Abstract
The recognition of Moraxella catarrhalis as an important cause of respiratory tract infections has been protracted, mainly because it is a frequent commensal organism of the upper respiratory tract and the diagnostic sensitivity of blood or pleural fluid culture is low. Given that the amount of M. catarrhalis bacteria in the upper respiratory tract may change during infection, quantification of these bacteria in nasopharyngeal secretions (NPSs) by real-time PCR may offer a suitable diagnostic approach. Using primers and a fluorescent probe specific for the copB outer membrane protein gene, we detected DNA from serial dilutions of M. catarrhalis cells corresponding to 1 to 106 cells. Importantly, there was no difference in the amplification efficiency when the same DNA was mixed with DNA from NPSs devoid of M. catarrhalis. The specificity of the reaction was further confirmed by the lack of amplification of DNAs from other Moraxella species, nontypeable Haemophilus influenzae, H. influenzae type b, Streptococcus pneumoniae, Streptococcus oralis, Streptococcus pyogenes, Bordetella pertussis, Corynebacterium diphtheriae, and various Neisseria species. The assay applied to NPSs from 184 patients with respiratory tract infections performed with a sensitivity of 100% and a specificity of up to 98% compared to the culture results. The numbers of M. catarrhalis organisms detected by real-time PCR correlated with the numbers detected by semiquantitative culture. This real-time PCR assay targeting the copB outer membrane protein gene provided a sensitive and reliable means for the rapid detection and quantification of M. catarrhalis in NPSs; may serve as a tool to study changes in the amounts of M. catarrhalis during lower respiratory tract infections or following vaccination against S. pneumoniae, H. influenzae, or N. meningitidis; and may be applied to other clinical samples.
The gram-negative bacillus Moraxella catarrhalis (previously Branhamella catarrhalis or Neisseria catarrhalis) has long been regarded as a harmless commensal organism of the upper respiratory tract (4). The bacterium is now considered an important cause of upper respiratory tract infections in otherwise healthy children and elderly people (30). In children, this organism is the third most frequent bacterial cause of acute otitis media (2, 5, 10, 19) and has been identified as a cause of sinusitis, bacterial tracheitis, and conjunctivitis in single cases (19). Furthermore, M. catarrhalis causes lower respiratory tract infections, particularly in adults with chronic obstructive pulmonary disease (28). Severe invasive infections with this organism, including bacteremia, meningitis, skeletal infections, and endocarditis, are rare and occur mainly in immunocompromised individuals (30). Reports of hospital outbreaks of respiratory disease caused by M. catarrhalis have established the bacterium as a nosocomial pathogen (23, 25).
The etiological diagnosis of infections due to M. catarrhalis can be established by isolation of the microorganism from otherwise sterile body fluids. However, M. catarrhalis causes mainly noninvasive infections and is therefore rarely recovered in blood or pleural fluid cultures. In adults with lower respiratory tract infection due to M. catarrhalis, the bacterium can be demonstrated in sputum samples, but such specimens are rarely obtainable from children, as they do not readily produce sputum. Although the etiological diagnosis of lower respiratory tract infections due to M. catarrhalis can be achieved through serologic methods, paired serum specimens from the acute phase and the convalescent phase are required to document increases in the titers of specific antibodies (17, 21). These obstacles, together with the fact that M. catarrhalis colonizes the upper respiratory tracts of children and adults in the absence of clinical signs of infection (30), have delayed the recognition that M. catarrhalis is an important respiratory tract pathogen; and consequently, this delay has hampered studies of its pathogenesis. Thus, the development of more appropriate diagnostic tools would be desirable to improve the etiological diagnosis of lower respiratory tract infections due to M. catarrhalis when clinical specimens from the anatomical site of infection cannot easily be obtained. Since nasopharyngeal colonization often precedes the development of M. catarrhalis-mediated disease (8, 11), there exists a possibility that a quantitative change in the bacteria in the upper respiratory tract may reflect a lower respiratory tract infection, suggesting that routine diagnostic quantification of bacteria from readily accessible nasopharyngeal secretions (NPSs) might be clinically relevant. However, classical semiquantitative culturing of NPSs is cumbersome and slow compared to the molecular diagnostic approaches used at present. Furthermore, the colony morphology of M. catarrhalis is difficult to distinguish from that of commensal Neisseria species, which also form part of the normal upper respiratory tract flora (28).
