Abstract
The ecology of virioplankton in tropical aquatic ecosystems is poorly documented, and in particular, there are no references concerning African continental waters in the literature. In this study, we examined virus-bacterium interactions in the pelagic and benthic zones of seven contrasting shallow inland waters in Senegal, including one hypersaline lake. SYBR Gold-stained samples revealed that in the surface layers of the sites, the numbers of viruses were in the same range as the numbers of viruses reported previously for productive temperate systems. Despite high bacterial production rates, the percentages of visibly infected cells (as determined by transmission electron microscopy) were similar to the lowest percentages (range, 0.3 to 1.1%; mean, 0.5%) found previously at pelagic freshwater or marine sites, presumably because of the local environmental and climatic conditions. Since the percentages of lysogenic bacteria were consistently less than 8% for pelagic and benthic samples, lysogeny did not appear to be a dominant strategy for virus propagation at these sites. In the benthic samples, viruses were highly concentrated, but paradoxically, no bacteria were visibly infected. This suggests that sediment provides good conditions for virus preservation but ironically is an unfavorable environment for proliferation. In addition, given the comparable size distributions of viruses in the water and sediment samples, our results support the paradigm that aquatic viruses are ubiquitous and may have moved between the two compartments of the shallow systems examined. Overall, this study provides additional information about the relevance of viruses in tropical areas and indicates that the intensity of virus-bacterium interactions in benthic habitats may lower than the intensity in the adjacent bodies of water.
The interest of plankto-ecologists in viruses has not faded since virus particles were shown to be at least as numerous as prokaryotes in fresh and marine waters (5, 49). To date, more than 100 sites have been investigated for the presence of viruses, and studies conducted worldwide have come to the same conclusion: with concentrations in most cases exceeding 107 particles per ml, viruses are actually the most abundant biological entities in aquatic systems (25, 53, 57, 69, 72).
Apart from being ubiquitous and numerous, viruses also play fundamental roles in structuring the microbial food webs, primarily by killing microbes (25), by governing microbial diversity and diversification (70), and, to a lesser extent, by being a potential food source for protists (10, 27). Natural phages are now presumed to be the largest reservoir of uncharacterized genetic diversity on Earth (31, 57), and theories regarding their role in shaping the diversity of prokaryotes have been proposed (61, 71). The term “viriosphere” has even been coined, illustrating the stake that an increasing number of scientists have in virus ecology (58).
Given the responsiveness of planktonic viruses and microbes to environmental conditions and more specifically to solar radiation and temperature (41, 48), investigating their relevance at all latitudes is fundamental. Geographically, most of the aquatic viruses that have been ecologically examined so far have been located in temperate regions. Studies of the occurrence of viruses in polar zones are rare (29, 39), and to our knowledge, tropical waters have been investigated only once previously, by Peduzzi and Schiemer (46).
During the past two decades, biophysicochemical data provided by multidisciplinary studies have consistently revealed that limnology in temperate zones is somewhat different from limnology in tropical systems (2, 59). For instance, because of the typically high bacterial growth rates mostly resulting from the temperatures in tropical regions, trophic pathways within microbial food webs are not readily homologous in the two zones (13, 62). The same conclusion is expected to be reached regarding virus-mediated processes. However, in the only study conducted with tropical waters (two freshwater reservoirs in Sri Lanka), the levels of viral abundance and activity preliminarily appeared to be in the same range as the levels reported for temperate regions (46). Thus, there is a severe lack of data concerning virioplankton “performance” at tropical latitudes, including the African continent, whose waters have not been studied previously.
Over the last few decades, West African inland waters have typically been diverted for irrigation and cattle ranching to promote development of the local human population. Thousands of reservoirs of different sizes have been built in most sub-Saharan countries, predominantly for agricultural and domestic purposes. The presence of dense populations and the by-products of these agriculture-oriented sites commonly result in rapid eutrophication of waters that are often identified as highly productive (12).
The objective of the present work was to examine virus-bacterium interactions at additional tropical sites where virus infectivity and decay may be affected by the large amount of local solar irradiation and by the typically elevated temperatures. The levels and activities of viruses and their bacterial hosts were determined for the surface waters and sediments of inland aquatic systems of Senegal, which is the westernmost country in continental Africa. Six contrasting freshwater sites located in the basin of the Senegal River covering a wide, representative spectrum of productivity in African aquatic environments, as well as one hypersaline lake, were sampled in November 2004. Below we discuss the effect of viruses on bacterial mortality in shallow freshwaters and speculate about the origin of viruses in the superficial sediment layers.
MATERIALS AND METHODS
Study sites and environmental context.
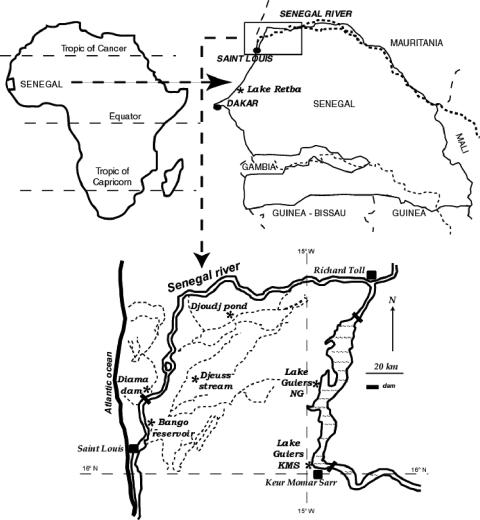
Samples were collected on 3, 4, and 5 November 2004, at the end of the flood season. Samples were obtained from seven contrasting aquatic systems in a ∼20,000-km2 area delimited in the south by the Cape Vert Peninsula and in the north by the Senegal River (Fig. 1). The seven sites were selected to cover a wide range of productivity and salinity in Senegal continental aquatic systems. The sites were designated as follows. Lake Retba (surface area, ∼400 ha) is located near Dakar (30 km), and it has shallow lagoon-like features and a maximum depth of 3 m and is close (400 m) to the Atlantic Ocean. Lake Retba is a hypersaline lake with a maximum salt concentration of 463 g liter−1. Djoudj is a pond located in the National Ornithological Park of Senegal. This site is a habitat for thousands of migratory birds from October to April and thus receives considerable amounts of bird excretion that makes it highly productive. Djeuss Stream is a narrow defluent of the Senegal River that occasionally provides water to Bango Reservoir. Diama Dam is located on the Senegal River, 27 km upstream of Saint Louis City. This dam was built in 1985 to prevent saltwater input into the drinking water reservoirs fed by the Senegal River. Bango Reservoir is located 30 km from St. Louis City and is the main drinking water reservoir for this city. Finally, Lake Guiers is a large shallow lake that is 50 km long; the average width is 7 km, the surface area is 240 km2, and the volume is approximately 390 × 106 m3. This lake receives water from the Senegal River and is a drinking water reservoir for the city of Dakar. Samples were obtained from two different sites in this lake; N′Gnith (NG) is located in the central zone near the pumping station, and Keur Momar Sarr (KMS) is located in the southern part, near the gate that regulates the water flow and level of the lake. Nutrient and chlorophyll concentrations at all sites would be considered eutrophic to hypereutrophic according to Carlson's criteria (16), compared to lakes in temperate regions.
FIG. 1.
Map of Senegal (West Africa) and locations of the seven sampling sites.
Sampling.
