Abstract
Novel strains of obligately chemolithoautotrophic, sulfur-oxidizing bacteria have been isolated from various depths of Lake Fryxell, Antarctica. Physiological, morphological, and phylogenetic analyses showed these strains to be related to mesophilic Thiobacillus species, such as T. thioparus. However, the psychrotolerant Antarctic isolates showed an adaptation to cold temperatures and thus should be active in the nearly freezing waters of the lake. Enumeration by most-probable-number analysis in an oxic, thiosulfate-containing medium revealed that the sulfur-oxidizing chemolithotroph population peaks precisely at the oxycline (9.5 m), although viable cells exist well into the anoxic, sulfidic waters of the lake. The sulfur-oxidizing bacteria described here likely play a key role in the biogeochemical cycling of carbon and sulfur in Lake Fryxell.
The role of sulfur-oxidizing bacteria (SOB) in carbon and sulfur cycling has been well studied in both temperate and geothermal aquatic environments (1, 17, 29). Many metabolically and phylogenetically diverse organisms involved in sulfur cycling have been isolated in pure culture from these environments and characterized in detail (6, 10, 32, 36). However, the same cannot be said for sulfur-oxidizing chemolithotrophs in permanently cold environments.
Although SOB have been identified in cold environments (25, 37), well-characterized pure cultures of these organisms are rare. One exception is in the work of Knittel et al. (19), in which two psychrophilic Thiomicrospira species, T. arctica and T. psychrophila, from sediments off the coast of Svalbard were described. These organisms were the first psychrophilic, chemolithoautotrophic, sulfur-oxidizing bacteria isolated in pure culture. In contrast to those studies, which focused on new species of SOB from marine sediments, our work describes planktonic SOB from the water column of the permanently ice-covered Lake Fryxell, Antarctica.
Lake Fryxell is one of the most productive of several lakes in the McMurdo Dry Valleys of southern Victoria Land, Antarctica (41). The lake is just over 18 m deep and has a length of about 5.5 km, a width of 2 km, and a surface area of approximately 7 km2 (21). A salinity gradient ranging from freshwater to about 0.8% NaCl near the sediments is also present in the lake (21). In addition to the increasing density of the water with depth, a thick ice cover that is currently just over 6 m contributes to a highly stratified and amictic water column (27, 41). Water directly beneath the ice cover is supersaturated with dissolved gases, including oxygen and carbon dioxide (31). However, dissolved oxygen levels decrease dramatically below 8 m, and the water column becomes anoxic below 10 m, soon after the appearance of sulfide at 9.5 m (15). The sulfide, a product of sulfate-reducing bacteria, diffuses up from the sediments and anoxic portions of the water column, with concentrations increasing with depth to more than 1 mM near the sediments (13).
The gradients of oxygen and sulfide in Lake Fryxell should provide an ideal environment for development of SOB (20). With this in mind, we report here on the vertical distribution of cultivable strains of SOB in this lake. Three strains of SOB resembling Thiobacillus thioparus were isolated from different depths of Lake Fryxell, and relative numbers of SOB were quantified using extinction dilution methods. Our results support the hypothesis that biological sulfur cycling is occurring in this polar lake.
MATERIALS AND METHODS
Sample collection and water analyses.
Water samples were collected from Lake Fryxell during November 2001 and November 2003 at global positioning system coordinates 77°36.570′S, 163°8.969′E and 77°36.604′S, 163°8.853′E, respectively, as previously described (14, 15). Briefly, a sampling hole about 0.6 m wide was drilled and melted into the ice cover of the lake by using a Jiffy auger and Hotsy furnace, respectively. Niskin bottles (2.5 and 5 liters) were used to retrieve samples from the water column in 0.5-m intervals from 8 to 13 m and in 1-m intervals elsewhere. All samples were dispensed into completely filled Nalgene polyvinyl chloride bottles and transported at approximately 4°C in darkness to Crary Laboratory in McMurdo for processing. Lake water temperature and dissolved oxygen concentrations were determined using a Yellow Springs Instrument Company model 57 precalibrated probe. Sulfide and sulfate concentrations were determined as previously described (14, 15), using colorimetric or turbidimetric chemical assays, respectively.
Growth media and culture incubation.
Enrichment cultures and most-probable-number (MPN) dilution sets were established under aerobic conditions in a 10 mM MOPS-buffered medium (pH 7.2) containing the following mineral salts (amounts are per liter of deionized water): NaCl, 2.0 g; MgSO4 · 7H2O, 0.1 g; CaCl2 · 2H2O, 0.05 g; NH4Cl, 0.5 g; KCl, 0.5 g; KH2PO4, 0.5 g; and trace elements (EDTA, 5.2 g; FeCl2 · 4H2O, 1.5 g, CoCl2 · 6H2O, 0.19 g; MnCl2 · 4H2O, 0.1 g; CuCl2 · 2H2O, 0.017 g; ZnSO4 · 7H2O, 0.15 g; H3BO3, 0.006 g; Na2MoO4 · 2H2O, 0.188 g; NiCl2 · 6H2O, 0.025 g; VOSO4 · 2H2O, 0.03 g; Na2WO4 · 2H2O, 0.002 g; and NaHSeO3, 0.002 g), 1.0 ml. Enrichment and MPN cultures were incubated in the dark in loosely capped, one-third-filled 10- to 40-ml screw cap tubes containing sodium thiosulfate (20 mM) as an electron donor. For comparative purposes, the type strain of Thiobacillus thioparus, obtained from the American Type Culture Collection as ATCC 8158, was cultured in the same thiosulfate-mineral salts medium as the Lake Fryxell strains.
