Abstract
Multilocus sequence typing of 151 Campylobacter coli isolates from swine reared in conventional (n = 74) and antimicrobial-free (n = 77) production systems revealed high genotypic diversity. Sequence type (ST) 1413 was predominant and observed among ciprofloxacin-resistant strains. We identified a C. coli ST 828 clonal complex consisting of isolates from both production systems.
Campylobacter is an important food-borne pathogen. Preliminary data estimated by FoodNet for diseases caused by enteric pathogens for the year 2004 show Campylobacter infection to have an incidence of 12.9 cases per 100,000 persons, second only to Salmonella (1). Various genotypic approaches have been used for outbreak investigations, source tracking, and determining the distribution of Campylobacter in humans, animals, and the environment (2, 3, 4). However, the Campylobacter genome has been shown to be hypervariable, which limits the use of methods like pulsed-field gel electrophoresis, flaA restriction fragment length polymorphism, and serotyping. It is therefore important to use a typing method that is based on indexing variation seen in the more conserved region of the genome, such as multilocus sequence typing (MLST) (7, 19, 23).
There is a paucity of information on the molecular epidemiology of Campylobacter coli, a species commonly identified in swine. The main objective of this study was to use MLST for determining the genotypic diversity and presence of clonal complexes and their association with antimicrobial resistance patterns in C. coli in swine in two distinct production systems. Swine reared in the conventional system were exposed to antimicrobials either for growth promotion or for therapeutic purposes. Under the antimicrobial-free (ABF) system, antimicrobials were not used for any purpose and animals exposed to antimicrobials due to sickness were removed from the group. We included 151 C. coli isolates, including 77 isolates from the ABF system (50 from the farm environment and 27 from the slaughter environment) and 74 from the conventional production system (46 from the farm environment and 28 from the slaughter environment) that were selected from a larger collection of 1,459 C. coli isolates (22). Selection of isolates was conducted systematically, representing the farm, slaughter, and antimicrobial resistance profiles and the total number of isolates recovered at each stage. This is represented by the higher number of isolates selected for genotyping from the farm environment than at slaughter. We used random-number-generator software to select among isolates with the same resistance profiles and isolated from the same farm and slaughter stages. Overall, the 151 C. coli isolates selected for typing using MLST satisfied the preset criteria for selection. Antimicrobial susceptibility testing against six antimicrobials was done using the agar dilution method as recommended by the Clinical Laboratory Standards Institute (CLSI) (18). MLST of the seven housekeeping genes (aspA, glnA, gltA, glyA, pgm, tkt, and uncA), sequence type (ST) generation, and analysis were done by following a method described previously (5, 6). Isolates with six or more shared alleles at each locus were considered members of the same clonal complex. The degree of clonality was determined using the index of association and phylogenetic analysis as shown previously (11, 12). A minimum spanning tree was created using Bionumerics software version 4.0 (Applied Maths, Kortrijik, Belgium). We used the ClustalW software (available at http://www.ebi.ac.uk/clustalw) to perform the sequence alignments.
A total of 33 different alleles were observed, and 51 (68.9%) out of 74 STs detected in this study are reported here for the first time. We detected 51 STs (31 from the farm environment and 20 from the slaughter environment) in the ABF and 49 STs (30 from the farm environment, 19 from the slaughter environment) in the conventional system. Overall, isolates that were temporally and spatially related were further differentiated based on STs, implying a higher discriminatory power of MLST than that of the phenotypic approaches. For example, erythromycin-resistant isolates 690 and 699 (conventional), isolated at the same sampling visit at slaughter, were associated with STs 1421 and 1443, respectively. We also found STs that were unique and observed only in a specific production system or processing stage, showing evidence of niche adaptation. For instance, ST 1413 (n = 9) was observed only in isolates from the conventionally reared pigs. Similarly, ST 1428 was isolated only at slaughter at different sampling visits and not in the farm environment. Cases of ST showing niche adaptation have been reported for swine and cattle (5, 17).