Triplicate samples were collected from the sediments and subsurface waters at the seven sampling sites. The water samples used for nutrient and chlorophyll analysis, as well as for determining bacterial and viral parameters, were obtained with acid-cleaned sterile bottles at locations 0.5 m below the surface. The samples used for measurement of dissolved inorganic nutrients (NO3-N, NH4-N, PO4-P) were filtered through Whatman GF/F fiberglass filters, stored at −20°C, and analyzed as described by Strickland and Parsons (56). Chlorophyll a concentrations were determined fluorometrically following filtration of samples onto Whatman GF/F fiberglass filters, storage in liquid nitrogen, and methanol extraction (72). Oxygen concentrations were determined in situ using a YSI probe with temperature correction. For bacterial and viral parameters, samples were fixed with prefiltered (0.02 μm) buffered formaldehyde (final concentration, 2%), stored at 4°C in the dark, and immediately analyzed when they arrived at the laboratory.
Sediment cores were taken using a corer (Uwitec no. 016001) equipped with polyvinyl chloride tubes having an inside diameter of 60 mm. Cores were processed immediately after collection, using three subsamples taken with 5-ml sterile syringes. The top centimeter of the core layer was carefully extracted and used for bacterial, viral, and chlorophyll analyses. Approximately 100 g (wet weight) of sediment was collected for determination of sediment structure and composition (42).
Enumeration of viruses and microorganisms.
Amounts of planktonic bacteria were determined by standard techniques using the fluorochrome 4′,6′-diamidino-2-phenylindole (DAPI) and epifluorescence microscopy (EM) (47). The numbers of virus-like particles (VLPs) in triplicate 50- to 200-μl samples were determined after retention of the particles on 0.02-μm-pore-size membranes (Anodisc) and staining with SYBR Gold (17). On each slide, 300 to 600 bacteria and VLPs were counted in 15 to 20 fields.
Concurrently, viruses were counted and their sizes were determined using transmission electron microscopy (TEM) (6). The viruses in 5-ml aliquots of formalin-fixed samples were harvested by ultracentrifugation onto grids (400-mesh Cu electron microscope grids with carbon-coated Formvar film) by using a Centrikon TST 41.14 swing-out rotor at 120,000 × g for 2 h. The grids were then stained for 30 s with uranyl acetate (2%, wt/wt), and viruses were counted and measured with a JEOL model 1200EX TEM operated at 80 kV and a magnification of ×40,000. The following three classes of virus capsid size were examined to characterize viral populations: <60 nm, 60 to 95 nm, >95 nm.
Benthic viruses and bacteria were extracted as recommended by Danovaro et al. (19) and were analyzed using the procedures described above. Briefly, aliquots of the fixed sediment samples (1 ml) were diluted with tetrasodium pyrophosphate (4 ml; final concentration, 10 mM) and incubated for 20 min at 4°C. Samples were then sonicated three times (100 W for 1 min) and diluted 200- to 1,000-fold with filtered (0.02 μm) formaldehyde (final concentration, 2%). This procedure has been shown to extract the majority of VLPs and bacteria from sediment (26), except silty sediment, in which both VLPs and bacteria are thought to be less efficiently extractable (26, 34).
Bacterial production.
For both sediments and overlaying waters, heterotrophic bacterial production (BP) was determined by the [methyl-3H]thymidine incorporation method (38). For water samples, duplicates and one control (zero time) were incubated with [methyl-3H]thymidine (47 Ci mmol−1; Amersham) in the dark at the in situ temperature. The incubation time was 15 min, and the final thymidine concentration was 20 nM (saturation conditions); we assumed that isotope dilution could be prevented at this concentration (50). Radioactivity was counted using the liquid scintillation procedure. Bacterial production was calculated from the radioactivity incorporated into a trichloroacetic acid precipitate, using a conversion factor of 2 × 1018 cells produced per mol of incorporated thymidine (4).
For the sediments, two replicates and one control were incubated with labeled thymidine used under saturation conditions at a final concentration of 1,000 nM (30). Subsamples (0.5 g) of wet sediment were incubated in 10-ml centrifugation tubes at the in situ temperature. Incubation was stopped with formaldehyde after 30 min. Samples were then centrifuged (8,500 × g) for 20 min, the supernatants were discarded, and the pellets were washed three times with 5 ml of 80% ethanol. Finally, the pellets were washed twice with 5 ml of ice-cold trichloroacetic acid (5%), filtered onto 0.2-μm-pore-size membrane filters, transferred to vials with 3 ml of 2 N NaOH, and heated for 2 h in a water bath at 100°C. After cooling, 1 ml of each supernatant was transferred to a scintillation vial, and scintillation cocktail was added. Bacterial production was estimated using the same conversion factor that was used for surface waters. This conversion factor has been used by several authors for lake sediment samples (30, 38).
Viral infection of prokaryotes.
Benthic bacteria were detached from the sediment by treatment with sodium pyrophosphate and sonication, as described above. The bacteria in duplicate 8-ml aliquots of formalin-fixed samples were harvested by ultracentrifugation at 70,000 × g for 20 min onto 400-mesh Cu grids, stained for 30 s with uranyl acetate (2%, wt/wt), and examined at a magnification of ×40,000 by using a TEM operated at 80 kV to distinguish between virus-infected and uninfected bacteria (66). The extraction procedure for benthic bacteria resulted in reliable observations by TEM since individual bacterial cells were clearly identifiable based on morphology and refringence. At least 600 bacterial cells were inspected per grid, and the number of infected bacteria encountered ranged from 15 to 20. To estimate virus-induced bacterial mortality (VIBM), the frequency of infected cells (FIC) was calculated from the frequency of visibly infected cells (FVIC) (expressed as a percentage) using the formula (67): FIC = 7.11 × FVIC. FIC was then converted to VIBM as described by Binder (11): VIBM = (FIC + 0.6FIC2)/(1 − 1.2FIC).
Viral production was estimated by multiplying the lysed bacterial production (BP × VIBM) by the burst size (BS) (i.e., the mean number of viruses in the infected cells which were filled with phages) (9, 66).
Fraction of lysogenic bacteria.
We used the approaches of Weinbauer et al. (68) and Mei and Danovaro (43) to initiate prophage induction in benthic and pelagic bacteria, respectively. Mitomycin C (final concentration, 1 μg ml−1; catalog no. M-0503; Sigma Chemical Co.) was added to duplicate 10-ml water or sediment samples (1 g of sediment with 9 ml of prefiltered [0.02 μm] overlaying water). Untreated samples served as controls. Both types of samples were formalin fixed after they were incubated for 12 h (43, 68). Prophage induction was calculated by determining the difference between the viral abundance in mitomycin C-treated incubation mixtures (Vm) and the viral abundance in control incubation mixtures (Vc). The fraction of lysogenic bacteria cells (FLC) (expressed as a percentage) was calculated as follows: FLC = 100[(Vm − Vc)/(BS × BACt0)], where BS is the burst size (number of virus particles bacterium−1) and BACto is the bacterial abundance at the start of the experiment (i.e., before the addition of mitomycin C) (68).
Statistical analyses.
Data were log transformed to satisfy the requirements of normality and homogeneity of variance necessary for parametric analyses. Differences in the means were tested using a t test. Simple relationships between original data sets were tested by Pearson correlation analysis. All statistical analyses were performed using the SigmaStat 2.0 software.
RESULTS
Environmental conditions.
