Abstract
A set of 118 strains of the species Lactobacillus rhamnosus was collected, including probiotic strains, research strains with potential probiotic properties, food starter cultures, and human isolates. The majority of the strains were collected from companies, hospitals, or culture collections or were obtained after contacting authors who reported clinical case studies in the literature. The present work aimed to reveal the genotypic relationships between strains of these diverse sources. All strains were initially investigated using fluorescent amplified fragment length polymorphism (FAFLP) with three different primer combinations. Numerical analysis of FAFLP data allowed (i) confirmation of the identification of all strains as members of L. rhamnosus and (ii) delineation of seven stable intraspecific FAFLP clusters. Most of these clusters contained both (potentially) probiotic strains and isolates of human origin. For each of the clusters, strains of different sources were selected for pulsed-field gel electrophoresis (PFGE) of macrorestriction fragments obtained with the enzymes NotI and AscI. Analysis of PFGE data indicated that (i) some (potentially) probiotic strains were indistinguishable from other probiotic strains, suggesting that several companies may use duplicate cultures of the same probiotic strain, and (ii) in a number of cases human isolates from sterile body sites were indistinguishable from a particular probiotic strain, suggesting that some of these isolates may be reisolations of commercial strains.
Lactobacillus species are members of the commensal microflora in the oral cavity and the gastrointestinal and genitourinary system in humans and animals and are usually considered nonpathogenic (1). Selected strains of some Lactobacillus species are being used as probiotics and are claimed to influence the health of the host in a beneficial manner (10). To a very limited extent, strains belonging to the same Lactobacillus species as those used in probiotic applications have also been isolated from clinical specimens and have been associated with rare cases of endocarditis, meningitis, deep abscesses, and bacteremia (1, 5, 11). It should be noted that in these types of infections underlying disease or immunosuppression is a common feature and that infection in previously healthy humans is extremely rare.
Among the lactobacilli, strains of Lactobacillus rhamnosus are commonly used as probiotics including the GG (Gorbach-Goldin) strain, which is one of the most widely used and well-documented probiotics (13, 25). On the other hand, L. rhamnosus has also been reported as one of the most common Lactobacillus taxa in human clinical cases (5). Harty et al. (8, 9) identified possible virulence factors in L. rhamnosus endocarditis strains compared to laboratory or oral isolates. Furthermore, L. rhamnosus strains have been shown to produce enzymes enabling the breakdown of glycoproteins and synthesis or lysis of fibrin clots (18).
Aggregate epidemiologic studies have not found a link between the number of clinical L. rhamnosus cases and the frequency of consumption of foods or probiotics containing L. rhamnosus. Salminen and colleagues (24) studied the relatedness among L. rhamnosus bacteremic isolates and commercial probiotic strains consumed in Finland by means of pulsed-field gel electrophoresis (PFGE) using four different enzymes. The authors reported that 11 out of 26 L. rhamnosus blood isolates could not be distinguished from the probiotic L. rhamnosus GG strain. However, Salminen and coworkers also stressed that no increase in the incidence of L. rhamnosus GG isolation or the proportion of all Lactobacillus bacteremia could be demonstrated in relation to the increased use of the probiotic strain in Finland from 1995 to 2000. Some other studies have speculated on the possible association between consumption of L. rhamnosus strain GG and subsequent bacterial infection (14, 15, 21). Land et al. (15) reported two pediatric cases of bacteremia where the blood isolates were indistinguishable by repetitive element sequence-based PCR DNA fingerprinting from probiotic L. rhamnosus strain GG, which was administered for treatment of antibiotic-associated diarrhea in severely ill and immunocompromised patients. In contrast, Ouwehand and coworkers (19) recently reported that the commercial GG strain differed significantly from L. rhamnosus blood isolates in one or more phenotypic properties. Overall, few studies have attempted to study the link between L. rhamnosus consumption and clinical infection. MacGregor and coworkers (16) reported a case of L. rhamnosus sepsis in a patient with corticoid immunosuppression in association with live yogurt biotherapy and highlight the need to proceed with caution with live therapy in heavily immunosuppressed individuals. MacKay and coworkers (17) reported a case of endocarditis due to L. rhamnosus. They concluded that patients who are immunosuppressed or have preexisting heart valve disease should avoid probiotic preparations containing L. rhamnosus.
In order to obtain more-reliable insights regarding the possible link between the use of L. rhamnosus probiotics and the incidence of L. rhamnosus infections, the scope of a typing study should be much broader than the data provided in literature. First, a larger number of geographically distributed and well-documented probiotic and clinical strains should be studied. Secondly, the typing approach is of crucial importance and only those techniques with the highest resolution should be applied. In the present paper, we collected a set of 118 L. rhamnosus strains comprising multiple strains intended for probiotic use distributed by companies all over Europe and a large number of human isolates from various infections or from the commensal microflora mostly recovered around Europe. The objective was to reveal genotypic relationships between strains (potentially) used as probiotics and isolates from human origin. For typing, two widely used high-resolution DNA fingerprinting techniques were used for determination of genotypic relatedness at the intraspecific and strain levels, respectively, i.e., fluorescent amplified fragment length polymorphism (FAFLP) and PFGE of macrorestriction fragments.
MATERIALS AND METHODS
Bacterial strains.
The list of 118 L. rhamnosus strains investigated in the present study is detailed in Table 1. Fourteen strains of 10 different companies are commercially used in probiotic products (P), 7 strains are research cultures under investigation for potential probiotic use (R), and 4 strains are nutritional isolates or industrial food starters (N). The L. rhamnosus GG strain (LMG 18243) was obtained from the BCCM/LMG Bacteria Collection. The majority of the other probiotic strains were received directly from companies; those that requested to remain anonymous are indicated with a depositor (D) number throughout this report. In cases where the strain was isolated from the probiotic product, a D-P number was assigned. Additionally, 92 human isolates (H), of which 58 are from sterile sites and 34 from commensal (mainly fecal, vaginal, and oral) human flora, were included. The human isolates were collected in hospitals or were obtained from culture collections or from authors who published clinical cases (2, 3, 6, 9, 19, 21, 23). The origin of the type strain of L. rhamnosus, LMG 6400, is not known.