The aim of the present study was to develop a method for the rapid and quantitative detection of M. catarrhalis with high degrees of sensitivity and specificity that could be applied as an investigational tool, e.g., in large-scale investigations of carriage, such as those encountered in vaccine development programs (26, 27) or in pathogenesis studies comparing the M. catarrhalis load in the nasopharynx with documented M. catarrhalis otitis media, the quantitative dynamics of M. catarrhalis in the upper respiratory tract during infections of the lower respiratory tract, or the quantitative dynamics of this pathogen during and after antimicrobial treatment. Here we describe a real-time PCR (TaqMan)-based assay that uses oligonucleotides targeting the copB outer membrane protein gene (1) for the detection and quantification of M. catarrhalis.
MATERIALS AND METHODS
Bacterial strains.
A clinical isolate of M. catarrhalis was used as the reference strain. To test the specificity of the real-time PCR assay, Moraxella lacunata, Moraxella lincolnii, Moraxella phenylpyruvarica, and Neisseria lactamica from the collection at the Institute for Medical Microbiology of the University of Zurich (all kindly provided by R. Zbinden, Zurich, Switzerland) and Neisseria meningitidis groups B and C, Haemophilus influenzae type b, nontypeable H. influenzae, and Bordetella pertussis (all from our own collection) were used as control strains of gram-negative bacteria. Streptococcus pneumoniae strain ATCC 49619 (American Type Culture Collection, Manassas, Va.), Streptococcus oralis strain ATCC 10557 (kindly provided by R. Gmür, Zurich), and Streptococcus pyogenes and Corynebacterium diphtheriae (both of which were from our own collection) served as representatives of gram-positive bacteria from the upper respiratory tract.
Clinical samples.
Surplus NPSs (n = 184) collected from children with respiratory tract infections and sent to the Infectious Diseases Laboratory of the University Children's Hospital of Zurich for a rapid test for respiratory syncytial virus were used for semiquantitative bacterial culturing and amplification of the copB outer membrane protein gene of M. catarrhalis. Immediately after the patient samples were plated for culture, the samples were stored at −20°C until DNA extractions were performed (see below). Data regarding the antimicrobial treatment of the patients were gathered retrospectively from the patients' records.
Semiquantitative bacterial cultures.
All 184 samples of NPSs were tested by semiquantitative bacterial culturing as described previously (13). Briefly, the samples were inoculated onto sheep blood agar, chocolate blood agar, and Columbia colistin-nalidixic acid agar by fractionation by using a calibrated wire loop (5 μl); and the plates were incubated at 37°C in 5% CO2 for 48 h. Growth only in the first fraction was defined as a low level of growth (+), corresponding to 103 to 104 microorganisms per μl; growth extending into the second fraction was defined as an intermediate level of growth (++), corresponding to 104 to 105 microorganisms per μl; and growth in all three fractions was defined as a high level of growth (+++), corresponding to 105 to 106 microorganisms per μl. Species identification was done by standard methods (20).
DNA extraction.
The extraction of DNA was performed as described previously (9). Briefly, 1 ml of liquid culture or patient sample was centrifuged at 12,000 × g for 10 min. The pellet was resuspended in 200 μl of digestion buffer (50 mM Tris-HCl [pH 8.5], 1 mM EDTA, 0.5% sodium dodecyl sulfate, 200 mg of proteinase K per ml) and incubated with shaking for 1 h at 55°C. The DNA was then purified with the QIAamp tissue kit (Qiagen, Basel, Switzerland) according to the instructions of the supplier. Extracts were stored at −20°C until they were required for analysis.
Real-time PCR.