With temperatures between 22 and 29°C and maximum depths of 5 m, the seven aquatic sites sampled were typical of shallow tropical systems (Table 1). The depths of light penetration which were determined using a Secchi disk were low, ranging from 19 cm in Lake Retba to 90 cm in the southern part of Lake Guiers (KMS). In the water column, the levels of dissolved oxygen ranged from 1.4 to 7.4 mg liter−1, and the lowest values were obtained for hypersaline Lake Retba. Among the freshwater sites, the most nutrient-rich site was Djoudj Stream, where the levels of dissolved inorganic nitrogen (nitrate, nitrite, and ammonium), as well as the chlorophyll concentrations, were highest. This was also true for orthophosphate when Lake Retba, in which the PO4 concentration was 3 to 72 times higher than the PO4 concentrations at the other sites, was excluded. At this single hypersaline site, the shallow water column (50 cm) favored high temperatures (28.9°C) (Table 1). Overall, no significant relationships between benthic and pelagic chlorophyll concentrations were found (P > 0.05; n = 7).
TABLE 1.
Geographical coordinates and physicochemical parameters of the seven inland aquatic systems in November 2004
| Site | Latitude north | Longitude west | Maximum depth (m) | Temp (°C)a | Secchi depth (cm) | Oxygen (mg liter−1) | PO4− (μM) | NO3− + NO2− (μM) | NH4+ (μM) | Chlorophyll
|
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pelagic (μg liter−1) | Benthic (μg cm−3) | ||||||||||
| Lake Retba | 14°49′30" | 17°14′30" | 0.5 | 28.9 (4.2) | 19 | 2.1 | 7.2 | 1.4 | 1.0 | 8.7 | 1.9 |
| Djoudj Pond | 16°24′95" | 16°18′18" | 3.1 | 22.0 (0.1) | 25 | 4.8 | 2.3 | 12.8 | 20.9 | 49.5 | 1.9 |
| Djeuss Stream | 16°06′10" | 16°23′25" | 1.6 | 23.6 (0.1) | 56 | 4.0 | 0.6 | 0.4 | 1.0 | 10.1 | 1.0 |
| Diama Dam | 16°12′51" | 16°24′59" | 4.1 | 23.9 (0.1) | 27 | 5.8 | 0.5 | 7.4 | 1.0 | 2.7 | 2.7 |
| Bango Reservoir | 16°04′03" | 16°27′04" | 3.4 | 25.2 (0.4) | 62 | 6.8 | 0.1 | 0.2 | 0.8 | 15.0 | 1.4 |
| Lake Guiers (NG) | 16°11′90" | 15°54420" | 3.0 | 23.5 (0.3) | 81 | 7.4 | 0.1 | 0.1 | 0.1 | 36.2 | 1.0 |
| Lake Guiers (KMS) | 15°56′15" | 15°56′56" | 1.2 | 23.4 (0.1) | 90 | 6.0 | 0.1 | 0.3 | 1.1 | 25.4 | 1.0 |
The values in parentheses are coefficients of variation for values measured every 10 cm in the water column.
Sediment characteristics.
The sites investigated were relatively comparable and were characterized by sediments consisting mostly of sand (fine and coarse), which accounted for 30 to 98% of the cores. An exception was Bango Reservoir, where the sediments were dominated by clay (41%) and, to a lesser extent, silt (29%) (Table 2).
TABLE 2.
Composition of sediments at the seven study sites
| Site | Sediment composition (%)
|
|||
|---|---|---|---|---|
| Clay (<2 μm) | Silt (2-50 μm) | Fine sand (50-200 μm) | Coarse sand (0.2-2 mm) | |
| Lake Retba | 7.8 | 1.8 | 41.0 | 49.4 |
| Djoudj Pond | 12.3 | 8.1 | 40.8 | 38.8 |
| Djeuss Stream | 21.8 | 27.8 | 33.7 | 16.7 |
| Diama Dam | 0.9 | 1.4 | 57.2 | 40.5 |
| Bango Reservoir | 41.2 | 28.8 | 13.8 | 16.2 |
| Lake Guiers (NG) | 2.5 | 3.9 | 66.9 | 26.7 |
| Lake Guiers (KMS) | 1.8 | 1.4 | 24.7 | 72.1 |
Size classes of viruses.
Viruses smaller than 60 nm were clearly dominant at all sites studied (Table 3). They accounted for 68% and 59% of the total community in the overlaying waters and in the sediments, respectively. Viruses larger than 95 nm were relatively underrepresented in both the pelagic and benthic compartments (means, 3% and 6%, respectively). Regardless of the site, the fractions of <60-nm viruses were comparable for the sediment (mean, 59.3%; coefficient of variation [CV], 10.5%) and the water column (mean, 68.1%; CV, 9.6%). The same was true for the middle size class (60 to 95 nm), which on average accounted for 32.3% (CV, 9.4%) of the total abundance. Conversely, the contribution of large viruses (>90 nm) was distinctly greater in the benthic environment (mean, 6.4%; CV, 66.9%) than in the pelagic environment (mean, 3.1%; CV, 117.9%).
TABLE 3.
Distribution of three virus size classes in the surface waters and sediments at the seven study sites in November 2004
| Site | % of virus size classes
|
|||||
|---|---|---|---|---|---|---|
| Water
|
Sediment
|
|||||
| <60 nm | 60-95 nm | >95 nm | <60 nm | 60-95 nm | >95 nm | |
| Lake Retba | 73.3 | 21.9 | 4.8 | 55.7 | 36.8 | 7.5 |
| Djoudj Pond | 71.5 | 27.8 | 0.7 | NDa | ND | ND |
| Djeuss Stream | 64.3 | 32.8 | 2.9 | 64.8 | 33.2 | 2 |
| Diama Dam | 75.4 | 24.5 | 0.1 | 54.1 | 31.7 | 4.2 |
| Bango Reservoir | 68.4 | 30.8 | 0.8 | 53.2 | 32.5 | 14.3 |
| Lake Guiers (NG) | 54.2 | 34.6 | 11.2 | 68.5 | 27.3 | 4.2 |
| Lake Guiers (KMS) | 69.5 | 29.4 | 1.1 | ND | ND | ND |
ND, not determined.
Standing stocks.
In the overlaying waters, enumeration of viruses by EM and TEM did not reveal significant differences (P = 0.62, as determined by a t test). The epifluorescence microscopy estimates of pelagic viral concentrations ranged from 1.1 × 107 VLPs ml−1 (Djeuss Stream) to 72.0 × 107 VLPs ml−1 (Lake Retba) (Table 4). Viruses were 3 to 14 times more abundant than bacteria. The highest densities were found at Lake Retba, where the concentrations of viruses and bacteria were more than 1 order of magnitude greater than the concentrations at the other sites. Viral abundance was highly correlated with bacterial abundance and dissolved phosphate concentrations (Table 5).
TABLE 4.
