Abstract
Linker for activation of T cells (LAT) is a scaffolding adaptor protein that is critical for T cell development and function. A mutation of LAT (Y136F) that disrupts phospholipase C-γ1 activation and subsequent calcium influx causes a partial block in T cell development and leads to a severe lymphoproliferative disease in homozygous knock-in mice. One possible contribution to the fatal disease of LAT Y136F knock-in mice could be from autoreactive T cells generated in these mice because of altered thymocyte selection. To examine the impact of the LAT Y136F mutation on thymocyte positive and negative selection, we bred this mutation onto the HY T cell receptor (TCR) transgenic, recombination activating gene-2 knockout background. Female mice with this genotype showed a severe defect in positive selection, whereas male mice exhibited a phenotype resembling positive selection (i.e., development and survival of CD8hi HY TCR-specific T cells) instead of negative selection. These results support the hypothesis that in non-TCR transgenic, LAT Y136F knock-in mice, altered thymocyte selection leads to the survival and proliferation of autoreactive T cells that would otherwise be negatively selected in the thymus.
The mature T cell repertoire contains a very large number of TCRs with the potential to bind foreign antigens with high affinity, but it is relatively devoid of TCRs that bind with high affinity to self-peptides. Positive selection accounts for survival and proliferation of T cells that are minimally reactive to self but potentially highly reactive to foreign antigens, whereas negative selection functions to eliminate (by programmed cell death) overtly autoreactive T cells. A wealth of information, some of it conflicting, has accumulated concerning what signals mediate positive and negative selection (for review see references 1–3). In general, ligands that induce weak TCR signaling and/or slow, sustained Erk activation promote positive selection, whereas ligands that induce strong TCR signaling including strong, transient Erk activation promote negative selection (3–5). Under these circumstances, quantitative differences in signaling account for the outcome of selection. Qualitative differences in signaling have also been suggested to contribute to the outcome of selection. For example, signaling through Ras/Raf/Mek/Erk pathways has been described to impact positive but not negative selection (2). Furthermore, differential contributions from phospholipase C (PLC)-γ1, Erk, p38, and Jnk signaling pathways may collectively determine the outcome of selection (1).
Linker for activation of T cells (LAT) is an adaptor protein that is critical for T cell signaling and T cell development (for review see references 6 and 7). LAT contains nine conserved tyrosines in its cytoplasmic domain, the distal four of which are absolutely required for both TCR signaling and T cell development (8–12). Tyrosine 136 (Y136) of mouse LAT is a docking site for PLC-γ1. The other three distal tyrosines of LAT bind the adaptor proteins Grb-2 and Gads and likely other molecules. Grb-2 can associate with the Ras GEF Sos and the ubiquitin ligase and adaptor protein Cbl. Gads associates with the adaptor SLP-76, which can stimulate actin remodeling through interactions with Vav and Nck (13). In addition, SLP-76 associates directly with PLC-γ1 and may participate in PLC-γ1 activation by recruiting the Tec family tyrosine kinase Itk (14, 15). PLC-γ1 activation results in Ca2+ release, which in turn activates the calcium-dependent phosphatase calcineurin. Calcineurin activation then results in activation of transcription factors for cytokine genes, resulting in T cell proliferation (16). Ras can potentially be stimulated by at least two LAT-dependent pathways: first, by association of Sos with LAT-associated Grb-2, and second, by PLC-γ1–mediated production of diacylglycerol, which activates the Ras GEF, RasGRP (17, 18). Ras signaling can then activate Erk and Jnk kinases. Coordinated activation of both calcium and Ras signaling pathways are thought to be required for full T cell activation and might be required for efficient thymocyte selection as well (19).
We and others have generated knock-in mice to study contributions of individual tyrosines of LAT to signal transduction and T cell development (20, 21). Mutation of Y136 (the PLC-γ1–binding tyrosine residue of LAT) results in a partial block in early T cell development. However, beginning at about weaning age, a fatal lymphoproliferative disease characterized by expansion of Th2 cell–type CD4+ cells ensues. Interestingly, TCR-mediated calcium mobilization in LAT Y136 knock-in T cells is drastically reduced, although TCR-induced Erk signaling is relatively intact. Therefore, LAT Y136 knock-in mice provide a useful system for assessing the effects of selectively disrupting PLC-γ1 activation in developing T cells. In this study, we interbred LAT Y136 knock-in mice and TCR transgenic mice to analyze the effect of disrupting calcium signaling on thymocyte selection in an in vivo model using endogenous ligands. The HY TCR transgenic system was chosen as a well-established TCR transgenic system whose TCR binds to a male-specific peptide allowing analysis of both positive and negative selection using female and male mice, respectively (22). Using this system, we demonstrate that both positive and negative selection are altered by the LAT Y136F mutation. A possible consequence of altered negative selection in LAT Y136F knock-in mice is survival and proliferation of autoreactive T cells that contribute to the fatal lymphoproliferative disease that develops in these mutant mice.
Results
The lymphoproliferative disorder in LATY136Fm/m mice is not abrogated by introduction of a TCR transgene
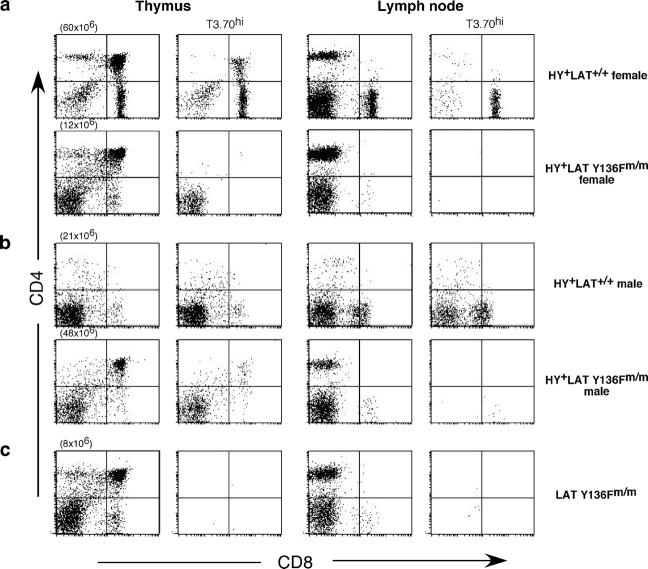
We and others previously described the phenotype of LAT knock-in mice in which tyrosine 136, a consensus binding site for PLC-γ1, is mutated to phenylalanine (20, 21). In brief, LAT Y136Fm/m mice exhibit a severe block in thymocyte development that is best documented early in life. Thymi from these mice are small and contain mostly CD4− CD8− (double negative [DN]) thymocytes. The block is at the DN3 (CD25+ CD44−) stage, confirming a requirement for wild-type LAT for pre-TCR signaling. As they age, virtually all LAT Y136Fm/m mice develop a lymphoproliferative disease evidenced by markedly enlarged spleens and lymph nodes and lymphocytic infiltration of multiple organs. T cells in LAT Y136Fm/m mice arise from polyclonal expansion and consist almost entirely of CD4+ CD3lo CD62Llo CD44hi cells, thus showing signs of previous activation. Thymocytes and T cells from LAT Y136Fm/m mice are defective in TCR-induced calcium flux, but have near normal levels of Erk activation. To assess the effect of this relatively specific defect in calcium signaling on positive and negative selection, the LAT knock-in mutants were interbred with HY TCR transgenic mice. The HY TCR transgene was chosen because the HY TCR reacts with the male-specific antigen HY, causing negative selection in male mice; however, in female HY TCR transgenic mice, T cells are positively selected by endogenous peptide(s) (22). Therefore, use of the HY model system allows us to analyze both positive and negative selection in one TCR transgenic system and to investigate if class I–restricted LAT mutant T cells would exhibit a similar phenotype to LAT Y136F CD4+ T cells. As shown in Fig. 1, HY+ LAT Y136Fm/m males and females exhibited a phenotype essentially identical to LAT Y136Fm/m mice. They also had enlarged spleens and lymph nodes (not depicted). Thymi from the mutant mice displayed varying degrees of infiltration by CD4+ CD3lo CD62Llo CD44hi T cells, characteristic of peripheral T cells seen in non-TCR transgenic LAT knock-in mice. These infiltrating peripheral T cells were the only T cells found in the thymus later in life (after ∼2 mo of age; not depicted). Lymph node T cells were largely CD4+ (i.e., they were not MHC class I restricted) and did not stain with antibody to the HY transgenic TCR (T3.70). In contrast, female control HY+ LAT+/+ mice contained T3.70+ CD8hi cells in the periphery, a result of successful positive selection of transgenic T cells (Fig. 1). Male control HY+ LAT+/+ mice lacked T3.70+ CD8hi T cells in the periphery, but had T3.70+ CD8lo transgenic T cells, which have been described previously (22).
