Abstract
Study Design
A cross sectional study of spinal stereo radiographs of adolescents with scoliosis to measure growth.
Objective
To determine the relative contributions of the vertebral bodies and the intervertebral discs to the increase in spinal length between T5 to L5 over the age range 7.5 to 20 years.
Summary of Background Data
The progression of spinal deformity (scoliosis) is associated with skeletal growth, but the relative roles of asymmetrical growth and remodelling of the vertebrae and discs during adolescent growth is unclear.
Methods
An existing database of 406 spinal stereo radiographic studies of 188 adolescents with idiopathic scoliosis, aged between 7.5 and 20 years was used to measure the heights of vertebral bodies and of intervertebral discs and the summation of both (spinal length).
Results
Spinal length was observed to increase from about 250 mm to 350 mm over this range of ages. Spinal growth was associated with increase in vertebral height after age 10 years, with minimal if any increase in disc height. The contribution of vertebral and discal height was estimated to be about 17 and 8 mm per year respectively at age 7.5 years, but discal height growth was estimated to be effectively zero after age 12.
Conclusion
Spinal growth of patients with scoliosis aged between 10 and 20 years occurs almost exclusively by height increases in the vertebrae, not the discs.
Mini-Abstract
Spinal length over the range T5 to L5, and its components due to vertebral height and discal height were measured from 406 spinal stereo radiographic studies of 188 adolescents with idiopathic scoliosis, aged between 7.5 and 20 years. This cross-sectional analysis indicated that after age 10 years spinal growth was associated with increase in vertebral height, with minimal if any increase in disc height.
Keywords: Growth, Adolescence, Scoliosis, Skeletal_maturity, Vertebra, Intervertebral_disc
INTRODUCTION
Growth occurs in the spine during adolescence as evidenced by the increase in sitting height [1,2]. The greatest annual growth in sitting height in a Taiwanese population [3] was 61 mm (in boys) and 63 mm (in girls) at age 8.5 years, with a second peak of 46 mm in boys (at age 12.5 years) and 32 mm in girls (at age 11.5 years). Sitting height increases are not necessarily synchronized with leg growth [4,5]. Howell et al. [6] reported continued growth (about 14 mm increase in sitting height) after skeletal maturity, indicating that the spine continues to grow after cessation of limb growth.
Human vertebrae grow in height by a mechanism similar to that in long bones, by endochondral ossification in growth plates adjacent to the discs, and they increase in diameter by appositional growth [7]. Growth of the neural canal dimensions, including increase in inter-pedicular distance is near complete in early childhood [8]. The growth in height can be modulated by sustained mechanical loading [9].
In contrast to the growth of bones that occurs locally in specialized growth plates and apophyses, discs probably grow by a distributed mechanism of cell proliferation and matrix synthesis, although little is apparently known about post natal growth of the discs [10,11]. Human vertebrae are unusual since they lack the ossified and vascularized secondary centers of ossification (epiphyses) that form between the growth plate and the discs of most other vertebrates. This may place the discs at additional metabolic risk during skeletal growth, since they must compete with the growth plates for the nutrition that originates in the vertebral body’s vasculature. Antoniou et al. [12] reported a steady decline in the rate of matrix synthesis in the disc over all decades of life, with collagen denaturation occurring at a relatively steady rate in the age range 5 to 25 years. Rat discs appear to continue growing throughout the first 18 months of life [11], but these radiographic measurements had limited accuracy because of difficulties with the radiographic method, as well as acute changes in disc height with in vivo loading conditions, etc.
It is not clear how differential growth of the spine is regulated in relationship to the coordinated control of skeletal growth. Scoliosis is apparently a result of inadequate control of spinal growth. The deformity progresses most rapidly during adolescent growth [13] and residual growth is a risk factor for scoliosis progression [14]. There is evidence that adolescents with idiopathic scoliosis have an earlier growth spurt [15]. The mechanism of progression of post-natal skeletal growth deformity such as scoliosis is often attributed to the ’Hueter-Volkmann Law’ of mechanically modulated endochondral growth in a ’vicious cycle’ [9,16]. Hence, scoliosis progression slows or ceases at skeletal maturity [17]. However, the simultaneous apparent acceleration of progression of intervertebral disc deformity at puberty, and slowing at skeletal maturity occurs in the absence of any known dramatic changes in intervertebral disc metabolism in the second decade.
Improved management of spinal deformity progression during adolescent growth may result from improved understanding of how spinal growth occurs in vertebrae and intervertebral discs. The present study made use of a pre-existing set of three-dimensional spinal measurements from stereo-radiographs of patients attending a scoliosis clinic [18] to document their relative contributions. The objective was to identify the relative contributions of the height increases in discs and vertebrae to the elongation of the spinal column.