Mean viral and microbial parameters for the pelagic and benthic zones of the seven study sites in November 2004
| Site | Virus concn (107 viruses ml−1)
|
Bacterial concn (106 cells ml−1) | VBR | BP (108 cells liter−1 h−1) | FVIC (%) | VIBM (%) | Burst size | Virus production (105 viruses ml−1 h−1) | FLC (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| EM | TEM | |||||||||
| Pelagic | ||||||||||
| Lake Retba | 72.0 (12.3)a | 32.0 | 134.0 (17.5) | 5.4 | 5.0 (8.1) | 0.5 (4.8) | 3.5 (5.1) | 60.2 (24.0) | 11.1 | 0.6 (16.7) |
| Djoudj Pond | 3.9 (8.6) | 2.5 | 5.3 (7.9) | 7.4 | 13.7 (6.8) | 0.4 (28.5) | 3.1 (29.9) | 30.0 (27.3) | 12.7 | NDb |
| Djeuss Stream | 1.1 (15.3) | 2.6 | 2.7 (14.4) | 4.1 | 4.8 (8.3) | 0.3 (20.6) | 2.5 (21.5) | 34.0 (23.5) | 4.1 | 7.1 (13.2) |
| Diama Dam | 1.5 (16.0) | 2.8 | 1.1 (4.3) | 13.6 | 0.8 (6.2) | 0.3 (0.9) | 2.5 (0.9) | 25.0 (22.4) | 0.5 | 0.3 (26.4) |
| Bango Reservoir | 1.9 (2.7) | 1.0 | 2.9 (9.0) | 6.6 | 1.7 (1.5) | 0.5 (27.3) | 4.0 (29.0) | 10.3 (20.4) | 0.7 | 2.5 (3.8) |
| Lake Guiers (NG) | 2.7 (9.3) | 1.5 | 7.7 (1.5) | 3.5 | 1.4 (16.7) | 1.1 (3.8) | 9.1 (4.4) | 23.8 (40.8) | 3.0 | 0.1 (14.8) |
| Lake Guiers (KMS) | 3.8 (7.6) | 2.5 | 8.5 (6.0) | 4.5 | 2.3 (32.5) | 0.6 (8.8) | 4.7 (9.5) | 36.8 (29.4) | 4.0 | 0.2 (11.4) |
| Benthic | ||||||||||
| Lake Retba | 56.2 (9.2) | 15.3 | 61.7 (9.6) | 9.1 | 0.4 (8.0) | <0.1 | NCc | NC | NC | 4.2 (12.2) |
| Djoudj Pond | 55.9 (20.7) | ND | 219.3 (12.1) | 2.5 | 0.7 (5.4) | <0.1 | NC | NC | NC | 1.0 (9.3) |
| Djeuss Stream | 15.5 (8.3) | 8.5 | 117.6 (17.8) | 1.3 | 201.1 (66.3) | <0.1 | NC | NC | NC | 1.9 (4.1) |
| Diama Dam | 74 (6.9) | 11.4 | 117 (2.5) | 6.3 | 257.8 (15.2) | <0.1 | NC | NC | NC | 0.8 (5.0) |
| Bango Reservoir | 21.2 (8.4) | 8.8 | 209.0 (2.5) | 1.0 | 35.6 (1.3) | <0.1 | NC | NC | NC | 0.9 (14.8) |
| Lake Guiers (NG) | 33.7 (5.2) | 10.2 | 406.3 (4.6) | 0.8 | 454.3 (6.3) | <0.1 | NC | NC | NC | 0.3 (3.4) |
| Lake Guiers (KMS) | 20.7 (8.7) | ND | 326.1 (12.9) | 0.6 | 72.8 (0.5) | <0.1 | NC | NC | NC | 0.7 (1.4) |
The values in parentheses are coefficients of variation (expressed as percentages).
ND, not determined.
NC, could not be calculated.
TABLE 5.
Correlations of basic parameters estimated for the surface water of the seven study sitesa
| Parameter | Correlationb
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [virus]-TEM | [bacteria] | [HNF] | BS | VP | <60-nm viruses | [oxygen] | [PO4−] | [NO2− + NO3−] | [NH4+] | |
| [virus]-EM | 0.99*** | 0.99*** | 0.99*** | 0.83** | 0.68* | −0.77** | 0.96*** | |||
| [bacteria] | 0.99*** | 0.84** | 0.68* | −0.76* | 0.95*** | |||||
| [HNF] | 0.82* | 0.68* | −0.77* | 0.96*** | ||||||
| BP | 0.71* | 0.93** | ||||||||
| FVIC | −0.78* | |||||||||
| BS | −0.85** | 0.829* | ||||||||
| VP | −0.74* | 0.824* | ||||||||
In the sediment, only EM counts of viruses were correlated with benthic chlorophyll concentrations (r = 0.92; P < 0.001). [virus]-EM, virus concentration determined by EM; [bacteria], concentration of bacteria; [HNF], concentration of heterotrophic nanoflagellates; VP, viral lytic production; [virus]-TEM, virus concentration determined by TEM; [oxygen], oxygen concentration; [PO4−], PO4− concentration; [NO2− + NO3−], concentration of NO2− plus NO3−; [NH4+], NH4+ concentration.
Significant correlations are indicated as follows: three asterisks, P < 0.001; two asterisks, P < 0.05; one asterisk, P < 0.05.
Epifluorescence microscopy counts revealed that benthic viruses were 5 to 50 times more abundant than planktonic viruses. The average benthic-to-pelagic ratios as calculated by EM and TEM were 15.3 and 4.7, respectively. The most viriobenthos-rich site (Diama Dam; mean, 7.4 × 108 VLPs ml−1) was different from the site at which the greatest densities in the water column were found (Lake Retba) (Table 4). The virus concentrations in the sediment were highly correlated with benthic chlorophyll contents (r = 0.92; P < 0.01).
The mean virus-to-bacterium ratio (VBR) was significantly higher in the water column (mean, 6.5; CV, 49.8%) than in the sediment (mean, 3.1; CV, 99.3%). Benthic bacteria were more numerous than viruses at both sites in Lake Guiers. The pattern of bacterial abundance in the overlaying waters was radically different from the pattern in the sediments. For example, Lake Retba was the site at which the concentration of planktonic bacteria was highest and, paradoxically, the site at which the concentration of benthic bacteria was lowest. Although the viral and bacterial concentrations were highly correlated with each other in the water column, this was not the case for the sediment (Table 5).
BP.
The mean value for BP was 35-fold higher for the sediments (146.1 × 108 cells liter−1 h−1) than for the overlaying waters (4.2 × 108 cells liter−1 h−1). However, benthic BP varied greatly among sites. The values were almost negligible in the sediments from Djoudj Pond and Lake Retba, but paradoxically, the water columns of these two bodies of water exhibited the highest pelagic BP (Table 4). Generally, pelagic BP was significantly and positively correlated with nitrogen content (NO2−, NO3−, and NH4+) (Table 5).
Lytic infection.
In the water column, the FVIC ranged from 0.3 to 1.1% (mean, = 0.5%). The maximum values were obtained for the two stations in Lake Guiers (mean for NG, 1.1% [CV, 3.8%]; mean for KMS 0.6% [CV, 8.8%]) (Table 4). The derived bacterial mortality rates due to lytic viruses were always less than 10% of the BP. Noticeably, the level of small viruses (<60 nm) was negatively correlated with the FVIC in the water column (Table 5).
In the various sediment samples, none of the 5,840 bacteria that were inspected were found to be infected. Therefore, we could not calculate VIBM, viral production, and BS for benthic habitats (Table 4).
The burst size in the pelagic samples varied substantially, ranging from 10.3 (Bango) to 60.2 (Retba) with an average of 31.4 (CV, 45.1%) (Table 3). Burst size was positively and significantly correlated with viral, bacterial, and phosphorus concentrations (Table 5).
The pelagic viral production ranged from 0.7 to 12.7 × 105 viruses ml−1 h−1 (mean, 5.2 × 105 viruses ml−1 h−1; CV, 87.0%). The highest values were obtained for Djouj Pond, while the lowest values were obtained for Diama Dam along with negligible bacterial production (Table 4).
Lysogenic infection.
In the water column, the FLC was between 0.1% (Lake Guiers NG) and 7.1% (Djeuss Stream) (Table 4). On average, 1.8% (CV, 139.3%) of pelagic bacteria were estimated to be lysogenic. FLC did not correlate with any of the parameters examined.