The primers and the fluorogenic probe for the copB outer membrane protein gene (1, 31) of M. catarrhalis (GenBank accession no. U69982) were designed by using Primer Express software (version 1.5; Perkin-Elmer, Applied Biosystems, Foster City, Calif.) and were synthesized by Microsynth GmbH (Balgach, Switzerland). The copB sequence was selected because the copB gene is largely conserved among M. catarrhalis strains (29). The nucleotide sequence of the forward primer was 5′-GTGAGTGCCGCTTTACAACC-3′ (positions 50 to 70), the sequence of the reverse primer was 5′-TGTATCGCCTGCCAAGACAA-3′ (121 to 102), and the sequence of the probe was 5′-TGCTTTTGCAGCTGTTAGCCAGCCTAA-3′ (73 to 99). The fluorescent reporter dye at the 5′ end of the probe was hexachloro-6-carboxyfluorescein (Biosearch Technologies Inc., Novato, Calif.); the quencher at the 3′ end was a dark quencher called QSY-7 (Biosearch Technologies Inc.). The principle of real-time PCR has been described extensively (13).
The real-time PCR amplifications were performed in 25-μl reaction volumes containing 2× PlatinumQuantitative PCR SuperMix-UDG (Life Technologies Inc., Gathersburg, Md.), which includes dUTP and uracil-N-glycosylase and to which Blue 636 (Invitrogen, Basel, Switzerland) was added as passive reference dye to a final concentration of 0.1 pmol/μl; 333 nM each primer; 200 nM fluorescence-labeled probe; and 1 μl of DNA extract. All reactions were performed in duplicate, and an ABI PRISM 7700 sequence detection system (Perkin-Elmer, Applied Biosystems) was used for amplification and detection. Each run contained negative and known positive controls. The standard amplification parameters used were as follows: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles comprising 95°C for 15 s and 60°C for 1 min. Real-time data were analyzed by using Sequence Detection Systems software (version 1.7).
Sensitivity, detection range, and specificity.
To determine the sensitivity and the detection range of the real-time PCR assay, a standard curve for M. catarrhalis was generated as follows: M. catarrhalis was grown aerobically on sheep blood agar plates at 37°C for 24 h. The bacteria were then displaced from the plates by using saline (0.9% NaCl) and diluted with saline until a McFarland standard of 0.5 (representing 108 microorganisms/ml) was reached. Starting from this concentration, 10-fold serial dilutions were prepared in physiological saline. One milliliter of each dilution was used for DNA extraction, followed by in vitro gene amplification, as described above. The calculated cycle threshold (CT) values were then plotted against the numbers of microorganisms per microliter.
To determine the specificity of the M. catarrhalis real-time PCR assay, serial dilutions of different bacteria including N. lactamica, N. meningitidis groups B and C, M. lacunata, M. phenylpyruvarica, M. lincolnii, nontypeable H. influenzae, H. influenzae type b, S. pneumoniae, S. pyogenes, S. oralis, B. pertussis, and C. diphtheriae were tested in the same manner as described above for M. catarrhalis. Finally, the real-time PCR assay was applied to clinical samples, i.e., NPSs, and the sensitivity of detection and quantification and the specificity were assessed by comparison of the results with those of the semiquantitative culture procedure.
Sequencing.
For sequencing of the region covering the TaqMan amplicon of the copB outer membrane protein, flanking amplimers upstream and downstream of the TaqMan system were designed by using Primer Express software (version 1.5; Perkin-Elmer, Applied Biosystems). The nucleotide sequence of the forward primer was 5′-TGGCGGTGAGTGCCG-3′ (positions 45 to 59), and that of the reverse primer was 5′-AGCCGTGCTTTCGTCTTTTTC-3′ (positions 196 to 176). Prior to sequencing, a conventional touchdown PCR with the amplimers described above was carried out with the following amplification parameters: 95°C for 10 min, followed by 10 cycles of 95°C for 15 s and 65°C for 1 min, with the temperature reduced by 0.5°C/cycle to reach 60°C, which was followed by 30 cycles of 95°C for 15 s and 60°C for 1 min. Cycle sequencing was done with 90 ng of PCR products, 25 pmol of either forward or reverse primer, and the BigDye Terminator cycle sequencing ready reaction kit (part no. 4303152; Applied Biosystems, Warrington, United Kingdom). After amplification (96°C for 30 s, followed by 25 cycles comprising 96°C for 10s, 50°C for 5 s, and 60°C for 4 min), ethanol precipitation was performed for purification. The dried pellet was resuspended in 12 μl of template suppression reagent (part no. 401674; Applied Biosystems) and was stored at 4°C until use. The sequence was read on an ABI PRISM 310 genetic analyzer (Applied Biosystems), according to the instructions of the supplier. The sequencing data were analyzed by comparison to sequences available in the National Center for Biotechnology Information database.
