PREFACE
I am honored to present the third annual Memorial Lecture to commemorate the achievements of the late Marian W. Fischman. Marian was a dedicated and creative scientist and an effective science advocate. She was also an outstanding mentor and very supportive of her trainees and junior collaborators. And Marian was a wonderful colleague and a delightful person to be with. All of us who knew her continue to mourn her loss.
Marian was a pioneer in clinical research on the behavioral and physiological effects of cocaine. Between 1976 and her untimely death in 2001, Marian published an outstanding series of ground-breaking clinical studies of the effects of cocaine. Marian’s research was reviewed by Chris Ellyn Johanson in the First Memorial Lecture (Johanson 2003). Briefly, in a series of important papers by Marian and her colleagues, it was shown that cocaine’s abuse-related effects can be systematically studied in humans in the clinical laboratory, that these effects of cocaine are dose-dependent, and the subjective effects of cocaine are highly correlated with the drug’s plasma concentration and physiological effects. Moreover, cocaine self-administration by humans is amenable to environmental manipulations including schedule of reinforcement, availability of alternative reinforcers and treatment with candidate medications. A few examples of some important papers follow: (Evans et al. 1999; Fischman 1984, 1987,1988; Fischman and Foltin 1992; Fischman and Schuster 1980,1981,1982; Fischman et al. 1976, 1990; Foltin and Fischman 1991, 1991a Foltin and Fischman 1992a and b, 1994a and b, 1996a and b, 1997a and b, 1998; Foltin et al. 1995; Javaid et al. 1978; Johanson and Fischman 1989; Ward et al. 1997a and b).
Marian was an active member of the Board of Directors of the College on Problems of Drug Dependence (CPDD) and our terms on the Board overlapped. With the sponsorship of the CPDD, Marian and I worked together to edit two monographs. The first was based on a CPDD-sponsored meeting in 1988, and resulted in a monograph entitled Testing for Abuse Liability of Drugs in Humans (Fischman and Mello 1989). The second monograph, published in 1991, was a review of the abuse liability of stimulants, opioid mixed agonist-antagonist drugs, anxiolytics and sedative hypnotics (Fischman and Mello 1991). It was a great pleasure to work with Marian on these monographs, and we felt that we had accomplished something important for CPDD and for the field.
Both of these CPDD monographs focused on drug abuse liability, and much of the preclinical research of that time was primarily concerned with predicting the abuse liability of novel compounds using drug self-administration and drug discrimination procedures. These behavioral procedures were also used to analyze the pharmacological mechanisms underlying the reinforcing effects of drugs, as well as tolerance and physical dependence. There was considerably less interest in using drug self-administration models to predict the effectiveness of drug abuse treatment medications. Yet, I thought that this was an obvious application of these powerful behavioral procedures, and I organized a symposium on this topic for the 1991 meeting of the CPDD. That symposium was entitled “Behavioral Strategies for the Evaluation of New Pharmacotherapies for Drug Abuse Treatment” (Mello 1992), and papers were presented by Bob Balster, Roger Spealman and Bill Woolverton. Evaluation of potential drug abuse treatment medications continues to be a dominant theme in my research. In the remainder of this paper, I will review and discuss some preclinical and clinical findings from my laboratory.
CHALLENGES IN PRECLINICAL EVALUATION OF CANDIDATE TREATMENT MEDICATIONS
The development and evaluation of new drug abuse treatment medications has been greatly facilitated by the availability of animal models of drug self-administration (Mello 1992; Mello and Negus 1996). Animals will reliably self-administer most drugs that are abused by man, and it is reasonable to expect that medications that decrease drug self-administration should be more effective clinically than medications that do not change or increase drug self-administration. Drug self-administration models can be used to evaluate medications that substitute for or antagonize the effects of abused drugs. These medication evaluation strategies do not depend on any particular conceptualization of the pathogenesis of drug abuse, and the effects of antagonists and agonists on drug self-administration can be interpreted in terms of pharmacological interactions with the target drug of abuse.
Animal drug self-administration models offer a number of methodological advantages for the systematic evaluation of new medications. Unlike clinical trials, compliance with the medication regimen is ensured, and there is no confounding influence of unreported polydrug abuse. The effects of a new treatment medication on an ongoing pattern of drug self-administration can be evaluated quantitatively under controlled experimental conditions. Accurate baseline measures of the daily dose and frequency of drug self-administration can be determined before, during, and after administration of the treatment medication, whereas in clinical studies, the drug abuse history and the usual pattern of drug use are often unknown. It is also possible to monitor medication safety during exposure to the abused drugs, and to detect any adverse side effects that compromise animal health. In addition, uncontrolled social factors such as expectancy or placebo effects and peer pressure cannot complicate the interpretation of data obtained in animal models. Finally, preclinical evaluations are more cost-effective than extensive clinical trials.
Despite these several methodological advantages, assessing the clinical relevance of animal models of drug self-administration is an ongoing challenge. Many of the potential treatment medications that selectively reduce drug self-administration in preclinical studies have not been approved by the FDA for evaluation in man. This limits opportunities for cross validation of medication effectiveness between preclinical studies and clinical trials. The schematic in Figure 1 shows an ongoing assessment of the concordance between medication evaluations in animal models and human drug abusers. Ideally, preclinical studies can be used to predict the effectiveness of medications in clinical trials, and confirmatory clinical results validate the predictive value and clinical relevance of animal models of drug self-administration. The result would be to accelerate identification of new medications for drug abuse treatment.
Figure 1.
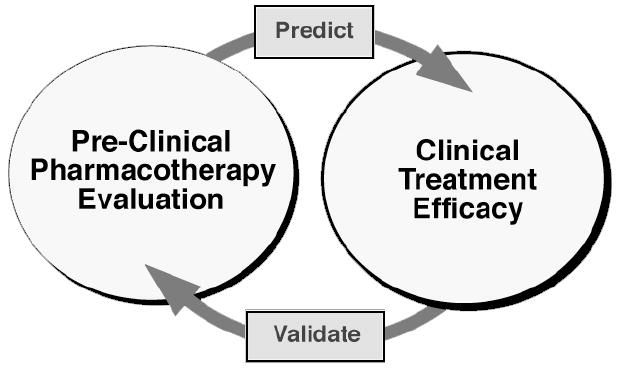
Cross validation of preclinical and clinical models. Validation of the effectiveness of animal models for preclinical evaluation of drug abuse treatment medications requires assessing the degree of concordance between preclinical studies and outpatient clinical studies. Eventually, the preclinical model should enable users to predict the potential effectiveness of new pharmacotherapies. This interactive process of cross-validation and prediction is essential for refinement of the preclinical model of drug self-administration. From Mello NK (1992); Behavioral Strategies for the Evaluation of New Pharmacotherapies for Drug Abuse Treatment. NIDA Research Monograph 119. Washington, DC: Government Printing Office, pp 150–154.
Fortunately, there are some instances in which the concordance between findings from clinical and preclinical evaluations has been clearly documented. The remainder of this review will describe some clinical and preclinical findings from my laboratory that illustrate the potential predictive value of animal models for medication evaluation. Much of my research has focused on the opioid mixed agonist antagonist buprenorphine, and I will describe some relevant findings from those studies. More detailed reviews of our research on buprenorphine have been published elsewhere (Mello and Mendelson 1985, 1992, 1993, 1995; Mendelson and Mello 1992; Mello et al. 1993).
BUPRENORPHINE FOR THE TREATMENT OF OPIOID ABUSE AND DEPENDENCE
Background
Buprenorphine was approved by the FDA for the outpatient treatment of opioid abuse in October, 2002. Importantly, buprenorphine can be prescribed by qualified physicians to private patients. The availability of buprenorphine, a safe and long-acting medication, provides another treatment option for physicians and patients (Jones 2004). The odyssey of buprenorphine from early clinical trials in the late 1970’s to FDA approval in 2002 was long, and fraught with many obstacles. Our research group played a small part in this journey, and a comprehensive review of the buprenorphine story has been published in a book entitled “Buprenorphine: Combating Drug Abuse with a Unique Opioid” (Cowan and Lewis 1995).
Buprenorphine was synthesized by John Lewis at Reckitt and Colman, Inc. (Lewis 1974, 1995; Lewis et al. 1983), and he received the Eddy Award from CPDD in recognition of this significant achievement. Buprenorphine is a congener of the potent mu opioid agonist, etorphine and an opioid antagonist, diprenorphine. Because buprenorphine is derived from a combination of these two compounds, it is often referred to as an opioid mixed agonist-antagonist (Schuster and Harris 1985). From a treatment perspective, buprenorphine combines the characteristics of opioid agonist and antagonist pharmacotherapies for opioid addiction and offers some advantages over using either agonists or antagonists alone. Unlike opioid agonists, abrupt discontinuation of buprenorphine treatment usually does not produce severe and protracted withdrawal signs and symptoms (Jasinski et al. 1978; Fudala et al. 1989). Buprenorphine is also safe, and its antagonist component prevents lethal overdose even at approximately 10 times the analgesic therapeutic dose (Banks 1979). This reduces the risk for drug overdose deaths, which are often associated with illicit methadone abuse (Kreek 1978), although buprenorphine in combination with benzodiazepines may be fatal (Jones 2004). Finally, the opioid agonist component of buprenorphine appears to be very important for patient acceptance and is the primary advantage of buprenorphine over treatment with the opioid antagonist, naltrexone. The risk for illicit diversion has been reduced by combining buprenorphine with the opioid antagonist naloxone (Jones 2004).