TABLE 1.
List of strains studied
| Cluster | PRSF no. | Other strain no. | Depositora | Originb | Source, geographical origin, yr of isolation | Reference(s) |
|---|---|---|---|---|---|---|
| I | PRSF-L002 | R-10688 | D35-P11 | P | Probiotic product | |
| I | PRSF-L006 | R-11420 | D30-P08 | P | Probiotic product | |
| I | PRSF-L014 | R-16072 | D36-P12 | P | Probiotic product | |
| I | PRSF-L175 | GG (Gorbach-Goldin); LMG 18243 | BCCM/LMG | P | Feces | |
| I | PRSF-L181 | D46 | P | |||
| I | PRSF-L235 | CCUG 28641 | CCUG | H | Heart valve, Sweden, 1991 | |
| I | PRSF-L236 | CCUG 30651 | CCUG | N | Industrial starter, Sweden, 1992 | |
| I | PRSF-L260 | AHP 27651 | Rautio, M. | H | Hepatic abscess (diabetes), Finland, 1998 | 21 |
| I | PRSF-L316 | T30914; LMG 23314 | Rautelin, H. | H | Blood | 19, 23 |
| I | PRSF-L317 | T31528; LMG 23315 | Rautelin, H. | H | Blood | 19, 23 |
| I | PRSF-L318 | T17221; LMG 23316 | Rautelin, H. | H | Blood | 19, 23 |
| I | PRSF-L321 | T4846; LMG 23317 | Rautelin, H. | H | Blood | 23 |
| I | PRSF-L329 | T31583; LMG 23322 | Rautelin, H. | H | Blood | 23 |
| I | PRSF-L331 | T33651; LMG 23324 | Rautelin, H. | H | Blood | 23 |
| I | PRSF-L332 | T33721; LMG 23325 | Rautelin, H. | H | Blood | 19 |
| I | PRSF-L336 | D46 | P | Feces, United States, 1986 | ||
| I | PRSF-L415 | 7.1a; LMG 23529 | D13 | H | Feces, Finland, 1999 | |
| I | PRSF-L416 | 5.5a; LMG 23530 | D13 | H | Feces, Finland, 1999 | |
| I | PRSF-L417 | 5.3a; LMG 23531 | D13 | H | Feces, Finland, 1999 | |
| I | PRSF-L418 | 5.1a; LMG 23532 | D13 | H | Feces, Finland, 1999 | |
| I | PRSF-L419 | 3.8b; LMG 23533 | D13 | H | Feces, Finland, 1999 | |
| I | PRSF-L420 | 3.7a; LMG 23534 | D13 | H | Feces, Finland, 1999 | |
| I | PRSF-L421 | 3.3a; LMG 23535 | D13 | H | Feces, Finland, 1999 | |
| I | PRSF-L422 | 3.1b; LMG 23536 | D13 | H | Feces, Finland, 1999 | |
| I | PRSF-L430 | 14.5a; LMG 23544 | D13 | H | Feces, Finland, 1999 | |
| I | PRSF-L436 | D39-P | P | Probiotic product? | ||
| I | PRSF-L482 | R | Feces | |||
| I | PRSF-L487 | R | Feces | |||
| II | PRSF-L373 | A77/87; LMG 23553 | Harty, D. | H | Blood (endocarditis) | 9 |
| II | PRSF-L375 | A103/70 | Harty, D. | H | Blood (endocarditis) | 9 |
| II | PRSF-L397 | LB21; LMG 23667 | Essum | N | Feces, Sweden, 1999 | |
| II | PRSF-L479 | R | Feces | |||
| III | PRSF-L036 | UCL 17; LMG 23279 | Wauters, G. | H | Blood, Belgium, 1995 | |
| III | PRSF-L042 | UCL 34; LMG 23281 | Wauters, G. | H | Blood, Belgium, 1997 | |
| III | PRSF-L050 | UCL 14; LMG 23285 | Wauters, G. | H | Pus diverticulitis, Belgium, 1995 | |
| III | PRSF-L051 | UCL 15; LMG 23286 | Wauters, G. | H | Blood, Belgium, 1995 | |
| III | PRSF-L079 | UCL 46; LMG 23303 | Wauters, G. | H | Blood, Belgium, 1998 | |
| III | PRSF-L082 | UCLF 2; LMG 23304 | Wauters, G. | H | Feces, Belgium | |
| III | PRSF-L100 | ULille | H | Feces (healthy person), France, 1998 | ||
| III | PRSF-L177 | Arpi 11233; LMG 19717 | BCCM/LMG | H | Blood (terminal ileitis), Denmark | 2 |
| III | PRSF-L261 | SIJD 2001 | Carretto, E. | H | Blood (organ transplantation), Italy | 6 |
| III | PRSF-L273 | CCUG 44276 | CCUG | H | Vaginal flora, Sweden, 2001 | |
| III | PRSF-L274 | CCUG 44667 | CCUG | H | Jaw (osteomyelitis), Sweden, 2000 | |
| III | PRSF-L286 | A64/87; LMG 23551 | Harty, D. | H | Blood (endocarditis) | 9 |
| III | PRSF-L287 | A65/87; LMG 23552 | Harty, D. | H | Blood (endocarditis) | 9 |
| III | PRSF-L325 | T21162; LMG 23320 | Rautelin, H. | H | Blood | 23 |
| III | PRSF-L393 | CJ 61; LMG 23327 | CHUV | H | Blood | |
| III | PRSF-L486 | R | Feces | |||
| IV | PRSF-L015 | R-16073 | D31-P09 | P | Probiotic product | |
| IV | PRSF-L170 | CCUG 23335; LMG 10768 | BCCM/LMG | H | Blood, Sweden, 1988 | |
| IV | PRSF-L303 | D10 | P | Dairy product, Canada, 1981 | ||
