Abstract
Hereditary multiple exostoses, a dominantly inherited genetic disorder characterized by multiple cartilaginous tumors, is caused by mutations in members of the EXT gene family, EXT1 or EXT2. The proteins encoded by these genes, EXT1 and EXT2, are endoplasmic reticulum-localized type II transmembrane glycoproteins that possess or are tightly associated with glycosyltransferase activities involved in the polymerization of heparan sulfate. Here, by testing a cell line with a specific defect in EXT1 in in vivo and in vitro assays, we show that EXT2 does not harbor significant glycosyltransferase activity in the absence of EXT1. Instead, it appears that EXT1 and EXT2 form a hetero-oligomeric complex in vivo that leads to the accumulation of both proteins in the Golgi apparatus. Remarkably, the Golgi-localized EXT1/EXT2 complex possesses substantially higher glycosyltransferase activity than EXT1 or EXT2 alone, which suggests that the complex represents the biologically relevant form of the enzyme(s). These findings provide a rationale to explain how inherited mutations in either of the two EXT genes can cause loss of activity, resulting in hereditary multiple exostoses.
Hereditary multiple exostoses (HME) is an autosomal dominant disorder characterized by the formation of cartilage-capped tumors (exostoses) that develop from the growth plate of endochondral bone (1). This condition can lead to skeletal abnormalities, short stature, and in some instances, malignant transformation from exostoses to chondrosarcomas (2, 3) or osteosarcomas (4, 5). Although genetic linkage analysis has identified three different loci for HME, EXT1 on 8q24.1, EXT2 on 11p11–13, and EXT3 on 19p (6–8), most HME cases have been attributed to missense or frameshift mutations in either EXT1 or EXT2 (9–15). EXT1 and EXT2 encode 746- and 718-aa proteins, respectively, that are expressed ubiquitously in human tissues (9, 16).
Previous studies using epitope-tagged constructs have demonstrated that EXT1 is a predominantly endoplasmic reticulum (ER)-localized glycoprotein whose expression enhances the synthesis of cell surface heparan sulfate (HS) (17). HS chains are composed of alternating residues of d-glucuronic acid (GlcA) and N-acetyl-d-glucosamine (GlcNAc) joined by 1→4 linkages, and a recent study has shown that both EXT1 and EXT2 harbor GlcA transferase (GlcA-T) and GlcNAc transferase (GlcNAc-T) activities that catalyze the polymerization of HS (18). EXT1 and EXT2 are structurally similar to previously identified glycosyltransferases in that they are type II transmembrane proteins comprising an N-terminal cytoplasmic tail, a transmembrane domain, a stalk, and a large globular domain that is likely to harbor enzymatic activity (19). Moreover, a truncated active form of EXT2 is secreted from cells and can be isolated from serum (18), which is a fate common to other ER and Golgi-localized glycosyltransferases, including EXTL2, which is an EXT homolog shown to encode an α1,4-N-acetylhexosaminyltransferase (20). However, several important questions are raised by these observations. EXT1, when overexpressed in a cell, appears to be localized predominantly to the ER (17, 21), whereas the biosynthesis of HS chains is thought to occur in the Golgi cisternae (22–25). Moreover, if the EXT1 and EXT2 genes encode functionally redundant HS polymerases (HS-Pol), it is not clear why mutations in either gene cause HME.
To address these questions, we overexpressed functional epitope-tagged and native forms of EXT1 and EXT2 in cells and examined their subcellular localization and enzymatic activity. By using a cell line, sog9, with a specific defect in EXT1, we show that EXT2 does not harbor significant glycosyltransferase activity in the absence of EXT1. Instead, it appears that EXT1 and EXT2 form a hetero-oligomeric complex in vivo that leads to an accumulation of both proteins in the Golgi apparatus. Remarkably, the Golgi-localized EXT1/EXT2 complex possesses substantially higher glycosyltransferase activity than EXT1 or EXT2 alone, which suggests that this complex represents the biologically relevant form of the enzyme(s). These findings provide a rationale to explain how inherited mutations in either of the two EXT genes can cause loss of activity, resulting in hereditary multiple exostoses.
Materials and Methods
EXT Constructs.