Statistics.
The results for the M. catarrhalis culture-positive and culture-negative subpopulations of NPSs were compared with those of the PCR assay by the chi-square test and the two-tailed Fisher exact test. The Mann-Whitney test was used for comparison of the mean ± standard deviation CT values for the groups. P values of <0.05 were considered statistically significant.
RESULTS
Detection range and sensitivity of the real-time PCR assay for M. catarrhalis.
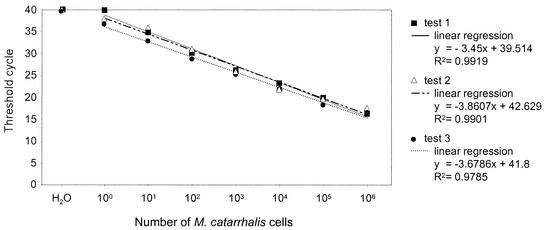
Tenfold serial dilutions starting from a culture of 108 M. catarrhalis microorganisms/ml were investigated to determine the sensitivity and the detection range of the real-time PCR assay. As shown in Fig. 1, the assay was able to detect bacterial DNA over a linear range of between 1 and 106 microorganisms per μl, with CT values ranging between 17 and 36. The intra- and interassay variabilities of the CT values obtained with replicates of the same DNA extracted from each dilution series or replicates from DNA extracted from different dilution series was <±1. To exclude the possibility that PCR-inhibiting substances were present in the clinical samples, one NPS sample with no M. catarrhalis organisms, as determined by culture, was spiked with DNA extracted from 10-fold serial dilutions of liquid cultures of M. catarrhalis. This demonstrated that the level of amplification of M. catarrhalis DNA from spiked samples was similar to that directly from liquid bacterial cultures (data not shown).
FIG. 1.
Sensitivity, detection range, and specificity of the real-time PCR assay for M. catarrhalis. The reproducibility of the assay was determined by testing a dilution series of M. catarrhalis, followed by DNA extraction, in three independent assays with independent DNA extractions.
Specificity of real-time PCR assay.
The specificity of the real-time PCR assay for M. catarrhalis with primers specific for the copB outer membrane protein gene was investigated by testing 10-fold serial dilutions of M. lacunata, M. lincolnii, M. phenylpyruvarica, N. lacunata, N. meningitidis groups B and C, H. influenzae type b, nontypeable H. influenzae, B. pertussis, C. diphtheriae, S. pyogenes, and the alpha-hemolytic streptococci S. pneumoniae and S. oralis. No amplification of any of the non-M. catarrhalis bacteria was achieved at any of the dilutions even after 40 cycles (data not shown).
Application of the real-time PCR assay for M. catarrhalis to NPSs.
Bacterial cultures of 49 of the 184 NPS samples demonstrated growth of M. catarrhalis. The concomitant real-time PCR assay applied to the 49 NPS samples growing M. catarrhalis resulted in CT values ranging between 15.0 and 37.8 (mean, 19.8; median, 17.9) and thus performed with a calculated sensitivity of 100% (Fig. 2). In contrast, when the real-time PCR assay was applied to the 135 NPS samples not growing M. catarrhalis, it resulted in CT values ranging between 15.5 and 40 (mean, 36.4; median, 37.9) (P < 0.0001) (Fig. 2). While 123 (91%) of these 135 NPS samples had CT values >30 (29 NPS samples had CT values of 40, i.e., no detectable amplification), 12 (9%) NPS samples had CT values ≤30, as did 46 (94%) of the M. catarrhalis culture-positive NPSs. The proportion of NPS samples that grew M. catarrhalis and that had CT values ≤30 versus the proportion of NPS samples that did not grow this bacterium and that had CT values ≤30 was statistically significantly different (P < 0.0001). By arbitrarily choosing a CT value of >30 as the cutoff for M. catarrhalis-negative clinical specimens, the calculated specificity in comparison to the results of culture was 91%.