Clinical Studies
The first clinical pharmacology studies of buprenorphine were published in 1978 by Donald Jasinski and co-workers (Jasinski et al. 1978). They found that buprenorphine antagonized the physiological and subjective effects of high doses of morphine for up to 29.5 hours (Jasinski et al. 1978). Buprenorphine’s long duration of action is an important advantage for drug abuse treatment. Don Jasinski and John Lewis made it possible for our group to study the effects of buprenorphine on heroin self-administration in heroin-dependent men (Mello and Mendelson 1980). Our subjects were 10 heroin-dependent men who had used heroin for over 10 years and had failed in conventional treatment programs. After admission to a dedicated clinical research ward and detoxification with methadone, they were given gradually ascending doses of buprenorphine or placebo, administered subcutaneously, over 14 days. Men were then maintained on placebo or 8 mg per day of buprenorphine for 10 days and given an opportunity to work on a simple operant task for points for heroin or money. After the 10-day buprenorphine or placebo treatment, men assigned to buprenorphine treatment were given gradually descending doses over five days and men assigned to placebo treatment were given gradually decreasing doses of methadone to prevent heroin withdrawal signs.
Men could work for either money or for heroin, and it took approximately five minutes to earn one point on a second order FR 300 (FI 1 sec:S) schedule. The operant manipulandum was a garage door opener, and each response was transmitted to computer-controlled circuitry. After 90 minutes of performance on the operant task, subjects could earn $1.50 or a single injection of heroin. Heroin was administered intravenously, and a maximum of three heroin injections was available each day (one injection every eight hours). During the first five days of buprenorphine or placebo treatment, a total of 21 mg of heroin was available each day. During the second five days of buprenorphine or placebo treatment, a total of 40.5 mg of heroin was available each day.
Heroin self-administration data for individual subjects are shown in Figure 2. Three men who were maintained on placebo treatment worked at the operant task for all the heroin that was available. In contrast, three men who were maintained on buprenorphine self-administered only one or two heroin injections over the entire 10 day treatment period. It is obvious that buprenorphine treatment dramatically reduced heroin self-administration. We also studied four subjects under both placebo and buprenorphine treatment conditions. Under placebo conditions, each subject took between 90 and 100 percent of the heroin available, whereas during buprenorphine treatment conditions, heroin self-administration was greatly reduced. One subject (number 8) could not tolerate the 8 mg dose of buprenorphine and was maintained on 4 mg per day. This subject took more heroin than the subjects maintained on 8 mg per day. We concluded that buprenorphine significantly reduced heroin self-administration by heroin-dependent men (Mello and Mendelson 1980). Buprenorphine was well tolerated and opioid side effects were mild and transient (Mello et al. 1982). A more complete review of these studies has been published (Mello and Mendelson 1995).
Figure 2.
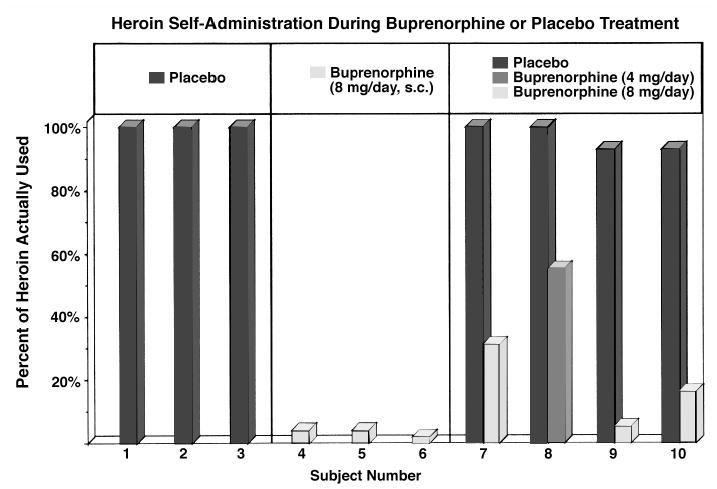
Percentage of available heroin used during 10 days of treatment with buprenorphine or placebo buprenorphine. Three subjects were maintained on placebo-buprenorphine (left columns); three subjects were maintained on buprenorphine (8 mg/day, s.c.) (middle columns), and four subjects were studied under both buprenorphine and placebo-buprenorphine conditions (right columns). Adapted from Mello and Mendelson, Science (1980).
Preclinical Studies
Although we have studied the effects of a number of candidate treatment medications on drug self-administration in rhesus monkeys, many of these have not been approved for clinical use (Mello and Negus 1996, 2000; Negus et al. 1999). Buprenorphine offered an opportunity to cross validate findings from clinical studies and preclinical studies in non-human primates. The drug self-administration procedures have been described in detail in our original reports (Mello et al. 1990a, 1992) and are briefly summarized here. Monkeys were trained to press a key on an operant response panel to earn food (1 g banana-flavored pellets) on a second order schedule that required 32 or 64 responses for a single reinforcer [FR 2 or FR 4 (VR 16:S)]. The response key was transilluminated with different colors to signal the availability of food, drug or a timeout period when responding had no scheduled consequences. After food-maintained responding was stable, monkeys were surgically implanted with a double lumen i.v. catheter in the jugular vein or the femoral vein under aseptic conditions. The double lumen catheter has the advantage that drugs can be administered through one catheter lumen and the treatment medication can be administered through the second catheter lumen, so it is not necessary to flush the catheter contents into the vein before administering a treatment.
In most of our studies, food availability was followed by cocaine availability and a two-hour timeout period, and four such sessions were run each day. Food sessions always were before drug sessions so that intoxication would not compromise food intake. We study both drug- and food-maintained responding so we can determine if medication effects are selective for drug reinforcement or reflect a general disruption of operant responding (Mello 1992; Mello and Negus 1996). In some studies, a long-acting treatment medication or saline control treatment was administered during a morning timeout period. In other studies, a short-acting treatment medication was administered every 20 minutes. We usually examined the effects of chronic medication treatment for seven or 10 days or longer, because this is most analogous to the way medications are administered clinically. Moreover, we have often found that the effects of the treatment medication may change with repeated administration.
BUPRENORPHINE’S EFFECTS ON HEROIN SELF-ADMINISTRATION
On the basis of our clinical studies of buprenorphine’s effects on heroin self-administration in heroin-dependent men (Mello and Mendelson 1980), we expected that buprenorphine would also decrease heroin self-administration by rhesus monkeys. Figure 3 shows one example of cross validation between clinical and preclinical studies of the effects of treatment medications. Heroin self-administration dose-effect curves were determined during saline treatment and during treatment with buprenorphine (0.075 or 0.237 mg/kg/day). Each treatment was studied for 10 days at each of four unit doses of heroin (0.0001–0.10). Each data point shown in Figure 3 is the average of the last three days of 10 days of treatment. It is apparent that buprenorphine (0.075 or 0.237 mg/kg) shifted the heroin dose-effect curve downwards and to the right, and had less effect on food-maintained responding in comparison to saline control treatment (Mello and Negus 1998).
Figure 3.
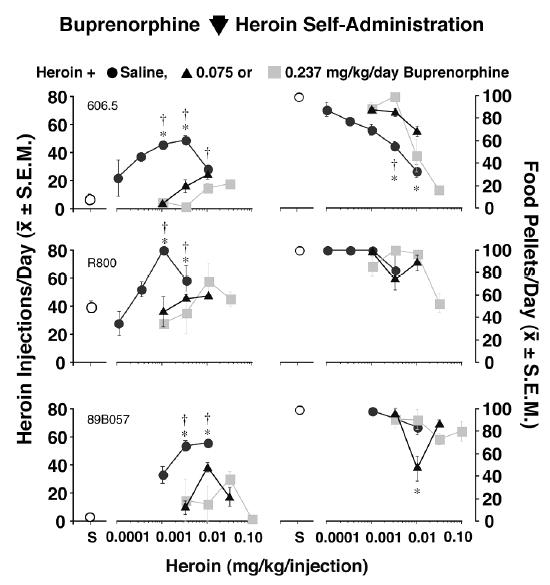
Effects of buprenorphine on heroin and food self-administration in rhesus monkeys. Dose-effect curves for heroin alone (0.0001–0.10 mg/kg/inj) are shown for individual monkeys in the left panel. The mean number of injections per day of heroin during saline treatment (closed circles) or buprenorphine treatment (0.075 mg/kg/day, triangles and 0.237 mg/kg/day, shaded squares) are shown on the left ordinate. Points above S show data from sessions when saline was available for self-administration (open circles). The dose of heroin available for self-administration is shown on the abscissa. In the right panel, food-maintained responding during saline self-administration (open circles) and heroin (0.0001–0.10 mg/kg/inj) self-administration during saline treatment (closed circles) and buprenorphine treatment [0.075 mg/kg/day, triangles and 0.237 mg/kg/day, shaded squares) is shown for individual monkeys. Each data point is the average of the last 3 days of 10 days during saline or buprenorphine treatment ( ± S.E.M.). Statistically significant differences between corresponding heroin doses during saline control treatment and buprenorphine treatment are indicated as follows: * 0.075 mg/kg/day buprenorphine different from saline, p<0.05; = = 0.237 mg/kg/day buprenorphine different from saline, p<0.05. Adapted from Mello and Negus, J. Pharmacol. Exp. Ther. (1998).