pEXT1 was isolated from a HeLa cell cDNA library in pcDNA3.1 (A550–26, Invitrogen) as described previously (17). pEXT1 myc-His, pG339DEXT1 myc-His, and pR340CEXT1 myc-His were constructed as described previously (17). All reagents were obtained from Life Technologies unless otherwise stated. pEXT1 GFP was constructed by excision of EXT1 from pEXT1 myc-His with BamHI and SstII, followed by ligation into the BglII and SstII sites in the pEGFP-N1 expression vector (CLONTECH). The bovine EXT2 constructs were constructed as previously described (18). pbEXT2 myc was constructed by PCR of the EXT2 coding region by using primers 5′-CGG GAT CCC GGT TTC ATT ATG TGT GCG TCA GTC AAG TCC AAC A-3′ and 5′-GCT CTA GAG CTC ACA GAT CCT CTT CTG AGA TGA GTT TTT GTT CTA AGC TGC CAA TGT TGG-3′. After digestion with BamHI and XbaI, the bEXT2 myc PCR product was then ligated into pcDNA3.1/myc-His B. A murine EXT2 (mEXT2) cDNA was a gift from M. Lovett (Washington University School of Medicine, St. Louis). pmEXT2 GFP was constructed by PCR using primers 5′-CGG GAT CCC GGT TTC ATT ATG TGT GCG TCA GTC AAG TCC AAC A-3′ and 5′-TCC CCG CGG GGA TAA GCT GCC AAT GTT GGG GAA-3′. The mEXT2 PCR product was ligated into T-tailed pBluescript (Stratagene), followed by digestion with HindIII and EcoRI and subsequent ligation into pEGFP-N1. pmEXT2 myc-His was constructed by digestion of pmEXT2 GFP with HindIII and SstII and ligation into the pcDNA3.1/myc-His B vector. To isolate human EXTL2, EXTL3, and murine N-deacetylase/N-sulfotransferase-2 (NDST2), total cellular RNA was isolated from confluent HeLa cell and L cell cultures by using Trizol reagent, reverse transcribed and amplified by PCR using primers 5′-CCG CTC GAG CGG AAT TAA ACT TCA ACA CAA TG-3′ and 5′-GGG GTA CCC CTA TTT TTC TTT TGT AGT TGG CAT-3′ for EXTL2, 5′-CCG CTC GAG CGG CAG GCT GCA GAG GAC TCA T-3′ and 5′-GGG GTA CCC CGA TGA ACT TGA AGC ACT TGG TCT-3′ for EXTL3 and 5′-GAA GAT CTT CCC ACC ATG CTC CAG CTG TGG AAG GT-3′ and 5′-CGG AAT TCC GCC CAC ACT GGA ATG TTG CAA T-3′ for NDST2. The digested PCR products were ligated into the appropriate sites in pEGFP-N1. G339DEXT1-GFP, R340CEXT1-GFP, and ΔNTMEXT1-GFP were constructed by digestion and subsequent ligation into pEGFP-N1.
Herpes Simplex Virus Type 1 (HSV-1) Infection Assay.
The procedure for the isolation of mutant sog9 cells was described previously (26), as was the HSV-1 infection assay (17).
Analysis of the mEXT1 Transcript in Cultured Mouse Fibroblast Cells.
Total cellular RNA was isolated from confluent murine L and sog9 cell cultures by using Trizol reagent, purified by using oligo(dT)-cellulose columns, reverse transcribed, and then amplified with the EXT1-specific primers 5′-CCG GAA TTC CGG AAG TCG TTC AAT GTC TCT G-3′ and 5′-CCG GAA TTC CGG AAG TCG CTC AAT GTC TCG GTA-3′. The amplified fragment was used to map a putative deletion observed for the sog9 mRNA transcript, and the identified region was then amplified by PCR from L and sog9 cells, using internal mEXT1-specific primers: 5′-ACC ATC CCT CCT CTC AGG AAG-3′ and 5′-CCA CAG AAC TAT GAT CTG CGC-3′, and sequenced. The original full-length reverse transcription–PCR products were cloned into pcDNA3.1/myc-HisA (Invitrogen), resulting in a Myc-His-tagged wild-type EXT1 and an untagged truncated 335-aa EXT1 protein. Both the wild-type and the mutant EXT1 coding regions were cloned into pEGFP-N1.
Fluorescence Microscopy.
Monolayers of BHK or sog9 cells were grown on glass coverslips to 70% confluence in DMEM/10% FBS and transfected with EXT constructs by using Lipofectamine Plus. At 30 h after transfection, the cells were rinsed with PBS and fixed in 4% paraformaldehyde for 15 min, followed by a 15-min incubation in PBS with 1% BSA. For indirect immunofluorescence experiments, cells were incubated with anti-His monoclonal antibody (Invitrogen) or anti-Myc monoclonal antibody (Invitrogen) at 1:100, and anti-Golgi 58K monoclonal antibody (Sigma) or anti-calnexin monoclonal antibody (Transduction Laboratories) at 1:50 in PBS/1% BSA with 0.25% saponin (Sigma) for 1 h. Cells were washed with PBS, then incubated with goat anti-mouse IgG conjugated to Texas red (Jackson Immunochemicals) at 1:200 in PBS/1% BSA for 30 min. Cells were washed with PBS and mounted in 30% glycerol in PBS. Fluorescence was observed with a Bio-Rad MRC 600 confocal epifluorescence microscope. Confocal images were rendered by using NIH image Version 1.60 and colorized with Adobe PhotoShop Version 5.0 (Adobe Systems).
Immunoprecipitations.
BHK cells (1 × 106) were transfected with green fluorescent protein (GFP) or Myc-His-tagged EXT constructs. After 20 h, cells were radiolabeled with 100 μCi/ml (1 μCi = 37 kBq) [35S]methionine (ICN) in methionine- and cysteine-free DMEM (ICN) for 1.5 h at 37°C. Cells were washed with PBS and lysed in Triton lysis buffer [2% Triton X-100/20 mM Tris⋅HCl pH 7.4/150 mM NaCl containing CØmplete protease inhibitors (Roche)] at 4°C for 15 min. The lysates were centrifuged at 12,000 × g for 15 min, and precleared for 30 min with 25 μl of protein G-Sepharose (Pharmacia) at 4°C. The lysates were then incubated with 0.5 μg of mouse anti-Myc monoclonal antibody (Invitrogen) or 0.5 μg of rabbit anti-GFP monoclonal antibody (CLONTECH) for 2 h, followed by incubation with 25 μl of protein G-Sepharose for 1 h. The lysates were centrifuged at 12,000 × g for 10 s, and washed two times with 10 mM Tris⋅HCl pH 7.4/150 mM NaCl/2 mM EDTA/0.2% Triton X-100, two times with 10 mM Tris⋅HCl, pH 7.4/500 mM NaCl/2 mM EDTA/0.2% Triton X-100, and two times with 10 mM Tris⋅HCl, pH 7.4. The pellet was suspended in 30 μl of SDS/PAGE sample buffer and boiled for 5 min before SDS/PAGE. Proteins were transferred to Immobilon-P membranes (Millipore) and exposed to BioMAX MR film (Kodak).