Since no infected bacteria were detected in the sediment, we estimated the fraction of lysogenic benthic bacteria by using a BS of 38, which was determined by a TEM examination of sediment samples from Lake Hallwil by Filippini et al. (21). To our knowledge, this value is the sole previously published value for TEM-measured BS for benthic bacteria and is relatively close to the mean pelagic BS (31.4) calculated in this study. Overall, with only 1.4% of the bacteria being lysogenic (range, 0.3 to 4.2%), virulent viruses distinctly seemed to prevail in the viriobenthos.
DISCUSSION
To our knowledge, this study was the first study dealing with aquatic viral ecology in Africa. We examined the viral compartment in benthic and pelagic environments of seven contrasting continental sites located in Senegal (West Africa) for evidence of differences and similarities in virus-bacterium interactions, compared to the interactions widely described for temperate regions. Overall, despite the characteristically elevated microbial activities observed in tropical freshwaters, we hypothesize that viral control of bacterial populations in these waters may not be as relevant as the control that has been found in temperate systems.
Viruses in surface waters. (i) Abundance and lytic activity.
Both EM and TEM counts of pelagic viruses (range, 1.1 × 107 to 72.0 × 107 VLPs ml−1) are in the classical range (107 to 108 VLPs ml−1) for temperate productive systems, such as estuaries or eutrophic lakes (53, 69). At all study sites, the water temperature was higher than 25°C. In tropical regions, such temperatures strongly favor bacterial activity (13), and this was also the case in this study, in which the heterotrophic bacterial production was about 1 order of magnitude higher than the production commonly found in temperate regions. One may therefore expect higher levels of viruses in tropical inland waters than in temperate inland waters. Apart from the hypersaline Lake Retba (see below), the absence of particularly high densities of planktonic viruses was associated with relatively low VBRs (3.5 to 13.7; mean, 6.5), which were similar to the lowest values recorded for temperate lakes (36). Samples were collected from the surface layers, where virus decay attributable to virucidal solar radiation is thought to occur at high rates (35, 41). Although the VBR is not a strong indicator of virus-host interactions, we suspect that the low values obtained for six of the seven study sites may indicate that the level of viral attack is low as a result of a low probability of contact between the viral pathogens and their bacterial hosts.
In this single-time study, the FVIC at the various sites were indeed extremely low (range, 0.3 to 1.1%; mean, 0.5%) and were among the lowest FVIC recorded for both marine and freshwater systems (69). As samples were collected from the surface layer at the sites (depth, ∼50 cm), the virucidal properties of solar radiation, especially UV wavelengths, were thus presumed to significantly affect the viral stocks and infectivity, which may help explain why not only the VBR but also the FVIC were so low. In the only previous report on virioplankton in tropical freshwaters (Sri Lanka) (46), the significance of viruses appeared to be somewhat different. Although the viral densities (3.1 × 107 to 7.7 × 107 VLPs ml−1) were comparable to those obtained in this study (except the value for Lake Retba), the FVIC (range, 1.6 to 4.4%; mean, 3.0%; n = 14) were clearly higher (sixfold higher than the values estimated in the present study). Our water samples were collected at a depth of 50 cm or less, while Peduzzi and Schiemer (46) collected their samples at depths between 1 and 25 m. At such depths, viruses may be protected from solar radiation more than they were in our surface water samples.
At all of the sites studied, the VIBM, as empirically derived from the FVIC, was not as high as 10% of the bacterial production. Such low levels of viral lysis are rare, as values in temperate lake ecosystems predominantly fluctuate between 10 and 50% (7, 8, 9, 52, 64, 66). Nevertheless, one has to keep in mind that the latent period (the time from infection to the appearance of mature phages) depends greatly on bacterial growth. In this case, because of high bacterial production, the VIBM may be of greater importance if this aspect is included in the calculation. One important issue is to examine whether the apparent poor contribution that viruses make to the control of bacterioplankton is associated with strong protistan or metazoan predation pressure.
(ii) Significance of lysogeny.
The low infection rates determined in this study may also be explained by high proportions of lysogenic bacteria, but this did not appear to be the case. A maximum of 7.1% lysogens was found at the Djeuss Stream site, while the values were less than 2.5% at the five other freshwater sites. In the only study of lysogeny conducted with freshwater (Lake Superior, United States), the workers reported similar values (0.1 to 7.4%) (60). Lysogeny is thought to be a strategy for virus propagation in systems with poor growth conditions for the bacterial hosts that would be unfavorable for their replication (25, 69). In tropical waters, temperature and nutrient availability are generally optimal for sustaining elevated growth rates of bacterioplankton. Lysogeny may therefore be circumstantial in tropical systems, although this hypothesis needs to be explored further.
(iii) Size of viruses.
The majority (68%) of pelagic viruses in this study were less than 60 nm in diameter. In temperate freshwater, viral head sizes are also usually in the size range from 30 to 60 nm (69). Similar results were reported for estuarine and marine waters at different latitudes, including the French Atlantic coast (3), the North Atlantic Ocean (5), Southern California (18), the Adriatic Sea (65), Norwegian fjords (5), and the Alboran Sea (1), indicating that there is relative homogeneity in viral capsid size on a global scale. Interestingly, we found a negative correlation between FVIC and the percentage of small dominant viruses (<60 nm). Thus, when the possibility of infection is low, small viruses, for undetermined reasons, may be more competitive than large viruses for infection of bacterial hosts.
(iv) Hypersaline Lake Retba.
To our knowledge, with an average salt concentration of 480 g per liter of water, Lake Retba is certainly one of the most saline environments that have been investigated from a microbiological perspective to date (55). The level of viruses (mean, 7.2 × 108 VLPs ml−1) is also among the highest levels recorded for natural aquatic systems (72). However, such concentrations are not unusual in hypersaline environments; for example, the values typically range from 0.1 × 108 to 10 × 108 VLPs ml−1 in the Dead Sea (45), in Mono Lake (15), in solar salterns (28), and in a deep hypersaline anoxic basin of the Mediterranean Sea (20). The results of previous studies seem to indicate that such extreme environments may be reservoirs for long-term preservation of planktonic viruses, although the reasons for this remain unclear. In Lake Retba, as well as in the two other hypersaline environments for which the viral impact on bacterial production has been estimated (15, 28), the high virus-host contact rates (as a consequence of high viral and bacterial densities) were paradoxically coupled with modest viral infection rates. Brum et al. (15) hypothesized that significant subpopulations of prokaryotes were very likely resistant to the cooccurring viruses. In Lake Retba, as in other hypersaline environments, oxygen availability is typically low because of poor solubility. However, low levels of oxygen in water generally provide advantageous conditions for enhanced lytic activity in freshwater systems (9, 66). Inexplicably, in Lake Retba, none of the large square halophilic bacteria that dominate the bacterioplankton assemblage showed signs of infection, whereas only unidentified cocci had intracellular phages (as determined by TEM analyses).
Excluding Lake Retba, the mean BS for the six other freshwater sites was 26.7. This value is very close to the value (mean, 24) that Wommack and Colwell (72) calculated from an inventory of BS from the literature in temperate zones. In Lake Retba, the BS was noticeably larger (average 60.2). Large burst sizes have also been reported for the hypersaline Mono Lake (mean, 100) by Brum et al. (15). Since the proportion of small planktonic viruses (<60 nm) was not higher in Lake Retba than in the other continental sites, the bacterial biovolume, which is typically larger in hypersaline environments (55), may contain a larger number of viruses.