Buprenorphine’s Effects on Cocaine Self-Administration
In the late 1980’s, we wondered if buprenorphine would also reduce cocaine self-administration. This notion was based in part on accumulating evidence of interactions between dopaminergic and endogenous opioid systems. Ron Hammer had just reported that cocaine increased opioid receptor density in brain areas associated with drug reinforcement (Hammer 1989). We found that cocaine stimulated release of LH, a gonadotropin hormone regulated in part by endogenous opioid inhibitory control of hypothalamic luteinizing-hormone-releasing-hormone (LHRH) (Mello et al. 1990b and c; Mendelson et al. 1992). This effect of cocaine was unexpected. It was well established that opioid antagonists stimulated LH release by antagonizing endogenous opioid inhibition of LHRH (Yen et al. 1985). There was considerable interest in the co-modulatory interactions between endogenous opioid and dopaminergic systems in brain (Herz and Shippenberg 1988; Koob and Bloom 1988; Watson et al. 1988). Nonetheless it was surprising to find that buprenorphine dose-dependently reduced cocaine self-administration by rhesus monkeys. Figure 4 shows that after only five days of buprenorphine treatment, cocaine injections per day decreased significantly in comparison to 15 days of saline treatment. At a higher dose of buprenorphine, fewer than 10 cocaine injections per day were self administered over 15 days. We published these data in Science in 1989, and this finding has been repeatedly replicated in our laboratory and in a number of other preclinical studies (Mello and Mendelson 1995). As described below, several outpatient clinical studies have also shown that buprenorphine decreases cocaine self-administration (Kosten et al. 1989a and b; Gastfriend et al. 1993a; Montoya et al. 2004).
Figure 4.
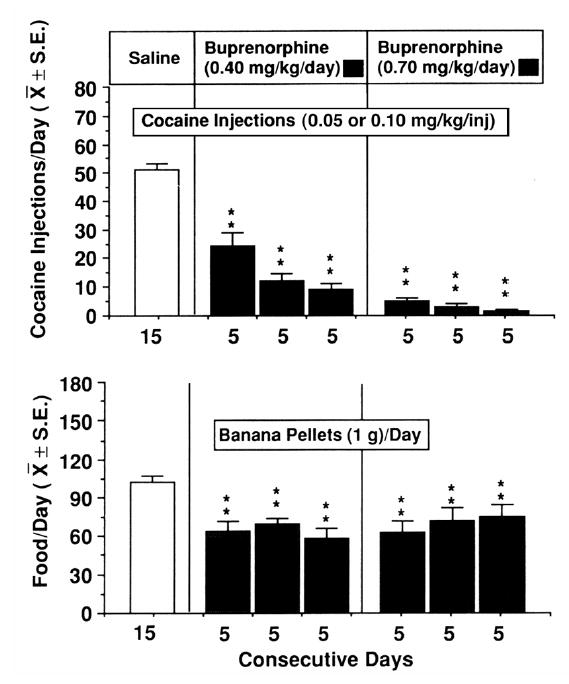
Buprenorphine reduces cocaine self-administration by rhesus monkeys. The effects of single daily infusions of buprenorphine or a saline control solution on cocaine and food self-administration are shown. Saline treatment is shown as an open bar in the left panel and buprenorphine treatment 0.40 mg/kg/day is shown as closed bars in the middle panel and 0.70 mg/kg/day is shown as closed bars in the right panel. The average number of cocaine injections self-administered is shown in the top row. The average number of food pellets self-administered is shown in the second row. The number of days that each treatment condition was in effect is shown on the abscissa. Each data point is the mean ±SEM for five subjects. The statistical significance of each change from the saline treatment as determined by analysis of variance for repeated measures and Dunnett’s tests for multiple comparisons is shown by asterisks (**p< 0.01). Reproduced from Mello et al., Science (1989), with permission of the publishers. Copyright 1989 by the A.A.A.S.
We were interested to learn if buprenorphine’s reduction of cocaine self-administration would persist over a long period of time, or if tolerance would develop to buprenorphine’s effects on cocaine self-administration (Mello et al. 1992). After a 15-day saline treatment baseline period, monkeys were treated with 0.32 mg/kg/day buprenorphine for four months. Figure 5 shows that cocaine self-administration was reduced throughout the 120 day period. Food-maintained responding was initially reduced but returned to and exceeded baseline levels. During the post-buprenorphine 15 day saline treatment period, cocaine self-administration recovered, although not to pretreatment baseline levels. These findings were encouraging insofar as they suggested that any effect of buprenorphine on cocaine self-administration would persist during a long period of treatment.
Figure 5.
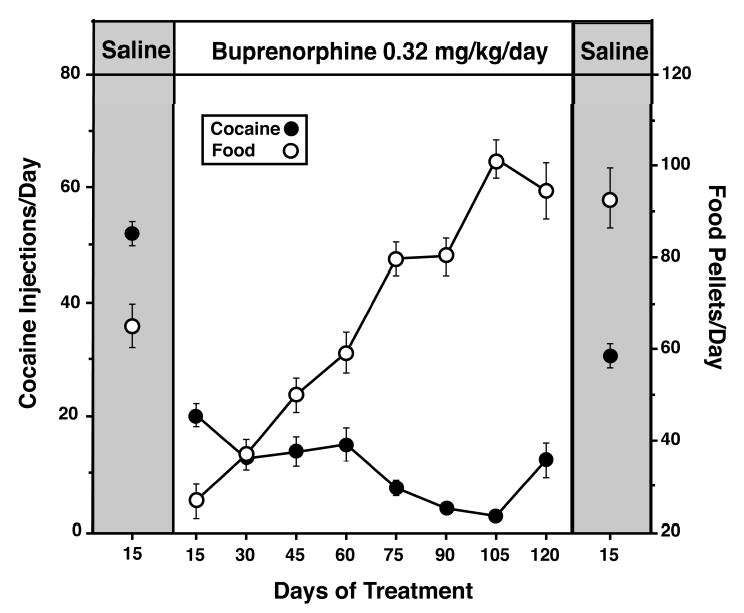
Effects of 3–4 months of daily buprenorphine treatment (0.32 mg/kg/day) on cocaine and food self-administration. Each of the data points for cocaine injections (filled circles) and food pellets (open circles) during the pre-buprenorphine saline control period is the average ±SE of four monkeys over 15 days. The first 100 days of buprenorphine treatment are an average of data from four monkeys and days 101–120 are an average of data from three monkeys. Adapted from Mello et al., J. Pharmacol. Exp. Ther. (1992).
The mechanisms underlying buprenorphine’s robust effects on cocaine self-administration are unknown. Because buprenorphine has both mu opioid agonist and antagonist effects, we tried to determine which component was most important for its interactions with cocaine. We administered ascending doses of the long-acting opioid antagonist naltrexone 20 minutes before buprenorphine, and compared the effects of the buprenorphine + naltrexone combination with the effects of buprenorphine alone on cocaine self-administration. In earlier studies, we found that naltrexone alone did not decrease cocaine self-administration by rhesus monkeys (Mello et al. 1990a). Figure 6 shows the percent change in cocaine injections from baseline during treatment with 0.40 mg/kg/day buprenorphine alone and with buprenorphine in combination with four doses of naltrexone. There was a significant naltrexone dose-dependent decrease in the effectiveness of buprenorphine in reducing cocaine self-administration. We interpreted these data to suggest that the mu opioid agonist component of buprenorphine was essential for its effects on cocaine self-administration in rhesus monkeys (Mello et al. 1993c). Subsequently, we learned that low efficacy mu agonists were more effective in reducing cocaine self-administration than high efficacy mu agonists (Negus and Mello 2002).
Figure 6.
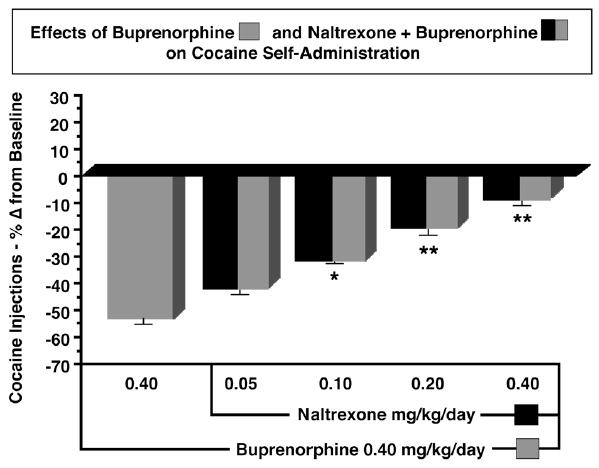
Comparison of the effects of buprenorphine only with buprenorphine-naltrexone combinations on cocaine self-administration. The average number of cocaine injections (mean ±SEM) self-administered are shown as percentage change from the saline treatment baseline before buprenorphine administration. A 10-day period of buprenorphine treatment (0.40 mg/kg/day) is shown at the left as a grey bar. Successive 10-day periods of ascending doses of naltrexone (0.05 – 0.40 mg/kg/day) administered 20 min before buprenorphine (striped and black bars) are shown at the right. Adapted from Mello et al., Neuropsychopharmacology (1993), Macmillan Publishers Ltd.