Figure 1.
LAT Y136F phenotype is dominant in HY+ LAT Y136Fm/m female and male mice. (a) Thymocytes and lymph node cells from HY+ LAT+/+ and HY+ LAT Y136Fm/m female mice were analyzed by flow cytometry using anti-CD4, anti-CD8, and T3.70 antibodies. CD4 versus CD8 dot plots are shown either ungated or gated on T3.70hi cells. HY+ LAT+/+ and HY+ LAT Y136Fm/m female mice were 41 and 8 wk of age, respectively. (b) Thymocytes and lymph node cells from HY+ LAT+/+ and HY+ LAT Y136Fm/m male mice were analyzed as described in (a). HY+ LAT+/+ and HY+ LAT Y136Fm/m male mice were 7 wk of age. (c) Thymocytes and lymph node cells from a LAT Y136Fm/m mouse (5 wk old) were analyzed as described in (a).
To investigate if the LATY136Fm/m lymphoproliferative phenotype would also manifest in other TCR transgenic mouse model systems, we interbred the LAT knock-in mutant with class II–restricted AND TCR transgenic mice (23). A similar phenotype was observed, i.e., expansion of CD4+ T cells not staining for the transgenic TCR (Vα11−) and the presence of large lymph nodes and spleens (not depicted). We speculated that either the LAT Y136F mutation drives all T cells to the same phenotype regardless of the TCR that they express, or that the specificity of the TCRs in these TCR transgenic model systems was changed by the pairing of transgenic TCRβ chains with endogenous TCRα chains, a phenomenon known to occur due to incomplete early allelic exclusion of the TCRα locus (24). To distinguish between these possibilities, we examined the HY TCR transgenic system in more detail by crossing the LAT mutation onto the RAG knockout background, where TCR specificity would be fixed because contributions from endogenous TCRα chains would be eliminated.
Lack of positive selection in HY+ LATY136Fm/m RAG−/− female mice
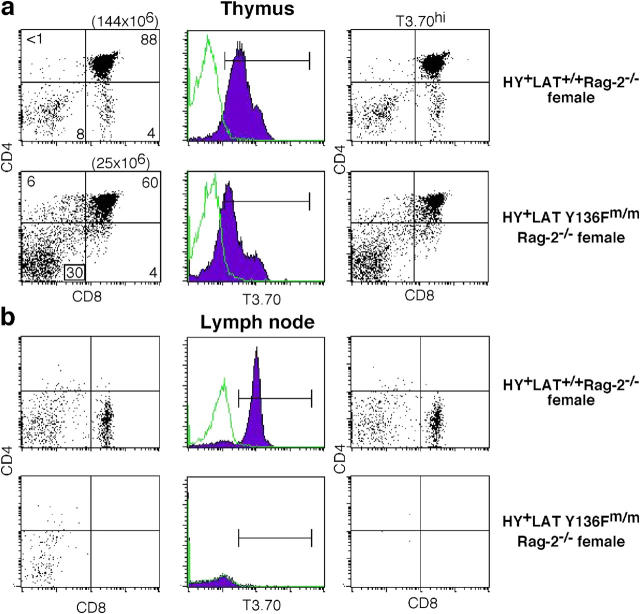
By breeding onto the RAG-2 null background, we were able to determine whether clonotypic CD8hi T cells could develop in HY+ LAT Y136F knock-in mice by preventing expression of endogenous TCR α or β chains, therefore eliminating competition from CD4+ T cells expressing endogenous TCRα chains. A much different phenotype was observed using the RAG-2 null background. In HY+ LAT Y136Fm/m RAG-2−/− female mice, thymi were smaller than those in their LAT+/+ counterparts, about one fifth to one tenth the normal size (Fig. 2 a). This decrease in thymic cellularity, especially in the number of CD4+ CD8+ (double positive [DP]) thymocytes is consistent with a block in the DN→DP transition (20). Although DP thymocytes are generated, a distinct population of CD8 single positive (SP) thymocytes is missing, indicating a defect in positive selection. DPs from HY+ LAT Y136Fm/m RAG-2−/− female mice express lower levels of the HY transgenic TCR as evidenced by staining with the antibody T3.70 (∼60% wild-type levels). Consistent with a lack of positive selection in the thymus, HY+ LAT Y136Fm/m RAG-2−/− female mice do not contain T3.70hi CD8hi T cells in the periphery (Fig. 2 b).
Figure 2.
Positive selection in HY+ LAT Y136Fm/m RAG-2 knockout female mice. (a) Thymocytes from HY+ LAT+/+ RAG-2−/− and HY+ LAT Y136Fm/m RAG-2−/− female mice were analyzed as described in Fig. 1. Total thymocyte numbers are given in parentheses. Unfilled histograms represent nonspecific antibody controls. HY+ LAT+/+ RAG-2−/− and HY+ LAT Y136Fm/m RAG-2−/− female mice were 22 and 4 wk of age, respectively. (b) Lymph node cells from HY+ LAT+/+ RAG-2−/− and HY+ LAT Y136Fm/m RAG-2−/− female mice were analyzed as described in (a).