Viruses in the sediments: virus-bacterium interactions.
The current view that viruses are more abundant (up to 3 orders of magnitude) in aquatic sediments than in the overlaying water columns (22, 33, 40, 43) was confirmed at our study sites. Mei and Danovaro (43) recently calculated using previously published data that the average ratio of benthic viral abundance to pelagic viral abundance was 20 in both marine and freshwater systems. In our study, the counts determined by EM resulted in a ratio of 15.3. However, the origin of high abundance of viriobenthos is still uncertain. On several occasions, it has been suggested that the mechanical import of viruses from overlaying waters due to (i) sinking of free viral particles and (ii) settling of viruses adsorbed to suspended material does not contribute significantly to benthic viral production (23, 43, 44). However, surprisingly, none of the nearly 6,000 bacteria inspected by TEM was infected by phages, regardless of the site. If benthic bacteria are not perceptibly susceptible to viral infection, then the high concentrations of viruses in the sediment may be due to sedimentation, accumulation, and persistence of viruses that originated from the open-water environment. Such a virtual absence of phage-infected cells in the benthos was also reported based on TEM analyses of three benthic microhabitats in a shallow wetland system (21). Filippini et al. found only a single infected bacterium in 4,269 cells from sediment samples that were inspected. They also reported negligible infection rates (FVIC, <0.1%) in two other nonpelagic microhabitats (plant biofilm and litter) despite high levels of free viruses. In another freshwater system, Lake Kühwörter, an oxbow lake, viruses were also shown to have little effect on bacterioplankton production in the sediment (22). Conversely, in marine environments, benthic viruses have often been reported to be as efficient bacterial killers as planktonic viruses (26, 34, 43). In these studies, viral production and activity were determined mainly by dilution approaches, which are known to dramatically stimulate benthic heterotrophic activity and de facto viral activity (32). Concurrently, the Würgler bag incubation technique used by Glud and Middelboe (26) can be used only for anoxic sediment and therefore may not be used for measurement of viral production in all benthic habitats. Among the different methods used to quantify virus-mediated processes, TEM observations of whole cells provide direct evidence of phage infection. In all sediment samples, bacteria were clearly identifiable on the basis of staining quality, cell transparency, and membrane refringence. In addition, given that phages could be detected within pelagic cells, it is reasonable to believe that the absence of visibly infected prokaryotes in the sediment may not be due to methodological bias. Nevertheless, there are uncertainties since the sonication procedure used to extract bacteria from sediment may result in disruption of cell membranes and may be more effective with weakened infected cells.
Even though it is recognized that bacteria are very active in sediments (30, 38), the reasons why they may be weakly susceptible to viral infection are unclear. Viral proliferation in sediments may be limited by physical or biochemical conditions that prevent high encounter rates (21, 23). Viruses adsorbed on sediment particles may also not be infectious for bacterioplankton (34), and attachment to sediment particles may also alter bacterial physiology and to an unknown extent may render cells more resistant to viral attack (22). In addition, a prevalence of temperate benthic viruses as opposed to virulent benthic viruses not only would elucidate the absence of intracellular mature phages but also would protect the host from a novel attack (21). However, this was not the case in this study since less than 5% of benthic bacteria were shown to be lysogenic. Similar negligible fractions of lysogenic bacteria (<3.5%) were reported previously for benthic habitats of the Mediterranean Sea (43). Alternatively, the highly diverse prokaryote communities in the sediment (63) may also not be the best vectors for virus propagation (21).
If the benthic environment does not provide favorable conditions for viral proliferation, then the consistently high concentrations of viriobenthos implies that viruses may be produced in the water column. Although this hypothesis is controversial and has no clear support, interestingly, the distributions of two of the three virus size classes (<60 nm and 60 to 95 nm) were similar in the sediments and the overlaying waters at all sites studied, suggesting that the viruses may have had the same origin. Nevertheless, no evidence supports this hypothesis given that many types of viruses are now known to be ubiquitous and capable of moving between different environments (14, 51, 57). Thus, in this scenario, a fraction of free or adsorbed planktonic viruses may be transported through water fluxes to the bottom, where the sediment functions as a filter. The typical shallow depth of these environments (<5 m) may facilitate the movement of virioplankton between the two compartments. Then, entrapped and concentrated in the matrix and the biofilm of the sediment, as suggested by Flood and Ashbolt (24), viruses may encounter conditions suitable for preservation by resisting decay, which is primarily caused by UV radiation and protease activities (20).
Finally, our preliminary analysis of virioplankton ecology in shallow inland waters of West Africa revealed the consistently low impact of viruses on prokaryote mortality in this region. The reasons that some bacterioplankton may be less susceptible to phage infection at these latitudes need to be elucidated. Are tropical viruses poorly adapted to the high levels of UV irradiance? Conversely, is the development of resistance to viral attack at such a large scale realistic? Overall, our results indicate the need to increase sampling efforts for a wide range of latitudes as viral processes may be very variable.
Acknowledgments
This work was part of a study dedicated to the Senegal River Estuary and was supported by the IRD Research Unit “CYROCO” (cyanobacterial occurrence in tropical regions).
We thank Maïmouna MBoup and Daniel Corbin for their technical assistance. We also appreciate the helpful comments of R. Arfi, D. W. Coats, and E. Verling.
REFERENCES
- 1.Alonso, M., F. Jimenez-Gomez, J. Rodriguez, and J. Borrego. 2001. Distribution of virus-like particles in an oligotrophic marine environment (Alborean Sea, western Mediterranean). Microb. Ecol. 42:407-415. [DOI] [PubMed] [Google Scholar]
- 2.Arfi, R., M. Bouvy, P. Cecchi, D. Corbin, and M. Pagano. 2003. Environmental conditions and phytoplankton assemblages in two shallow reservoirs of Ivory Coast (West Africa). Arch. Hydrobiol. 156:511-534. [Google Scholar]
- 3.Auguet, J.-C., H. Montanié, and P. Lebaron. 2006. Structure of virioplankton in the Charente Estuary (France): transmission electron microscopy versus pulsed field gel electrophoresis. Microb. Ecol. 51:197-208. [DOI] [PubMed] [Google Scholar]
- 4.Bell, R. T. 1993. Estimating production of heterotrophic bacterioplankton via incorporation of tritiated thymidine, p. 495-503. In P. F. Kemp, B. F. Sherr, E. B. Sherr, and J. J. Cole (ed.), Handbook of methods in aquatic microbial ecology. Lewis Publishers, Boca Raton, Fla.