Other electron donors used to test substrate utilization in pure cultures included elemental sulfur treated at 100°C for 15 min on 3 successive days (0.2% [wt/vol] in shaken 125-ml Erlenmeyer flasks), sodium thiocyanate (10 mM), sodium sulfite (20 mM), and sodium sulfide. Cultures containing sulfide as an electron donor were grown in three different ways: (i) in tubes having 4 ml of a 4 mM sulfide agar (agar at 1.5%; Becton, Dickinson and Co., Sparks, MD) plug underneath 8 ml of a mineral salts thin agar (0.7%) overlay, (ii) on petri plates containing 12 ml of 10 mM sulfide agar underneath 12 ml of a mineral salts thin agar (0.7%) overlay, or (iii) in tubes of liquid (5 ml) or plates of mineral salts medium placed in sealed jars containing a sulfide-evolving solution (11). Agar used in solid growth media was washed three times in distilled water before use to remove residual organic materials. Incubations of primary enrichment cultures and subcultures were established aerobically at 4, 10, or 18°C; isolated strains were routinely maintained at 4 or 10°C.
Anaerobic growth using nitrate as electron acceptor was tested in crimp-top butyl rubber-stoppered tubes of 0.5 mM sulfide-reduced liquid thiosulfate medium containing 10 mM KNO3. Manipulations of anoxic media were carried out in an anoxic glove bag, giving the tubes a headspace of N2-H2 (95:5); the addition of resazurin to the medium confirmed anoxic conditions. Heterotrophic growth was tested in both complex media (tryptic soy and nutrient broths) and the defined mineral salts medium (without thiosulfate) supplemented with the organic substrates glucose (5 mM), fructose (5 mM), galactose (5 mM), lactose (3 mM), pyruvate (10 mM), lactate (10 mM), acetate (10 mM), succinate (10 mM), ethanol (10 mM), and propanol (10 mM).
MPN experiments were performed in precooled (10°C) thiosulfate-mineral salts medium with undiluted, 10−1-diluted, and 10−2-diluted Lake Fryxell water from depths of 8, 8.5, 9, 9.5, 10, 10.5, 11, 11.5, 12, and 13 m as inocula. Inocula from each dilution from each depth were maintained at 4°C and added to triplicate tubes of media in 10-, 1-, and 0.1-ml aliquots (a total of 27 tubes per depth). Cultures were then incubated at 18°C in darkness. Scoring of positive tubes was determined visually with the development of turbidity over a 16-day incubation period, and MPN values were obtained using standard tables.
Microscopy.
Photomicrographs were taken with an Olympus B-Max 60 photomicroscope by using the method of Pfennig and Wagener (24). Cell counts for growth of isolates in response to temperature were determined microscopically using a Petroff-Hausser counting chamber. Electron micrographs of mid-exponential-phase cells were obtained by the method described by Kimble et al. (18), in which cells were fixed first in 2% glutaraldehyde and then in OsO4 (transmission electron microscopy), or via dehydration by ethanol washing followed by critical-point drying (scanning electron microscopy).
Molecular analyses.
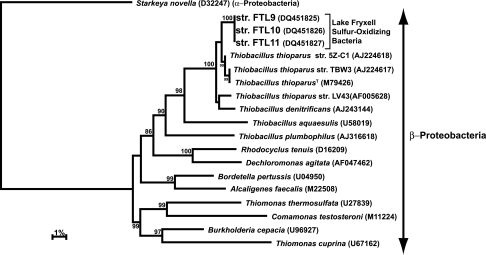
Extractions of genomic DNA from liquid cultures were performed using the Puregene DNA purification system according to the manufacturer's instructions (Gentra Systems, Minneapolis, MN). PCR amplification of small-subunit (SSU) rRNA genes was performed as previously described (15), using bacterial primers 8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1525R (5′-AAGGAGGTGATCCAGCC-3′) and the following cycling parameters: an initial denaturation at 94°C for 30 s; then 30 cycles of 30 s at 94°C, 30 s at 54°C, and 60 s at 72°C; and then a 10-min extension at 72°C. PCR products were purified using the QIAquick PCR purification kit according to the manufacturer's instructions (QIAGEN Sciences, Germantown, MD). Gene products of SSU rRNA were sequenced either manually using the ThermoSequenase cycle sequencing kit (U.S. Biochemicals, Cleveland, OH) or with an automated DNA sequencer. Sequence fragments of the SSU rRNA genes of cultured strains were assembled using either SeqApp (7) or the CAP3 sequence assembly program (9). Assembled sequences were aligned, and a phylogenetic tree was constructed using MacVector (version 7.2.3). Details of the tree construction are described in the legend to Fig. 2. Accession numbers for all organisms used in the phylogenetic analysis are indicated in Fig. 2.
FIG. 2.
Phylogenetic tree generated from 1,419 nucleotide positions of the 16S rRNA gene by using the Kimura two-parameter distance algorithm in a heuristic search. Only cultured strains were included in the analysis. GenBank accession numbers are shown in parentheses. Organisms shown are members of the Betaproteobacteria, with the exception of the outgroup organism, Starkeya novella (formerly Thiobacillus novellus), a member of the Alphaproteobacteria. Tree topology was maintained with all analyses, and high bootstrap values (>70%) based on 1,000 replicates are represented.