FIG. 2.
Sensitivity and specificity of the real-time PCR assay for M. catarrhalis in clinical samples (NPSs) compared with culture results. The dashed line indicates the arbitrary cutoff CT value. CT values 30 or below were regarded as positive for M. catarrhalis. Horizontal bars indicate medians, which are also given as absolute values.
None of the three M. catarrhalis culture-positive NPSs with CT values >30 harbored PCR-inhibiting substances when they were spiked with DNA extracted from 10-fold serial dilutions of liquid cultures of M. catarrhalis (data not shown). DNA from two of these three NPS samples could be amplified by conventional PCR and subsequently sequenced. No mismatches in the sequence targeted by the real-time PCR system in comparison to the sequence of M. catarrhalis in the National Center for Biotechnology Information database were found. Conventional PCR of DNA from the third NPS sample (CT = 37.8) yielded no detectable amplicon.
Three of the 12 patients whose NPS samples did not grow M. catarrhalis in culture but that had CT values <30 in the real-time PCR assay had been treated with β-lactams before the samples were collected. Cultures of the NPSs from these three patients showed an absence of bacterial growth. The other nine NPSs were from patients not treated with antimicrobials and grew normal oral and nasal flora other than M. catarrhalis. Sequencing of the DNA from these 12 NPS samples for the region of the copB gene targeted by the real-time PCR assay revealed a mismatch in 1 of the 3 NPS samples from patients receiving antimicrobials before sample collection and in 2 of the other 9 NPS samples. All three mismatches detected were identical. DNA from 10 randomly selected NPS samples among the 46 that grew M. catarrhalis in culture and that had CT values <30 were also sequenced for the region on the copB gene targeted by the real-time PCR assay, and no mismatches were found. Thus, when the antecedent antimicrobial treatment of the patients and the sequencing results for the amplicons of the samples positive by real-time PCR but negative by culture are taken into consideration, the calculated specificity of the real-time PCR assay was, indeed, 98%. The calculated positive predictive value of the real-time PCR assay was 79%, and after consideration of the sequencing results the positive predictive value was 95%; the calculated negative predictive value was 98%.
Comparison of the real-time PCR assay with semiquantitative cultures.
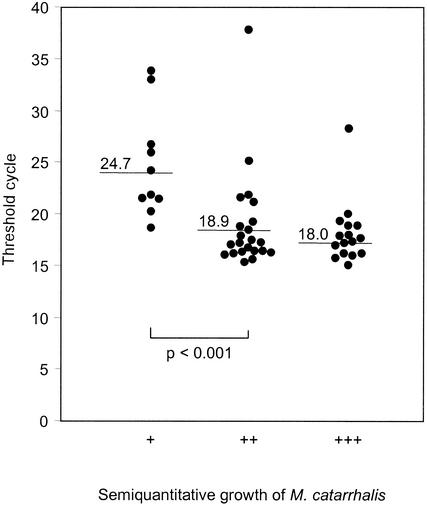
Ten of the 49 cultures growing M. catarrhalis showed a low level of growth (+), 23 showed an intermediate level of growth (++), and 16 showed a high level of growth (+++). The mean CT values obtained by the real-time PCR assay for these three levels of growth were 24.7 (range, 18.6 to 26.7), 18.9 (range, 15.3 to 25.1), and 18.0 (range, 15.0 to 28.2), respectively (Fig. 3). The difference in CT values between a low level of growth and intermediate or high levels of growth was statistically significant (P < 0.001).
FIG. 3.
Comparison of M. catarrhalis quantification by real-time PCR with that by semiquantitative culture. The samples were inoculated onto sheep blood agar by fractionation with a calibrated wire loop (5 μl). Growth only in the first fraction was defined as a low level of growth (+), growth in the second fraction as well was defined as an intermediate level of growth (++), and growth in all three fractions was defined as abundant growth (+++).