Assay of Cellular Glycosyltransferase Activities.
BHK or mutant sog9 cells were transfected with EXT constructs. At 30 h after transfection, cells were washed in PBS and lysed in Triton/glycerol lysis buffer (2% Triton X-100/50% glycerol/20 mM Tris⋅HCl, pH 7.4/150 mM NaCl containing CØmplete protease inhibitors) with gentle agitation at 4°C for 15 min. The lysates were centrifuged at 12,000 × g for 15 min, and a portion of the supernatant representing 5 × 105 cell equivalents was subjected to immunoprecipitation as described above. Prior to the final wash, the beads were split into two equal fractions and centrifuged. Each pellet was suspended in 10 μl of either GlcNAc-T reaction mix [20 μg of (GlcA-GlcNAc)n acceptor, 0.04 μCi of UDP-[3H]GlcNAc, 10 mM MnCl2, 0.04% Triton X-100, and 70 mM Hepes, pH 7.2] or GlcA-T reaction mix [40 μg of GlcNAc-(GlcA-GlcNAc)n acceptor, 0.032 μCi of UDP-[14C]GlcA, 10 mM MgCl2, 5 mM CaCl2, 0.04% Triton X-100, and 70 mM Hepes, pH 7.2], and incubated for 30 min at 37°C as described previously (18). The reaction products were suspended in 1 ml of H2O and centrifuged at 12,000 × g for 1 min before loading on a 50-cm Sepharose G-25 column. Labeled oligosaccharides were eluted in 50 mM Tris⋅HCl, pH 7.4/1 M NaCl/1% Triton X-100 and quantified by liquid scintillation spectroscopy.
Results
Sog9 Cells Are Defective in EXT1.
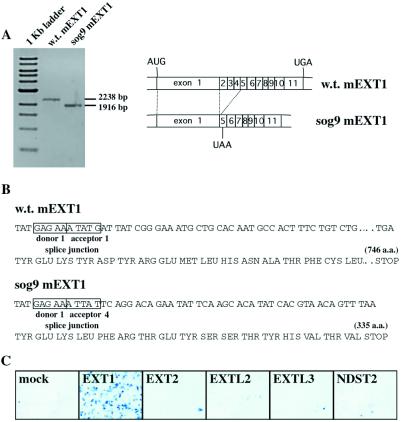
Previous studies have shown that expression of wild-type or epitope-tagged EXT1 cDNA in a glycosaminoglycan-deficient murine L cell mutant, sog9, results in an increase in the expression of HS (17). To determine whether sog9 cells contain a specific defect in the EXT1 gene, EXT1 mRNAs from sog9 and control L cells were amplified by reverse transcription–PCR and characterized. The EXT1 cDNA amplified from sog9 cells was 322 bp shorter than the full-length sequence (Fig. 1A), and sequence analysis revealed that this shortening was caused by splicing exon 1 to exon 5 in a +1 reading frame. This predicts that sog9 cells synthesize a truncated EXT1 protein of 335 amino acids (Fig. 1B). The lack of HS-Pol activity in sog9 cells (17) indicates that this truncated protein is not functional. Moreover, overexpression of the truncated protein in sog9 cells did not restore any functional EXT activity (data not shown).
Figure 1.
Sequence and rescue of the EXT1 defect in sog9 cells. (A) The coding region of murine EXT1 cDNA was amplified from wild-type L cells and HS-deficient sog9 cells by reverse transcription-PCR and analyzed on a 0.8% agarose gel. (B) Sequence analysis indicates that sog9 cells contain a mutation that results in splicing of exon 1 to exon 5 in a +1 reading frame, predicting a truncated 335-aa EXT1 protein. (C) sog9 cells were transfected with the indicated constructs and infected with HSV-1 to detect the presence of newly synthesized cell surface HS. Infected cells stain blue in the presence of 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal).
EXT1 and EXT2 Are Functionally Distinct Proteins.
To test the in vivo function of the EXT proteins, we used an assay based on the ability of HSV-1 to infect cells by attaching to cell surface HS (17). The target cells, sog9 cells, are 99.5% resistant to HSV-1 infection compared with control cells because of their defect in HS biosynthesis (26). Transfection of sog9 cells with native forms (17) or tagged forms (Fig. 1C) of human EXT1 resulted in a 200-fold enhancement of HSV-1 infection because of a restoration of HS biosynthesis and subsequent expression of HS on the cell surface, as measured by anion-exchange chromatography of glycosaminoglycans (17). By contrast, the related protein EXT2 displayed no activity in this assay (Fig. 1C). Because sog9 cells lack a functional EXT1, these results indicate that EXT2 alone does not possess significant HS-Pol activity in the absence of full-length EXT1. Additional experiments also established that the EXT homologs EXTL3 (27) and EXTL2 (20), and the HS-modifying enzyme N-deacetylase/N-sulfotransferase-2 (NDST2)(28) were inactive in this assay (Fig. 1C), indicating that they could not compensate for a lack of EXT1 activity.
EXT1 and EXT2 Accumulate in the Golgi Apparatus.
It has been shown previously that epitope-tagged human and murine EXT1 proteins are localized predominantly to the ER when overexpressed in cells (17, 21). To determine the intracellular localization of EXT2, a GFP-tagged murine EXT2 was expressed in BHK and sog9 cells and analyzed by confocal microscopy (Fig. 2). EXT2, like EXT1, was localized predominantly to the ER (Fig. 2 A and B). Moreover, pulse–chase experiments showed that the majority of newly synthesized EXT2 remained sensitive to endoglycosidase H (endoH) for at least 5 h after synthesis (data not shown). Thus, EXT2 appeared to be modified by high-mannose N-linked oligosaccharide moieties characteristic of proteins retained in the ER or cis-Golgi elements. The presence of N-linked glycans is also consistent with the type II membrane topology predicted for EXT2.