Nucleotide sequence accession numbers.
Sequences of the SSU rRNA genes from strains FTL9 (ATCC, BAA-1341) to -11 were deposited in GenBank under accession numbers DQ451825 to DQ451827, respectively.
RESULTS
Enrichment and isolation.
Liquid thiosulfate mineral salts media were inoculated with Lake Fryxell water from depths of 9, 10, and 11 m and incubated aerobically at 4, 10, and 18°C. Positive cultures for SOB became turbid after 1 to 3 weeks, depending upon incubation temperature, and were streaked for isolation on 1.5% washed agar plates of the same medium. Pinpoint, yellowish colonies were picked and restreaked repeatedly until pure cultures of three strains were obtained. Microscopic observation, colony characteristics, and the lack of growth in complex media verified that cultures of the FTL strains were axenic.
Morphology.
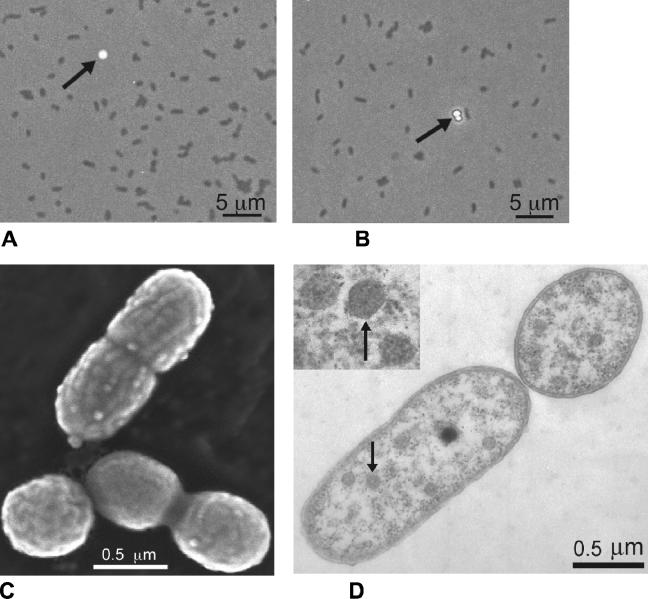
Strains FTL9, FTL10, and FTL11 (FTL, Fryxell Thiobacillus-like) were isolated from 9, 10, and 11 m, respectively. All strains showed similar morphological characteristics. Therefore, strain FTL9 was chosen as a representative strain for detailed studies. Cells of strain FTL9 were small cocci to short rods measuring 0.6 to 0.9 μm by 0.7 to 1.9 μm (Fig. 1A, C, and D). Like Thiobacillus thioparus (Fig. 1 B), strain FTL9 was motile, stained gram negatively, and existed as single cells or cell pairs. As is typical of sulfur-oxidizing Thiobacillus species grown on thiosulfate as an electron donor, cultures of strain FTL9 showed a milky turbidity, largely due to accumulation of elemental sulfur in the medium (Fig. 1A) (20). In older cultures, this turbidity disappeared as elemental sulfur was oxidized to sulfate and cells began to settle and form a white sediment. A sharp drop in pH of approximately 1.8 units accompanied this disappearance of sulfur, and cell viability was greatly diminished when cultures reached this stage of growth.
FIG. 1.
Cell morphology of Thiobacillus strain FTL9 versus that of T. thioparus ATCC 8158T. (A) Phase-contrast photomicrograph of strain FTL9. (B) Phase-contrast photomicrograph of T. thioparus ATCC 8158T, shown for comparison. Note the elemental sulfur globules (arrows) that accumulate in the growth media of both organisms as thiosulfate is oxidized. (C) Scanning electron micrograph of strain FTL9, showing different stages of dividing cells. (D) Transmission electron micrograph of strain FTL9, showing longitudinal and cross-sectioned cells. Arrows indicate polyhedral carboxysomes within cells of strain FTL9, shown at higher magnification in the inset.
Scanning and transmission electron microscopy of cells of strain FTL9 revealed an undulating outer membrane (Fig. 1C and D), with thin sections of cells showing polyhedral inclusion bodies that appear to be carboxysomes (Fig. 1D). These structures, found in cyanobacteria and many chemolithotrophic prokaryotes, contain ribulose 1,5-bisphosphate carboxylase, a key enzyme of the Calvin cycle (3).
Phylogeny.
Phylogenetic analyses were performed on all three strains of Lake Fryxell SOB. Distance analysis placed the strains within the Betaproteobacteria, specifically, within a clade containing strains of Thiobacillus thioparus (Fig. 2). The three Antarctic strains showed >99.9% identical rRNA gene sequence homology among themselves over the 1,419 bases included in the analysis. T. thioparus strains 5Z-C1 and TBW3 were most closely related to the FTL strains, at 99.0 and 98.6%, respectively. These organisms were isolated from microcosms prepared from rice field soils of northern Italy and are both described as obligately chemolithoautotrophic SOB (34). The FTL strains showed 98.4% rRNA sequence homology to T. thioparus ATCC 8158T and 97.8% homology to T. thioparus strain LV43, an organism isolated from a groundwater cave ecosystem in Romania (42). The topology of the neighbor-joining distance tree was robust and remained unchanged whether left uncorrected or corrected using the Jukes-Cantor or Kimura two-parameter method. High bootstrap values (≥72%) from 1,000 replicates provided strong support of the clade positions (Fig. 2).