| IV | PRSF-L341 | D10 | P | Dairy product, Canada, 1976 | ||
| IV | PRSF-L455 | LRh5; LMG 23525 | Sacco | P | Cheese, Italy | |
| V | PRSF-L088 | UCLF 8; LMG 23307 | Wauters, G. | H | Feces, Belgium | |
| V | PRSF-L113 | Wauters, G. | H | Feces, Belgium | ||
| V | PRSF-L116 | UCLF 20; LMG 23309 | Wauters, G. | H | Feces, Belgium | |
| V | PRSF-L272 | CCUG 41464 | CCUG | H | Cerebrospinal fluid, Norway, 1998 | |
| V | PRSF-L324 | T19557; LMG 23319 | Rautelin, H. | H | Blood | 23 |
| V | PRSF-L330 | T33620; LMG 23323 | Rautelin, H. | H | Blood | 23 |
| V | PRSF-L335 | VTT E-97800 | R | |||
| VI | PRSF-L060 | M6/09/0580; LMG 23294 | UIA | H | Blood, Belgium, 1996 | |
| VI | PRSF-L258 | CCUG 44070 | CCUG | H | Vaginal flora, Sweden, 1999 | |
| VII | PRSF-L034 | UCL 7; LMG 23277 | Wauters, G. | H | Blood, Belgium, 1993 | |
| VII | PRSF-L035 | UCL12; LMG 23278 | Wauters, G. | H | Pleural fluid, Belgium, 1994 | |
| VII | PRSF-L038 | UCL 23; LMG 23280 | Wauters, G. | H | Blood, Belgium, 1995 | |
| VII | PRSF-L043 | UCL 37; LMG 23282 | Wauters, G. | H | Blood, Belgium, 1997 | |
| VII | PRSF-L047 | UCL10; LMG 23283 | Wauters, G. | H | Blood, Belgium, 1994 | |
| VII | PRSF-L048 | UCL 11; LMG 23284 | Wauters, G. | H | Blood, Belgium, 1994 | |
| VII | PRSF-L052 | UCL 16; LMG 23287 | Wauters, G. | H | Blood, Belgium, 1995 | |
| VII | PRSF-L053 | UCL 19; LMG 23288 | Wauters, G. | H | Throat, Belgium, 1995 | |
| VII | PRSF-L054 | UCL 20; LMG 23289 | Wauters, G. | H | Blood, Belgium, 1995 | |
| VII | PRSF-L055 | UCL 21; LMG 23290 | Wauters, G. | H | Sterile site (mediastinum), Belgium, 1995 | |
| VII | PRSF-L057 | UCL 29; LMG 23291 | Wauters, G. | H | Abcess, Belgium, 1997 | |
| VII | PRSF-L058 | M1/11/0657; LMG 23292 | UIA | H | Blood, Belgium, 1991 | |
| VII | PRSF-L059 | M5/01/0582; LMG 23293 | UIA | H | Blood, Belgium, 1995 | |
| VII | PRSF-L061 | MI/9802/663; LMG 23295 | UIA | H | Blood, Belgium, 1998 | |
| VII | PRSF-L063 | MI/9806/5998; LMG 23296 | UIA | H | Bronchoalveolar Lavage fluid, Belgium, 1999 | |
| VII | PRSF-L068 | UCL 5; LMG 23298 | Wauters, G. | H | Blood, Belgium, 1993 | |
| VII | PRSF-L069 | UCL 6; LMG 23299 | Wauters, G. | H | Throat, Belgium, 1993 | |
| VII | PRSF-L070 | UCL 8; LMG 23300 | Wauters, G. | H | Blood, Belgium, 1993 | |
| VII | PRSF-L071 | Wauters, G. | H | Bile, Belgium, 1996 | ||
| VII | PRSF-L083 | UCLF 3; LMG 23305 | Wauters, G. | H | Feces, Belgium | |
| VII | PRSF-L099 | ULille | H | Feces, France, 1998 | ||
| VII | PRSF-L102 t2 | ULille | H | |||
| VII | PRSF-L105 | ULille | H | Feces, France, 1998 | ||
| VII | PRSF-L115 | UCLF 19; LMG 23308 | Wauters, G. | H | Feces, Belgium | |
| VII | PRSF-L117 | UCLF 21; LMG 23310 | Wauters, G. | H | Feces, Belgium | |
| VII | PRSF-L119 | UCLF 23; LMG 23311 | Wauters, G. | H | Feces, Belgium | |
| VII | PRSF-L121 | UCLF 25; LMG 23312 | Wauters, G. | H | Feces, Belgium | |
| VII | PRSF-L128 | UCL 49; LMG 23313 | Wauters, G. | H | Blood, Belgium, 2002 | |
| VII | PRSF-L172 | CCUG 25594; LMG 10770 | BCCM/LMG | H | Feces, bowel drain, Sweden, 1989 | |
| VII | PRSF-L173 | CCUG 27333; LMG 10775 | BCCM/LMG | H | Hip, Sweden, 1990 | |
| VII | PRSF-L234 | CCUG 27772 | CCUG | H | Blood, Sweden, 1991 | |
| VII | PRSF-L252 | CCUG 33698 | CCUG | H | Blood, Sweden, 1994 | |
| VII | PRSF-L255 | CCUG 36679 | CCUG | H | Blood, Sweden, 1996 | |
| VII | PRSF-L257 | CCUG 44047 | CCUG | H | Vaginal flora, Sweden, 1999 | |
| VII | PRSF-L259 | CCUG 44260 | CCUG | H | Vaginal flora, Sweden, 2001 | |
| VII | PRSF-L271 | CCUG 37262 | CCUG | H | Blood, Sweden, 1996 | |
| VII | PRSF-L290 | A544/84; LMG 23550 | Harty, D. | H | Blood | 9 |
| VII | PRSF-L309 | DSM 6594 | D14 | N | Intestinal mucosa (human), Sweden, 1991 | |