The higher frequency of unique STs observed in the slaughter environment than in the farm environment indicates that pigs can be exposed to new strains in the harvesting process. This also implies that not all strains detected at slaughter originated from the farm environment, and other factors, such as cross-contamination during trucking and in holding pens, remain a concern. It is possible that the different management and environmental factors may create selective pressure that enables specific STs to persist in a diverse environment. However, it is important to point out that although we tested three isolates per sample, it is possible that we may have missed sampling these genotypes in the farm environment. We observed different MLST profiles even for isolates that had the same antimicrobial resistance profile and were from the same sample. In addition, we also observed a larger number of STs at the farm level, which could be attributed to many reasons. Among the conventionally reared pigs, one potential reason could be the commingling of pigs from different farms at the finishing farms. In the case of the ABF pigs, in addition to commingling, horizontal transmission via exposure to the environment could be another reason why the pigs were infected with this pathogen. Horizontal transmission of Campylobacter from environmental sources has been suggested to be an important route for the spread of infection in food animals (8, 10, 13).
The high genotypic diversity of the C. coli population was reflected by the low index-of-association value for the overall population (0.37) and also at the individual production level, ranging from 0.34 for the ABF system to 0.62 for the conventional system. High divergence was observed in the alleles, including those with ST 1415 (47 nucleotide changes), when all seven housekeeping gene sequences were concatenated and compared to other STs displaying extreme sequence divergence. This could be an indication of a recombinational event, which in turn contributes to the weak clonality of the population (15). Hume et al. reported the absence of shared genotypes from isolates from sows, their respective piglets, and their littermates, highlighting the extent of the diversity of this pathogen (9). Similar observations were made when we analyzed the STs with respect to the resistance patterns. Barring a few STs that were restricted to specific resistance patterns, most of the STs were found to be associated with different resistance patterns, again highlighting the diverse genetic makeup of this species (Table 1). However, it is important to highlight that the multidrug-resistant C. coli isolates that were resistant to ciprofloxacin from the conventional farms were associated only with ST 1413. This ST is reported here for the first time and could represent an ST associated with ciprofloxacin-resistant C. coli strains.
TABLE 1.
ST 828 lineage, sequence types, and resistance patterns observed in C. coli from swine in the farm environment and at slaughter
| ST | Allele no. for:
|
No. of isolates with ST | Production system(s)a | Resistance profileb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| aspA | glnA | gltA | glyA | pgm | tkt | uncA | ||||
| 825 | 33 | 39 | 30 | 82 | 113 | 47 | 17 | 4 | ABF (F), conv (F) | E, T, ChET |
| 828 | 33 | 39 | 30 | 82 | 104 | 43 | 17 | 2 | ABF (S) | Pansusceptible, T |
| 854 | 33 | 38 | 30 | 82 | 104 | 43 | 17 | 7 | ABF (F), conv (F, S) | E, T, ET |
| 887 | 33 | 38 | 30 | 82 | 104 | 85 | 68 | 4 | Conv (S) | T, ChET, ChEGNaT |