A PRECLINICAL MODEL OF SPEEDBALL SELF-ADMINISTRATION
It is increasingly rare to find persons who use only heroin or only cocaine exclusively. Dual dependence on cocaine and heroin or heroin dependence with occasional cocaine use are common patterns (Condelli et al. 1991; Kreek 1991; NIDA 2002). A survey of over 900 heroin-dependent persons found that 63 percent reported using cocaine with heroin in a speedball over a six-month period (Schütz et al. 1994). In 1995, we reported the development of a polydrug abuse model in the rhesus monkey (Mello et al. 1995). We found that monkeys would avidly self-administer speedballs that consisted of a simultaneous injection of cocaine and heroin in a 3 to 1 dose ratio. We then began to explore various strategies for reducing speedball self-administration. We hypothesized that a combination of medications that targeted both the cocaine and the heroin component of the speedball might be more effective in reducing speedball self-administration than treatment with only one of the same medications alone.
In one study, we examined the effects of treatment with a combination of the dopamine antagonist flupenthixol and the mu antagonist quadazocine (Mello and Negus 1999). We found that those two antagonist drugs in combination reduced speedball self-administration far more effectively than either drug administered alone. These data are shown in the left panel of Figure 7. The highest dose of flupenthixol + quadazocine produced a three-fold rightward shift in the speedball dose-effect curve. However, the effects of the flupenthixol + quadazocine combination were transient and associated with sedation (Mello and Negus 1999). In contrast, treatment with a combination of the monoamine reuptake inhibitor indatraline and buprenorphine produced a sustained downshift in the speedball dose-effect curve that persisted throughout all ten days of treatment (Mello and Negus 2001). The buprenorphine and indatraline combination was also more effective in reducing speedball self-administration than treatment with indatraline alone, or a low dose of buprenorphine alone (0.18 mg/kg). These data are shown in the right panel of Figure 7.
Figure 7.
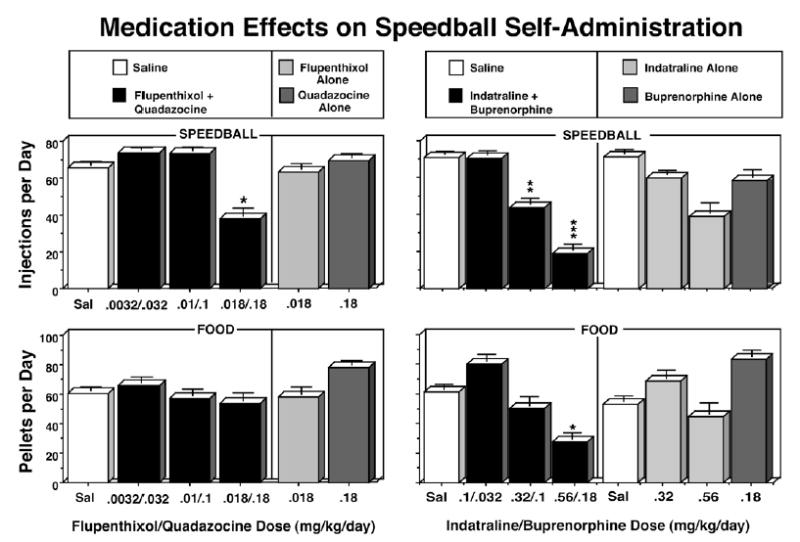
Effects of saline, ascending doses of flupenthixol + quadazocine combinations and flupenthixol or quadazocine alone [left panel] or ascending doses of indatraline + buprenorphine combinations and indatraline or buprenorphine alone on speedball- and food-maintained responding [right panel]. Saline and doses of flupenthixol + quadazocine (mg/kg/day) [left panel] or indatraline + buprenorphine [right panel] are shown on the abscissa. The number of speedball injections per day (row 1) or food pellets per day (row 2) are shown on the left ordinate. Speedballs consisted of a unit dose of cocaine (0.01 mg/kg/inj) and heroin (0.0032 mg/kg/inj) in combination. Speedball- and food-maintained responding during saline treatment are shown as open rectangles, and as black rectangles during treatment with each medication combination. Speedball- and food-maintained responding during treatment with each medication alone are shown as light gray rectangles during treatment with flupenthixol alone [left panel] or indatraline alone [right panel], and as dark gray rectangles during treatment with quadazocine alone [left panel] or buprenorphine alone [right panel]. Each data point represents the average number of injections or food pellets ( ± S.E.) during 10 consecutive days of saline or drug treatment in a group of four monkeys [left panel] or three to five monkeys [right panel]. The asterisks indicate a significant change from the saline treatment baseline (*=p<0.05; **=p<0.01; ***=p<0.001). Adapted from Mello and Negus, Neuropsychopharmacology (1999 and 2001), Macmillan Publishers Ltd.
Given that buprenorphine alone significantly reduced both heroin and cocaine self-administration by rhesus monkeys, we anticipated that buprenorphine would also reduce speedball self-administration. Over a dose range of 0.075 to 0.75 mg/kg/day, buprenorphine dose-dependently reduced speedball self-administration. Figure 8 shows that at a dose of 0.237 mg/kg/day, buprenorphine treatment reduced speedball self-administration and shifted the speedball dose-effect curve one log unit to the right (Mello and Negus 1998). These effects were selective for speedball self-administration, because food-maintained responding were equivalent to or higher during buprenorphine treatment than during saline treatment. It appeared that buprenorphine antagonized the suppressive effects of speedballs on food-maintained responding. Interestingly, 0.237 mg/kg/day was the lowest buprenorphine dose that reduced cocaine self-administration in our earlier studies (Mello et al. 1990a).
Figure 8.
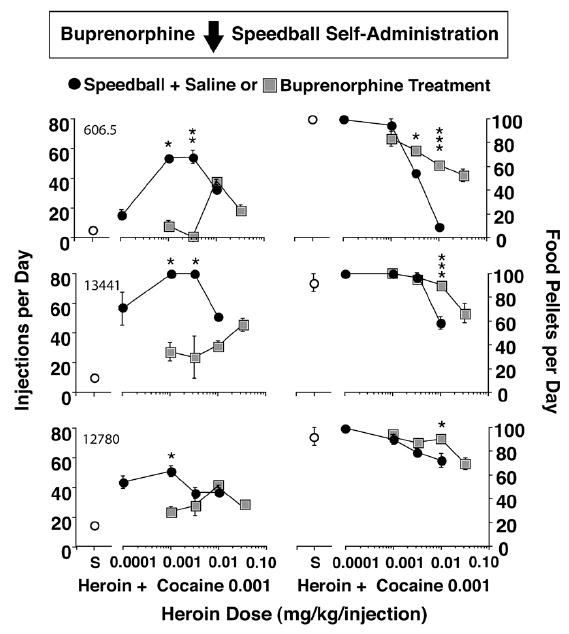
Effects of Buprenorphine (0.237) mg/kg/day on speedball dose-effect curves for individual monkeys. Dose-effect curves for a low dose of cocaine (0.001 mg/kg/inj) in combination with one of four doses of heroin (0.0001–0.032 mg/kg/inj) are shown for individual monkeys (left panel). The unit dose of heroin in combination with cocaine is shown on the abscissa. Points above S show data from saline treatment sessions when saline was the solution available for self-administration (open circles). Self-administration of each heroin + cocaine speedball combination during saline treatment (closed circles) and during buprenorphine treatment (0.237 mg/kg/day) (shaded squares) is shown on the left ordinate as injections per day. Each data point is the average of the last 3 days of 10 days of self-administration of each heroin + cocaine combination for each monkey ( ± S.E.M.). Food-maintained responding during saline self-administration (open circles), self-administration of heroin + cocaine combinations during saline treatment (closed circles) and during buprenorphine treatment (0.237 mg/kg/day) (shaded squares) is shown in the right panel. The number of 1g banana-flavored pellets per day earned during each condition is shown on the right ordinate. Statistically significant differences between corresponding speedball doses during saline control treatment and buprenorphine treatment are indicated by asterisks (*=p<0.05; **=p<0.01; ***=p<0.001). From Mello and Negus, J. Pharmacol. Exp. Ther. (1998).
CLINICAL STUDIES OF BUPRENORPHINE FOR TREATMENT OF HEROIN + COCAINE
The effectiveness of buprenorphine in reducing cocaine self-administration by rhesus monkeys led to clinical laboratory studies to determine the safety of buprenorphine in combination with single doses of cocaine, morphine or saline in human drug abusers (Teoh et al. 1993). Twenty men who fulfilled DSM-III-R criteria for concurrent cocaine and opioid dependence were admitted to a clinical research ward and treated with single daily doses of 4 or 8 mg sublingual buprenorphine for 21 days. Challenge doses of cocaine (30 mg, i.v.), morphine (10 mg, i.v.) and saline were given before and during buprenorphine treatment. There were no adverse interactions between buprenorphine and cocaine or morphine on measures of cardiovascular function, respiration or temperature. Moreover, buprenorphine tended to decrease the respiratory depressant effects of morphine. We concluded that buprenorphine should be safe for treatment of persons with dual cocaine and opioid dependence (Teoh et al. 1993).
The effects of buprenorphine on speedball self-administration by rhesus monkeys are consistent with clinical reports of the effects of buprenorphine on concurrent heroin and cocaine dependence. In 1993, David Gastfriend in our group published the results of an open trial with buprenorphine (4 or 8 mg s.l.) which showed that both heroin and cocaine self-administration were reduced in 22 men with a ten-year history of drug dependence. Needle use, needle sharing and measures of addiction severity were also reduced over 12 weeks (Gastfriend et al. 1993b). In 2004, scientists at the NIDA Intramural Program reported a randomized trial of buprenorphine for the treatment of concurrent opiate and cocaine dependence. They concluded that sublingual buprenorphine at a dose of 16 mg/day was well tolerated and effective in reducing concurrent opiate and cocaine dependence (Montoya et al. 2004).