Impaired negative selection in male HY+ LAT Y136Fm/m mice
Because of the extensive negative selection of HY clonotypic T cells in male HY+ LAT+/+ mice, thymi are small and contain mostly DN thymocytes (Fig. 3 a). In HY+ LAT Y136Fm/m RAG-2−/− male mice, thymi are also small; however, T3.70hi DP and CD8 SP are evident, much like in HY+ LAT+/+ RAG-2−/− female mice (Fig. 3 a), indicating a failure of negative selection and possible conversion to positive selection. In the lymph nodes of HY+ LAT Y136Fm/m RAG-2−/− male mice, T3.70+ CD8hi T cells are evident, again suggesting a failure of negative selection and possible conversion to positive selection (Fig. 3 b). In HY+ LAT+/+ RAG-2−/− male mice, T3.70+ DN and CD8lo T cells are also observed. These T cells have been hypothesized to be aberrant populations from the γδ T cell lineage (25). In HY+ LAT Y136Fm/m RAG-2−/− male mice, these populations are not present.
Figure 3.
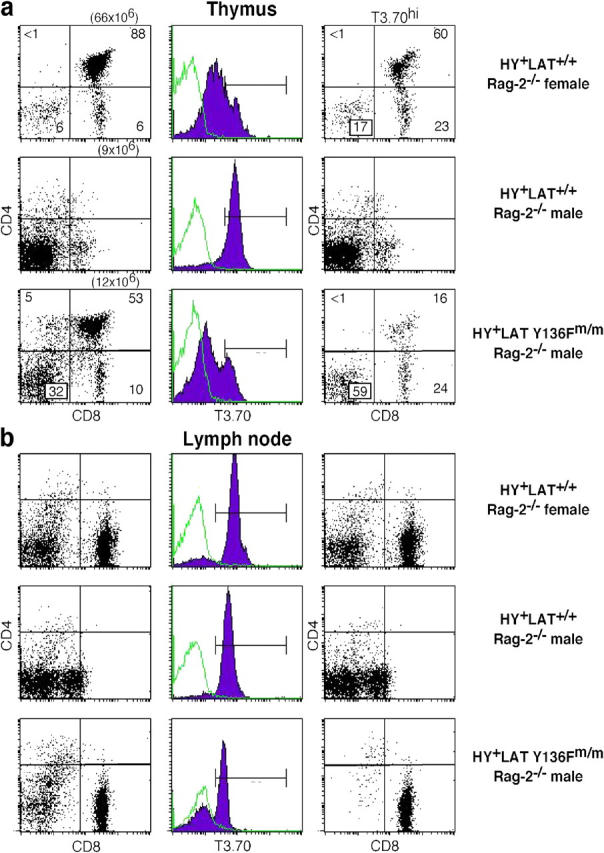
Negative selection in HY+ LAT Y136Fm/m RAG-2 knockout male mice. (a) Thymocytes from HY+ LAT+/+ RAG-2−/− and HY+ LAT Y136Fm/m RAG-2−/− male mice were analyzed as described in Fig. 1. Thymocytes from HY+ LAT+/+ RAG-2−/− female mice are shown for comparison. Total thymocyte numbers are given in parentheses. HY+ LAT+/+ RAG-2−/− female, HY+ LAT+/+ RAG-2−/− male, and HY+ LAT Y136Fm/m RAG-2−/− male mice were 24, 11, and 21 wk of age, respectively. (b) Lymph node cells from HY+ LAT+/+ RAG-2−/− and HY+ LAT Y136Fm/m RAG-2−/− male mice were analyzed as described in (a).
A possible contribution to the lack of negative selection in HY+ LAT Y136Fm/m RAG-2−/− male mice could derive from defects in activation-induced cell death in DP thymocytes. Plate-bound T3.70 and anti-CD28 were used to mimic signals known to induce cell death in DP thymocytes (26). DP thymocytes from HY+ LAT+/+ RAG-2−/− female mice showed an increase from 22 to 79% annexin V+ under these antibody stimulation conditions (Table I). However, DP thymocytes from HY+ LATm/m RAG-2−/− male mice showed no increase in the percentage of annexin V+ T cells under these antibody stimulation conditions. As a control, etoposide treatment resulted in 100% annexin V+ DPs in both HY+ LAT+/+ RAG-2−/− female and HY+ LAT Y136Fm/m RAG-2−/− male mice.
Table I.
Activation-induced cell death is defective in HY+ LATY136Fm/m RAG-2 knockout male and female thymocytes
| Percent of annexin V+ DP thymocytes | |||||
|---|---|---|---|---|---|
| Control | T3.70+αCD28 | APC control | APC+HY peptide | VP16 | |
| HY+ LAT+/+ RAG−/− femalea | 22 | 79 | ndb | nd | 100 |
| HY+ LATY136Fm/m RAG−/− malea | 39 | 40 | nd | nd | 100 |
| HY+ LAT+/+ RAG−/− femalec | 31 | 90 | 68 | 91 | 99 |
| HY+ LATY136Fm/m RAG−/− femalec | 38 | 22 | 66 | 58 | 99 |
Thymocytes from HY+ LATY136Fm/m RAG−/− male and HY+ LATY136Fm/m RAG−/− female mice were stimulated with 5 ug/ml plate-bound T3.70 and 50 ug/ml anti-CD28 or APCs preloaded with 1 uM HY peptide. As a positive control for apoptosis, thymocytes were also treated with etoposide (VP16). Annexin V staining was determined by flow cytometry after 24 h of incubation.
Experiment 1.
Not done.
Experiment 2.
We also tested the response of DP thymocytes from HY+ LAT Y136Fm/m RAG-2−/− female mice to antibody stimulation. DP thymocytes from HY+ LAT Y136Fm/m RAG-2−/− females showed no increase in annexin V staining in response to T3.70 and anti-CD28 treatment. In addition, incubation with HY peptide-loaded APCs did not result in increased annexin V staining above APC controls in the mutants, whereas DP thymocytes from HY+ LAT+/+ RAG-2−/− female mice did have increased annexin V staining. Again, etoposide treatment resulted in high rates of cell death in both HY+ LAT+/+ RAG-2−/− and HY+ LAT Y136Fm/m RAG-2−/− female mice. These data indicate a failure of TCR-mediated induction of cell death in thymocytes from both male and female mutant mice.