| 890 | 33 | 38 | 30 | 82 | 104 | 35 | 36 | 5 | ABF (F, S), conv (F, S) | T, ET, NaT, ChET |
| 1055 | 33 | 39 | 30 | 82 | 104 | 47 | 17 | 1 | ABF (F) | CENaT |
| 1056 | 33 | 39 | 30 | 82 | 104 | 43 | 36 | 2 | ABF (S), conv (F) | E, ET |
| 1058 | 33 | 39 | 30 | 82 | 104 | 35 | 17 | 1 | ABF (S) | Pansusceptible |
| 1059 | 33 | 153 | 30 | 82 | 104 | 35 | 17 | 1 | Conv (F) | ET |
| 1068 | 33 | 39 | 30 | 78 | 104 | 43 | 17 | 2 | ABF (F), conv (S) | NAL, E |
| 1096 | 33 | 38 | 30 | 82 | 104 | 35 | 17 | 9 | ABF (F, S), conv (F, S) | E, ET, ChET, ChEG, CENaT, ENaT |
| 1102 | 33 | 38 | 30 | 82 | 152 | 173 | 68 | 1 | ABF (F) | E |
| 1112 | 33 | 39 | 30 | 82 | 104 | 43 | 68 | 7 | ABF (F, S), conv (F) | Pansusceptible, E, T, ET, NaT, ChET |
| 1113 | 33 | 38 | 30 | 78 | 104 | 35 | 17 | 1 | ABF (S) | T |
| 1123 | 53 | 38 | 44 | 82 | 118 | 35 | 36 | 6 | ABF (F), conv (S) | Pansusceptible, T, ET, ChENaT |
| 1130 | 33 | 38 | 30 | 82 | 104 | 43 | 68 | 2 | ABF (F), conv (S) | T, ET |
| 1134 | 33 | 38 | 30 | 82 | 104 | 173 | 68 | 5 | ABF (F), conv (F) | Pansusceptible, NaT, ChET |
| 1142 | 33 | 153 | 30 | 82 | 104 | 43 | 36 | 1 | Conv (S) | T |
| 1177 | 33 | 38 | 30 | 82 | 104 | 85 | 17 | 3 | ABF (F), conv (F) | ENa |
| 1185 | 33 | 38 | 44 | 82 | 104 | 35 | 36 | 1 | ABF (S) | ET |
| 1200 | 53 | 38 | 30 | 82 | 118 | 35 | 36 | 1 | ABF (F) | T |
| 1413 | 33 | 38 | 30 | 82 | 104 | 117 | 17 | 9 | Conv (F) | T, ChET, CENaT |
| 1414 | 33 | 39 | 37 | 82 | 104 | 43 | 68 | 2 | ABF (F) | Pansusceptible, ET |
| 1415 | 33 | 39 | 47 | 82 | 104 | 43 | 36 | 1 | ABF (S) | E |
| 1417 | 33 | 39 | 44 | 82 | 104 | 35 | 36 | 3 | Conv (F, S) | E, ET |
| 1419 | 33 | 38 | 46 | 82 | 104 | 117 | 17 | 1 | Conv (F) | CENaT |
| 1421 | 33 | 38 | 44 | 82 | 104 | 117 | 36 | 1 | Conv (F) | E |
| 1422 | 33 | 38 | 46 | 82 | 104 | 173 | 17 | 1 | Conv (F) | ET |
| 1423 | 33 | 39 | 46 | 82 | 104 | 47 | 17 | 1 | Conv (F) | ET |
| 1424 | 33 | 39 | 132 | 82 | 104 | 44 | 17 | 1 | ABF (F) | ChNaT |
| 1426 | 53 | 38 | 30 | 81 | 118 | 85 | 36 | 1 | ABF (F) | Pansusceptible |
| 1428 | 53 | 38 | 30 | 81 | 118 | 43 | 36 | 4 | Conv (S) | E, ET |
| 1429 | 33 | 39 | 44 | 82 | 189 | 35 | 36 | 1 | Conv (F) | ET |
| 1436 | 33 | 39 | 44 | 82 | 104 | 44 | 17 | 1 | ABF (F) | T |
| 1437 | 33 | 39 | 46 | 82 | 104 | 43 | 68 | 1 | ABF (F) | ENaT |
| 1438 | 33 | 153 | 30 | 82 | 104 | 44 | 17 | 3 | ABF (F), conv (F, S) | ET, CNaT |
| 1439 | 33 | 38 | 30 | 82 | 104 | 173 | 17 | 2 | Conv (F) | ENaT |
| 1441 | 33 | 38 | 37 | 82 | 104 | 117 | 17 | 1 | Conv (F) | CENaT |
| 1442 | 33 | 38 | 132 | 82 | 104 | 85 | 17 | 1 | Conv (F) | ET |
| 1444 | 33 | 39 | 30 | 82 | 104 | 171 | 17 | 1 | ABF (F) | E |
| 1445 | 33 | 39 | 30 | 82 | 104 | 85 | 17 | 4 | ABF (S), conv (F) | Pansusceptible, T, ET |
| 1446 | 33 | 38 | 30 | 82 | 118 | 35 | 17 | 2 | ABF (F) | ET |
| 1449 | 33 | 38 | 37 | 78 | 104 | 35 | 17 | 1 | ABF (S) | T |
| 1450 | 53 | 39 | 44 | 82 | 104 | 35 | 36 | 4 | ABF (S) | Pansusceptible, Na, T, ET |
| 1452 | 33 | 39 | 46 | 82 | 104 | 44 | 17 | 2 | ABF (F) | Pansusceptible, CNaT |
| 1455 | 33 | 38 | 37 | 82 | 104 | 85 | 68 | 1 | Conv (S) | T |
| 1463 | 33 | 153 | 30 | 82 | 104 | 85 | 17 | 1 | ABF (F) | NaT |
| 1464 | 33 | 39 | 30 | 82 | 104 | 117 | 17 | 1 | Conv (F) | EGT |
| 1465 | 33 | 39 | 30 | 82 | 113 | 117 | 17 | 1 | Conv (F) | EGT |
ABF (F), antimicrobial-free production system (finishing farm); ABF (S), antimicrobial-free production system (slaughter); conv (F), conventional production system (finishing farm); conv (S), conventional production system (slaughter).