THE ROLE OF AGONIST THERAPIES IN THE TREATMENT OF DRUG ABUSE
There is an ongoing debate concerning the optimal approach to the development of drug abuse treatment medications. It now appears that agonist medications are more effective for drug abuse treatment then antagonist medications. Although antagonists are pharmacologically effective in reducing the effects of an illicit drug, treatment with antagonist medications usually is not acceptable to the client population. The opioid antagonist naltrexone is one compelling case in point. Naltrexone, like buprenorphine, significantly reduced heroin self-administration by heroin dependent men (Mello et al. 1981), but naltrexone was not generally accepted by patients for outpatient treatment (Meyer and Mirin 1979; Schecter 1980). Naltrexone has proved most useful for treatment of former opioid-dependent professionals who must remain drug free to maintain their medical license. In contrast, the mu opioid agonist methadone, and more recently, the mixed opioid agonist-antagonist buprenorphine, are well accepted by patients in treatment.
Recent preclinical findings from our laboratory are consistent with the notion that agonist medications can be very effective in reducing cocaine self-administration. We have found that d-amphetamine selectively reduces cocaine self-administration in several behavioral procedures (Negus 2003; Negus and Mello 2003a and b). Using the same operant behavioral procedures described earlier for our preclinical studies of buprenorphine, we compared the effects of three doses of d-amphetamine with saline treatment in monkeys trained to self-administer cocaine on a second-order schedule [FR 2 (VR 16:S)]. Each treatment condition was in effect for seven days. d-Amphetamine was administered once every 20 minutes to ensure a constant dose level. Figure 9 shows that during saline treatment and low dose d-amphetamine treatment (0.01 mg/kg/hr), there was no effect on either cocaine- or food-maintained responding. However, at higher doses of d-amphetamine (0.032 and 0.1 mg/kg/hr) there was a significant decrease in cocaine self-administration, and food-maintained responding was less affected (Negus and Mello 2003b).
Figure 9.
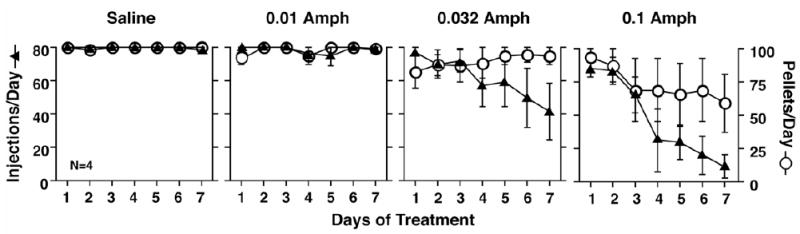
Time course of effects of saline or d-amphetamine (0.01–0.1 mg/kg/hr) on responding for 0.01 mg/kg/injection cocaine and food pellets. Abscissae: Consecutive days of treatment. Left ordinates: Number of cocaine injections (0.01 mg/kg/injection) delivered on each day of treatment (filled triangles, maximum=80). Right ordinates: Number of food pellets delivered on each day of treatment (open circles, maximum=100). Each point shows mean data from four monkeys, and error bars show the SEM. Statistical analysis indicated a significant effect of d-amphetamine dose [F(3,9)=7.93; p=0.0088] and treatment day [F(3,18)=21.96; p<0.0001] on cocaine self-administration and a significant interaction between d-amphetamine dose and treatment day [F(3,54)=4.17; p<0.0001]. A high dose of 0.1 mg/kg/hr d-amphetamine significantly decreased cocaine self-administration relative to saline control (p<0.01, Duncan post hoc test). In contrast, there was not a significant effect of d-amphetamine dose [F(3,9)=2.09; p=0.17] or treatment day [F(3,18)=0.70; p=0.59] on food-maintained responding, although the interaction between d-amphetamine dose and treatment day approached significance [F(3,54)=1.77; p=0.055]. Adapted from Negus and Mello, Drug Alc. Depend. (2003b).
As in our earlier studies of buprenorphine (Mello et al. 1992), we were interested to see if the effects of d-amphetamine on cocaine self-administration were sustained during long-term chronic treatment. Figure 10 shows the effects of 28 days of d-amphetamine treatment on cocaine- and food-maintained responding by rhesus monkeys. Cocaine self-administration remained significantly reduced throughout the entire period of d-amphetamine treatment, whereas food-maintained responding returned to baseline levels within nine days after treatment began. After d-amphetamine treatment was discontinued, food-maintained responding remained at baseline levels, and cocaine self-administration gradually increased over seven days. Recovery of cocaine self-administration indicated that the sustained reduction in cocaine self-administration was a d-amphetamine effect and not due to uncontrolled variables.
Figure 10.
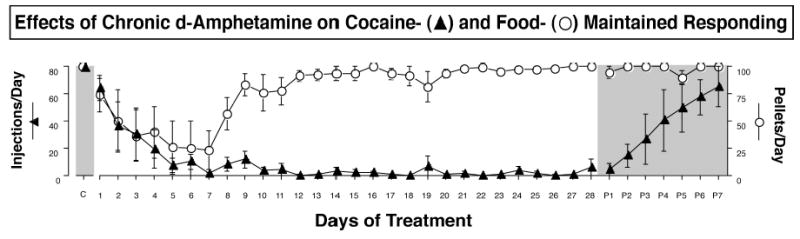
Effects of 28-day treatment with 0.1 mg/kg/hr d-amphetamine on responding for 0.01 mg/kg/injection cocaine and food pellets. Abscissa: Consecutive days of treatment. Left ordinate: Number of cocaine injections (0.01 mg/kg/injection) delivered on each day of treatment (filled triangles, maximum=80). Right ordinate: Number of food pellets delivered on each day of treatment (open circles, maximum=100). Each point shows mean data from four monkeys, and error bars show the SEM. Points in the shaded area on the far left over “C” show average numbers of cocaine injections and food pellets per day when 0.01 mg/kg/injection cocaine was available during saline treatment for 7 days in a separate experiment. Points in the shaded area to the far right over “P1-P7” show post-treatment data collected during the first seven days after d-amphetamine treatment was discontinued. During this time, the same unit dose of cocaine (0.01 mg/kg/injection) was available, and saline was substituted for 0.1 mg/kg/hr d-amphetamine as the treatment. Adapted from Negus and Mello, Drug Alc. Depend. (2003b).
We also determined cocaine dose-effect curves during saline treatment and examined the effects of two doses of d-amphetamine on cocaine- and food-maintained responding. As shown in Figure 11, 0.032 and 0.1 mg/kg/hr d-amphetamine produced dose-dependent and significant rightward and downward shifts in the cocaine self-administration dose-effect curve, with no significant effects on food-maintained responding.
Figure 11.
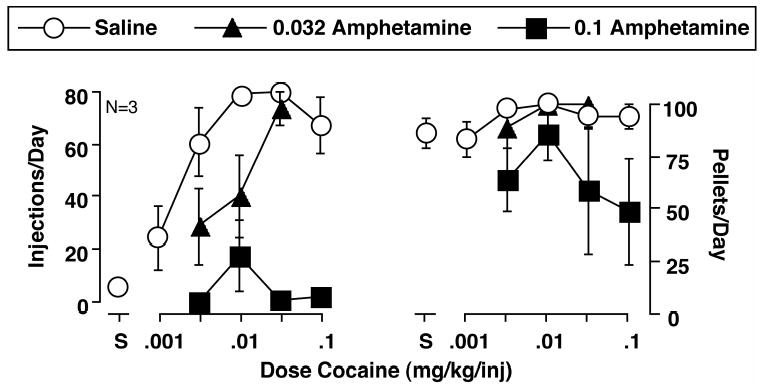
Effects of d-amphetamine on the cocaine self-administration dose-effect curve and concurrent food-maintained responding. Abscissae: Unit dose of cocaine available during daily drug components in mg/kg/injection. Points above S show data collected when saline was the solution available for self-administration. Ordinate (left panel): Mean number of cocaine injections delivered per day during the last three days of each treatment. Ordinate (right panel): Mean number of food pellets (1g) delivered per day during the last three days of each treatment. All points show mean data from three monkeys, and error bars show the SEM. Statistical analysis indicated significant effects on cocaine self-administration of cocaine dose [F(2,4)=10.31; p=0.026] and d-amphetamine dose [F(2,4)=139.8; p=0.0002], but not a significant interaction between cocaine dose and d-amphetamine dose [F(2,8)=1.21; p=0.38]. Both 0.032 and 0.1 mg/kg/hr d-amphetamine produced effects significantly different from saline treatment (p<0.01; Duncan post hoc test). In contrast, there was not a significant effect on food-maintained responding of cocaine dose [F(2,4)=5.37; p=0.074] or d-amphetamine dose [F(2,4)=2.21; p=0.23], and there was not a significant interaction between cocaine dose and d-amphetamine dose [F(2,8)=0.94; p=0.49]. Adapted from Negus and Mello, Drug Alc. Depend. (2003b).
d-Amphetamine’s selective reduction of cocaine self-administration by rhesus monkeys is a robust effect, observed when cocaine self-administration was maintained under a progressive ratio schedule (Negus and Mello 2003a), and in a food vs. cocaine choice procedure (Negus 2003) as well as under a second-order schedule (Negus and Mello 2003b). There was no evidence of increases in cocaine-maintained responding during d-amphetamine treatment as is sometimes observed during continuous cocaine infusion (Panlilio et al. 1998). In that study, when the cocaine infusions began 30 min before the session and the total cocaine treatment dose was approximately twice the amount usually self-administered in a session, only two of seven monkeys decreased cocaine-maintained responding. The other five monkeys increased cocaine self-administration at some cocaine infusion durations (Panlilio et al. 1998). When cocaine was administered as a pre-session bolus, followed by a continuous cocaine infusion during the session, cocaine self-administration was decreased at a moderate unit dose but not at a high unit dose (Glowa and Fantegrossi 1997). d-Amphetamine is longer acting than cocaine, and administration every 20 min resulted in more consistent effects on cocaine self-administration (Figures 9, 10 and 11) than continuous cocaine infusions in previous studies. Under the conditions of our experiments, d-amphetamine, a non-selective monoamine releaser, was similar to GBR-12909, a long-acting dopamine reuptake inhibitor, in its effects on cocaine-maintained responding (Rothman and Glowa 1995).