Biochemical characterization of T cells from male HY+ LAT Y136Fm/m mice
Because defects in TCR-induced calcium influx were described in thymocytes and peripheral T cells from LAT Y136Fm/m mice (20), we also measured calcium influx in T cells from HY+ LAT Y136Fm/m RAG-2−/− mice. DP thymocytes and CD8+ lymph node T cells from HY+ LAT Y136Fm/m RAG-2−/− male mice had reduced TCR-induced calcium influx compared with DP thymocytes and CD8+ lymph node T cells from C57BL/6 and HY+ LAT+/+ RAG-2−/− female mice (Fig. 4 a). DP thymocytes from HY+ LAT Y136Fm/m RAG-2−/− female mice also had diminished levels of calcium influx compared with DP thymocytes from C57BL/6 and HY+ LAT+/+ RAG-2−/− female mice (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20041869/DC1). However, initial calcium influx in DP thymocytes from HY+ LAT Y136Fm/m RAG-2−/− male and female mice and in CD8+ lymph node T cells from HY+ LAT Y136Fm/m RAG-2−/− male mice was higher than in DP thymocytes and CD4+ lymph node T cells from non-TCR transgenic LAT Y136F knock-in mice (Fig. 4 a and Fig. S1). This heightened initial calcium influx could be characteristic of the strength of signal from the HY TCR (compared with the diversity of TCRs found in T cells in LAT Y136F knock-in mice) or due to the higher levels of TCR in HY+ LAT Y136Fm/m RAG-2−/− T cells compared with non-TCR transgenic LAT Y136Fm/m T cells. In the case of peripheral cells, the difference could also be related to differences between CD4+ and CD8+ T cells. We were unable to obtain data for TCR-induced calcium influx in CD8+ T cells from LAT Y136F knock-in mice to make a direct comparison because so few of those cells exist.
Figure 4.
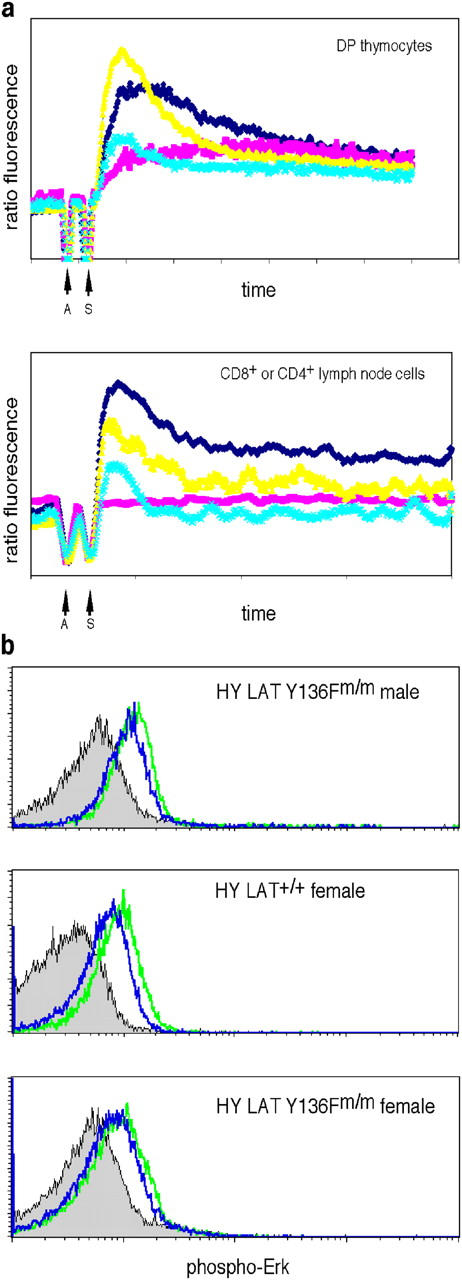
TCR-induced calcium flux and Erk activation in T cells from HY+ LATm/m RAG-2−/− male mice. (a) TCR-induced calcium flux of CD4+ CD8+ thymocytes (top) or CD8+ lymph node cells (bottom) was measured as described in Materials and methods. T cells were from C57BL/6 (dark blue), LAT Y136Fm/m (pink), HY+ LAT+/+ RAG-2−/− female (yellow) or HY+ LATm/m RAG-2−/− male mice (light blue). For LAT Y136Fm/m mice, T cells were stimulated with anti-CD3 and anti-CD4 (instead of anti-CD8), and CD4+ lymph node cells are pictured in the bottom instead of CD8+ lymph node cells because there are only very few CD8+ peripheral T cells in LAT Y136Fm/m mice. Antibodies (A; anti-CD3 and anti-CD8 or anti-CD3 and anti-CD4 in the case of LAT Y136Fm/m mice) were added at 30 s and streptavidin (S) was added at 1 min. Cells were analyzed for a total of 7 min. (b) Thymocytes from HY+ LAT Y136Fm/m RAG-2−/− male and female mice and HY+ LAT+/+ RAG-2−/− female mice were analyzed for levels of phospho-Erk by flow cytometry as described in Materials and methods. Data are shown for Thy1.2+ CD4+ (DP) thymocytes. Histograms represent unstimulated T cells (filled gray), anti-CD3 and anti-CD8 stimulation for 1 min (green), and anti-CD3 and anti-CD8 stimulation for 5 min (blue).
We also examined Erk activation in T cells from HY+ LAT Y136Fm/m RAG-2−/− mice by measuring levels of phospho-Erk1/2 by flow cytometry. The extent and kinetics of Erk activation was approximately the same in DP thymocytes (Fig. 4 b) and CD8+ lymph node T cells (not depicted) from HY+ LAT Y136Fm/m RAG-2−/− male mice and HY+ LAT+/+ RAG-2−/− female mice. Erk activation in HY+ LAT Y136Fm/m RAG-2−/− female DP thymocytes was observed, although the increase in phospho-Erk levels at 1 min of TCR activation was less pronounced in mutant female (1.4-fold increase) than in mutant male thymocytes (2.2-fold increase). This may reflect differences in levels of ongoing positive selection in the male and female mutant mice. The increase in phospho-Erk staining in male mutant thymocytes at 1 min of activation was similar to that seen in HY+ LAT+/+ RAG-2−/− female thymocytes (2.4-fold). These results are consistent with the small impact of the LAT Y136F mutation on Erk activation in T cells from LAT Y136F non-TCR transgenic mice as observed previously (20).
Evidence of conversion of negative selection to positive selection in male HY+ LAT Y136Fm/m mice
To examine the differentiation state of CD8 SP thymocytes and CD8+ lymphocytes from HY+ LAT Y136Fm/m RAG-2−/− mice, we measured the expression of several cell surface markers by flow cytometry (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20041869/DC1). Thymocyte maturation is accompanied by down-regulation of CD24 (HSA) and, in the CD8 lineage, up-regulation of β7 integrin (27, 28). CD24 is down-regulated and β7 integrin is up-regulated in mature T cells from HY+ LAT Y136Fm/m RAG-2−/− male mice as in mature T cells from HY+ LAT+/+ RAG-2−/− female mice. CD69, a marker of maturation and activation, is up-regulated in CD8 SP thymocytes compared with DP thymocytes from both kinds of mice and, in fact, is up-regulated to a slightly greater extent in the mutant male mice, consistent with functional activation of the Ras/Erk pathway in these mice (29). TCR (T3.70) levels are consistently lower in T cells from the male mutant mice than in female wild-type mice.