Ch, chloramphenicol; C, ciprofloxacin; E, erythromycin; G, gentamicin; Na, nalidixic acid; T, tetracycline.
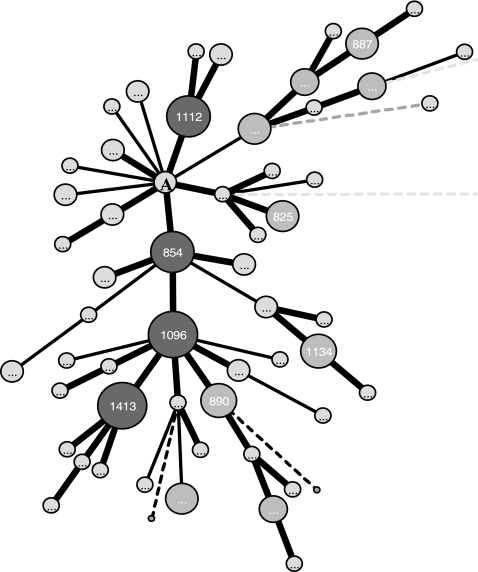
Phylogenetic comparison of C. coli isolates from the two production systems revealed that the two populations of C. coli had no fixed differences between them and had 67 shared mutations, indicating similarity at the sequence level. A minimum spanning tree constructed using the 74 STs revealed close clustering of isolates irrespective of their origin (Fig. 1). A single clonal complex (ST 828 complex) which included STs (n = 53) representing isolates from both of the production systems was identified (Table 1 and Fig. 1). The identification of clonal complexes in C. jejuni and their use as an epidemiological unit have tremendously helped in comparing isolates from different parts of the world (2, 4, 5, 14, 20, 21). Miller et al. recently reported a C. coli clonal complex comprising isolates from diverse sources, including humans, swine, chickens, sheep, a marmoset, and manure (16). The potential progenitor strain ST 828 has been reported for humans, swine, and cattle from different parts of the world (5, 17). This indicates that ST 828 is an important ST that has potential to infect both humans and animals and is an important finding from a food-borne-disease perspective. We acknowledge that typing of more isolates, including isolates from diverse sources, will help in better defining this apparent lineage.
FIG. 1.
Minimum spanning tree constructed using the STs from 151 C. coli isolates from the two production systems. The ST 828 complex shown is the putative founder of the major clonal complex. The sizes of the circles are proportional to the numbers of isolates with the indicated STs. The ST 828 complex is represented by the letter A.
In conclusion, MLST of C. coli isolates from swine highlighted the overall weak clonal population and the diverse genetic makeup of this species. However, the high proportion of STs being shared and the close clustering of STs in the two production systems provide evidence that these genotypically diverse C. coli strains are commonly shared between pigs reared in different production systems. This finding might potentially explain the reason for the high prevalence of antimicrobial-resistant C. coli in the ABF production system (22). Given the potential of pig isolates to cause disease in humans (14), we recommend testing of more C. coli isolates from diverse geographic sources and time points to better define the clonal relationships and lineages.
Acknowledgments
The work was supported by a research grant funded by the United States Department of Agriculture (2002-51110-01508).