| VII | PRSF-L326 | T24029; LMG 23321 | Rautelin, H. | H | Blood | 23 |
| VII | PRSF-L358 | L-1198; LMG 23576 | Avlami, A. | H | Blood | 3 |
| VII | PRSF-L368 | D19 | N | Dairy product, Switzerland, before 1984 | ||
| VII | PRSF-L370 | T32154; LMG 23326 | Rautelin, H. | H | Blood | 23 |
| VII | PRSF-L376 | D10 | P | Nonhuman, France, 1998 | ||
| VII | PRSF-L413 | 14.4a; LMG 23527 | D13 | H | Feces, Finland, 1999 | |
| VII | PRSF-L423 | 4.17a; LMG 23537 | D13 | H | Feces, Finland, 1999 | |
| VII | PRSF-L434 | D13 | H | Blood, United Kingdom, 1999 | ||
| VII | PRSF-L440 | T-25865 | D13 | H | Blood, Finland | |
| VII | PRSF-L477 | R | Feces | |||
| VII | PRSF-L488 | R | Feces | |||
| VII | LMG 6400T | BCCM/LMG | ||||
| Sc | PRSF-L016 | R-16076 | D40-P15 | P | Probiotic product | |
| S | PRSF-L072 | UCL 40; LMG 23301 | Wauters, G. | H | Blood, Belgium, 2000 | |
| S | PRSF-L086 | UCLF 6; LMG 23306 | Wauters, G. | H | Feces, Belgium | |
| S | PRSF-L169 | El Soda 42; LMG 18030 | BCCM/LMG | N | Zabady (yogurt), Egypt, 1992 | |
| S | PRSF-L323 | T4813; LMG 23318 | Rautelin, H. | H | Blood | 23 |
| S | PRSF-L400 | MR870 | Danisco | P | Human, United States |
BCCM/LMG, BCCM/LMG Bacteria Collection, Laboratorium voor Microbiologie, Ghent University, Gent, Belgium; CCUG, Culture Collection University of Göteborg, Department of Clinical Bacteriology, Göteborg, Sweden; CHUV, Centre de Collection de Type Microbien, Centre Hospitalier Universitaire Vaudois, Institut de Microbiologie, Université de Lausanne, Lausanne, Switzerland; Danisco, Danisco USA Inc., Madison, WI; Dx, probiotic strain collected from company x; Dx-Py, probiotic strain collected from product y from company x; Essum, Essum AB, Umea, Sweden; Rautelin H., The Haartman Institute, University of Helsinki, and Helsinki University Central Hospital Diagnostic Laboratory, Helsinki, Finland; Sacco, Sacco SRL, Cadorago, Italy; UIA, University Hospital Antwerp, Antwerp, Belgium; ULille, Laboratoire de Bactériologie, Faculté des Sciences Pharmaceutiques et Biologiques, Lille, France; Wauters G., Microbiology Unit, University of Louvain, Brussels, Belgium.
P, strain commercially used as probiotic; R, research strain investigated as potential probiotic strain; N, food isolates or industrial starters; H, human isolates.
S, single strains that remained ungrouped in numerical analysis of FAFLP profiles.
FAFLP.
FAFLP is a PCR-based technique for whole-genome DNA fingerprinting via the selective amplification of restriction fragments. Total chromosomal DNA was prepared using a modification of the method described by Pitcher et al. (20). Template preparation was carried out essentially as described previously (12). Purified genomic DNA is digested by two restriction enzymes, 4-base cutter EcoRI and 6-base cutter TaqI. Small double-stranded DNA molecules (adaptors; 15 to 20 bp) containing one compatible end are ligated to the corresponding “sticky end” of each restriction fragment. These adaptors serve as binding sites for selective amplification with primer combinations E01/T01 (primers extended with an additional A), E01/T03 (primers extended with an additional A and G, respectively), and E03/T03 (primers extended with an additional G). PCR products are separated according to their lengths on a high-resolution polyacrylamide gel using a DNA sequencer (ABI 377). Fragments that contain an adaptor specific for the restriction half site created by the 6-bp cutter are visualized due to the 5′-end labeling of the corresponding primer with the fluorescent dye 6-carboxyfluorescein. The resulting electrophoretic patterns are numerically analyzed with Bionumerics software, version 4.01 (Applied Maths, Belgium) using the Dice coefficient and unweighted pair group method linkage cluster analysis.
PFGE.