DISCUSSION
A real-time PCR-based assay targeting the copB outer membrane protein gene was developed that proved to be highly sensitive (100%) and specific (up to 98%) for the detection of M. catarrhalis in NPSs. The numbers of M. catarrhalis organisms quantified by the real-time PCR assay corresponded to the numbers detected by semiquantitative culture. Since the assay can be completed en mass and within 1 working day, it is considerably faster than conventional culturing and subsequent identification.
The linear detection range of the real-time PCR assay was from 1 to 106 organisms per reaction mixture, encompassing 7 orders of magnitude above the background. In comparison to previously reported real-time PCR assays for the detection of bacteria including S. pneumoniae (13), Mycoplasma pneumoniae (14), Porphyromonas gingivalis (18), Borrelia burgdorferi (22), and Mycobacterium tuberculosis (6), the detection range and the sensitivity of the assay described here were similar, as were the intra- and interexperimental reproducibilities.
All except 12 of the 135 NPS samples shown to be devoid of M. catarrhalis following culture had CT values above 30. Consequently, by using a CT value of >30 as the cutoff level for M. catarrhalis-negative NPSs, the calculated specificity would amount to 91%. Notably, of these 12 NPS samples, 3 originated from patients pretreated with β-lactam antimicrobials. It seems likely that the real-time PCR assay may have detected DNA from dead bacteria in these three samples. Nevertheless, for all 12 NPS samples the sequence in the region of the copB gene targeted by the real-time PCR assay was determined. Three samples (one from a patient pretreated with antimicrobials) had identical mismatches in common. Thus, for the seven samples without mismatches from patients who had not previously received antimicrobial treatment, one must assume, as is well known, that colonies in culture were misinterpreted as Neisseria species (28) or as normal flora but had been correctly identified by the real-time PCR. In addition to the measures taken to degrade previously formed PCR products, the use of flanking primers for sequence verification also confirms that the PCR results were not false positive due to carryover contamination with previously amplified TaqMan amplicons. For the three samples with the one identical mismatch, it can be assumed that the identification by the real-time PCR assay was correct for several reasons. First, the mismatch does not lead to a change in the amino acid sequence. Second, the closest relatives to the copB outer membrane protein gene from M. catarrhalis are the tonB-dependent outer membrane proteins and especially the frpB outer membrane protein gene (3, 24) from N. meningitidis. However, the homology of the known gene sequences between the copB outer membrane protein gene and frpB makes it very unlikely that this assay amplifies the frpB outer membrane protein gene. In addition, N. meningitidis group B and C strains were tested by the real-time PCR assay, and no amplification was seen (data not shown). Thus, by taking the sequencing results into consideration when a CT value of >30 was used as the cutoff for M. catarrhalis-negative NPS samples, the calculated specificity increased to 98%. Furthermore, the sequencing results suggest a higher degree of specificity of the real-time PCR assay compared to the specificity of colony interpretation by culturing.
To the best of our knowledge, the PCR assay for the detection of M. catarrhalis reported here is the first one based on real-time (TaqMan) technology. Previously reported PCR assays were either conventional end-point assays (7, 12, 32) or multiplex assays (15, 16). When applied to clinical samples, some of these assays yielded significantly more positive results than the number obtained by culturing, suggesting the superior sensitivities of the DNA amplification assays (7). Our sequencing results described above are in line with these observations.
In summary, we developed a new diagnostic assay based on real-time PCR that allows the fast, sensitive, specific, reproducible, and simple medium- to high-throughput detection and quantification of the copB gene of M. catarrhalis. This assay is faster and more precise than conventional culture and identification procedures and therefore provides a potentially reliable tool for the diagnosis of M. catarrhalis infection. This in turn may become a tool that can be used to evaluate lower respiratory tract disease and otitis media, but those relationships are yet to be determined.
Acknowledgments
We thank Pia Beck and Sibylle Gunziger for superb organization of logistics, support, and technical expertise.
The study was partly supported by the Swiss National Foundation (grant 31-55553.98) and by an unrestricted grant of AstraZeneca, Zug, Switzerland.