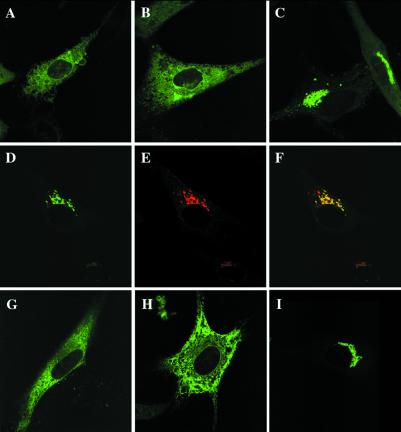
Figure 2.
EXT1 and EXT2 comigrate to the Golgi apparatus. Monolayers of BHK cells were transfected with EXT1-GFP (A), mEXT2-GFP (B), or both (C). When transfected into the same cell, EXT1-GFP and EXT2-GFP relocated to the Golgi (D), while the Golgi apparatus was immunolabeled with an anti-Golgi 58K monoclonal antibody and a Texas red-conjugated secondary antibody (E). When overlaid, they show excellent colocalization (yellow) (F). GFP fusion constructs of the EXT homologs EXTL2 (G) and EXTL3 (H) were also localized, as well as the murine N-deacetylase/N-sulfotransferase (NDST2), a key enzyme in HS biosynthesis (I).
To investigate the pattern of EXT2 expression in the presence of EXT1, GFP-tagged versions of each protein, which retained full activity, were overexpressed in the same cell. Remarkably, the EXT proteins redistributed to the Golgi apparatus from the ER (Fig. 2 A–C). The Golgi accumulation of the EXT1/EXT2 complex was confirmed by colocalization with a Golgi marker protein (Fig. 2 D–F). Moreover, all combinations of epitope-tagged and untagged EXT polypeptides behaved in this manner (data not shown). Thus, it appears that accumulation of EXT1 and EXT2 in the Golgi complex resulted from the concomitant expression of both proteins.
As a control for these studies, we determined whether several other closely linked enzymes in the HS biosynthesis pathway, EXTL2 and NDST2, as well as the EXT-like protein EXTL3, behaved in this manner. When overexpressed individually in cells, GFP-tagged EXTL2 and EXTL3 were localized predominantly to the ER, whereas NDST2 localized to the Golgi (Fig. 2 G–I). The intracellular distribution of EXTL2, EXTL3, or NDST2 was not altered by concomitant overexpression of EXT1 and/or EXT2 (data not shown). Thus, these proteins were transported to their respective intracellular locations without interaction with EXT1 or EXT2.
EXT1 and EXT2 Form Homo- and Hetero-oligomeric Complexes.
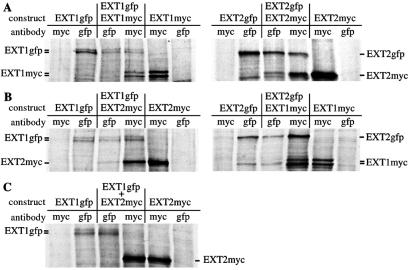
The data so far suggested that EXT1 and EXT2 form a complex that accumulates in the Golgi. To determine directly whether EXT1 and EXT2 form homo- and/or hetero-oligomeric complexes in vivo, different combinations of Myc- or GFP-tagged EXT constructs were transfected into BHK cells, radiolabeled, and immunoprecipitated. Liposome-mediated transfection of BHK cells is very efficient (transfection rate = 20–40%), and cotransfections usually result in >95% of cells expressing both EXT1 and EXT2. SDS/PAGE analysis revealed that anti-GFP antibody brought down two forms of EXT1-GFP, 113-kDa and 115-kDa, and a single 112-kDa polypeptide representing EXT2-GFP, whereas anti-Myc antibody brought down two forms of EXT1-Myc-His, 88-kDa and 91-kDa, and a single 85-kDa polypeptide representing Myc-His-tagged EXT2 (Fig. 3). When EXT1-GFP and EXT1-Myc-His were coexpressed and EXT1-Myc-His was immunoprecipitated with anti-Myc, EXT1-GFP coprecipitated, indicating that EXT1 was capable of forming homo-oligomers in vivo (Fig. 3A). The corresponding experiment using EXT2-Myc-His and EXT2-GFP revealed that EXT2 is also able to form homo-oligomers in vivo, which can be immunoprecipitated by either the anti-Myc or the anti-GFP antibody (Fig. 3A). With regard to hetero-oligomer formation, SDS/PAGE analysis revealed that when EXT1-GFP and mEXT2-Myc-His, or EXT1-Myc-His and EXT2-GFP were coexpressed and immunoprecipitated with antibody against one of the tags, the oppositely tagged protein coprecipitated (Fig. 3B). These results indicate that EXT1 and EXT2 form a complex in vivo. To ensure that the observed association between EXT1 and EXT2 was not an artifact of our experiments, lysates from singly transfected cells were mixed and immunoprecipitated (Fig. 3C). In this case no complex between EXT1 and EXT2 was detected, indicating that the two proteins probably cannot associate ex vivo. Taken together, these data show that both EXT1 and EXT2 are capable of forming homo- and hetero-oligomeric complexes in vivo.
Figure 3.