Physiology. (i) Electron donors and acceptors.
Like for T. thioparus, heterotrophic growth was not observed among the FTL strains on either complex media or defined media containing any of 10 different carbon sources; thus, they are likely obligate chemolithoautotrophs. Electron donors that supported chemolithotrophic growth of strain FTL9 included thiosulfate, sulfide, elemental sulfur, and thiocyanate; sulfite did not support growth. Growth was observed in cultures containing up to 0.2 M thiosulfate (data not shown). Cultures grown using elemental sulfur required shaking and a longer incubation time to show turbidity, and growth was confirmed by a microscopic count. Like their closest relatives, T. thioparus strains 5Z-C1 and TBW3, none of the FTL strains were able to grow anaerobically with nitrate as an electron acceptor. This is in contrast to the case for T. thioparus ATCC 8158T, however, which has been shown to respire nitrate to nitrite during anaerobic growth (20).
(ii) pH and salt.
T. thioparus is capable of growth within a broad pH range, with an optimum near pH 7 (20, 34, 42). The pH ranges supporting growth of the FTL strains were similarly broad, but the midpoint of their pH ranges was shifted slightly to the alkaline side in comparison to that of T. thioparus (data not shown). While T. thioparus ATCC 8158T grew between pH 5 and 9, strain FTL9 grew at pH 6.5 to 9.5, and strains FTL10 and FTL11 grew at pH 6.25 to 9.5. The pH optimum for growth of the FTL strains was between pH 7.75 and 8.0.
Neither T. thioparus ATCC 8158T nor the Lake Fryxell strains showed a salt requirement. All strains grew in medium lacking NaCl, but the addition of 0.05% (wt/vol) NaCl stimulated growth among the FTL strains (data not shown). The Antarctic strains showed a higher salt tolerance than the type strain. While growth of T. thioparus ATCC 8158T was completely inhibited in media containing more than 1.3% NaCl, strain FTL9 grew well in media containing up to 2.4% NaCl, and strains FTL10 and FTL11 grew in media containing up to 2.6% NaCl.
(iii) Temperature.
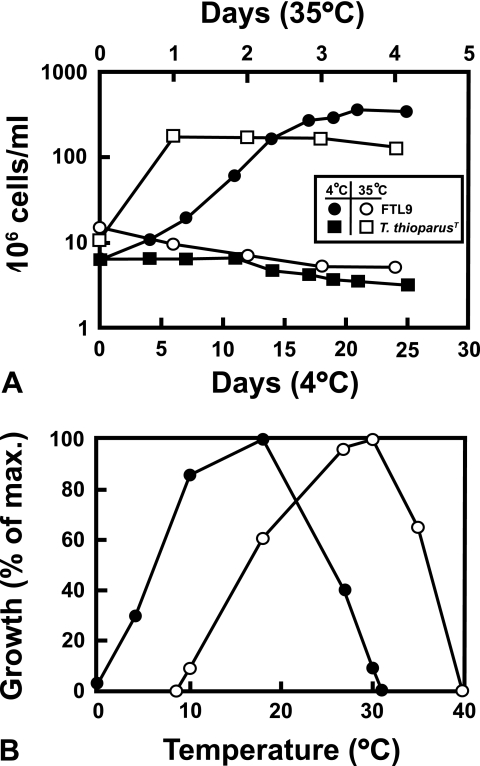
The temperature range supporting growth showed a clear distinction between the Antarctic Thiobacillus strains and T. thioparus ATCC 8158T. Strain FTL9 showed a clear adaptation to cold and an intolerance of warmer temperatures in comparison to T. thioparus ATCC 8158T when cultures of each were incubated at 4°C and 35°C. Strain FTL9 grew at 4°C but not at 35°C, while the opposite was true for strain ATCC 8158T (Fig. 3A). In addition, the FTL strains were capable of growth to −2°C and exhibited a temperature range and a temperature optimum approximately 10°C cooler than those of strain ATCC 8158T (Fig. 3B). The FTL strains grew optimally at 18°C, with an upper limit of 31°C (Fig. 3B). By contrast, T. thioparus ATCC 8158T showed no growth at 8°C, grew optimally at 30°C, and had an upper limit of nearly 40°C (Fig. 3B). The FTL Thiobacillus strains are thus best described as psychrotolerant.
FIG. 3.
Temperature relationships of strain FTL9 and T. thioparus ATCC 8158T. (A) Graph showing cold adaptation of strain FTL9 compared to that of the mesophilic T. thioparus ATCC 8158T. (B) Graph showing growth temperature ranges and optima for strain FTL9 (closed circles) and T. thioparus ATCC 8158T (open circles) after 8 days of incubation.
With each organism incubated at its respective optimum temperature and all other factors being the same, T. thioparus ATCC 8158T demonstrated a higher growth rate than strain FTL9. Cells of strain ATCC 8158T displayed a doubling time of approximately 12 h when incubated at 30°C, while cells of strain FTL9 showed a doubling time of about 20 h when incubated at 18°C (data not shown).
Distribution of SOB in Lake Fryxell.