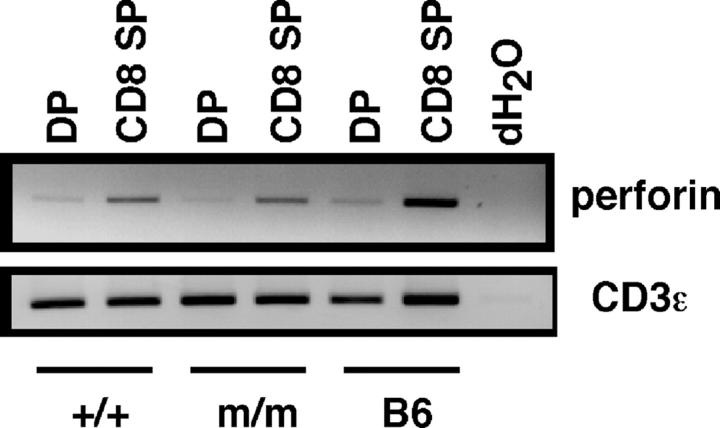
To investigate whether bona fide CD8+ T cells develop in HY+ LAT Y136Fm/m RAG-2−/− male mice by positive selection, we analyzed the expression of perforin, a differentiation marker of CD8+ cytotoxic T cells. CD8 SP thymocytes were sorted and analyzed by RT-PCR for expression of perforin. As shown in Fig. 5, CD8 SP thymocytes from HY+ LAT Y136Fm/m RAG-2−/− male mice (as well as from HY+ LAT+/+ RAG-2−/− female mice and control C57BL/6 mice) expressed perforin. Perforin expression was also detected by RT-PCR in CD8+ splenocytes from these mice (not depicted).
Figure 5.
Perforin and CD3ɛ RT-PCR. Thymocytes from HY+ LAT+/+ RAG-2−/− female and HY+ LAT Y136Fm/m male mice were sorted and analyzed by RT-PCR for expression of perforin and CD3ɛ. C57BL/6 (B6) thymocytes are included for comparison.
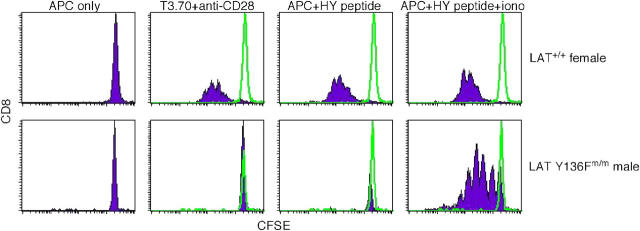
To investigate whether CD8+ T cells in HY+ LAT Y136Fm/m RAG-2−/− male mice were functional (as might be expected if they resulted from normal positive selection), we examined the ability of peripheral CD8+ T cells to proliferate in response to TCR stimulation. Purified CD8+ T cells from HY+ LAT Y136Fm/m RAG-2−/− male mice did not proliferate in response to antibody stimulation or treatment with HY peptide-loaded APCs as did CD8+ T cells from HY+ LAT+/+ RAG-2−/− female mice (Fig. 6). Even use of high concentrations of antibodies or HY peptide for stimulation did not result in a proliferative response in CD8+ T cells from HY+ LAT Y136Fm/m RAG-2−/− male mice (not depicted). Because this proliferation defect could be due to the effects of the LAT Y136F mutation, we included ionomycin in an attempt to bypass the PLC-γ1–mediated Ca2+ defect in these cells. When ionomycin was included in cultures with CD8+ T cells from HY+ LAT Y136Fm/m RAG-2−/− male mice and HY peptide-loaded APCs, proliferation did occur (Fig. 6). Ionomycin alone did not induce proliferation (not depicted). Therefore, mutant cells were able to respond to antigen if calcium influx was pharmacologically induced. Under the same conditions, however, interferon γ and IL-2 could not be detected from supernatants of cultures of HY+ LAT Y136Fm/m RAG-2−/− male CD8+ T cells and HY peptide-loaded APCs, even in the presence of ionomycin, whereas cytokine secretion was detected after treatment with PMA and ionomycin (not depicted). This suggests that although CD8+ T cells from mutant male mice can respond to antigen, they are not fully functional in vivo, possibly due to defects caused by the LAT Y136F mutation.
Figure 6.
In vitro proliferation of CD8+ lymph node T cells from HY+ LAT Y136Fm/m RAG-2 knockout male mice. CD8+ T cells were purified by magnetic bead separation from lymph node preparations from HY+ LAT+/+ RAG-2−/− female and HY+ LAT Y136Fm/m RAG-2−/− male mice. Cells were loaded with CFSE and stimulated with 5 ug/ml of plate-bound T3.70 and 50 ug/ml anti-CD28 or with APCs loaded with 1,000 nM HY peptide in the absence or presence of 1,300 nM ionomycin. After 18 h, cells were analyzed by flow cytometry. CFSE staining is shown gated on CD8+ T cells. Unfilled histograms depict treatment with APCs only.
Discussion
The LAT Y136F mutation impairs TCR-mediated PLC-γ1 activation in thymocytes and T cells resulting in decreased TCR-induced calcium influx (20, 21). We used this LAT mutant to investigate the effect of dampening calcium signaling on thymocyte positive and negative selection in a system that does not use broad-range inhibitors of calcium-dependent enzymes or agents that alter calcium influx triggered by non-LAT–mediated signaling pathways. We examined HY TCR transgenic, RAG-2 knockout mice to evaluate the effect of the LAT Y136F mutation on positive and negative selection. In HY female mice, thymocytes normally undergo positive selection resulting in the generation of HY TCR-specific CD8hi T cells; however, HY LAT mutant female mice showed a complete absence of positive selection. In HY LAT+/+ male mice, thymocytes undergo negative selection and HY TCR-specific CD8hi T cells are absent because the HY antigen is male specific. In contrast, T cells from HY LAT mutant male mice do not undergo negative selection, but instead resemble positively selected T cells found in LAT+/+ HY females. The effect of dampening calcium signaling in this system can be interpreted according to a quantitative model of thymocyte differentiation in which very low levels of TCR signal result in nonselection (also called death by neglect), intermediate signals result in positive selection, and strong signals result in negative selection. According to this model, the “window” for positive and negative selection has been shifted in the LAT mutant mice such that thymocytes from male mice, which would otherwise undergo negative selection, undergo positive selection (or a process very much like positive selection), and thymocytes from female mice, which would otherwise undergo positive selection, most likely undergo death by neglect.
PLC-γ1, whose activation is defective in LAT Y136F knock-in mice, catalyzes the conversion of phosphatidylinositol (4,5) bisphosphate to diacylglycerol and inositol (1,4,5) trisphosphate. An increase in inositol (1,4,5) tris phosphate levels results in release of calcium from intracellular stores, whereas increased diacylglycerol results in stimulation of PKC and RasGRP (for review see reference 30). Therefore, the effects on selection seen in this study could be because of decreased signaling through PKC and RasGRP as a result of decreased PLC-γ1 activity. However, the downstream endpoint of Erk activation is relatively normal in LAT Y136F mutant T cells, implying that Erk can be activated through mechanisms other than those mediated by RasGRP in these cells. Pharmacologic disruptions of TCR-mediated PLC-γ1 signaling pathways have previously been shown to affect thymocyte selection. Use of inhibitors that block PKC (31) or of inhibitors of the calcium-dependent phosphatase calcineurin (32) results in less thymocyte deletion, an in vitro correlate of negative selection. Our results are consistent with the idea that inhibition of downstream mediators of PLC-γ1 action can block negative selection.