Strains were grown in MRS broth (Oxoid, United Kingdom) for 3 to 4 h until an optical density at 600 nm (OD600) of 0.3 to 0.4 was obtained. Cells were washed in 50 mM EDTA (pH 8.5) and resuspended in 11 ml of buffer until an OD600 of 0.5 to 0.8 was obtained. Of this suspension 125 μl was mixed with 750 μl of 1.1% (wt/vol) low-melting-point agarose (Bio-Rad) in buffer and pipetted into plug molds. The solidified plugs were incubated overnight at 37°C in 1 ml lysozyme solution (2 mg lysozyme per ml of buffer, with addition of 0.05% N-lauorylsarcosine and 12.5 U ml−1 mutanolysine). The agarose plugs were suspended in 4 ml NDS buffer with 2 mg ml−1 proteinase K (100 ml of NDS buffer contains 1 ml 1 M Tris-HCl [pH] 8.0], 10 ml 100% sodium duodecyl sulfate, and 89 ml 0.5 M EDTA [pH 8.5]) and incubated at 50°C overnight. After removal of the upper phase, the plugs were washed six times by gently shaking them in 50 mM EDTA (pH 8.5). The plugs were suspended in 400 μl of appropriate restriction buffer: for NotI 1× buffer D (6 mM Tris-HCl, 6 mM MgCl2, 150 mM NaCl, 1 mM dithiothreitol, pH 7.9) with addition of 0.1 mg ml−1 bovine serum albumin and for AscI 1× NEBuffer 4 (New England Biolabs). The restriction reactions with NotI (Promega, Belgium) and AscI (New England Biolabs) were carried out as specified by the respective manufacturers overnight at 37°C by adding 30 units of enzyme. The digestion was stopped by adding 0.4 ml of 0.05 M EDTA (pH 8.5), and the plugs were stored at 4°C. The restriction fragments were separated by PFGE in a contour-clamped homogeneous electric field MAPPER system (Bio-Rad) by loading plug fractions in 1.1% (wt/vol) pulsed-field-certified agarose (Bio-Rad) gel prepared with a 0.5× TBE buffer (45 mM Tris-HCl, 45 mM boric acid, 1 mM EDTA). Electrophoresis was performed in 2 liters 0.5× TBE at 14°C for 24 h at 5.3 V/cm and an angle of 120°, with pulse times ramping linearly from 1 to 15 s.
The low-range PFGE marker (Westburg, The Netherlands) was used as a molecular weight marker. The gels were stained with ethidium bromide. Conversion, normalization, and analysis of the band patterns were performed using Bionumerics software, version 4.01. Correlation coefficients and levels of similarity were calculated using the Dice coefficient, and cluster analysis used the unweighted pair group method.
RESULTS
Delineation of clusters.
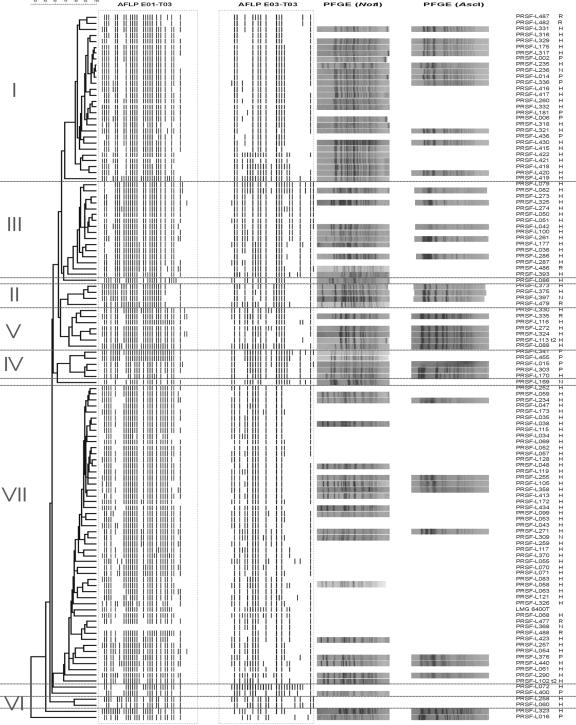
Three separate dendrograms were obtained from cluster analysis of FAFLP data generated with the primer combinations E01/T01, E01/T03, and E03/T03 (data not shown). The structures of the three dendrograms were highly congruent and allowed delineation of seven stable intraspecific FAFLP clusters in each of these trees. Six strains did not belong to any of these clusters and remained ungrouped. In Fig. 1, a combined numerical analysis of digitized FAFLP profiles obtained with the three primer combinations E01/T01, E01/T03, and E03/T03 in which the seven FAFLP clusters are indicated with roman numerals (I to VII) is visualized. For each of these clusters (except cluster VI), a selection of strains of different sources were subjected to macrorestriction analysis with NotI (69 strains) and AscI (37 strains) in order to reveal possible clonal relationships.
FIG. 1.
Dendrogram based on the combined numerical analysis of AFLP patterns of the three primer combinations E01/T01, E01/T03, and E03/T03, with visualization of the banding patterns for the last two primer combinations, and PFGE profiles of selected strains using NotI and/or AscI.
Cluster I (n = 28).
This is one of the largest clusters, comprising seven probiotic strains that are commercially used by five different companies. Strain PRSF-L175 corresponds to the L. rhamnosus strain GG (ATCC 53103, LMG 18243). In addition, two potentially probiotic research strains and one industrial starter culture also group in this cluster. Finally, cluster I also comprises 18 human isolates including 9 fecal isolates and 7 blood isolates. Two other human isolates originated from infected sites, i.e., a hepatic abscess (21) and a heart valve. PFGE analysis revealed that all tested strains (23 with NotI and 11 with AscI) within cluster I, except PRSF-L235, exhibited indistinguishable NotI and AscI macrorestriction profiles independent of their source or geographical origin.
Cluster II (n = 4).
This small cluster comprises one food starter culture, one potentially probiotic research strain, and two human blood isolates which were isolated after endocarditis (9). PFGE analysis revealed that three strains (not PRSF-L479) displayed indistinguishable patterns.
Cluster III (n = 16).
The third cluster comprises 1 potentially probiotic research strain and 15 human isolates, of which 10 were isolated from blood samples. The latter group represented published cases of L. rhamnosus isolation after organ transplantation (6), terminal ileitis (2), or endocarditis (9). A jaw isolate was isolated after a case of osteomyelitis. Furthermore, cluster III also contains four human commensal isolates from fecal, oral (throat pus), or vaginal microflora. PFGE analysis revealed a significant variation (in particular with the enzyme NotI) among the strains of this cluster.