CONCLUSIONS
Figure 12 summarizes the concordance between clinical and preclinical evaluations of buprenorphine and d-amphetamine as drug abuse treatment medications. Buprenorphine consistently reduces cocaine self-administration in both clinical and preclinical studies (Mello and Mendelson 1995). Buprenorphine also reduces heroin self-administration in both clinical and preclinical studies (Mello and Mendelson 1995; Mello and Negus 1998). Finally, when cocaine and heroin are used concurrently, buprenorphine reduces both cocaine and heroin use in clinical studies (Gastfriend et al. 1993b; Montoya et al. 2004) and speedball self-administration in preclinical studies (Mello and Negus 1998, 2004).
Figure 12.
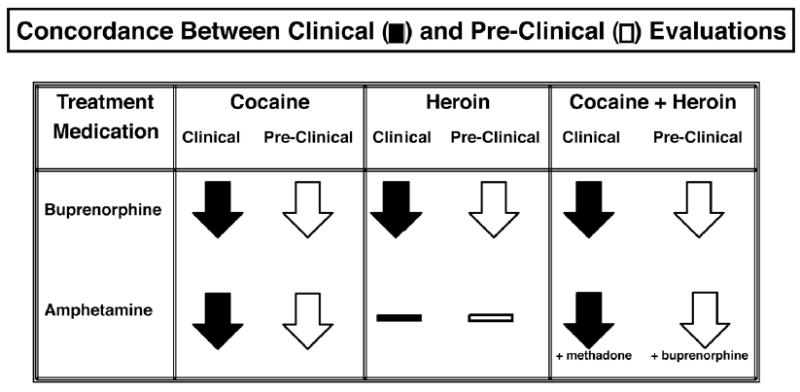
Concordance between clinical and preclinical evaluations of the effects of buprenorphine and of amphetamine on self-administration of cocaine, heroin and cocaine + heroin speedballs. The treatment medication is shown in the far left column. The effects of each treatment medication on cocaine (second column), heroin (third column) and cocaine + heroin speedballs (fourth column) are shown as downward pointing arrows (indicating decreases in drug self-administration) or as horizontal bars (indicating not studied). Clinical studies are shown as black arrows and preclinical studies are shown as white arrows. In row two, column four, amphetamine was studied with methadone or with buprenorphine.
The non-selective monoamine releaser, d-amphetamine also shows a promising profile for cocaine abuse treatment. d-Amphetamine selectively reduces cocaine self-administration by rhesus monkeys with minimal effects on food-maintained responding (Negus 2003; Negus and Mello 2003a and b). John Grabowski and co-workers systematically examined the effectiveness of d-amphetamine for the treatment of cocaine abuse in stimulant abusers and in polydrug abusers (Grabowski et al. 2001, 2004a and b). Recently, Grabowski’s group reported that the combination of d-amphetamine and methadone effectively reduced both cocaine and heroin use in polydrug abusers (Grabowski et al. 2004a and b). We are currently studying the effects of treatment with d-amphetamine in combination with buprenorphine on speedball self-administration in our non-human primate polydrug abuse model. Data obtained thus far indicate that a combination of d-amphetamine + buprenorphine significantly reduced speedball self-administration and shifted the speedball dose effect curve downwards and to the right (Mello and Negus 2004). Of course, we recognize that the introduction of d-amphetamine as a cocaine abuse treatment agent may be somewhat problematic, but these data encourage the development of other long-acting monoamine releasers with less abuse liability (see Grabowski et al. 2004b; Rothman et al. 2004, submitted).
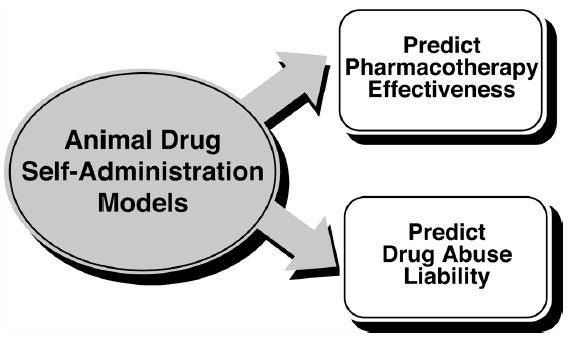
In summary, there is compelling evidence that preclinical drug self-administration models can predict the effectiveness of pharmacotherapies for drug abuse treatment and there is considerable concordance between the results of clinical treatment trials and preclinical findings (Figure 12). Moreover, drug self-administration is a powerful behavioral technique for assessing both medication effectiveness and drug abuse liability. This conclusion is shown graphically in Figure 13. Drug self-administration procedures are a productive merger between the operant tradition of behavioral science and pharmacology. The basic procedures have been repeatedly refined and can be used to ask a number of experimental questions that are relevant to medications development (Mello and Negus 1996) as well as to examine the effects of drugs on physiological and neuroendocrine endpoints (Mello and Mendelson 2002). Some of the pioneers in the development of intravenous drug self-administration procedures in non-human primates are members of the CPDD today. Some of the earliest studies were published by Tomoji Yanagita (Yanagita et al. 1965; Deneau et al. 1969; Yanagita 1973, 1975, 1977), Bob Schuster (Schuster and Thompson 1969; Schuster and Balster 1972; Schuster 1976) and Joe Brady (Brady and Griffiths 1977). Those of us who have followed and built on their original innovations remain very much in their debt. Preclinical evaluation of drug abuse treatment medications is an ongoing challenge, and we continue to refine our models to address the new questions posed in this ever evolving and inherently fascinating field.
Figure 13.
Acknowledgments
I would like to thank my many colleagues and collaborators who have contributed to and influenced my research at the Alcohol and Drug Abuse Research Center at McLean Hospital. I especially thank S. Stevens Negus, S. Barak Caine and Jack Bergman for their many valuable contributions to this research program and for their friendship and encouragement. I am very grateful to my husband, Jack H. Mendelson, who has been my friend, colleague and collaborator for most of my scientific career, and a very important role model for me. All the research described in this review has been supported by the National Institute on Drug Abuse and is currently supported by the following awards: K05-DA00101, P01-DA14528 and R01-DA02519 from NIDA, NIH.
References for the Preface
- Evans SM, Haney M, Fischman MW, Foltin RW. Limited sex differences in response to “binge” smoked cocaine use in humans. Neuropsychopharmacology. 1999;21:445–454. doi: 10.1016/S0893-133X(98)00120-1. [DOI] [PubMed] [Google Scholar]
- Fischman MW. The behavioral pharmacology of cocaiine in humans. NIDA Res Monogr. 1984;50:72–91. [PubMed] [Google Scholar]
- Fischman, M. W. Cocaine and the amphetamines, in Psychopharmacology, The Third Generation of Progress (Meltzer, H. Y.; ed) New York: Raven Press, 1987, pp. 1543–1553.
- Fischman MW. Human drug taking under controlled laboratory conditions. NIDA Res Monogr. 1988;84:196–211. [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. A laboratory model for evaluating potential treatment medications in humans. NIDA Res Monogr. 1992;119:165–169. [PubMed] [Google Scholar]
- Fischman MW, Foltin RW, Nestadt G, Pearlson GD. Effects of desipramine maintenance on cocaine self-administration by humans. J Pharmacol Exp Ther. 1990;253:760–770. [PubMed] [Google Scholar]
- Fischman, M. W., and Mello, N. K. eds Testing for Abuse Liability of Drugs in Humans. NIDA Research Monograph Series No. 92 Rockville, MD: National Institute on Drug Abuse, DHHS Publ. No. (ADM) 89–1613, 1989.