EXT1 and EXT2 form homo- and hetero-oligomers in vivo. BHK cells were transfected with various combinations of Myc-His- or GFP-tagged EXT1 and EXT2 constructs, radiolabeled with [35S]methionine and immunoprecipitated with the indicated anti-Myc or anti-GFP antibodies. (A) EXT1 and EXT2 homo-oligomer analysis. (B) EXT1/EXT2 hetero-oligomer analysis. (C) To determine whether EXT1/EXT2 complex formation was able to occur outside of the cell, lysates containing EXT1-GFP or EXT2-Myc-His were mixed ex vivo and immunoprecipitated with either anti-GFP or anti-Myc antibody.
EXT1/EXT2 Complexes Possess Enhanced Glycosyltransferase Activity.
Recent evidence indicates that EXT1 and EXT2 possess GlcA-T and GlcNAc-T activities in vitro (18). To explore the possibility that the Golgi-resident EXT1/EXT2 complex represented the active form of HS-Pol, GFP-tagged EXT forms were expressed in BHK or sog9 cells, purified by immunoprecipitation, and assayed for GlcNAc-T and GlcA-T activities (Table 1). Both cell lines were analyzed because BHK cells express HS and therefore have some endogenous HS-Pol activity, whereas sog9 cells, which are deficient in HS biosynthesis, harbor a specific defect in EXT1 (Fig. 1 A and B). In all cases when EXT1 was overexpressed in cells, a high level of HS-Pol activity was observed. By contrast, when EXT2 was overexpressed in BHK cells it exhibited low levels of GlcA-T activity and essentially no GlcNAc-T activity. Moreover, there was no detectable HS-Pol activity when EXT2 was overexpressed in sog9 cells, indicating that under these experimental conditions EXT2 overexpressed on its own does not exhibit HS-Pol activity. The highest enzymatic activity was isolated from cells overexpressing both EXT1 and EXT2 (Table 1, GlcA-T activity), which suggests that the Golgi-resident EXT1/EXT2 complex represents the active form of HS-Pol in the cell.
Table 1.
GlcNAc-T and GlcA-T activities of immunoprecipitated EXT1–EXT2 complexes
| Cell line | EXT1 construct | EXT2 construct | [3H]GlcNAc-T activity, cpm* | [14C]GlcA-T activity, cpm* |
|---|---|---|---|---|
| BHK | — | — | 9 | 4 |
| EXT1-GFP | — | 349 | 770 | |
| — | mEXT2-GFP | 19 | 63 | |
| sog9 | — | — | 8 | 29 |
| EXT1-GFP | — | 691 | 592 | |
| — | mEXT2-GFP | 20 | 20 | |
| EXT1-GFP | mEXT2-Myc | 219 | 2538 | |
| EXT1-GFP | mEXT2-GFP | 303 | 3018 | |
| EXT1-GFP | bEXT2-Myc | 573 | 1862 | |
| EXT1 | mEXT2-GFP | 750 | 3381 | |
| G339DEXT1-Myc | mEXT2-GFP | 106 | 12 | |
| R340CEXT1-Myc | mEXT2-GFP | 76 | 2 | |
| G339DEXT1-GFP | mEXT2-Myc | 846 | 14 | |
| R340CEXT1-GFP | mEXT2-Myc | 543 | 22 | |
| ΔNTMEXT1-GFP | — | 11 | 5 |
EXT proteins were immunoprecipitated with the rabbit anti-GFP antibody. The prefixes m and b indicate mouse and bovine, respectively. GlcA-T and GlcNAc-T activities were calculated as cpm of incorporated [3H]GlcNAc or [14C]GlcA per immunoprecipitate. When multiple experiments were performed the values were averaged.
HME-Linked Mutant Constructs Lack Glycosyltransferase Activities.
The data so far indicated that EXT1 and EXT2 form a hetero-oligomeric complex in the cell that, following isolation, possesses significantly more GlcA transferase activity, and therefore HS-Pol activity, than either polypeptide alone. These data are consistent with the etiology of the disease in which patients having a hereditary mutation in either EXT1 or EXT2 present with multiple exostoses. To determine which portions of the EXT1 polypeptide were important for activity, several mutant forms of EXT1 were generated and tested for glycosyltransferase activity and subcellular localization. In general, glycosyltransferases are type II membrane proteins comprising four regions: an N-terminal cytoplasmic tail, a transmembrane domain, a stalk, and a globular catalytic domain in the C-terminal portion. When a truncated 335-aa EXT1 polypeptide (identified in sog9 cells) missing the C-terminal half was overexpressed on its own in cells, it was ER-localized (Fig. 4A). However, when coexpressed with EXT2, it failed to redirect EXT2-Myc-His (Fig. 4 B–D) or EXT2-GFP (data not shown) to the Golgi. This failure indicated that some portion of the relatively conserved C-terminal half of EXT1 was required for proper distribution to the Golgi.
Figure 4.
Mutant EXT1 proteins have different intracellular localizations. Monolayers of BHK cells were tranfected with 335-aa-EXT1-GFP (A), 335–EXT1-GFP and mEXT2-Myc-His (B–D), G339DEXT1-GFP (E), or G339DEXT1-Myc-His and mEXT2-GFP (F–H). After 30 h of expression, Myc-His-tagged EXT1 or EXT2 proteins were detected with an anti-His monoclonal antibody and a Texas red-conjugated secondary antibody.