REFERENCES
- 1.Aebi, C., L. D. Cope, J. L. Latimer, S. E. Thomas, C. A. Slaughter, G. H. McCracken, Jr., and E. J. Hansen. 1998. Mapping of a protective epitope of the CopB outer membrane protein of Moraxella catarrhalis. Infect. Immun. 66:540-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aspin, M. M., A. Hoberman, J. McCarty, S. E. McLinn, S. Aronoff, D. J. Lang, and A. Arrieta. 1994. Comparative study of the safety and efficacy of clarithromycin and amoxicillin-clavulanate in the treatment of acute otitis media in children. J. Pediatr. 125:136-141. [DOI] [PubMed] [Google Scholar]
- 3.Beucher, M., and P. F. Sparling. 1995. Cloning, sequencing, and characterization of the gene encoding FrpB, a major iron-regulated, outer membrane protein of Neisseria gonorrhoeae. J. Bacteriol. 177:2041-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catlin, B. W. 1990. Branhamella catarrhalis: an organism gaining respect as a pathogen. Clin. Microbiol. Rev. 3:293-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Beccaro, M. A., P. M. Mendelman, A. F. Inglis, M. A. Richardson, N. O. Duncan, C. R. Clausen, and T. L. Stull. 1992. Bacteriology of acute otitis media: a new perspective. J. Pediatr. 120:81-84. [DOI] [PubMed] [Google Scholar]
- 6.Desjardin, L. E., Y. Chen, M. D. Perkins, L. Teixeira, M. D. Cave, and K. D. Eisenach. 1998. Comparison of the ABI 7700 system (TaqMan) and competitive PCR for quantification of IS6110 DNA in sputum during treatment of tuberculosis. J. Clin. Microbiol. 36:1964-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dingman, J. R., M. G. Rayner, S. Mishra, Y. Zhang, M. D. Ehrlich, J. C. Post, and G. D. Ehrlich. 1998. Correlation between presence of viable bacteria and presence of endotoxin in middle-ear effusions. J. Clin. Microbiol. 36:3417-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faden, H., J. Stanievich, L. Brodsky, J. Bernstein, and P. L. Ogra. 1990. Changes in nasopharyngeal flora during otitis media of childhood. Pediatr. Infect. Dis. J. 9:623-626. [PubMed] [Google Scholar]
- 9.Fischer-Romero, C., J. Lüthy-Hottenstein, and M. Altwegg. 2000. Development and evaluation of a broad-range PCR-ELISA assay with Borrelia burgdorferi and Streptococcus pneumoniae as model organisms for reactive arthritis and bacterial meningitis. J. Microbiol. Methods 40:79-88. [DOI] [PubMed] [Google Scholar]
- 10.Gan, V. N., H. Kusmiesz, S. Shelton, and J. D. Nelson. 1991. Comparative evaluation of loracarbef and amoxicillin-clavulanate for acute otitis media. Antimicrob. Agents Chemother. 35:967-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gehanno, P., G. Lenoir, B. Barry, J. Bons, I. Boucot, and P. Berche. 1996. Evaluation of nasopharyngeal cultures for bacteriologic assessment of acute otitis media in children. Pediatr. Infect. Dis. J. 15:329-332. [DOI] [PubMed] [Google Scholar]
- 12.Gok, U., Y. Bulut, E. Keles, S. Yalcin, and M. Z. Doymaz. 2001. Bacteriological and PCR analysis of clinical material aspirated from otitis media with effusions. Int. J. Pediatr. Otorhinolaryngol. 60:49-54. [DOI] [PubMed] [Google Scholar]
- 13.Greiner, O., P. J. R. Day, P. P. Bosshard, F. Imeri, M. Altwegg, and D. Nadal. 2001. Quantitative detection of Streptococcus pneumoniae in nasopharyngeal secretions by real-time PCR. J. Clin. Microbiol. 39:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardegger, D., D. Nadal, W. Bossart, M. Altwegg, and F. Dutly. 2000. Rapid detection of Mycoplasma pneumoniae in clinical samples by real-time PCR. J. Microbiol. Methods 41:45-51. [DOI] [PubMed] [Google Scholar]