METHODS
Vertebral and discal heights measured from 406 stereo-radiographs [18] of 188 patients with a diagnosis of idiopathic adolescent or juvenile idiopathic scoliosis were studied. The stereo-radiographs had been made during the period 1981 to 1986. Data were included in the present study for patients 7.5–20 years old, prior to any surgery. There were 33 boys and 155 girls. Ninety-eight of the 406 radiographs were recorded as being of patients undergoing brace treatment at the time of radiography. Eight were of patients while wearing their brace, according to the records. Of the 188 patients, 104 had one radiographic record, 32 two, and 52 had more than three (maximum 11) radiographic studies. The distribution of the ages of these patients at the times of stereo-radiography are shown in Figure 1. Cobb angle of the largest curve had been recorded for 319 radiographs. The mean was 24°, range 3–75°, mode 20°.
Figure 1.
Histogram of the ages of patients when the 406 stereo-radiographs were made.
The patients had been radiographed in a controlled standing posture with supports contacting the anterior superior iliac spines and clavicles, with arms to the sides, to minimize patient motion during radiography. Two radiographic projections were made with a 3 m film-to-focus distance, using 36-inch (914 mm) cassettes with low-dose intensifying screen and film combinations. A postero-anterior (PA) and an oblique view were made. The oblique view used an x-ray tube either at 20 degrees on the patient’s right side, or at 15 degrees above the horizontal.
For each stereo spine reconstruction, vertebral landmarks (vertebral endplates and bases of pedicles) had been previously identified, marked and digitized from each radiograph according to the methods described [18] to obtain the 3-D coordinates of each landmark. The height of each vertebra and disc was determined as the pythagorean distance between the 3-D coordinates of the corresponding endplate centers. Spinal length was defined as the sum of the heights of all vertebrae and discs. The contributions of the vertebrae and intervertebral discs was defined as the sum of all vertebral and disc heights respectively. Although all vertebral landmarks from T1 to L5 had been measured, the vertebrae and discs above T5 were omitted from this study because they were frequently unclear in the original films as a result of exposure or projectional problems.
For each of the measurements (spinal length, total vertebral height and total disc height), a nonlinear mathematical relationship with age was determined by regression analysis. Since growth relationships are asymptotic to a final dimension, logistic regression analysis (c.f. Tanner et al. [19]) was used to obtain an analytical curve fit to observed spinal dimensions and patient age, using the following equation:
| (1) |
where h = summated height (mm) of the vertebrae or discs or both
y = age of patient (years)
a, m, s are parameters in the curve fit
The parameter a represents the asymptote of the curve, thus it is a measure of the final height (of the spine, and its components), and values of m and s provide a fit to the amount of growth and rate of growth respectively.
The parameters a, m and s were estimated by least-squares fit to the observed data points, using the function fminsearch.m in the Matlab programming language (Mathworks, Natick MA). Subsequently, estimates of the growth (increase in height) per year was derived from the slope (first derivative) of the non-linear regression. A linear regression analysis was employed to identify significant trends in vertebral height, disc height and spinal length increases with time.
RESULTS
The total spinal length (T5-L5) was found to increase in this population from approximately 250 mm at age 7.5 years to 380 mm at age 20. There was little contribution from intervertebral disc height increase to this spinal growth after age about 10 years (Figure 2). In Figure 2 the solid lines that correspond to the logistic regression fit to observed data indicate that vertebral growth continued during the second decade, but that there was a decline to apparently negligible rates of disc height increase during the same period. The parameter values obtained from the logistic regressions for each curve are listed in Table 1. Based on linear regression analysis there was a statistically significant (p < 0.001) trend of increasing total vertebral height with age, while no such significant increase existed for disc height.
Figure 2.
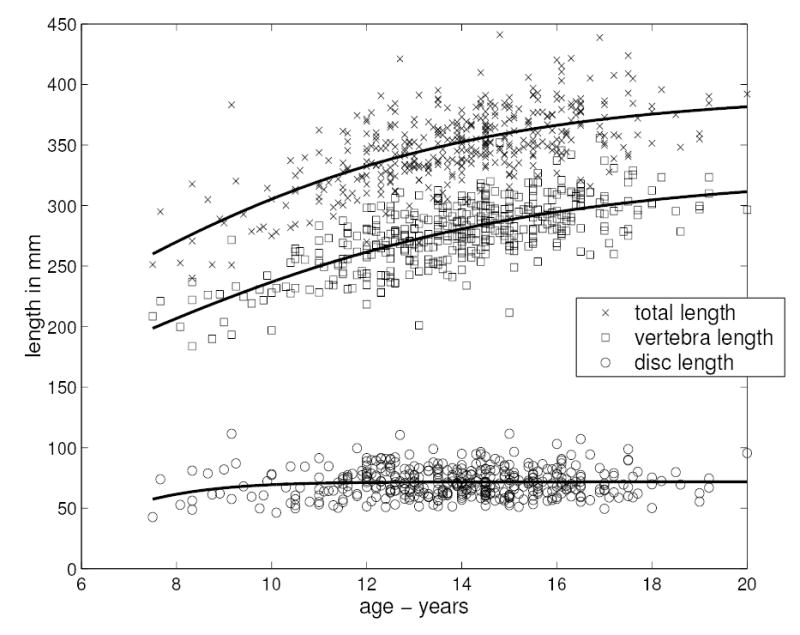
The measured spinal dimensions plotted against age, (x= total spinal length T5-L5; squares: summated heights of vertebrae; circles: summated heights of intervertebral discs). The solid lines correspond to curves fitted to the observed data by logistic regression (Equation 1).