Interestingly, immunofluorescence analysis of EXT1 constructs containing HME-linked missense mutations showed that G339D (Fig. 4E) and R340C (data not shown) were localized predominantly to the Golgi apparatus when transfected alone. Moreover, EXT2 was translocated to the Golgi when coexpressed with these mutant forms of EXT1 (Fig. 4 F–H). Taken together, these data suggest that the underlying basis for disease in patients harboring these specific EXT1 mutations is not a failure of EXT1/EXT2 complexes to form or to redistribute to the Golgi. However, the observation that mutant forms of EXT1 containing a single amino acid change show altered trafficking when expressed on their own may be indicative of a perturbation in folding or processing after synthesis in the ER, or may result from a structural defect in the enzyme itself. Interestingly, missense mutations in EXT1 completely eliminated GlcA-T activity from the EXT1/EXT2 complex (Table 1). In light of the previous data showing an enhancement of GlcA-T activity by EXT2, it appears that EXT1 and EXT2 are both required for full HS-Pol activity.
Discussion
The putative tumor suppressors EXT1 and EXT2 were first identified because of their role in HME, which is an autosomal dominant disorder characterized by the formation of multiple, cartilage-capped tumors (exostoses) that develop from the growth plate of endochondral bone (1). This condition can lead to skeletal abnormalities, short stature, and in some instances, malignant transformation from exostoses to chondrosarcomas (2, 3) or osteosarcomas (4, 5). Our demonstration that EXT1 and EXT2 form a stable complex comprising the GlcA-T and GlcNAc-T activities required for the polymerization of HS (Table 1) provides compelling evidence that HME is caused by a deficiency in HS-Pol. Moreover, by using sog9 cells, which lack functional EXT1, we show that EXT1 and EXT2 cannot substitute for each other in vivo. Furthermore, regardless of their measured GlcNAc-T and GlcA-T activities in vitro, it is clear that EXT1 and EXT2 exhibit different activities in the cell. These results support a model in which EXT1 works in concert with EXT2 to provide the HS-Pol activity in the cell. It is also possible that other polypeptides involved in HS biosynthesis may associate with the EXT proteins in the Golgi.
An analysis of enzymatic activities associated with different EXT constructs (Table 1) suggests that a complex of EXT1 and EXT2 is required to elicit the maximal GlcA-T activity observed. By contrast, EXT1 overexpressed in our cell lines possesses both GlcNAc-T and some GlcA-T activity. Because a cell line deficient in EXT2 is not available, it is not yet possible to examine EXT1 expression in the absence of EXT2. However, it is evident from the data that the examined mutant forms of EXT1 abrogate the expression of GlcA-T activity (Table 1). This finding suggests a model in which EXT1 and EXT2 cooperate to generate the active enzyme responsible for the polymerization of HS. Although HS polymerization is not directly demonstrated by the in vitro assays, the HSV-1 adsorption assay (Fig. 1) detects the expression of cell surface HS moieties, and this has been shown to be caused by the restoration of EXT1 activity in sog9 cells. Additional studies on purified polypeptides will be required to sort out the roles of individual components of the enzyme complex.
These conclusions were facilitated by the analysis of HS-deficient sog9 cells, which harbor a specific defect in the EXT1 gene that results in the absence of HS-Pol activity. As such, sog9 cells represent the only stable cell line for which an EXT1 defect has been characterized, and they provide a useful tool with which to determine the activity of EXT2 expressed on its own. Also critical to this study was the use of functional assays to assess EXT1 activity. This allowed for the development of epitope- and GFP-tagged constructs that remained fully functional in vivo. By expressing combinations of functional tagged and untagged constructs in the sog9 cells, we were able to correlate localization data with activity. In this manner, we determined that EXT2 expression in sog9 cells does not rescue HS biosynthesis, as measured in the highly sensitive HSV-1 infection assay or in HS-Pol enzyme assays. In fact, the highest amount of HS-Pol activity was observed when EXT1 and EXT2 were coexpressed. Because we have observed that the EXT1/EXT2 hetero-oligomeric complex is localized predominantly to the Golgi cisternae, our data suggest that the Golgi is the site of cellular HS-Pol activity. The relatively small amount of HS-Pol activity that results from the expression of EXT1 alone in sog9 cells is likely the result of complex formation between transfected EXT1 and endogenous EXT2. We have shown that EXT2 in sog9 cells accumulates in the ER because of the inability of the truncated EXT1 to facilitate its redistribution to the Golgi. Although we have observed some Golgi-localized EXT1 in sog9 cells, most of the mass appears to be either retained in or recycled to the ER. It is interesting that EXT1 contains a motif, KKR, in its short cytoplasmic tail that is similar to the consensus diarginine ER retrieval signal described for several type II membrane proteins (29). A similar motif, KXR, is present in EXT2. Although preliminary data suggest that these motifs are important for ER localization (unpublished data), additional experiments will be required to elucidate the steps in the formation of functional EXT complexes.
Glycosyltransferases are commonly secreted into the extracellular medium in truncated form, and a number of these enzymes have been cloned on the basis of sequence information derived from soluble forms isolated from serum or milk (30, 31). In the case of EXT, it has been shown previously that a 70-kDa truncated form of EXT2 isolated from bovine serum harbors the two transferase activities required for the biosynthesis of HS (18, 32). In light of our demonstration that EXT1 and EXT2 form a functional hetero-oligomer, it may be that the soluble ectodomains of EXT1 and EXT2 are sufficient to maintain the oligomeric complex through a number of purification steps. Alternatively, it is also possible that EXT2 harbors some enzymatic activity that is not detectable in any of our cell-based assays, or that EXT2 is somehow activated after its exposure to EXT1 in the cell. In either case, the data support a model in which EXT1 and EXT2 possess distinct activities that are not functionally redundant in mammalian cells. Moreover, the EXT enzyme complex must traverse the secretory organelles while exiting the cell, which is consistent with our demonstration of Golgi localization for the complex. Additional experiments to identify and characterize the putative extracellular EXT1-EXT2 complex should help to resolve the activities of EXT1 and EXT2 in this complex.