- 15.Hendolin, P. H., A. Markkanen, J. Ylikoski, and J. J. Wahlfors. 1997. Use of multiplex PCR for simultaneous detection of four bacterial species in middle ear effusions. J. Clin. Microbiol. 35:2854-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendolin, P. H., L. Paulin, and J. Ylikoski. 2000. Clinically applicable multiplex PCR for four middle ear pathogens. J. Clin. Microbiol. 38:125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juvén, T., J. Mertsola, M. Waris, M. Leinonen, O. Meurman, M. Roivainen, J. Eskola, P. Saikku, and O. Ruuskanen. 2000. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr. Infect. Dis. J. 19:293-298. [DOI] [PubMed] [Google Scholar]
- 18.Lyons, S. R., A. L. Griffen, and E. J. Leys. 2000. Quantitative real-time PCR for Porphyromonas gingivalis and total bacteria. J. Clin. Microbiol. 38:2362-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchant, C. D. 1990. Spectrum of disease due to Branhamella catarrhalis in children with particular reference to acute otitis media. Am. J. Med. 88:15S-19S. [DOI] [PubMed] [Google Scholar]
- 20.Murray, P. R., E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.). 1999. Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 21.Nohynek, H., J. Eskola, M. Kleemola, E. Jalonen, P. Saikku, and M. Leinonen. 1995. Bacterial antibody assays in the diagnosis of acute lower respiratory tract infection in children. Pediatr. Infect. Dis. J. 14:478-484. [DOI] [PubMed] [Google Scholar]
- 22.Pahl, A., U. Kuhlbrandt, K. Brune, M. Rollinghoff, and A. Gessner. 1999. Quantitative detection of Borrelia burgdorferi by real-time PCR. J. Clin. Microbiol. 37:1958-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson, T. F., J. E. Patterson, B. L. Masecar, G. E. Barden, W. J. Hierholzer, Jr., and M. J. Zervos. 1988. A nosocomial outbreak of Branhamella catarrhalis confirmed by restriction endonuclease analysis. J. Infect. Dis. 157:996-1001. [DOI] [PubMed] [Google Scholar]
- 24.Pettersson, A., A. Maas, D. van Wassenaar, P. van der Ley, and J. Tommassen. 1995. Molecular characterization of FrpB, the 70-kilodalton iron-regulated outer membrane protein of Neisseria meningitidis. Infect. Immun. 63:4181-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards, S. J., A. P. Greening, M. C. Enright, M. G. Morgan, and H. McKenzie. 1993. Outbreak of Moraxella catarrhalis in a respiratory unit. Thorax 48:91-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samukawa, T., N. Yamanaka, S. Hollingshead, K. Klingman, and H. Faden. 2000. Immune responses to specific antigens of Streptococcus pneumoniae and Moraxella catarrhalis in the respiratory tract. Infect. Immun. 68:1569-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samukawa, T., N. Yamanaka, S. Hollingshead, T. F. Murphy, and H. Faden. 2000. Immune response to surface protein A of Streptococcus pneumoniae and to high-molecular-weight outer membrane protein A of Moraxella catarrhalis in children with acute otitis media. J. Infect. Dis. 181:1842-1845. [DOI] [PubMed] [Google Scholar]
- 28.Sethi, S., and T. F. Murphy. 2001. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin. Microbiol. Rev. 14:336-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sethi, S., J. M. Surface, and T. F. Murphy. 1997. Antigenic heterogeneity and molecular analysis of copB of Moraxella (Branhamella) catarrhalis. Infect. Immun. 65:3666-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verduin, C. M., C. Hol, A. Fleer, H. van Dijk, and A. van Belkum. 2002. Moraxella catarrhalis: from emerging to established pathogen. Clin. Microbiol. Rev. 15:125-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker, J. A., R. L. Allen, P. Falmagne, M. K. Johnson, and G. J. Boulnois. 1987. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect. Immun. 55:1184-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf, B., M. Kools-Sijmons, C. Verduin, L. C. Rey, A. Gama, J. Roord, J. Verhoef, and A. van Belkum. 2000. Genetic diversity among strains of Moraxella catarrhalis cultured from the nasopharynx of young and healthy Brazilian, Angolan and Dutch children. Eur. J. Clin. Microbiol. Infect. Dis. 19:759-764. [DOI] [PubMed] [Google Scholar]