the data in Fig. 2 show the presence of an indigenous population of fungi that belong to the Neocallimastigales. This link between the detection of Neocallimastigales DNA and the presence of an active population of cellulose-degrading fungi receives further support from the analysis of Brogborough test cell samples. Only one of the six test cell DNA extracts could be amplified with the Neocallimastigales specific PCR primers (data not shown), and that test cell had been used to study the effect of excessive loading of cellulose, in the form of paper and cardboard.
FIG. 1.
PCR amplification products of the 18S rRNA gene obtained after amplification using different primer sets. (a) Primers NS1-Euk and Univ-1390, designed to target the eukaryotic18S rRNA gene. These products were subsequently diluted and used as a template in the following amplification reaction. (b) Primers Neocal-forward and Neocal-reverse, designed to target the Neocallimastigales 18S rRNA gene. Lane 1, GeneRuler 100-bp DNA Ladder Plus (MBI Fermentas); lane 2, Neocallimastix frontalis; lane 3, Rhizopus stolonifera; lane 4, Saccharomyces cerevisiae; lane 5, Cunninghamella elegans; lane 6, bovine rumen fluid.
FIG. 2.
18S rRNA PCR amplification products obtained from landfill sites. Lane 1, GeneRuler 100-bp DNA Ladder Plus (MBI Fermentas); lane 2, Neocallimastix frontalis; lane 3, Rhizopus stolonifera; lane 4, dH2O; lanes 5 to 9, samples taken from five of the Drigg gas vent pipes.
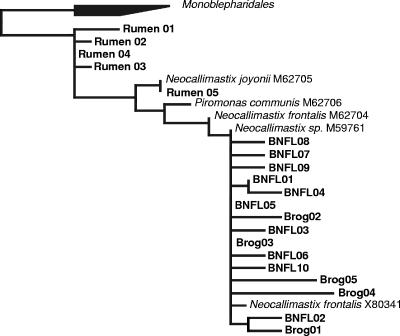
The amplified DNA was purified with a QIAquick PCR purification kit (QIAGEN) according to the manufacturer's instructions. The DNA was then ligated into the pGEM-T vector system and transformed into competent Escherichia coli (JM109) cells, before plasmid isolation using a QIAprep spin miniprep kit (QIAGEN). The nucleotide sequences of the clones were then determined. Alignments of 18S rRNA gene sequences were constructed using ARB (8) and the 2003 16S and 18S rRNA gene database (http://www.arb-home.de/). A maximum likelihood tree was constructed using AxML in ARB over 608 bp of sequence corresponding to nucleotides 651 to 1259 of the E. coli 16S ARB reference sequence, and all of the 18S rRNA gene sequences cloned from the PCR amplification products clustered with members of the Neocallimastigales (Fig. 3). The high degree of conservation of the 18S rRNA gene sequences in this phylogenetic group does not permit genus assignment, and for that, a typing system based on the internally transcribed spacer regions of anaerobic fungal rRNA genes (10) would need to be applied to cultured representatives or incorporated into a direct-PCR-based system that can be applied to environmental DNA extracts. The sequence analysis (Fig. 3) confirms that the primers designed in this study are specific for Neocallimastigales and thus provide a tool to identify environmental samples that can be targeted for the isolation of new strains that will provide material for both evolutionary and biotechnological studies. It is our contention that these “gut” fungi will be found to occur, and contribute to cellulose degradation, in a number of environments, such as sediments, anaerobic compartments of freshwater lakes, and waterlogged soils.
FIG. 3.
A maximum likelihood tree showing the relatedness of environmentally derived clones (GenBank accession numbers DQ449579 to DQ449598) and database sequences of cultivated members of the Neocallimastigales. Clones BNFL 01 to BNFL 10 are from the Drigg landfill site, clones Brog 01 to Brog 05 are from the Brogborough landfill test cell, and clones Rumen 01 to Rumen 05 are from bovine rumen. The tree is rooted with another order of the same phylum, the Monoblepharidales.
Acknowledgments
Funding for this research was provided by the Engineering and Physical Sciences Research Council United Kingdom (EPSRC), the Natural Environment Research Council United Kingdom (NERC), and British Nuclear Fuels Ltd. (BNFL).
REFERENCES
- 1.Akin, D. E., and L. L. Rigsby. 1987. Mixed fungal populations and lignocellulose tissue degradation in the bovine rumen. Appl. Environ. Microbiol. 53:1987-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brookman, J. L., G. Mennim, A. P. J. Trinci, M. K. Theodorou, and D. S. Tuckwell. 2000. Identification and characterization of anaerobic gut fungi using molecular methodologies based on ribosomal ITS1 and 18S rRNA. Microbiology 146:393-403. [DOI] [PubMed] [Google Scholar]
- 3.Bullerwell, C. E., and B. F. Lang. 2005. Fungal evolution: the case of the vanishing mitochondrion. Curr. Opin. Microbiol. 8:362-369. [DOI] [PubMed] [Google Scholar]
- 4.Caine, M., D. Campbell, and A. Van Santen. 1999. The landfill gas timeline: the Brogborough test cells. Waste Manag. Res. 17:430-442. [Google Scholar]
- 5.Daly, K., R. J. Sharp, and A. J. McCarthy. 2000. Development of oligonucleotide probes and PCR primers for detecting phylogenetic subgroups of sulfate-reducing bacteria. Microbiology 146:1693-1705. [DOI] [PubMed] [Google Scholar]
- 6.Humphreys, P., R. McGarry, A. Hoffmann, and P. Binks. 1997. DRINK: a biogeochemical source term model for low level radioactive waste disposal sites. FEMS Microbiol. Rev. 20:557-571. [DOI] [PubMed] [Google Scholar]
- 7.Kemp, P., D. J. Lander, and C. G. Orpin. 1984. The lipids of the rumen fungus Piromonas communis. J. Gen. Microbiol. 130:27-37. [DOI] [PubMed] [Google Scholar]
- 8.Kumar. Y., R. Westram, S. Behrens, B. Fuchs, F. O. Glöckner, R. Amann, H. Meier, and W. Ludwig. 2005. Graphical representation of ribosomal RNA probe accessibility data using ARB software package. BMC Bioinformatics 6:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orpin, C. G. 1975. Studies on the rumen flagellate Neocallimastix frontalis. J. Gen. Microbiol. 91:249-262. [DOI] [PubMed] [Google Scholar]
- 10.Tuckwell, D. S., M. J. Nicholson, C. S. McSweeney, M. K. Theodorou, and J. L. Brookman. 2005. The rapid assignment of ruminal fungi to presumptive genera using ITS1 and ITS2 RNA secondary structures to produce group-specific fingerprints. Microbiology 151:1557-1567. [DOI] [PubMed] [Google Scholar]
- 11.Van Dyke, M. I., and A. J. McCarthy. 2002. Molecular biological detection and characterization of Clostridium populations in municipal landfill sites. Appl. Environ. Microbiol. 68:2049-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White, T. J., T. Burns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols. Academic Press, San Diego, Calif.
- 13.Wood, T. M., and C. A. Wilson. 1995. Studies on the capacity of the cellulase of the anaerobic fungus Piromonas communis P to degrade hydrogen bond-ordered cellulose. Appl. Microbiol. Biotechnol. 43:572-578. [DOI] [PubMed] [Google Scholar]
- 14.Zheng, D. D., E. W. Alm, D. A. Stahl, and L. Raskin. 1996. Characterization of universal small-subunit rRNA hybridization probes for quantitative molecular microbial ecology studies. Appl. Environ. Microbiol. 62:4504-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]