The Golgi localization for the EXT1/EXT2 complex is consistent with what is known for other glycosyltransferases that modify proteins traversing the secretory organelles. Hetero-oligomer formation has been observed for other glycosyltransferases, and it may represent a common mechanism by which protein complexes are retained in a particular cellular compartment required for their activity. The kin recognition hypothesis of Warren and colleagues (33, 34) suggests that enzymes residing in the same Golgi cisternae could form hetero-oligomers. In the case of the EXT proteins, overexpression of either one does not lead to substantial Golgi localization, which suggests that EXT homo-oligomers, which may form in the ER, are not sufficient to signal movement to or retention in Golgi cisternae. It may be the case that uncomplexed EXT1 and EXT2 cycle between the ER and the Golgi, and that hetero-oligomer formation in the cis-Golgi cisternae, or an earlier compartment, leads to Golgi retention. This model does not preclude an important role for EXT homo-oligomers or of other members of the putative enzyme complex, nor does it eliminate the possibility that the complexes form in the ER and move to the Golgi. It is likely, however, that the highly active hetero-oligomer retains its function after cleavage and transport into the extracellular space. This would indicate that the complex is stable, and that it could retain at least partial activity in several different cellular compartments. In this manner, cells could control the expression of HS-Pol by down-regulating a single component of the complex. It would also resolve the issue of why mutations in either EXT1 or EXT2 alone cause HME, as both EXT1 and EXT2 appear to be necessary to produce the fully active enzyme complex.
HME is inherited in an autosomal dominant fashion, such that affected individuals should have one mutated copy of EXT1 or EXT2 and one normal copy. In light of our findings that the etiologic missense mutations in EXT1, G339D, and R340C do not prevent EXT1/EXT2 complex formation or transport to the Golgi apparatus, it is likely that these mutations alter the conformation of the HS-Pol heterocomplex, thereby inactivating the enzyme. Therefore, individuals with G339D or R340C mutations in one copy of EXT1 would likely have two pools of EXT1/EXT2 complexes, one active and one inactive. The presence of the inactive EXT complexes in the Golgi may be sufficient to cause deficiencies in the processing of proteoglycans destined for the cell surface or extracellular matrix. It will be interesting to investigate other HME-associated mutations to determine whether any of them are defective in complex formation or Golgi localization. It will also be useful to investigate whether modifications to cell surface HS occur in chondrocytes isolated from patients with HME, and whether other EXT genes represent additional constituents of glycosaminoglycan biosynthesis pathways. Taken together, these results should prove extremely useful for elucidating the role of EXT genes and perhaps other families of glycosyltransferase genes in the development of human tumors.
Acknowledgments
We thank U. Lindahl, M. Kushe-Gullberg, K. Lidholt, T. Lind, J. Esko, and K. Sugahara for valuable discussions. We also thank S. Déléhouzée, Y. Leduc, and J. Nomellini for expert technical assistance. This work was supported by grants to F.T. from the Medical Research Council of Canada and the Canadian Genetic Diseases Network. C.M. is supported by a scholarship from the Natural Sciences and Engineering Research Council in Canada.
Abbreviations
- HME
hereditary multiple exostoses
- ER
endoplasmic reticulum
- HS
heparan sulfate
- GlcA
d-glucuronic acid
- GlcNAc
N-acetyl-d-glucosamine
- GlcA-T
glucuronyltransferase
- GlcNAc-T
N-acetyl-d-glucosaminyltransferase
- HS-Pol
heparan sulfate polymerase
- HSV-1
herpes simplex virus type I
- GFP
green fluorescent protein
- endoH
endoglycosidase H
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Solomon L. J Bone Joint Surg. 1963;45:292–304. [Google Scholar]
- 2.Leone N C, Shupe J L, Gardner E J, Millar E A, Olson A E, Phillips E C. J Hered. 1987;78:171–177. doi: 10.1093/oxfordjournals.jhered.a110351. [DOI] [PubMed] [Google Scholar]
- 3.Hennekam R C. J Med Genet. 1991;28:262–266. doi: 10.1136/jmg.28.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmale G A, Conrad E U, Raskind W H. J Bone Joint Surg. 1994;76:986–992. doi: 10.2106/00004623-199407000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Luckert-Wicklund C, Pauli R, Johnston D, Hecht J. Am J Med Genet. 1995;55:43–46. doi: 10.1002/ajmg.1320550113. [DOI] [PubMed] [Google Scholar]