Mutations in mice that alter PLC-γ1–mediated signaling have also been shown to affect positive and negative thymocyte selection in ways consistent with results from this study. A spontaneous mutation in ZAP-70, the kinase that phosphorylates LAT, results in decreased LAT phosphorylation, decreased PLC-γ1 phosphorylation, and decreased calcium flux (33). Erk and p38 activities are also dampened. When this mutation is crossed into the HY TCR transgenic background, positive selection is abrogated in female mice, and in male mice peripheral transgenic CD8+ T cells are observed, similar to the results observed here for the LAT Y136F mutation (33). In addition, null mutation of TCRζ (which would preclude docking of ZAP-70 to the TCR complex and prevent ZAP-70 activation) resulted in a phenotype resembling positive selection in male HY TCR transgenic mice and nonselection in female HY TCR transgenic mice (34). The Tec family kinases Itk and Rlk/Txk regulate PLC-γ1 activity in T cells (15). Null mutation of both Itk and Rlk/Txk results in decreased positive selection in HY female mice and a phenotype suggestive of conversion of negative to positive selection in HY male mice (35). Null mutation of Itk and Rlk/Txk also resulted in decreased PLC-γ1 activation, decreased calcium flux, and dampened Erk activation (35). Like the LAT Y136F mutation, all of these mutations decrease activation of PLC-γ1 and activation-induced calcium influx. Unlike the examples above, in HY LAT Y136F knock-in mice, we were able to show antigen-induced proliferation in peripheral CD8+ male T cells by partially correcting for the LAT mutant phenotype by treatment with the calcium ionophore ionomycin, supporting the critical role of calcium in thymocyte selection and T cell function.
We have speculated that the lymphoproliferative disease in LAT Y136F knock-in mice could be due to expansion of autoreactive T cells that have escaped negative selection (20). A similar phenotype was observed in null mutants of the calcium-regulated NFAT transcription family members NFATc2 and NFATc3 (36, 37). NFAT transcription factors are downstream targets of calcium-mediated TCR signaling. Recent examples in the literature indicate that point mutations in T cell signaling molecules could result in defects in central (and not just peripheral) tolerance that could eventually lead to autoimmune disease. Examples of point mutations in T cell signaling molecules leading to autoimmune disease, presumably by failure of negative selection, include a spontaneous mutation in the Rasgrp1 gene, which results in a lymphoproliferative disease with symptoms resembling systemic lupus erythematosus (38). Also, the ZAP-70 spontaneous mutant mentioned earlier (33) has a syndrome with symptoms similar to autoimmune arthritis. A knock-in mutation of the phosphatase CD45 (E613R) results in a lupus-like nephritis (39). Possibly, mutations in different molecules will skew the TCR repertoire in different ways, manifesting as different autoimmune syndromes.
T cell hybridomas made from LAT Y136F peripheral T cells were autoreactive as reported by Aguado et al. (21). In this study, we have also provided evidence that aberrant negative selection might be contributing to a skewed TCR repertoire in LAT Y136F knock-in mice, which could ultimately lead to proliferation of autoreactive T cells. This conclusion is based on the fact that HY TCR+ CD8hi T cells can develop in LAT Y136Fm/m male mice. Lymphoproliferative disease does not ensue, however, in the HY model system, as evidenced by an absence of lymphadenopathy, splenomegaly, and lymphocytic infiltration to lung in HY+ LAT Y136Fm/m RAG-2−/− male mice (not depicted). This absence of lymphoproliferative disease from HY+ LAT Y136Fm/m RAG-2−/− T cells could be because the strength of signal transduced through the HY TCR may not be sufficient to cause this response, or the HY (self-) antigen may not be presented in the tissues examined. Alternatively, the lymphoproliferative phenotype may only manifest in CD4+ MHC class II–restricted T cells. Future experiments will be aimed at trying to distinguish between these possibilities.
Materials and Methods
Mice.
LAT Y136F knock-in mice were described previously (20). HY mice, which express an MHC class I–restricted TCR for the male antigen HY (22), were obtained from NIAID Taconic exchange. RAG-2 knockout mice were also obtained from NIAID Taconic exchange. All mice used in this study were on a C57BL/6 (H-2Db) background and were housed under specific pathogen-free conditions.
Flow cytometry.
Single cell suspensions were analyzed by standard flow cytometry as described previously (40). Flow cytometry was performed using a FACSCalibur and CELLQuest software (Becton Dickinson). Fluorochrome-conjugated antibodies were purchased from BD Biosciences.
Thymocyte cell death assays.
Thymocytes were harvested in Cellgro complete serum-free medium (Mediatech), washed, and incubated with plate-bound antibodies (5 ug/ml T3.70 and 50 ug/ml anti-CD28) or with APCs and HY peptide. APCs were prepared from ACK-treated splenocytes by treatment with anti-Thy antibody followed by treatment with complement. The T cell–depleted splenocytes were then irradiated at 500 rads to yield APCs. HY peptide (KCSRNRQYL) was synthesized by the FDA core facility. Cells were harvested for flow cytometry after 18 h of incubation. Annexin V-FITC was purchased from BD Biosciences and was used according to the manufacturer's specifications.
Proliferation assays.
CD8+ T cells from lymph nodes were purified by negative magnetic bead separation using biotinylated anti-B220, anti–Mac-1, and anti-CD4 antibodies (BD Biosciences) as well as magnetic streptavidin beads (Miltenyi Biotec). Purified cells were loaded with CFSE (Molecular Probes), washed, and incubated with plate-bound antibodies (5 ug/ml T3.70 and 50 ug/ml anti-CD28) or with APCs and HY peptide. Cells were harvested for flow cytometry after 3 d of incubation.
ELISA.
Supernatants from the proliferation assays described above were analyzed by ELISA for the presence of interferon γ and IL-2 using reagents and the protocol from BD Biosciences.
RT-PCR.
Thymocytes were stained with anti-CD4 FITC and anti-CD8 PE and were sorted for DP and SP populations using a FACS Vantage SE cell sorter equipped with TurboSort (Becton Dickinson). RNA was prepared from sorted populations using TriZol reagent (Invitrogen). cDNA was synthesized using the SuperScript cDNA synthesis system (Invitrogen). Dilutions of cDNA were made and tested so that amounts used for PCR were in the linear range. Primers for CD3ɛ were (sense) CTGAGAGGATGCGGTGGAACA and (antisense) GACCATCAGCAAGCCCAGAGT. PCR conditions for CD3ɛ were 95°C for 5 min and 30 cycles of 95°C for 45 s, 56°C for 45 s, and 72°C for 1 min. Primers for perforin were (sense) CAAGCAGAAGCACAAGTTCGT and (antisense) CGTGATAAAGTGCGTGCCATA. PCR conditions for perforin were 95°C for 5 min and 30 cycles of 95°C for 45 s, 50°C for 45 s, and 72°C for 1 min.