- Fischman MW, Mello NK. Methods for Testing the Abuse Liability of Stimulant, Opioid Mixed Agonist-Antagonist and Anxiolytic and Sedative Hypnotic Drugs in Humans. Drug Alc Depend. 1991;28:1–111. [Google Scholar]
- Fischman MW, Schuster CR. Cocaine effects in sleep-deprived humans. Psychopharmacology. 1980;72:1–8. doi: 10.1007/BF00433800. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR. Acute tolerance to cocaine in humans. NIDA Res Monogr. 1981;34:241–242. [PubMed] [Google Scholar]
- Fischman MW, Schuster CR. Cocaine self-administration in humans. Fed Proc. 1982;41:241–246. [PubMed] [Google Scholar]
- Fischman MW, Schuster CR, Resnekov L, Schick JFE, Krasnegor NA, Fennell W, Freedman DX. Cardiovascular and subjective effects of intravenous cocaine administration in humans. Arch Gen Psychiatry. 1976;33:983–989. doi: 10.1001/archpsyc.1976.01770080101010. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Christiansen I, Levin FR, Fischman MW. Effects of single and multiple intravenous cocaine injections in humans maintained on methadone. J Pharmacol Exp Ther. 1995;275:38–47. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Smoked and intravenous cocaine in humans: Acute tolerance, cardiovascular and subjective effects. J Pharmacol Exp Ther. 1991;257:247–261. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Assessment of abuse liability of stimulant drugs in humans: A methodological survey. Drug Alcohol Depend. 1991a;28:3–48. doi: 10.1016/0376-8716(91)90052-z. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. The cardiovascular and subjective effects of intravenous cocaine and morphine combinations in humans. J Pharmacol Exp Ther. 1992a;261:623–632. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Self-administration of cocaine by humans: Choice between smoked and intravenous cocaine. J Pharmacol Exp Ther. 1992b;231:841–849. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Cocaine self-administration research: treatment implications. NIDA Res Monogr. 1994a;145:139–162. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Effects of buprenorphine on the self-administration of cocaine by humans. Behavioral Pharmacology. 1994b;5:79–89. doi: 10.1097/00008877-199402000-00009. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Effects of methadone or buprenorphine maintenance on the subjective and reinforcing effects of intravenous cocaine in humans. J Pharmacol Exp Ther. 1996;278:1153–1164. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. A laboratory model of cocaine withdrawal in humans: intravenous cocaine. Exp Clin Psychopharmacol. 1997;5:404–411. doi: 10.1037//1064-1297.5.4.404. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Effects of “binge” use of intravenous cocaine in methadone-maintained individuals. Addiction. 1998;93:825–836. doi: 10.1046/j.1360-0443.1998.9368254.x. [DOI] [PubMed] [Google Scholar]
- Javaid JI, Fischman MW, Schuster CR, Dekirmenjian H, Davis JM. Cocaine plasma concentration: Relation to physiological and subjective effects in humans. Science. 1978;202:227–228. doi: 10.1126/science.694530. [DOI] [PubMed] [Google Scholar]
- Johanson, C. E. Marian Fischman: Research Legacy. NIDA Research Monograph, No. 183: 2003.
- Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol Rev. 1989;41:3–52. [PubMed] [Google Scholar]
- Mello, N. K. Behavioral strategies for the evaluation of new pharmacotherapies for drug abuse treatment., in Proceedings, Problems of Drug Dependence 1991 (Harris, L. S., ed) Washington, D.C.: U.S. Government Printing Office, 1992, pp. 150–154. [PubMed]
- Ward AS, Haney M, Fischman MW, Foltin R. Binge cocaine self-administration in humans: Intravenous cocaine. Psychopharmacology. 1997a;132:375–381. doi: 10.1007/s002130050358. [DOI] [PubMed] [Google Scholar]
- Ward AS, Haney M, Fischman MW, Foltin RW. Binge cocaine self-administration by humans: smoked cocaine. Behavioral Pharmacology. 1997b;8:736–744. doi: 10.1097/00008877-199712000-00009. [DOI] [PubMed] [Google Scholar]
References
- Banks CD. Overdose of buprenorphine: Case report. NZ Med J. 1979;89:255–256. [PubMed] [Google Scholar]
- Brady, J. V., and Griffiths, R. R. Drug-maintained performance procedures and the assessment of drug-abuse liability, in Predictiing Dependence Liability of Stimulant and Depressant Drugs (Thompson, T.; and Unna, K. R.; eds), Baltimore, MD: University Park Press, 1977, pp. 165–184.
- Condelli WS, Fairbank JA, Dennis ML, Rachal JV. Cocaine use by clients in methadone programs: Significance, scope, and behavioral interventions. J Subst Abuse Treat. 1991;8:203–212. doi: 10.1016/0740-5472(91)90040-h. [DOI] [PubMed] [Google Scholar]
- Cowan, A., and Lewis, J. W. eds Buprenorphine: Combatting Drug Abuse with a Unique Opioid New York: Wiley-Liss, Inc., 1995.
- Deneau G, Yanagita T, Seevers MH. Self-administration of psychoactive substances by the monkey. Psychopharmacologia. 1969;16:30–48. doi: 10.1007/BF00405254. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Johnson RE, Bunker E. Abrupt withdrawal of buprenorphine following chronic administration. Clin Pharmacol Ther. 1989;45:186. [Google Scholar]
- Gastfriend DR, Mendelson JH, Mello NK, Teoh SK, Reif S. Buprenorphine pharmacotherapy for concurrent heroin and cocaine dependence. American Journal on Addictions. 1993a;2:269–278. [Google Scholar]
- Gastfriend, D. R., Wapler, M., Teoh, S. K., Reif, S., Mendelson, J. H., and Mello, N. K. Effects of buprenorphine on needle sharing, reported drug use and craving in heroin and cocaine dependent men., in Problems of Drug Dependence 1992, NIDA Research Monograph (Harris, L. S.; ed) Washington, D.C.: U.S. Government Printing Office, 1993b, p 312.
- Glowa JR, Fantegrossi WE. Effects of dopaminergic drugs on food- and cocaine-maintained responding IV. Continuous cocaine infusions. Drug Alc Depend. 1997;45:71–79. doi: 10.1016/s0376-8716(97)01350-1. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004a;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004b;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Hammer RP., Jr Cocaine alters opiate receptor binding in critical brain reward regions. Synapse. 1989;3:55–60. doi: 10.1002/syn.890030108. [DOI] [PubMed] [Google Scholar]
- Herz, A., and Shippenberg, T. S. Neurochemical aspects of addiction, in Molecular and Cellular Aspects of the Drug Addictions (Goldstein, A.; ed) New York: Springer-Verlag, 1988, pp. 111–141.
- Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine. Arch Gen Psychiatry. 1978;35:601–616. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- Jones HE. Practical considerations for the clinical use of buprenorphine. Science & Practice Perspectives. 2004;2:4–19. doi: 10.1151/spp04224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kleber HD, Morgan C. Role of opioid antagonists in treating intravenous cocaine abuse. Life Sci. 1989a;44:887–892. doi: 10.1016/0024-3205(89)90589-4. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kleber HD, Morgan C. Treatment of cocaine abuse with buprenorphine. Biol Psychiatry. 1989b;26:170–172. doi: 10.1016/0006-3223(89)90090-5. [DOI] [PubMed] [Google Scholar]
- Kreek, M. J. Medical complications in methadone patients, in Recent Developments in Chemotherapy of Narcotic Addiction (Kissin, B.; Lowinson, J.; and Millman, R.; eds), New York: Ann. Acad. Sci., 1978, pp. 110–134. [DOI] [PubMed]
- Kreek, M. J. Multiple drug abuse patterns: Recent trends and associated medical consequences, in Advances in Substance Abuse, Behavioral and Biological Research (Mello, N. K.; ed) London: Jessica Kingsley Publishers, 1991, pp. 91–112.
- Lewis, J. W. Ring C-Bridged derivatives of thebaine and oripavine., in Narcotic Antagonists. Advances in Biochemical Psychopharmacology (Braude, M. C.; Harris, L. S.; May, E. L.; Smith, J. P.; and Villarreal, J. E.; eds), New York: Raven Press, 1974, pp. 123–136. [PubMed]
- Lewis, J. W. Clinical pharmacology of buprenorphine in relation to its use as an analgesic., in Buprenorphine: Combating Drug Abuse with a Unique Opioid (Cowan, A.; and Lewis, J. W.; eds), New York: Wiley-Lis, Inc., 1995, pp. 151–163.
- Lewis, J. W., Rance, M. J., and Sanger, D. J. The pharmacology and abuse potential of buprenorphine: A new antagonist analgesic., in Advances in Substance Abuse: Behavioral and Biological Research (Mello, N. K.; ed) Greenwich, CT.: JAI Press, 1983, pp. 103–154.
- Mello, N. K. Behavioral strategies for the evaluation of new pharmacotherapies for drug abuse treatment., in Proceedings, Problems of Drug Dependence 1991 (Harris, L. S.; ed) Washington, D.C.: U.S. Government Printing Office, 1992, pp. 150–154. [PubMed]
- Mello NK, Lukas SE, Kamien JB, Mendelson JH, Cone EJ. The effects of chronic buprenorphine treatment on cocaine and food self-administration by rhesus monkeys. J Pharmacol Exper Ther. 1992;260:1185–1193. [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Mendelson JH, Drieze J. Naltrexone-buprenorphine interactions: Effects on cocaine self-administration. Neuropsychopharmacology. 1993c;9:211–224. doi: 10.1038/npp.1993.57. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Buprenorphine suppresses heroin use by heroin addicts. Science. 1980;27:657–659. doi: 10.1126/science.7352279. [DOI] [PubMed] [Google Scholar]
- Mello, N. K., and Mendelson, J. H. Behavioral pharmacology of buprenorphine, in Mixed Agonist-Antagonist Analgesics (Schuster, C. R.; and Harris, L. S.; eds): Drug Alcohol Depend, 1985, pp. 283–303. [DOI] [PubMed]
- Mello, N. K., and Mendelson, J. H. Primate studies of the behavioral pharmacology of buprenorphine, in Buprenorphine: An Alternative Treatment for Opioid Dependence (Blaine, J. D.; ed) Rockville, MD: U.S. Department of Health and Human Services, DHHS Publ. No. (ADM)92–1912, 1992, pp. 61–100.