Cluster IV (n = 5).
This group comprises four commercial probiotic strains used by three different companies and one human blood isolate. PFGE analysis demonstrated that all four probiotic strains are indistinguishable. In comparison, the human isolate harbors one additional band in the top region of its NotI macrorestriction profile.
Cluster V (n = 7).
This cluster comprises one potentially probiotic research strain and six human isolates, including three fecal isolates, two blood isolates, and one isolate from cerebrospinal fluid. PFGE analysis demonstrated that the human isolates PRSF-L116, PRSF-L272, and PRSF-L324 share identical NotI and AscI macrorestriction patterns and that other human and probiotic research strains analyzed differ in only one or two bands.
Cluster VI (n = 2).
This small cluster groups two human isolates, one from a blood sample and one from vaginal flora. The two strains of cluster VI were not tested in PFGE analysis.
Cluster VII (n = 50).
This is the largest cluster, comprising only 1 commercial probiotic strain, 2 potentially probiotic research strains, 2 food strains, and 45 human isolates. The latter group included 30 isolates from sterile sites (i.e., 23 from blood samples, 1 from an abscess, 1 from pleural fluid, 1 from bronchoalveolar lavage fluid, 1 from a hip, 1 from mediastinum, 1 from bile, and 1 not specified) and 15 from commensal flora (i.e., 10 of fecal origin, 2 from throat, 2 from vaginal flora, and 1 not specified). Within this cluster, a significant heterogeneity is observed among the PFGE profiles. The food starter strain PRSF-L309 was not distinguishable on the basis of its NotI profile from the human isolates PRSF-L099 and PRSF-L271.
DISCUSSION
Diversity of the strain collection.
The strains mainly originated from Belgium, Finland, Sweden, and The Netherlands and date from 1976 to 2001. To our knowledge, they represent the largest L. rhamnosus strain set studied to date for genotyping. The set includes not only commercial probiotic strains but also research strains with potential probiotic properties and food starter cultures. The largest part of the collection consisted of human isolates from normally sterile and nonsterile body sites (Table 1).
Intraspecific resolution of FAFLP versus PFGE analysis.
FAFLP is a powerful tool to reveal intraspecies diversity when based on the use of multiple primer combinations (29). In the present study, the very high congruency between the cluster analyses obtained with three different primer combinations was remarkable and resulted in the delineation of seven stable intraspecific groups (Fig. 1). Due to the highly selective amplification conditions, FAFLP primers perfectly match their target site and mismatches are not expected to participate in the amplification process (12). Although the observed strain-to-strain variations in the FAFLP patterns within a given cluster may reflect strain-specific differences, such variations are in most cases introduced during data processing in which one fixed threshold setting is used for automatic band recognition. For this reason, overall patterns instead of individual band positions should be considered in numerical analysis of FAFLP data. For strain typing, FAFLP should be complemented by other fingerprinting techniques such as PFGE.
PFGE is considered to offer the highest resolution for strain differentiation of lactic acid bacteria (4, 7, 22) and was in this study applied to a selection of probiotic and human strains to verify whether members of a given FAFLP cluster represent different strains or may be considered clones of the same parent strain. Because PFGE is a laborious and expensive method, only a limited number of strains were analyzed. It has been suggested that PFGE analysis with two well-chosen enzymes should be used for strain differentiation of lactobacilli, although in some studies on L. rhamnosus three or four enzymes were used (21, 24, 28). These studies all make use of NotI, which has been proven to generate strain-specific fingerprints (28), and additionally one or more of the following enzymes: AscI, SfiI, and FseI. Unfortunately, the difference in resolution between the last three enzymes is not discussed in any of these papers. In the present study, the enzymes NotI and AscI were selected for macrorestriction analysis, as they produced profiles with sufficient well-separated bands. Overall, NotI profiles were more discriminatory between strains than AscI patterns. Strains were considered to be indistinguishable if exactly the same number of bands occurred at the same positions upon visual comparison.
Delineation of intraspecies clusters and correlation between probiotic strains and human isolates.
Within the set of 118 L. rhamnosus strains considered for this study, seven stable intraspecific FAFLP clusters were delineated and six strains occupied separate positions. Given the fact that new members of existing clusters and/or additional intraspecific clusters are likely to be identified when more strains are added to our collection, it is clear that the grouping discussed below provides only a restricted insight in the intraspecific genotypic diversity of L. rhamnosus.