REFERENCES
- 1.Centers for Disease Control. 2005. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 sites, United States, 2004. Morb. Mortal. Wkly. Rep. 54:352-356. [PubMed] [Google Scholar]
- 2.Colles, F. M., K. Jones, R. M. Harding, and M. C. Maiden. 2003. Genetic diversity of Campylobacter jejuni isolates from farm animals and the farm environment. Appl. Environ. Microbiol. 69:7409-7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dingle, K. E., F. M. Colles, D. R. Wareing, R. Ure, A. J. Fox, F. E. Bolton, H. J. Bootsma, R. J. Willems, R. Urwin, and M. C. Maiden. 2001. Multilocus sequence typing for Campylobacter jejuni. J. Clin. Microbiol. 39:14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dingle, K. E., F. M. Colles, R. Ure, J. A. Wagenaar, B. Duim, F. J. Bolton, A. J. Fox, D. R. A. Wareing, and M. J. C. Maiden. 2002. Molecular characterization of Campylobacter jejuni clones: a basis for epidemiologic investigation. Emerg. Infect. Dis. 8:949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dingle, K. E., F. M. Colles, D. Falush, and M. C. Maiden. 2005. Sequence typing and comparison of population biology of Campylobacter coli and Campylobacter jejuni. J. Clin. Microbiol. 43:340-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feil, E. J., B. C. Lei, D. M. Aanensen, W. P. Hanage, and B. G. Spratt. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington, C. S., F. M. Thomson-Carter, and P. E. Carter. 1997. Evidence for recombination in the flagellin locus of Campylobacter jejuni: implications for the flagellin gene typing scheme. J. Clin. Microbiol. 35:2386-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heuer, O. E., K. Pedersen, J. S. Andersen, and M. Madsen. 2001. Prevalence and antimicrobial susceptibility of thermophilic Campylobacter in organic and conventional broiler flocks. Lett. Appl. Microbiol. 33:269-274. [DOI] [PubMed] [Google Scholar]
- 9.Hume, M. E., R. E. Droleskey, C. L. Sheffield, and R. B. Harvey. 2002. Campylobacter coli pulsed field gel electrophoresis genotypic diversity among sows and piglets in a farrowing barn. Curr. Microbiol. 45:128-132. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs-Reitsma, W. F., A. W. van de Giessen, N. M. Bolder, and R. W. Mulder. 1995. Epidemiology of Campylobacter spp. at two Dutch broiler farms. Epidemiol. Infect. 114:413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jolley, K. A., E. J. Feil, M. S. Chan, and M. C. Maiden. 2001. Sequence type analysis and recombinational tests (START). Bioinformatics 17:1230-1231. [DOI] [PubMed] [Google Scholar]
- 12.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 13.Leatherbarrow, A. J., C. A. Hart, R. Kemp, N. J. Williams, A. Ridley, M. Sharma, P. J. Diggle, E. J. Wright, J. Sutherst, and N. P. French. 2004. Genotypic and antibiotic susceptibility characteristics of a Campylobacter coli population isolated from dairy farmland in the United Kingdom. Appl. Environ. Microbiol. 70:822-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manning, G., C. G. Dowson, M. C. Bagnall, I. H. Ahmad, M. West, and D. G. Newell. 2003. Multilocus sequence typing for comparison of veterinary and human isolates of Campylobacter jejuni. Appl. Environ. Microbiol. 69:6370-6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meinersmann, R. J., C. M. Patton, G. M. Evins, I. K. Wachsmuth, and P. I. Fields. 2002. Genetic diversity and relationships of Campylobacter species and subspecies. Int. J. Syst. Evol. Microbiol. 52:1789-1797. [DOI] [PubMed] [Google Scholar]
- 16.Miller, W. G., S. L. On, G. Wang, S. Fontanoz, A. J. Lastovica, and R. E. Mandrell. 2005. Extended multilocus sequence typing system for Campylobacter coli, C. lari, C. upsaliensis, and C. helveticus. J. Clin. Microbiol. 43:2315-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller, W. G., M. D. Englen, S. Kathariou, I. V. Wesley, G. Wang, L. Pittenger-Alley, R. M. Siletz, W. Muraoka, P. J. Federka-Cray, and R. E. Mandrell. 2006. Identification of host-associated alleles by multilocus sequence typing of Campylobacter coli strains from food animals. Microbiology 152:245-255. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. 1999. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. Approved standard M31-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 19.On, S. L. W. 1998. In vitro genotypic variation of Campylobacter coli documented by pulsed-field gel electrophoretic DNA profiling implications for epidemiological studies. FEMS Microbiol. Lett. 165:341-346. [DOI] [PubMed] [Google Scholar]
- 20.Sails, A. D., B. Swaminathan, and P. I. Fields. 2003. Clonal complexes of Campylobacter jejuni identified by multilocus sequence typing correlate with strain associations identified by multilocus enzyme electrophoresis. J. Clin. Microbiol. 41:4058-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sails, A. D., B. Swaminathan, and P. I. Fields. 2003. Utility of multilocus sequence typing as an epidemiological tool for investigation of outbreaks of gastroenteritis caused by Campylobacter jejuni. J. Clin. Microbiol. 41:4733-4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thakur, S., and W. A. Gebreyes. 2005. Prevalence and antimicrobial resistance of Campylobacter in antimicrobial-free and conventional pig production systems. J. Food Prot. 68:2402-2410. [DOI] [PubMed] [Google Scholar]
- 23.Weijtens, M. J., R. D. Reinders, H. A. Urlings, and J. Van der Plas. 1999. Campylobacter infections in fattening pigs; excretion pattern and genetic diversity. J. Appl. Microbiol. 86:63-70. [DOI] [PubMed] [Google Scholar]