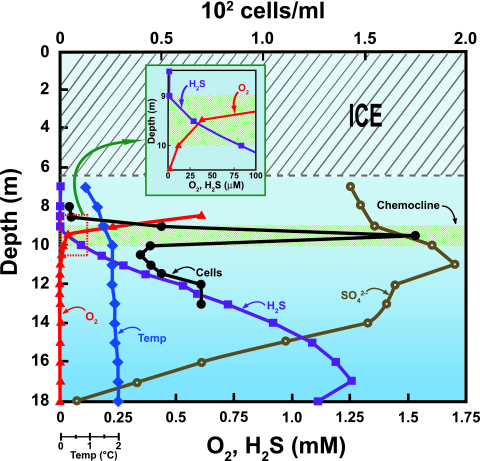
The distribution of viable SOB in the Lake Fryxell water column was explored by MPN culture analyses in thiosulfate-containing autotrophic media. Cell numbers of SOB peaked at 9.5 m, precisely within the narrow zone where both dissolved oxygen and sulfide coexist in the water column (Fig. 4). Numbers of SOB appeared to be more limited by a lack of sulfide than by a lack of dissolved oxygen. For example, above 9 m, where sulfide was not present, SOB were virtually undetectable using MPN methods. By contrast, below peak numbers at 9.5 m, numbers of cultivable SOB dropped quickly between 10 and 11 m, coinciding with the disappearance of dissolved oxygen. However, populations of SOB were still detectable to a depth of 13 m, well within the anoxic zone (Fig. 4). Absolute numbers of SOB were low at all depths tested, with a maximum number of slightly less than 2 × 102 cells/ml recorded at the chemocline of 9.5 m (Fig. 4).
FIG. 4.
Lake diagram showing relevant physiochemical parameters and cell numbers of thiosulfate-oxidizing bacteria based on MPN analyses. The expanded scale (inset) shows concentrations of sulfide and dissolved oxygen at the chemocline (oxycline).
DISCUSSION
Sulfur-oxidizing chemolithotrophic bacteria play a prominent role in biogeochemical sulfur cycling (2, 28). These organisms are a phylogenetically and metabolically diverse group that are capable of using a wide variety of compounds as carbon and energy sources (28). They are typically found in habitats where both oxygen and reduced inorganic sulfur compounds, such as sulfide, are present (2). To position themselves optimally within these microenvironments, many of these organisms exhibit chemotactic motility (4, 20, 38, 39).
Lake Fryxell provides a rare opportunity for the study of SOB and sulfur cycling in general in a permanently cold environment. A large volume of the Lake Fryxell basin is anoxic due to the production of sulfide by sulfate-reducing bacteria, which have been shown to occupy both the sediments and the water column of the lake (15). By contrast, the mixolimnion of Lake Fryxell is supersaturated with oxygen due to the activities of phytoplankton and the permanent ice cover; the latter inhibits diffusion of dissolved gases to the atmosphere (43). Considering this chemical profile, as well as the observation that sulfate concentrations peak just below the oxycline (Fig. 4), we suspected the presence of SOB in the water column of the lake.
Enrichment cultures for SOB in mineral salts thiosulfate medium yielded pure cultures of obligate chemolithoautotrophs from water samples taken from Lake Fryxell at depths of 9, 10, and 11 m. These isolates were close relatives of Thiobacillus thioparus but clearly differed from this organism in several physiological properties, most notably, growth in response to temperature. With the capability of growth at below 0°C and a growth temperature optimum shifted some 12°C lower than that of T. thioparus ATCC 8158T, the Lake Fryxell SOB show a clear adaptation to cold.
The Lake Fryxell SOB showed the same growth temperature minimum (−2°C) as two Arctic marine Thiomicrospira species, T. arctica and T. psychrophila (19). However, the Arctic SOB have slightly lower growth temperature optima (∼15°C) than the FTL strains and show maximum growth temperatures near 20°C; they are therefore true psychrophiles. By contrast, the FTL strains have a broader temperature range for growth, being able to grow at up to 31°C.
The ability of the FTL strains to grow at temperatures exceeding 20°C as well as in the cold is a characteristic that has been observed for other Lake Fryxell isolates, including anoxygenic phototrophic and sulfate-reducing bacteria and other chemoorganotrophic bacteria (5, 12, 15, 23, 26, 40). Although truly psychrophilic organisms have been isolated from Lake Fryxell (33), the ability to grow at above 20°C appears to be common among cultured strains, despite constant in situ temperatures of 2°C or lower. Whether the psychrotolerant nature of many Lake Fryxell isolates represents the predominant physiology of organisms in the lake remains unknown.
In addition to cold adaptation, other distinguishing properties of the FTL strains include the lack of growth at below pH 6.25, which was unexpected considering that all strains of T. thioparus studied in this regard are capable of growth at a pH of as low as 5 (20, 34, 42). Moreover, the fact that the pH of Lake Fryxell waters remains constant at just over pH 7 beneath 9 m (22) suggests that the FTL strains are subsisting at nearly a full pH unit below their optimum of pH 7.75 to 8.