Calcium flux experiments.
Measurements of calcium flux were performed essentially as described previously (41). Thymocytes or lymph node cells were stained for CD4 and CD8 and preloaded with indo-1 (Molecular Probes). At 30 s, biotinylated anti-CD3 and anti-CD8 (10 μg each) were added and at 60 s, 25 μg streptavidin was added. Calcium flux, measured as the ratio of FL5 to FL4, was recorded on an LSR I (Becton Dickinson) and is displayed as a function of time.
Phospho-Erk assays.
Measurement of levels of phospho-Erk was performed using a modification of the protocol of Priatel et al. (42). 2 × 106 cells were stimulated by the addition of biotinylated anti-CD3 and anti-CD8 (10 ug/ml) followed by the addition of 20 ug/ml streptavidin and incubation at 37°C for the indicated times. Reactions were stopped by the addition of an equal volume of 4% paraformaldehyde followed by cold methanol fixation (43). Cells were stained with 3 ug/ml anti–p-Erk (no. 9106S; Cell Signaling Technology) followed by staining with goat anti–mouse Ig-PE, anti-CD4 PerCP, and anti–Thy 1.2 APC (BD Biosciences). Flow cytometry was performed using a FACSCalibur and CELLQuest software (Becton Dickinson). Induction of phospho-Erk could be blocked by the addition of 10 uM MEK inhibitor U0126 (Calbiochem).
Online supplemental material.
Fig. S1 shows diminished calcium flux in DP thymocytes from female HY+ SM88m/m RAG-2−/− mice. Fig. S2 shows the cell surface expression of T3.70, CD24 (HSA), CD69, and β7 integrin on DP thymocytes, CD8 SP thymocytes, and CD8+ lymph node T cells from HY+ LAT+/+ RAG-2−/− female and HY+ LAT Y136Fm/m RAG-2−/− male mice. Figs. S1 and S2 are available at http://www.jem.org/cgi/content/full/jem.20041869/DC1.
Acknowledgments
We acknowledge the expert assistance of Barbara Taylor of the NCI Flow Cytometry Core Facility, Bethesda, MD for help with sorting and Valarie Barr (NCI) for help with histological analysis.
The authors have no conflicting financial interest.
Abbreviations used: DN, double negative; DP, double positive; LAT, linker for activation of T cells; PLC, phospholipase C; SP, single positive.
References
- 1.Starr, T.K., S.C. Jameson, and K.A. Hogquist. 2003. Positive and negative selection of T cells. Annu. Rev. Immunol. 21:139–176. [DOI] [PubMed] [Google Scholar]
- 2.Alberola-Ila, J., and G. Hernandez-Hoyos. 2003. The Ras/MAPK cascade and the control of positive selection. Immunol. Rev. 191:79–96. [DOI] [PubMed] [Google Scholar]
- 3.Mariathasan, S., R.G. Jones, and P.S. Ohashi. 1999. Signals involved in thymocyte positive and negative selection. Semin. Immunol. 11:263–272. [DOI] [PubMed] [Google Scholar]
- 4.Mariathasan, S., S.S. Ho, A. Zakarian, and P.S. Ohashi. 2000. Degree of ERK activation influences both positive and negative thymocyte selection. Eur. J. Immunol. 30:1060–1068. [DOI] [PubMed] [Google Scholar]
- 5.Mariathasan, S., A. Zakarian, D. Bouchard, A.M. Michie, J.C. Zuniga-Pflucker, and P.S. Ohashi. 2001. Duration and strength of extracellular signal-regulated kinase signals are altered during positive versus negative thymocyte selection. J. Immunol. 167:4966–4973. [DOI] [PubMed] [Google Scholar]
- 6.Wange, R.L. 2000. LAT, the linker for activation of T cells: a bridge between T cell-specific and general signaling pathways. Sci STKE 2000:RE1. [DOI] [PubMed]
- 7.Samelson, L.E. 2002. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu. Rev. Immunol. 20:371–394. [DOI] [PubMed] [Google Scholar]
- 8.Zhu, M., E. Janssen, and W. Zhang. 2003. Minimal requirement of tyrosine residues of linker for activation of T cells in TCR signaling and thymocyte development. J. Immunol. 170:325–333. [DOI] [PubMed] [Google Scholar]
- 9.Zhang, W., R.P. Trible, M. Zhu, S.K. Liu, C.J. McGlade, and L.E. Samelson. 2000. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell antigen receptor-mediated signaling. J. Biol. Chem. 275:23355–23361. [DOI] [PubMed] [Google Scholar]
- 10.Lin, J., and A. Weiss. 2001. Identification of the minimal tyrosine residues required for linker for activation of T cell function. J. Biol. Chem. 276:29588–29595. [DOI] [PubMed] [Google Scholar]
- 11.Paz, P.E., S. Wang, H. Clarke, X. Lu, D. Stokoe, and A. Abo. 2001. Mapping the Zap-70 phosphorylation sites on LAT (linker for activation of T cells) required for recruitment and activation of signalling proteins in T cells. Biochem. J. 356:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sommers, C.L., R.K. Menon, A. Grinberg, W. Zhang, L.E. Samelson, and P.E. Love. 2001. Knock-in mutation of the distal four tyrosines of linker for activation of T cells blocks murine T cell development. J. Exp. Med. 194:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clements, J.L. 2003. Known and potential functions for the SLP-76 adapter protein in regulating T-cell activation and development. Immunol. Rev. 191:211–219. [DOI] [PubMed] [Google Scholar]
- 14.Yablonski, D., T. Kadlecek, and A. Weiss. 2001. Identification of a phospholipase C-gamma1 (PLC-gamma1) SH3 domain-binding site in SLP-76 required for T-cell receptor-mediated activation of PLC-gamma1 and NFAT. Mol. Cell. Biol. 21:4208–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucas, J.A., A.T. Miller, L.O. Atherly, and L.J. Berg. 2003. The role of Tec family kinases in T cell development and function. Immunol. Rev. 191:119–138. [DOI] [PubMed] [Google Scholar]
- 16.Macian, F., C. Lopez-Rodriguez, and A. Rao. 2001. Partners in transcription: NFAT and AP-1. Oncogene. 20:2476–2489. [DOI] [PubMed] [Google Scholar]
- 17.Ebinu, J.O., S.L. Stang, C. Teixeira, D.A. Bottorff, J. Hooton, P.M. Blumberg, M. Barry, R.C. Bleakley, H.L. Ostergaard, and J.C. Stone. 2000. RasGRP links T-cell receptor signaling to Ras. Blood. 95:3199–3203. [PubMed] [Google Scholar]