- 6.Cook A, Raskind W, Blanton S H, Pauli R M, Gregg R G, Francomano C A, Puffenberger E, Conrad E U, Schmale G, Schellenberg G, et al. Am J Hum Genet. 1993;53:71–79. [PMC free article] [PubMed] [Google Scholar]
- 7.LeMerrer M, Legeai-Mallet L, Jeannin P M, Horsthemke B, Schinzel A, Plauchu H, Toutain A, Achard F, Munnich A, Maroteaux P. Hum Mol Genet. 1994;3:717–722. doi: 10.1093/hmg/3.5.717. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y Q, Heutink P, de Vries B B, Sandkuijl L A, van den Ouweland A M, Niermeijer M F, Galjaard H, Reyniers E, Willems P J, Halley D J. Hum Mol Genet. 1994;3:167–171. doi: 10.1093/hmg/3.1.167. [DOI] [PubMed] [Google Scholar]
- 9.Ahn J, Ludecke H J, Lindow S, Horton W A, Lee B, Wagner M J, Horsthemke B, Wells D E. Nat Genet. 1995;11:137–143. doi: 10.1038/ng1095-137. [DOI] [PubMed] [Google Scholar]
- 10.Hecht J T, Hogue D, Wang Y, Blanton S H, Wagner M, Strong L C, Raskind W, Hansen M F, Wells D. Am J Hum Genet. 1997;60:80–86. [PMC free article] [PubMed] [Google Scholar]
- 11.Philippe C, Porter D E, Emerton M E, Wells D E, Simpson A H, Monaco A P. Am J Hum Genet. 1997;61:520–528. doi: 10.1086/515505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells D E, Hill A, Lin X, Ahn J, Brown N, Wagner M J. Hum Genet. 1997;99:612–615. doi: 10.1007/s004390050415. [DOI] [PubMed] [Google Scholar]
- 13.Raskind W H, Conrad E U, Matsushita M, Wijsman E M, Wells D E, Chapman N, Sandell L J, Wagner M, Houck J. Hum Mutat. 1998;11:231–239. doi: 10.1002/(SICI)1098-1004(1998)11:3<231::AID-HUMU8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 14.Wuyts W, Van Hul W, De Boulle K, Hendrickx J, Bakker E, Vanhoenacker F, Mollica F, Ludecke H J, Sayli B S, Pazzaglia U E, et al. Am J Hum Genet. 1998;62:346–354. doi: 10.1086/301726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park K J, Shin K H, Ku J L, Cho T J, Lee S H, Choi I H, Phillipe C, Monaco A P, Porter D E, Park J G. J Hum Genet. 1999;44:230–234. doi: 10.1007/s100380050149. [DOI] [PubMed] [Google Scholar]
- 16.Stickens D, Clines G, Burbee D, Ramos P, Thomas S, Hogue D, Hecht J T, Lovett M, Evans G A. Nat Genet. 1996;14:25–32. doi: 10.1038/ng0996-25. [DOI] [PubMed] [Google Scholar]
- 17.McCormick C, Leduc Y, Martindale D, Mattison K, Esford L E, Dyer A P, Tufaro F. Nat Genet. 1998;19:158–161. doi: 10.1038/514. [DOI] [PubMed] [Google Scholar]
- 18.Lind T, Tufaro F, McCormick C, Lindahl U, Lidholt K. J Biol Chem. 1998;273:26265–26268. doi: 10.1074/jbc.273.41.26265. [DOI] [PubMed] [Google Scholar]
- 19.Colley K J. Glycobiology. 1997;7:1–13. doi: 10.1093/glycob/7.1.1-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitagawa H, Shimakawa H, Sugahara K. J Biol Chem. 1999;274:13933–13937. doi: 10.1074/jbc.274.20.13933. [DOI] [PubMed] [Google Scholar]
- 21.Lin X, Gan L, Klein W H, Wells D. Biochem Biophys Res Commun. 1998;248:738–743. doi: 10.1006/bbrc.1998.9050. [DOI] [PubMed] [Google Scholar]
- 22.Nuwayhid N, Glaser J H, Johnson J C, Conrad H E, Hauser S C, Hirschberg C B. J Biol Chem. 1986;261:12936–12941. [PubMed] [Google Scholar]
- 23.Hirschberg C B, Snider M D. Annu Rev Biochem. 1987;56:63–87. doi: 10.1146/annurev.bi.56.070187.000431. [DOI] [PubMed] [Google Scholar]
- 24.Silbert J E, Sugumaran G. Biochim Biophys Acta. 1995;1241:371–384. doi: 10.1016/0304-4157(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez C J, Warren G. J Biol Chem. 1998;273:19030–19039. doi: 10.1074/jbc.273.30.19030. [DOI] [PubMed] [Google Scholar]
- 26.Banfield B W, Leduc Y, Esford L, Schubert K, Tufaro F. J Virol. 1995;69:3290–3298. doi: 10.1128/jvi.69.6.3290-3298.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Hul W, Wuyts W, Hendrickx J, Speleman F, Wauters J, De Boulle K, Van Roy N, Bossuyt P, Willems P J. Genomics. 1998;47:230–237. doi: 10.1006/geno.1997.5101. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson I, Sandback D, Ek B, Lindahl U, Kjellen L. J Biol Chem. 1994;269:10438–10443. [PubMed] [Google Scholar]
- 29.Teasdale R D, Jackson M R. Annu Rev Cell Dev Biol. 1996;12:27–54. doi: 10.1146/annurev.cellbio.12.1.27. [DOI] [PubMed] [Google Scholar]
- 30.Paulson J C, Colley K J. J Biol Chem. 1989;264:17615–17618. [PubMed] [Google Scholar]
- 31.Joziasse D H. Glycobiology. 1992;2:271–277. doi: 10.1093/glycob/2.4.271. [DOI] [PubMed] [Google Scholar]
- 32.Lind T, Lindahl U, Lidholt K. J Biol Chem. 1993;268:20705–20708. [PubMed] [Google Scholar]
- 33.Nilsson T, Slusarewicz P, Hoe M H, Warren G. FEBS Lett. 1993;330:1–4. doi: 10.1016/0014-5793(93)80906-b. [DOI] [PubMed] [Google Scholar]
- 34.Nilsson T, Hoe M H, Slusarewicz P, Rabouille C, Watson R, Hunte F, Watzele G, Berger E G, Warren G. EMBO J. 1994;13:562–574. doi: 10.1002/j.1460-2075.1994.tb06294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]