The FTL strains also distinguished themselves from T. thioparus by an increased salt tolerance. The FTL strains tolerated nearly twice the concentration of NaCl that T. thioparus ATCC 8158T did. It is possible that this is a reflection of different cell membrane properties among the two strains, such as a decreased permeability to NaCl in the Antarctic strains. This trait may be a legacy of the proximity of Lake Fryxell to the sea. Because the lake is situated only about 7 km from McMurdo Sound, its brackish waters, and possibly some of the organisms within them, may have their origins in seawater (8). Halotolerant thiobacilli, such as Halothiobacillus neapolitanus (formerly Thiobacillus neapolitanus) and Halothiobacillus kellyi, have been isolated from seawater (16, 30), and Halothiobacillus hydrothermalis was isolated from a hydrothermal vent in the North Fiji basin (30). Although these organisms belong to the Gammaproteobacteria, they are all marine species and are physiologically similar to our Antarctic isolates in that they show an increased salt tolerance (in comparison to T. thioparus) but do not absolutely require NaCl. However, halothiobacilli are all mesophilic or mildly thermophilic (16, 30), with temperature optima ranging from 28 to 42°C, a difference of 10 to 24°C warmer than the temperature optima of our Antarctic isolates.
The distribution of cultivable SOB in Lake Fryxell was investigated by using MPN dilution cultures. Lake water used for these inoculations ranged from depths of 8 to 13 m. The choice of these depths reflected the range of physiochemical parameters of the water column that are critical for growth of SOB, that is, where SOB were expected to proliferate. As expected, maximum numbers of SOB occurred at 9.5 m, the midpoint of the narrow zone containing both oxygen and sulfide. The presence of SOB at between 10.5 and 13 m is intriguing, considering that at these depths conditions are anoxic (Fig. 4), mixing occurs only by molecular diffusion, and alternative electron acceptors, such as nitrate and ferric iron, are virtually undetectable (8, 44). It is therefore unclear whether these cells are maintaining active metabolism at these depths or are existing in a dormant state until conditions become more favorable.
We acknowledge that the cell numbers of Lake Fryxell SOB reported here are likely underestimates. This may be due to culture biases introduced by the composition of the growth medium and the incubation conditions. The medium used in our MPN analyses discriminates against SOB that are unable to use thiosulfate as an electron donor, for example. Although absolute numbers of SOB in Lake Fryxell were low at all depths tested, Vlasceanu et al. (42) reported similarly low cell numbers of approximately 102 to 103 cells/ml in surface and groundwater springs near the Black Sea in a study that used a fluorescent antibody specific for T. thioparus. Therefore, our estimates of cell numbers may not be that far from actual numbers. Regardless, we believe that our MPN study provides an accurate profile of cultivable, thiosulfate-oxidizing, chemolithoautotrophic bacteria in relevant depths of Lake Fryxell.
Sulfur-chemolithotrophy is one of several autotrophic metabolisms that are active in Lake Fryxell. These include oxygenic photosynthesis (41), anoxygenic photosynthesis (12, 14, 23), methanogenesis (13), and homoacetogenesis (W. M. Sattley and M. T. Madigan, unpublished results). Sulfur-oxidizing chemolithotrophs, such as the FTL strains described here, function to both close the sulfur cycle (by producing substrate for sulfate-reducing bacteria) and supply organic carbon to the Lake Fryxell ecosystem. Since Lake Fryxell is a totally microbial ecosystem (35), it is likely that even these small inputs of organic carbon are important for supporting heterotrophic processes throughout the water column and sediments of this constantly cold polar lake.
Acknowledgments
This work was supported by U.S. National Science Foundation grants OPP0085481 and MCB0237576.
We acknowledge Raytheon Polar Services, Petroleum Helicopters, Inc., and John Priscu and the McMurdo LTER limnology team for excellent logistical support in the Antarctic. Special thanks go to Steven Schmitt (IMAGE, SIUC) for electron microscopy, Laurie Achenbach for technical advice, and Deborah Jung for technical assistance.
REFERENCES
- 1.Brinkhoff, T., S. M. Sievert, J. Kuever, and G. Muyzer. 1999. Distribution and diversity of sulfur-oxidizing Thiomicrospira spp. at a shallow-water hydrothermal vent in the Aegean Sea (Milos, Greece). Appl. Environ. Microbiol. 65:3843-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brock, T. D., and H. G. Schlegel. 1989. Introduction, p. 1-15. In H. G. Schlegel and B. Bowien (ed.), Autotrophic bacteria. Springer-Verlag, Madison, Wis.
- 3.Cannon, G. C., C. E. Bradburne, H. C. Aldrich, S. H. Baker, S. Heinhorst, and J. M. Shively. 2001. Microcompartments in prokaryotes: carboxysomes and related polyhedra. Appl. Environ. Microbiol. 67:5351-5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenchel, T. 1994. Motility and chemosensory behavior of the sulfur bacterium Thiovulum majus. Microbiology 140:3109-3116. [Google Scholar]
- 5.Frühling, A., P. Schumann, H. Hippe, B. Sträubler, and E. Stackebrandt. 2002. Exiguobacterium undae sp. nov. and Exiguobacterium antarcticum sp. nov. Int. J. Syst. Evol. Microbiol. 52:1171-1176. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh, W., and P. Roy. 2006. Mesorhizobium thiogangeticum sp. nov., a novel sulfur-oxidizing chemolithoautotroph from rhizosphere soil of an Indian tropical leguminous plant. Int. J. Syst. Evol. Microbiol. 56:91-97. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert, D. G. 1993. SeqApp 1.9a157 ed. Biocomputing Office, Biology Department, Indiana University, Bloomington.