Clusters I, II, IV, and VII represent clusters that contain both human isolates and commercial probiotic strains or food starters. In cluster I, it was remarkable to find that all selected strains (23 out of 28 tested with NotI and 11 with AscI) except the heart valve isolate PRSF-L235 were indistinguishable based on PFGE typing. This cluster also comprises one of the best-known probiotic strains of L. rhamnosus, i.e., strain GG. All seven commercial probiotic strains, the two probiotic research strains, and the industrial starter culture showed indistinguishable PFGE profiles, suggesting that all these cultures might be commercial replicates of the GG strain. Taken together, these data could suggest that strain GG might be commercially used by at least five different companies. Cluster I also contains a liver abscess isolate that was previously found to be indistinguishable from L. rhamnosus strain GG based on PFGE analysis using the restriction enzymes NotI, AscI, and SfiI (21). Likewise, multiple human blood isolates (i.e., PRSF-L316, PRSF-L317, PRSF-L318, and PRSF-L332) associated with bacteremia could not be distinguished from L. rhamnosus strain GG using PFGE with four different enzymes (24). On the basis of phenotypic tests, adhesion properties, and respiration burst, Ouwehand and colleagues (19) were able to distinguish some of these blood isolates in one or more properties from the probiotic GG strain. In our opinion, however, results from this phenotypic typing approach should be interpreted with great caution as these tests have not been sufficiently validated and should not be used for strain typing purposes in Lactobacillus. Apart from the blood isolates, cluster I also grouped nine indistinguishable fecal isolates obtained from the same depositor. Unfortunately, no further information on the history of these isolates, e.g., whether they were isolated in the course of a probiotic intervention study, was available. Cluster II comprises one food starter culture, one potentially probiotic research strain, and two endocarditis isolates, three of them indistinguishable by PFGE. Both endocarditis isolates were studied for their surface-associated properties by Harty and coworkers (9). Cluster IV contained four indistinguishable probiotic strains commercially used by three different companies. In addition, this cluster also contained one blood isolate displaying only minor band differences in their PFGE profiles and may have the same clonal origin as the other strains of cluster IV. Cluster VII, the largest cluster containing both commercial and human strains, showed a higher degree of heterogeneity by PFGE. As shown for the members of cluster I, also in this cluster associations between commercial strains and human isolates could be observed. For instance, it was found that the food starter strain PRSF-L309 is indistinguishable from PRSF-L099 (a fecal isolate from France) and PRSF-L271 (a vaginal isolate from Sweden) based on NotI and AscI PFGE profiling.
Clusters III, V, and VI mainly contain human isolates occasionally joined by one or more potentially probiotic research strains. Clusters III and V contain only human isolates joined by one potentially probiotic research strain. It was remarkable to find indistinguishable patterns among cluster III members from diverse geographical origins. For instance, highly similar PFGE profiles were observed for strains PRSF-L042 (a blood isolate from Belgium), PRSF-L100 (a fecal isolate from France), and PRSF-L325 (a blood isolate from a nonspecified country) and for the blood isolates PRSF-L177 and PRSF-L286, previously included in the studies of Arpi et al. (2) and Harty et al. (9), respectively. Likewise, indistinguishable NotI and AscI PFGE profiles were generated for cluster V members PRSF-L116, PRSF-L272, and PRSF-L324 isolated in different European countries.
Conclusions.
The collection of 118 L. rhamnosus cultures included in this study consists of seven stable intraspecific FAFLP clusters, most of which are mixed groups with (potentially) probiotic and human strains. In several of these clusters, commercial strains were indistinguishable in PFGE from human isolates. Although the definition of clonal relatedness remains a matter of debate (26, 27), our findings do suggest that several companies use duplicate cultures of the same probiotic strain (e.g., L. rhamnosus GG) and that some human isolates may be reisolations of commercial strains. Also, the methodological concept of our study provides a frame for the integration of genotyping in epidemiological studies that aim to evaluate the safety of commercial probiotic cultures. The data presented in this study do not contradict the conclusion of Salminen and coworkers (24) that there is currently no clear evidence to anticipate an increased probability for acquiring an infection by the consumption of probiotics. On the other hand, it has been reported that bacteremia caused by strains of L. rhamnosus including isolates indistinguishable from the GG strain based on PFGE typing was associated with a higher mortality rate than Lactobacillus bacteremia caused by other species (23). However, despite the ubiquitous presence of lactobacilli, there are very few published cases of these clinically significant infections, and poor outcome is usually associated with severe underlying diseases. Collectively, it can thus be argued that risk of (co)infection with probiotic strains of L. rhamnosus might be higher in diseased and immunocompromised patients, but this hypothesis clearly needs further evaluation.
Acknowledgments
This work was supported by a grant from the European Commission (PROSAFE: Biosafety Evaluation of Lactic Acid Bacteria Used for Human Consumption; QLRT-2001-01273). G.H. is a postdoctoral fellow of the Fund for Scientific Research, Flanders, Belgium (F.W.O.-Vlaanderen).
We acknowledge all depositors of strains.
REFERENCES
- 1.Aguirre, M., and M. D. Collins. 1993. Lactic acid bacteria and human clinical infection. J. Appl. Bacteriol. 75:95-107. [DOI] [PubMed] [Google Scholar]