- 18.Roose, J., and A. Weiss. 2000. T cells: getting a GRP on Ras. Nat. Immunol. 1:275–276. [DOI] [PubMed] [Google Scholar]
- 19.Sommers, C.L., L.E. Samelson, and P.E. Love. 2004. LAT: a T lymphocyte adapter protein that couples the antigen receptor to downstream signaling pathways. Bioessays. 26:61–67. [DOI] [PubMed] [Google Scholar]
- 20.Sommers, C.L., C.S. Park, J. Lee, C. Feng, C.L. Fuller, A. Grinberg, J.A. Hildebrand, E. Lacana, R.K. Menon, E.W. Shores, et al. 2002. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science. 296:2040–2043. [DOI] [PubMed] [Google Scholar]
- 21.Aguado, E., S. Richelme, S. Nunez-Cruz, A. Miazek, A.M. Mura, M. Richelme, X.J. Guo, D. Sainty, H.T. He, B. Malissen, and M. Malissen. 2002. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science. 296:2036–2040. [DOI] [PubMed] [Google Scholar]
- 22.von Boehmer, H. 1990. Developmental biology of T cells in T cell-receptor transgenic mice. Annu. Rev. Immunol. 8:531–556. [DOI] [PubMed] [Google Scholar]
- 23.Kaye, J., M.L. Hsu, M.E. Sauron, S.C. Jameson, N.R. Gascoigne, and S.M. Hedrick. 1989. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 341:746–749. [DOI] [PubMed] [Google Scholar]
- 24.Borgulya, P., H. Kishi, Y. Uematsu, and H. von Boehmer. 1992. Exclusion and inclusion of alpha and beta T cell receptor alleles. Cell. 69:529–537. [DOI] [PubMed] [Google Scholar]
- 25.Bruno, L., H.J. Fehling, and H. von Boehmer. 1996. The alpha beta T cell receptor can replace the gamma delta receptor in the development of gamma delta lineage cells. Immunity. 5:343–352. [DOI] [PubMed] [Google Scholar]
- 26.Amsen, D., and A.M. Kruisbeek. 1996. CD28-B7 interactions function to co-stimulate clonal deletion of double-positive thymocytes. Int. Immunol. 8:1927–1936. [DOI] [PubMed] [Google Scholar]
- 27.Gabor, M.J., D.I. Godfrey, and R. Scollay. 1997. Recent thymic emigrants are distinct from most medullary thymocytes. Eur. J. Immunol. 27:2010–2015. [DOI] [PubMed] [Google Scholar]
- 28.Boursalian, T.E., J. Golob, D.M. Soper, C.J. Cooper, and P.J. Fink. 2004. Continued maturation of thymic emigrants in the periphery. Nat. Immunol. 5:418–425. [DOI] [PubMed] [Google Scholar]
- 29.D'Ambrosio, D., D.A. Cantrell, L. Frati, A. Santoni, and R. Testi. 1994. Involvement of p21ras activation in T cell CD69 expression. Eur. J. Immunol. 24:616–620. [DOI] [PubMed] [Google Scholar]
- 30.Acuto, O., and D. Cantrell. 2000. T cell activation and the cytoskeleton. Annu. Rev. Immunol. 18:165–184. [DOI] [PubMed] [Google Scholar]
- 31.Page, D.M., L.P. Kane, T.M. Onami, and S.M. Hedrick. 1996. Cellular and biochemical requirements for thymocyte negative selection. Semin. Immunol. 8:69–82. [DOI] [PubMed] [Google Scholar]
- 32.Kane, L.P., and S.M. Hedrick. 1996. A role for calcium influx in setting the threshold for CD4+CD8+ thymocyte negative selection. J. Immunol. 156:4594–4601. [PubMed] [Google Scholar]
- 33.Sakaguchi, N., T. Takahashi, H. Hata, T. Nomura, T. Tagami, S. Yamazaki, T. Sakihama, T. Matsutani, I. Negishi, S. Nakatsuru, and S. Sakaguchi. 2003. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 426:454–460. [DOI] [PubMed] [Google Scholar]
- 34.Yamazaki, T., H. Arase, S. Ono, H. Ohno, H. Watanabe, and T. Saito. 1997. A shift from negative to positive selection of autoreactive T cells by the reduced level of TCR signal in TCR-transgenic CD3 zeta-deficient mice. J. Immunol. 158:1634–1640. [PubMed] [Google Scholar]
- 35.Schaeffer, E.M., C. Broussard, J. Debnath, S. Anderson, D.W. McVicar, and P.L. Schwartzberg. 2000. Tec family kinases modulate thresholds for thymocyte development and selection. J. Exp. Med. 192:987–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranger, A.M., M. Oukka, J. Rengarajan, and L.H. Glimcher. 1998. Inhibitory function of two NFAT family members in lymphoid homeostasis and Th2 development. Immunity. 9:627–635. [DOI] [PubMed] [Google Scholar]
- 37.Rengarajan, J., B. Tang, and L.H. Glimcher. 2002. NFATc2 and NFATc3 regulate T(H)2 differentiation and modulate TCR-responsiveness of naive T(H)cells. Nat. Immunol. 3:48–54. [DOI] [PubMed] [Google Scholar]
- 38.Layer, K., G. Lin, A. Nencioni, W. Hu, A. Schmucker, A.N. Antov, X. Li, S. Takamatsu, T. Chevassut, N.A. Dower, et al. 2003. Autoimmunity as the consequence of a spontaneous mutation in Rasgrp1. Immunity. 19:243–255. [DOI] [PubMed] [Google Scholar]
- 39.Majeti, R., Z. Xu, T.G. Parslow, J.L. Olson, D.I. Daikh, N. Killeen, and A. Weiss. 2000. An inactivating point mutation in the inhibitory wedge of CD45 causes lymphoproliferation and autoimmunity. Cell. 103:1059–1070. [DOI] [PubMed] [Google Scholar]
- 40.Shores, E., V. Flamand, T. Tran, A. Grinberg, J.P. Kinet, and P.E. Love. 1997. Fc epsilonRI gamma can support T cell development and function in mice lacking endogenous TCR zeta-chain. J. Immunol. 159:222–230. [PubMed] [Google Scholar]
- 41.Sommers, C.L., R.L. Rabin, A. Grinberg, H.C. Tsay, J. Farber, and P.E. Love. 1999. A role for the Tec family tyrosine kinase Txk in T cell activation and thymocyte selection. J. Exp. Med. 190:1427–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Priatel, J.J., S.J. Teh, N.A. Dower, J.C. Stone, and H.S. Teh. 2002. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity. 17:617–627. [DOI] [PubMed] [Google Scholar]
- 43.Chow, S., H. Patel, and D.W. Hedley. 2001. Measurement of MAP kinase activation by flow cytometry using phospho-specific antibodies to MEK and ERK: potential for pharmacodynamic monitoring of signal transduction inhibitors. Cytometry. 46:72–78. [DOI] [PubMed] [Google Scholar]