- 8.Green, W. J., T. J. Gardner, T. G. Ferdelman, M. P. Angle, L. C. Varner, and P. Nixon. 1989. Geochemical processes in the Lake Fryxell basin (Victoria Land, Antarctica). Hydrobiologia 172:129-148. [Google Scholar]
- 9.Huang, X., and A. Madan. 1999. CAP3: a DNA sequence assembly program. Genome Res. 9:868-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inagaki, F., K. Takai, K. H. Nealson, and K. Horikoshi. 2004. Sulfurovum lithotrophicum gen. nov., sp. nov., a novel sulfur-oxidizing chemolithoautotroph within the ɛ-Proteobacteria isolated from Okinawa Trough hydrothermal sediments. Int. J. Syst. Evol. Microbiol. 54:1477-1482. [DOI] [PubMed] [Google Scholar]
- 11.Irgens, R. L. 1983. Thioacetamide as a source of hydrogen sulfide for colony growth of purple sulfur bacteria. Curr. Microbiol. 8:183-186. [Google Scholar]
- 12.Jung, D. O., L. A. Achenbach, E. A. Karr, S. Takaichi, and M. T. Madigan. 2004. A gas vesiculate planktonic strain of the purple non-sulfur bacterium Rhodoferax antarcticus isolated from Lake Fryxell, Dry Valleys, Antarctica. Arch. Microbiol. 182:236-243. [DOI] [PubMed] [Google Scholar]
- 13.Karr, E. A., J. M. Ng, S. M. Belchik, W. M. Sattley, M. T. Madigan, and L. A. Achenbach. 2006. Biodiversity of methanogenic and other Archaea in the permanently frozen Lake Fryxell, Antarctica. Appl. Environ. Microbiol. 72:1663-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karr, E. A., W. M. Sattley, D. O. Jung, M. T. Madigan, and L. A. Achenbach. 2003. Remarkable diversity of phototrophic purple bacteria in a permanently frozen Antarctic lake. Appl. Environ. Microbiol. 69:4910-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karr, E. A., W. M. Sattley, M. R. Rice, D. O. Jung, M. T. Madigan, and L. A. Achenbach. 2005. Diversity and distribution of sulfate-reducing bacteria in permanently frozen Lake Fryxell, McMurdo Dry Valleys, Antarctica. Appl. Environ. Microbiol. 71:6353-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly, D. P., and A. P. Wood. 2000. Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. nov., Halothiobacillus gen. nov. and Thermithiobacillus gen. nov. Int. J. Syst. Evol. Microbiol. 50:511-516. [DOI] [PubMed] [Google Scholar]
- 17.Kelly, D. P., J. K. Shergill, W. P. Lu, and A. P. Wood. 1997. Oxidative metabolism of inorganic sulfur compounds by bacteria. Antonie Leeuwenhoek 71:95-107. [DOI] [PubMed] [Google Scholar]
- 18.Kimble, L. K., L. Mandelco, C. R. Woese, and M. T. Madigan. 1995. Heliobacterium modesticaldum, sp. nov., a thermophilic heliobacterium of hot springs and volcanic soils. Arch. Microbiol. 163:259-267. [Google Scholar]
- 19.Knittel, K., J. Kuever, A. Meyerdierks, R. Meinke, R. Amann, and T. Brinkhoff. 2005. Thiomicrospira arctica sp. nov. and Thiomicrospira psychrophila sp. nov., psychrophilic, obligately chemolithoautotrophic, sulfur-oxidizing bacteria isolated from marine Arctic sediments. Int. J. Syst. Evol. Microbiol. 55:781-786. [DOI] [PubMed] [Google Scholar]
- 20.Kuenen, J. G., L. A. Robertson, and O. H. Tuovinen. 1992. The genera Thiobacillus, Thiomicrospira, and Thiosphaera, p. 2638-2657. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed. Springer, New York, N.Y.
- 21.Lawrence, M. J. F., and C. H. Hendy. 1985. Water column and sediment characteristics of Lake Fryxell, Taylor Valley, Antarctica. N. Zealand J. Geol. Geophys. 28:543-552. [Google Scholar]
- 22.Lee, P. A., J. A. Mickucki, C. M. Foreman, J. C. Priscu, G. R. DiTullio, S. F. Riseman, S. J. deMora, C. R. Wolf, and L. Kester. 2004. Thermodynamic constraints on microbially mediated processes in lakes of the McMurdo Dry Valleys, Antarctica. Geomicrobiol. J. 21:221-237. [Google Scholar]
- 23.Madigan, M. T., D. O. Jung, C. R. Woese, and L. A. Achenbach. 2000. Rhodoferax antarcticus sp. nov., a moderately psychrophilic purple nonsulfur bacterium isolated from an Antarctic microbial mat. Arch. Microbiol. 173:269-277. [DOI] [PubMed] [Google Scholar]
- 24.Pfennig, N., and S. Wagener. 1986. An improved method of preparing wet mounts for photomicrographs of microorganisms. J. Microbiol. Methods 4:303-306. [Google Scholar]
- 25.Ravenschlag, K., K. Sahm, J. Pernthaler, and R. Amann. 1999. High bacterial diversity in permanently cold marine sediments. Appl. Environ. Microbiol. 65:3982-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rees, G. N., P. H. Janssen, and C. G. Harfoot. 1986. An unusual strain of Desulfovibrio sp. from an Antarctic lake. FEMS Microbiol. Lett. 37:363-366. [Google Scholar]
- 27.Roberts, E. C., J. Laybourn-Parry, D. M. McKnight, and G. Novarinos. 2000. Stratification and dynamics of microbial loop communities in Lake Fryxell, Antarctica. Freshwater Biol. 44:649-661. [Google Scholar]
- 28.Robertson, L. A., and J. G. Kuenen. 1992. The colorless sulfur bacteria, p. 385-413. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed. Springer, New York, N.Y.