- 5.Bergh, Ø., K. Y. Børsheim, G. Bratbak, and M. Heldal. 1989. High abundance of viruses found in aquatic environments. Nature 340:467-468. [DOI] [PubMed] [Google Scholar]
- 6.Bettarel, Y., T. Sime-Ngando, C. Amblard, and H. Laveran. 2000. A comparison of methods for counting viruses in aquatic systems. Appl. Environ. Microbiol. 66:2283-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bettarel, Y., J. R. Dolan, K. Hornak, R. Lemee, M. Masin, M.-L. Pedrotti, E. Rochell-Newell, K. Simek, and T. Sime-Ngando. 2002. Strong, weak, and missing links in a microbial community of the N.W. Mediterranean Sea. FEMS Microbiol. Ecol. 42:451-462. [DOI] [PubMed] [Google Scholar]
- 8.Bettarel, Y., C. Amblard, T. Sime-Ngando, J.-F. Carrias, D. Sargos, F. Garabetian, and P. Lavandier. 2003. Viral lysis versus flagellate grazing as factors regulating bacterial abundance in a oligomesotrophic lake: a short-term study. Microb. Ecol. 45:119-127. [DOI] [PubMed] [Google Scholar]
- 9.Bettarel, Y., T. Sime-Ngando, C. Amblard, and J. Dolan. 2004. Viral activity in two contrasting lake ecosystems. Appl. Environ. Microbiol. 70:2941-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettarel, Y., T. Sime-Ngando, M. Bouvy, R. Arfi, and C. Amblard. 2005. Low consumption of virus-sized particles by heterotrophic nanoflagellates in two lakes of the French Massif Central. Aquat. Microb. Ecol. 39:205-209. [Google Scholar]
- 11.Binder, B. 1999. Reconsidering the relationship between virally induced bacterial mortality and frequency of infected cells. Aquat. Microb. Ecol. 18:207-215. [Google Scholar]
- 12.Bouvy, M., R. Arfi, P. Cecchi, D. Corbin, M. Pagano, L. Saint-Jean, and S. Thomas. 1998. Trophic coupling between bacterial and phytoplanktonic compartments in shallow tropical reservoirs (Ivory Coast, West Africa). Aquat. Microb. Ecol. 15:25-37. [Google Scholar]
- 13.Bouvy, M., M. Troussellier, P. Got, and R. Arfi. 2004. Bacterioplankton responses to bottom-up and top-down controls in a West African reservoir (Selingue, Mali). Aquat. Microb. Ecol. 34:301-307. [Google Scholar]
- 14.Breitbart, M., and F. Rohwer. 2005. Here a virus, there a virus, everywhere the same virus? Trends Microbiol. 13:278-284. [DOI] [PubMed] [Google Scholar]
- 15.Brum, J. R., G. F. Steward, S. C. Jiang, and R. Jellison. 2005. Spatial and temporal variability of prokaryotes, viruses, and viral infections of prokaryotes in an alkaline, hypersaline lake. Aquat. Microb. Ecol. 41:261-270. [Google Scholar]
- 16.Carlson, R. E. 1977. A trophic state index for lakes. Limnol. Oceanogr. 22:361-369. [Google Scholar]
- 17.Chen, F., J.-R. Lu, B. Binder, B., Y.-C. Liu, and R. E. Hodson. 2001. Application of digital image analysis and flow cytometry to enumerate marine viruses stained with SYBR Gold. Appl. Environ. Microbiol. 67:539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cochlan, W. P., J. Wikner, G. F. Steward, D. C. Smith, and F. Azam. 1993. Spatial distribution of viruses, bacteria and chlorophyll a in neritic, oceanic and estuarine environments. Mar. Ecol. Prog. Ser. 92:77-87. [Google Scholar]
- 19.Danovaro, R., A. Dell'anno, A. Trucco, M. Serresi, and S. Vanucci. 2001. Determination of virus abundance in marine sediments. Appl. Environ. Microbiol. 67:1384-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danovaro, R., C. Corinaldesi, A., Dell'Anno, M., Fabiano, and C. Corselli. 2005. Viruses, prokaryotes and DNA in the sediments of a deep-hypersaline anoxic basin (DHAB) of the Mediterranean Sea. Environ. Microbiol. 7:586-592. [DOI] [PubMed] [Google Scholar]
- 21.Filippini, M., N. Buesing, Y. Bettarel, T. Sime-Ngando, and M. Gessner. 2006. Infection paradox: high abundance but low impact of freshwater benthic viruses. Appl. Environ. Microbiol. 72:4893-4898. [DOI] [PMC free article] [PubMed]
- 22.Fischer, U. R., C. Wieltschnig, A. K. T. Kirschner, and B. Velimirov. 2003. Does virus-induced lysis contribute significantly to bacterial mortality in the oxygenated sediment layer of shallow oxbow lakes? Appl. Environ. Microbiol. 69:5281-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer, U. R., W. Weisz, C. Wieltschnig, A. K. T. Kirschner, and B. Velimirov. 2004. Benthic and pelagic viral decay experiments: a model-based analysis and its applicability. Appl. Environ. Microbiol. 70:6706-6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flood, J. A., and N. J. Ashbolt. 1999. Virus-sized particles can be entrapped and concentrated one hundred fold within wetland biofilms. Adv. Environ. Res. 3:1225-1228. [Google Scholar]
- 25.Fuhrman, J. A. 1999. Marine viruses and their biogeochemical and ecological effects. Nature 399:541-548. [DOI] [PubMed] [Google Scholar]
- 26.Glud, R. N., and M. Middelboe. 2004. Virus and bacteria dynamics of a coastal sediment: implication for benthic carbon cycling. Limnol. Oceanogr. 49:2073-2081. [Google Scholar]
- 27.Gonzàlez, J. M., and C. A. Suttle. 1993. Grazing by marine nanoflagellates on viruses and virus-sized particles: ingestion and digestion. Mar. Ecol. Prog. Ser. 94:1-10. [Google Scholar]
- 28.Guixa-Boixareu, N., J. I. Calderon-Paz, M. Heldal, G. Bratbak, and C. Pedros-Alio. 1996. Viral lysis and bacterivory as prokaryotic loss factors along a salinity gradient. Aquat. Microb. Ecol. 11:215-227. [Google Scholar]
- 29.Guixa-Boixareu, N., D. Vaqué, J. M. Gasol, J. Sanchez-Camara, and C. Pedros-Alio. 2002. Viral distribution and activity in Antarctic waters. Deep-Sea Res. II 49:827-845. [Google Scholar]
- 30.Haglund, A.-L., P. Lantz, E. Törnblom, and L. Tranvik. 2003. Depth distribution of active bacteria and bacterial activity in lake sediment. FEMS Microbiol. Ecol. 46:31-38. [DOI] [PubMed] [Google Scholar]
- 31.Hambly, E., and C. A. Suttle. 2005. The viriosphere, diversity, and genetic exchange within phage communities. Curr. Opin. Microbiol. 8:444-450. [DOI] [PubMed] [Google Scholar]
- 32.Hansen, J. W., B. Thampdrup, and B. B. Jorgensen. 2000. Anoxic incubation of sediment in gas-tight plastic bags: a method for biogeochemical process studies. Mar. Ecol. Prog. Ser. 208:273-282. [Google Scholar]
- 33.Hewson, I., J. M. O'Neil, J. A. Fuhrman, and W. C. Dennison. 2001. Virus-like particle distribution and abundance in sediments and overlaying waters along eutrophication gradients in two subtropical estuaries. Limnol. Oceanogr. 46:1734-1746. [Google Scholar]
- 34.Hewson, I., and J. A. Fuhrman. 2003. Viriobenthos production and virioplankton sorptive scavenging by suspended sediment particles in coastal and pelagic water. Microb. Ecol. 46:337-347. [DOI] [PubMed] [Google Scholar]
- 35.Hofer, J. S., and R. Sommaruga. 2001. Seasonal dynamics of viruses in an alpine lake: importance of filamentous forms. Aquat. Microb. Ecol. 26:1-11. [Google Scholar]
- 36.Jacquet, S., I. Domaizon, S. Personnic, A. S. Pradeep Ram, M. Heldal, S. Duhamel, S., and T. Sime-Ngando. 2005. Estimates of protozoan- and viral-mediated mortality of bacterioplankton in Lake Bourget (France). Freshw. Biol. 50:627-645. [Google Scholar]
- 37.Reference deleted.