- 2.Arpi, M., M. Vancanneyt, J. Swings, and J. J. Leisner. 2003. Six cases of Lactobacillus bacteraemia: identification of organisms and antibiotic susceptibility and therapy. Scand. J. Infect. Dis. 35:404-408. [DOI] [PubMed] [Google Scholar]
- 3.Avlami, A., T. Kordossis, N. Vrizidis, and N. V. Sipsas. 2001. Lactobacillus rhamnosus endocarditis complicating colonoscopy. J. Infect. 42:283-285. [DOI] [PubMed] [Google Scholar]
- 4.Björkroth, J., J. Ridell, and H. Korkeala. 1996. Characterization of Lactobacillus sake strains associating with production of ropy slime by randomly amplified polymorphic DNA (RAPD) and pulsed-field gel electrophoresis (PFGE) patterns. Int. J. Food Microbiol. 31:59-68. [DOI] [PubMed] [Google Scholar]
- 5.Cannon, J. P., T. A. Lee, J. T. Bolanos, and L. H. Danziger. 2005. Pathogenic relevance of Lactobacillus: a retrospective review of over 200 cases. Eur. J. Clin. Microbiol. Infect. Dis. 24:31-40. [DOI] [PubMed] [Google Scholar]
- 6.Carretto, E., D. Barbarini, F. C. Marzani, P. Fumagalli, V. Monzillo, P. Marone, and V. Emmi. 2001. Catheter-related bacteremia due to Lactobacillus rhamnosus in a single-lung transplant recipient. Scand. J. Infect. Dis. 33:780-782. [DOI] [PubMed] [Google Scholar]
- 7.Ferrero, M., C. Cesena, L. Morelli, G. Scolari, and M. Vescovo. 1996. Molecular characterization of Lactobacillus casei strains. FEMS Microbiol. Lett. 140:215-219. [Google Scholar]
- 8.Harty, D. W., M. Patrikakis, E. B. Hume, H. J. Oakey, and K. W. Knox. 1993. The aggregation of human platelets by Lactobacillus species. J. Gen. Microbiol. 139:2945-2951. [DOI] [PubMed] [Google Scholar]
- 9.Harty, D. W. S., M. Patrikakis, and K. W. Knox. 1993. Identification of Lactobacillus strains isolated from patients with infective endocarditis and comparison of their surface-associated properties with those of other strains of the same species. Microb. Ecol. Health Dis. 6:191-201. [Google Scholar]
- 10.Holzapfel, W. H., P. Haberer, J. Snel, U. Schillinger, and J. H. J. Huis in't Veld. 1998. Overview of gut flora and probiotics. Int. J. Food Microbiol. 41:85-101. [DOI] [PubMed] [Google Scholar]
- 11.Husni, R. N., S. M. Gordon, J. A. Washington, and D. L. Longworth. 1997. Lactobacillus bacteremia and endocarditis: review of 45 cases. Clin. Infect. Dis. 25:1048-1055. [DOI] [PubMed] [Google Scholar]
- 12.Janssen, P., R. Coopman, G. Huys, J. Swings, M. Bleeker, P. Vos, M. Zabeau, and K. Kersters. 1996. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology 142:1881-1893. [DOI] [PubMed] [Google Scholar]
- 13.Kalliomäki, M., S. Salminen, H. Arvilommi, P. Kero, P. Koskinen, and E. Isolauri. 2001. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357:1076-1079. [DOI] [PubMed] [Google Scholar]
- 14.Kunz, A. N., J. M. Noel, and M. P. Fairchok. 2004. Two cases of Lactobacillus bacteremia during probiotic treatment of short gut syndrome. J. Pediatr. Gastroenterol. Nutr. 38:457-458. [DOI] [PubMed] [Google Scholar]
- 15.Land, M. H., K. Rouster-Stevens, C. R. Woods, M. L. Cannon, J. Cnota, and A. K. Shetty. 2005. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 115:178-181. [DOI] [PubMed] [Google Scholar]
- 16.MacGregor, G., A. J. Smith, B. Thakker, and J. Kinsella. 2002. Yoghurt biotherapy: contraindicated in immunosuppressed patients? Postgrad. Med. J. 78:366-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacKay, A. D., M. B. Taylor, C. C. Kibbler, and J. M. Hamilton-Miller. 1999. Lactobacillus endocarditis caused by a probiotic organism. Clin. Microbiol. Infect. 5:290-292. [DOI] [PubMed] [Google Scholar]
- 18.Oakey, H. J., D. W. Harty, and K. W. Knox. 1995. Enzyme production by lactobacilli and the potential link with infective endocarditis. J. Appl. Bacteriol. 78:142-148. [DOI] [PubMed] [Google Scholar]
- 19.Ouwehand, A. C., M. Saxelin, and S. Salminen. 2004. Phenotypic differences between commercial Lactobacillus rhamnosus GG and L. rhamnosus strains recovered from blood. Clin. Infect. Dis. 39:1858-1860. [DOI] [PubMed] [Google Scholar]
- 20.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 21.Rautio, M., H. Jousimies-Somer, H. Kauma, I. Pietarinen, M. Saxelin, S. Tynkkynen, and M. Koskela. 1999. Liver abscess due to a Lactobacillus rhamnosus strain indistinguishable from L. rhamnosus strain GG. Clin. Infect. Dis. 28:1159-1160. [DOI] [PubMed] [Google Scholar]
- 22.Roussel, Y., C. Colmin, J. M. Simonet, and B. Decaris. 1993. Strain characterization, genome size and plasmid content in the Lactobacillus acidophilus group (Hansen and Mocquot). J. Appl. Bacteriol. 74:549-556. [PubMed] [Google Scholar]
- 23.Salminen, M. K., H. Rautelin, S. Tynkkynen, T. Poussa, M. Saxelin, V. Valtonen, and A. Jarvinen. 2004. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin. Infect. Dis. 38:62-69. [DOI] [PubMed] [Google Scholar]
- 24.Salminen, M. K., S. Tynkkynen, H. Rautelin, M. Saxelin, M. Vaara, P. Ruutu, S. Sarna, V. Valtonen, and A. Jarvinen. 2002. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin. Infect. Dis. 35:1155-1160. [DOI] [PubMed] [Google Scholar]
- 25.Saxelin, M. 1997. Lactobacillus GG—a human probiotic strain with thorough clinical documentation. Food Rev. Int. 13:293-313. [Google Scholar]
- 26.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomayko, J. F., and B. E. Murray. 1995. Analysis of Enterococcus faecalis isolates from intercontinental sources by multilocus enzyme electrophoresis and pulsed-field gel electrophoresis. J. Clin. Microbiol. 33:2903-2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tynkkynen, S., R. Satokari, M. Saarela, T. Mattila-Sandholm, and M. Saxelin. 1999. Comparison of ribotyping, randomly amplified polymorphic DNA analysis, and pulsed-field gel electrophoresis in typing of Lactobacillus rhamnosus and L. casei strains. Appl. Environ. Microbiol. 65:3908-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vancanneyt, M., A. Lombardi, C. Andrighetto, E. Knijff, S. Torriani, K. J. Björkroth, C. M. A. P. Franz, M. R. Foulquié Moreno, H. Revets, L. De Vuyst, J. Swings, K. Kersters, F. Dellaglio, and W. H. Holzapfel. 2002. Intraspecies genomic groups in Enterococcus faecium and their correlation with origin and pathogenicity. Appl. Environ. Microbiol. 68:1381-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]