- Mello, N. K., and Mendelson, J. H. Buprenorphine’s effects on cocaine and heroin abuse., in The Biological Basis of Substance Abuse (Korenman, S. B. a. B., J.; ed) New York: Oxford University Press, 1993, pp. 463–485.
- Mello, N. K., and Mendelson, J. H. Buprenorphine treatment of cocaine and heroin abuse, in Buprenorphine:Combating Drug Abuse With A Unique Opioid (Cowan, A.; and Lewis, J. W.; eds), New York: Wiley-μLiss, Inc., 1995, pp. 241–287.
- Mello, N. K., and Mendelson, J. H. Cocaine, hormones and behavior: Clinical and preclinical studies, in Hormones, Brain and Behavior (Pfaff, D. W.; Arnold, A. P.; Etgen, A. M.; Fahrbach, S. E.; and Rubin, R. T.; eds), New York: Academic Press, 2002, pp. 665–745.
- Mello NK, Mendelson JH, Bree MP, Lukas S. Buprenorphine and naltrexone effects on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1990a;254:926–939. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Drieze J, Kelly M. Acute effects of cocaine on prolactin and gonadotropins in female rhesus monkey during the follicular phase of the menstrual cycle. J Pharmacol Exp Ther. 1990b;254:815–823. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Drieze J, Kelly M. Cocaine effects on luteinizing hormone-releasing hormone-stimulated anterior pituitary hormones in female rhesus monkey. J Clin Endocrinol Metab. 1990c;71:1434–1441. doi: 10.1210/jcem-71-6-1434. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC. Buprenorphine effects on human heroin self-administration: An operant analysis. J Pharmacol Exp Ther. 1982;223:30–39. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC, Sellers ML. Operant analysis of human heroin self-administration and the effects of naltrexone. J Pharmacol Exp Ther. 1981;216:45–54. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Lukas SE, Gastfriend D, Teoh SK, Holman BL. Buprenorphine treatment of opiate and cocaine abuse: Clinical and preclinical studies. Harvard Rev Psychiatry. 1993;1:168–183. doi: 10.3109/10673229309017075. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. The effects of buprenorphine on self-administration of cocaine and heroin “speedball” combinations and heroin alone by rhesus monkeys. J Pharmacol Exp Ther. 1998;285:444–456. [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of flupenthixol and quadazocine on self-administration of speedball combinations of cocaine and heroin by rhesus monkeys. Neuropsychopharmacology. 1999;21:575–588. doi: 10.1016/S0893-133X(99)00056-1. [DOI] [PubMed] [Google Scholar]
- Mello, N. K., and Negus, S. S. Interactions between kappa opioid agonists and cocaine: Preclinical studies, in The Archer Conference on Drug Abuse: New Medications (Glick, S. D.; and Maisonneuve, I. M.; eds), New York: New York Academy of Sciences, 2000, pp. 104–132. [DOI] [PubMed]
- Mello NK, Negus SS. Effects of indatraline and buprenorphine on self-administration of speedball combinations of cocaine and heroin by rhesus monkeys. Neuropsychopharmacology. 2001;25:104–117. doi: 10.1016/S0893-133X(00)00247-5. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of buprenorphine and d-amphetamine on “speedball” self-administration by rhesus monkeys. ACNP 43rd Annual Meeting, San Juan, PR, December 2004. Neuropsychopharmacology. 2004;29:S137. [Google Scholar]
- Mello NK, Negus SS, Lukas SE, Mendelson JH, Sholar JW, Drieze J. A primate model of polydrug abuse: Cocaine and heroin combinations. J Pharmacol Exp Ther. 1995;274:1325–1337. [PubMed] [Google Scholar]
- Mendelson, J. H., and Mello, N. K. eds Human laboratory studies of buprenorphine. Rockville, MD: U.S. Department of Health and Human Services, DHHS Publ. No. (ADM)92–1912, 1992.
- Mendelson JH, Teoh SK, Mello NK, Ellingboe J, Rhoades E. Acute effects of cocaine on plasma adrenocorticotropic hormones, luteinizing hormone and prolactin levels in cocaine-dependent men. J Pharmacol Exp Ther. 1992;263:505–509. [PubMed] [Google Scholar]
- Meyer, R. E., and Mirin, S. M. (1979) The Heroin Stimulus, in p 254, Plenum Press, New York.
- Montoya ID, Gorelick DA, Preston KL, Schroeder JR, Umbricht A, Cheskin LJ, Lange WR, Contoreggi C, Johnson RE, Fudala PJ. Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clin Pharmacol Ther. 2004;75:34–48. doi: 10.1016/j.clpt.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: Effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS, Brandt MR, Mello NK. Effects of the long-acting monoamine reuptake inhibitor indatraline on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 1999;291:60–69. [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of μ-opioid agonists on cocaine- and food-maintained responding and cocaine discrimination in rhesus monkeys: Role of μ-agonist efficacy. J Pharmacol Exp Ther. 2002;300:111–1121. doi: 10.1124/jpet.300.3.1111. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology. 2003a;167:324–332. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alc Depend. 2003b;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- NIDA. Epidemiologic Trends in Drug Abuse. NIH Publ. No. 3-5109A 1: 71, 2002.
- Panlilio LV, Goldberg SR, Gilman P, Jufer R, Cone EJ, Schindler CW. Effects of delivery rate and non-contingent infusion of cocaine on cocaine self-administration in rhesus monkeys. Psychopharmacology. 1998;137:253–258. doi: 10.1007/s002130050618. [DOI] [PubMed] [Google Scholar]
- Rothman, R. B., Blough, B. E., Woolverton, W. L., Anderson, K. G., Negus, S. S., Mello, N. K., Roth, B. L., and Baumann, M. H. Successful development of a rationally-designed, low abuse potential biogenic amine releaser that suppresses cocaine self-administration. 2004 (submitted). [DOI] [PubMed]
- Rothman RB, Glowa JR. A review of the effects of dopaminergic agents on humans, animals, and drug-seeking behavior, and its implications for medication development: Focus on GBR 12909. Molecular Neurobiol. 1995:1–19. doi: 10.1007/BF02740680. [DOI] [PubMed] [Google Scholar]
- Schecter A. The role of narcotic antagonists in the rehabilitation of opiate addicts: A review of naltrexone. Am J Drug Alcohol Abuse. 1980;7:1–18. doi: 10.3109/00952998009028406. [DOI] [PubMed] [Google Scholar]
- Schuster CR. Drugs as reinforcers in monkey and man. Pharmacol Rev. 1976;27:511–521. [PubMed] [Google Scholar]
- Schuster, C. R., and Balster, R. I. Self-administration of agonists., in Agonist and Antagonist Actions of Narcotic Analgesic Drugs (Kosterlitz, H. W.; Collier, H. l. J.; and Villarreal, J. E.; eds), London: MacMillan, 1972, pp. 243–254.
- Schuster CR, Harris LS. Mixed Agonist-Antagonist Analgesics. Drug Alcohol Depend. 1985;14:221–431. doi: 10.1016/0376-8716(85)90057-2. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Thompson T. Self-administration of and behavioral dependence on drugs. Ann Rev Pharmacol. 1969;9:483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- Schütz CG, Vlahov D, Anthony JC, Graham NMH. Comparison of self-reported injection frequencies for past 30 days and 6 months among intravenous drug users. J Clin Epidemiol. 1994;47:191–195. doi: 10.1016/0895-4356(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Teoh SK, Mendelson JH, Mello NK, Kuehnle J, Sintavanarong P, Rhoades EM. Acute interactions of buprenorphine with intravenous cocaine and morphine: An IND phase I safety evaluation. J Clin Psychopharmacology. 1993;13:87–99. [PubMed] [Google Scholar]
- Watson, S. J., Trujillo, K. A., Herman, J. P., and Akil, H. Neuroanatomical and neurochemical substrates of drug-seeking behavior: Overview and future directions, in Molecular and Cellular Aspects of the Drug Addictions (Goldstein, A.; ed) New York: Springer-Verlag, 1988, pp. 29–91.
- Yanagita T. An experimental framework for evaluation of dependence liability of various types of drugs in monkeys. Bull Narc. 1973;25:57. [Google Scholar]
- Yanagita T. Some methodological problems in assessing dependence-producing properties of drugs in animals. Pharmacol Rev. 1975;27:503–509. [PubMed] [Google Scholar]
- Yanagita, T. Brief review on the use of self-administration techniques for predicting drug dependence potential, in Predicting Dependence Liability of Stimulant and Depressant Drugs (Thompson, T.; and Unna, K. R.; eds), Baltimore, MD: University Park Press, 1977, pp. 231–242.
- Yanagita T, Deneau GA, Seevers MH. Evaluation of pharmacologic agents in monkey by long term intravenous self or programmed administration. Excerpta Med Inl Congr Ser. 1965;87:450. [Google Scholar]
- Yen SSC, Quigley ME, Reid RL, Ropert JF, Cetel NS. Neuroendocrinology of opioid peptides and their role in the control of gonadotropin and prolactin secretion. Am J Obstet Gynecol. 1985;152:485–493. doi: 10.1016/s0002-9378(85)80162-9. [DOI] [PubMed] [Google Scholar]