- 29.Shulz, H. N., and B. B. Jorgensen. 2001. Big bacteria. Annu. Rev. Microbiol. 55:105-137. [DOI] [PubMed] [Google Scholar]
- 30.Sievert, S. M., T. Heidorn, and J. Kuever. 2000. Halothiobacillus kellyi sp. nov., a mesophilic, obligately chemolithoautotrophic, sulfur-oxidizing bacterium isolated from a shallow-water hydrothermal vent in the Aegean Sea, and emended description of the genus Halothiobacillus. Int. J. Syst. Evol. Microbiol. 50:1229-1237. [DOI] [PubMed] [Google Scholar]
- 31.Smith, R. L., L. G. Miller, and B. L. Howes. 1993. The geochemistry of methane in Lake Fryxell, an amictic, permanently ice-covered, Antarctic lake. Biogeochemistry 21:95-116. [Google Scholar]
- 32.Sorokin, D. Y., T. P. Tourova, E. M. Spiridonova, F. A. Rainey, and G. Muyzer. 2005. Thioclava pacifica gen. nov., sp. nov., a novel facultatively autotrophic, marine, sulfur-oxidizing bacterium from a near-shore sulfidic hydrothermal area. Int. J. Syst. Evol. Microbiol. 55:1069-1075. [DOI] [PubMed] [Google Scholar]
- 33.Spring, S., B. Merkhoffer, N. Weiss, R. M. Kroppenstedt, H. Hippe, and E. Stackebrandt. 2003. Characterization of novel psychrophilic clostridia from an Antarctic microbial mat: description of Clostridium frigoris sp. nov., Clostridium lacusfryxellense sp. nov., Clostridium bowmanii sp. nov. and Clostridium psychrophilum sp. nov. and reclassification of Clostridium laramiense as Clostridium estertheticum subsp. laramiense subsp. nov. Int. J. Syst. Evol. Microbiol. 53:1019-1029. [DOI] [PubMed] [Google Scholar]
- 34.Stubner, S., T. Wind, and R. Conrad. 1998. Sulfur oxidation in rice field soil: activity, enumeration, isolation and characterization of thiosulfate-oxidizing bacteria. Syst. Appl. Microbiol. 21:569-578. [DOI] [PubMed] [Google Scholar]
- 35.Takacs, C. D., and J. C. Priscu. 1998. Bacterioplankton dynamics in the McMurdo dry valley lakes, Antarctica: production and biomass loss over four seasons. Microb. Ecol. 36:239-250. [DOI] [PubMed] [Google Scholar]
- 36.Takai, K., B. J. Campbell, S. C. Cary, M. Suzuki, H. Oida, T. Nunoura, H. Hirayama, S. Nakagawa, Y. Suzuki, F. Inagaki, and K. Horikoshi. 2005. Enzymatic and genetic characterization of carbon and energy metabolisms by deep-sea hydrothermal chemolithoautotrophic isolates of Epsilonproteobacteria. Appl. Environ. Microbiol. 71:7310-7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teske, A., T. Brinkhoff, G. Muyzer, D. P. Moser, J. Rethmeier, and H. W. Jannasch. 2000. Diversity of thiosulfate-oxidizing bacteria from marine sediments and hydrothermal vents. Appl. Environ. Microbiol. 66:3125-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thar, R., and T. Fenchel. 2001. True chemotaxis in oxygen gradients of the sulfur-oxidizing bacterium Thiovulum majus. Appl. Environ. Microbiol. 67:3299-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thar, R., and T. Fenchel. 2005. Survey of motile microaerophilic bacterial morphotypes in the oxygen gradient above a marine sulfidic sediment. Appl. Environ. Microbiol. 71:3682-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Trappen, S., I. Vandecandelaere, J. Mergaert, and J. Swings. 2004. Gillisia limnaea gen. nov., sp. nov., a new member of the family Flavobacteriaceae isolated from a microbial mat in Lake Fryxell, Antarctica. Int. J. Syst. Evol. Microbiol. 54:445-448. [DOI] [PubMed] [Google Scholar]
- 41.Vincent, W. F. 1981. Production strategies in Antarctic inland waters: phytoplankton eco-physiology in a permanently ice-covered lake. Ecology 62:1215-1224. [Google Scholar]
- 42.Vlasceanu, L., R. Popa, and B. K. Kinkle. 1997. Characterization of Thiobacillus thioparus LV43 and its distribution in a chemoautotrophically based groundwater ecosystem. Appl. Environ. Microbiol. 63:3123-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wharton, R. A., C. P. McKay, G. D. Clow, and D. T. Andersen. 1993. Perennial ice covers and their influence on Antarctic lake ecosystems. Antarctic Res. Ser. Am. Geophys. Union 59:53-70. [Google Scholar]
- 44.Wharton, R. A., W. B. Lyons, and D. J. Des Marais. 1993. Stable isotopic biogeochemistry of carbon and nitrogen in a perennially ice-covered Antarctic lake. Chem. Geol. 107:159-172. [DOI] [PubMed] [Google Scholar]