- 38.Kirschner, A. K. T., and B. Velimirov. 1999. Benthic bacterial secondary production measured via simultaneous 3H-thymidine and 14C-leucine incorporation, and its implication for the carbon cycle of a shallow macrophyte-dominated backwater system. Limnol. Oceanogr. 44:1871-1881. [Google Scholar]
- 39.Madan, N. J., W. A. Marshall, and J. Laybourn-Parry. 2005. Virus and microbial loop dynamics over an annual cycle in three contrasting Antarctic lakes. Freshw. Biol. 50:1291-1300. [Google Scholar]
- 40.Maranger, R., and D. F. Bird. 1996. High concentrations of viruses in the sediments of Lac Gilbert, Québec. Microb. Ecol. 31:141-151. [DOI] [PubMed] [Google Scholar]
- 41.Maranger, R., P. A. Del Gorgio, and D. F. Bird. 2002. Accumulation of damaged bacteria and viruses in lake exposed to solar radiation. Aquat. Microb. Ecol. 28:213-227. [Google Scholar]
- 42.Mathieu, C., and F. Pieltain. 2003. Analyse chimique des sols. Tec/Doc, Cachan, France.
- 43.Mei, M. L., and R. Danovaro. 2004. Virus production and life strategies in aquatic sediments. Limnol. Oceanogr. 49:459-470. [Google Scholar]
- 44.Middelboe, M., R. N. Glud, and K. Finster. 2003. Distribution of viruses and bacteria in relation to diagenetic activity in an estuarine sediment. Limnol. Oceanogr. 48:1447-1456. [Google Scholar]
- 45.Oren, A., G. Bratbak, and M. Heldal. 1997. Occurrence of virus-like particles in the Dead Sea. Extremophiles 1:143-149. [DOI] [PubMed] [Google Scholar]
- 46.Peduzzi, P., and F. Schiemer. 2004. Bacteria and viruses in the water column of tropical freshwater reservoirs. Environ. Microbiol. 6:707-715. [DOI] [PubMed] [Google Scholar]
- 47.Porter, K. G., and Y. S. Feig. 1980. The use of DAPI for identification and enumeration of bacteria and and blue-green algae. Limnol. Oceanogr. 25:943-9480. [Google Scholar]
- 48.Pradeep Ram, A. S., D. Boucher, T. Sime-Ngando, D. Debroas, and J.-C. Romagoux. 2005. Phage bacteriolysis, prostistan bacterivory potential, and bacterial production in a freshwater reservoir: coupling with temperature. Microb. Ecol. 50:64-72. [DOI] [PubMed] [Google Scholar]
- 49.Proctor, L. M., and J. A. Fuhrman. 1990. Viral mortality of marine bacteria and cyanobacteria. Nature 343:60-62. [Google Scholar]
- 50.Robarts, R. D., and T. Zohary. 1993. Fact or fiction—bacterial growth rates and production as determined by (methyl-3H)-thymidine? Adv. Microb. Ecol. 13:371-425. [Google Scholar]
- 51.Sano, E., S. Carlson, L. Wegley, and F. Rohwer. 2004. Movement of viruses between biomes. Appl. Environ. Microbiol. 70:5842-5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Šimek, K., J. Pernthaler, M. G. Weinbauer, K. Horňák, J. R. Dolan, J. Nedoma, M. Mašín, and R. Amann. 2001. Changes in bacterial community composition and dynamics and viral mortality rates associated with enhanced flagellate grazing in a mesoeutrophic reservoir. Appl. Environ. Microbiol. 67:2723-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reference deleted.
- 54.Sime-Ngando, T., and A. S. Pradeep Ram. 2005. Grazers effects on prokaryotes and viruses in a freshwater microcosm experiment. Aquat. Microb. Ecol. 41:115-124. [Google Scholar]
- 55.Sorokin, D. Y., and J. G. Kuenen. 2005. Haloalkaliphilic sulphur-oxidizing bacteria in soda lakes. FEMS Microbiol. Rev. 29:685-702. [DOI] [PubMed] [Google Scholar]
- 56.Strickland, J. D. H., and T. R. Parson. 1968. A practical handbook of seawater analysis. Fisheries Research Board of Canada bulletin. Fisheries Research Board of Canada, Ottawa, Ontario, Canada.
- 57.Suttle, C. A. 2005. Viruses in the sea. Nature 437:356-361. [DOI] [PubMed] [Google Scholar]
- 58.Suttle, C. A. 2005. The viriosphere: the greatest biological diversity on Earth and driver of global processes. Environ. Microbiol. 7:481-482. [DOI] [PubMed] [Google Scholar]
- 59.Talling, J. F., and J. Lemoalle. 1998. Ecological Dynamics of tropical inland waters. Cambridge University Press, Cambridge, United Kingdom.
- 60.Tapper, M. A., and R. E. Hicks. 1998. Temperate viruses and lysogeny in Lake Superior bacterioplankton. Limnol. Oceanogr. 43:95-103. [Google Scholar]
- 61.Thingstad, T. F., and R. Lignell. 1997. Theoretical models for the control of bacterial growth rate, abundance, diversity and carbon demand. Aquat. Microb. Ecol. 13:19-27. [Google Scholar]
- 62.Torréton, J.-P., J. Pagès, and V. Talbot. 2002. Bacterioplankton and phytoplankton biomass and production in Tuamotu atoll lagoons. Aquat. Microb. Ecol. 28:267-277. [Google Scholar]
- 63.Torsvik, V., L. Øvreas, and T. F. Thingstad. 2002. Prokaryotic diversity-magnitude, dynamics, and controlling factors. Science 296:1064-1066. [DOI] [PubMed] [Google Scholar]
- 64.Vrede, K., U. Stensdotter, and E. S. Lindstrom. 2003. Viral and bacterioplankton dynamics in two lakes with different humic contents. Microb. Ecol. 46:406-415. [DOI] [PubMed] [Google Scholar]
- 65.Weinbauer, M. G., and P. Peduzzi. 1994. Frequency, size and distribution of bacteriophages in different marine bacterial morphotypes. Mar. Ecol. Prog. Ser. 108:11-20. [Google Scholar]
- 66.Weinbauer, M. G., and M. G. Höfle. 1998. Significance of virus lysis and flagellate grazing as factors controlling bacterioplankton production in a eutrophic lake. Appl. Environ. Microbiol. 64:431-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weinbauer, M. G., C. Winter, and M. G. Höfle. 2002. Reconsidering transmission electron microscopy based estimates of viral infection of bacterio-plankton using conversion factors derived from natural communities. Aquat. Microb. Ecol. 27:103-110. [Google Scholar]
- 68.Weinbauer, M. G., I. Brettar, and M. G. Höfle. 2003. Lysogeny and virus-induced mortality of bacterioplankton in surface, deep, and anoxic marine waters. Limnol. Oceanogr. 48:1457-1465. [Google Scholar]
- 69.Weinbauer, M. G. 2004. Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 28:127-181. [DOI] [PubMed] [Google Scholar]
- 70.Weinbauer, M. G., and F. Rassoulzadegan. 2004. Are viruses driving microbial diversification and diversity? Environ. Microbiol. 6:1-11. [DOI] [PubMed] [Google Scholar]
- 71.Winter, C., A. Smit, G. J. Herndl, and M. G. Weinbauer. 2005. Linking bacterial richness with abundance and prokaryotic activity. Limnol. Oceanogr. 50:968-977. [Google Scholar]
- 72.Wommack, K. E., and R. R. Colwell. 2000. Virioplankton: viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 64:69-114. [DOI] [PMC free article] [PubMed] [Google Scholar]