Table 1.
Values of parameters obtained by non-linear regression (Equation 1) of measured spinal length, and the contributions of vertebrae and discs, versus age.
| Spinal length (T5-L5) | Sum of vertebral heights | Sum of discal heights | |
|---|---|---|---|
| a (asymptote, mm) | 392.27 | 325.0 | 71.69 |
| s | 4.31 | 4.66 | 1.25 |
| m (years) | 4.59 | 5.39 | 5.75 |
The growth in spinal length estimated by taking the first derivative of the height-age non-linear regression relationship declined from about 20 mm per year to about 3 mm per year over the age range 7.5 to 20 years (Figure 3). Prior to age 10 years, the height increases of both vertebrae and discs contributed to this spinal growth, but subsequently, after age 10, the estimated contribution of disc height growth declined to a negligible rate.
Figure 3.
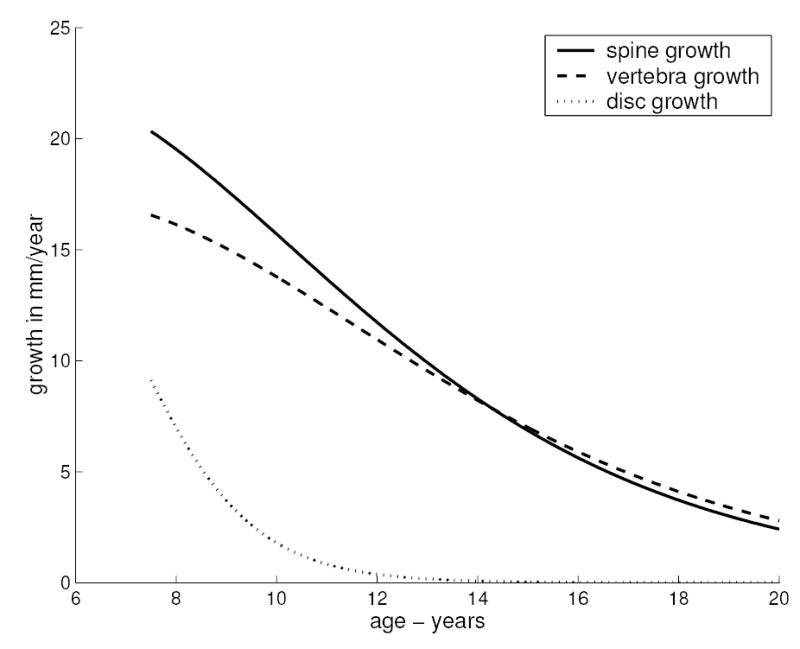
Growth rates (mm/year) obtained as the slope (first derivative) of the non-linear regression curve fits as shown in Figure 2.
The 308 radiographs of patients not undergoing treatment were analyzed separately by differentiation of the logistic regression fits to determine the spinal growth rate. The growth rate of the spine in this subset was found to decline from 15 mm per year at age 7.5 years to 4.2 mm/year at age 20 years. Thus it was found to be slower at age 7.5 and more rapid at age 20 than in the full sample. Assuming that the braced patients were those with more progressive scoliosis, this suggests that the subset (untreated patients) had a later growth spurt than the braced patients, consistent with the observation of Hagglund et al. [15]. In the untreated subset there was minimal growth (less than 2 mm/year) attributable to the discs at all ages.
DISCUSSION
This aim of this study was to determine the relative contributions of height increases of the vertebra and discs to growth in spinal length during adolescence in patients with scoliosis. It was found that there was little to no intrinsic growth in height of the intervertebral discs after age 10 years, hence almost all of the spinal growth occurred in the vertebrae.
The regression analyses included multiple (longitudinal) observations of some patients, and also included both male and female patients. The measurements corresponded to the part of the spine between T5 and L5, and the vertebral and discal height measures were based on distances between points at the centers of the vertebral endplates. The findings of this study were obtained from a group of patients with idiopathic scoliosis. However, the overall finding of growth occurring predominantly in the vertebrae can probably be generalized to adolescents without scoliosis, although the timing of the growth spurt may differ [15]. There is little gross disturbance of overall skeletal growth and sitting/standing ratio in scoliosis [20]. About a quarter of the radiographs were of patients undergoing brace treatment. While braces may alter the evolution of the Cobb angle (vertebral plus disc wedging), they probably do not substantially alter overall spinal loading, nor longitudinal growth. Also, the duration of brace wearing prior to x-rays being taken was probably insufficient to make significant difference in accumulated growth. It is not known whether the wedging of vertebrae and discs in scoliosis affects their overall height as measured here along the central axis of the spine.
There was a small number of observations at the extremes of the age range studied here. Therefore, true values for the increase in disc heights up to age 10, as well as the apparent continued growth of the spine at age 20 are not certain. Howell et al. [6] reported continued spinal growth of about 4 mm per year for three years after apparent skeletal maturity. A similar rate of growth after age 17 years was observed in the present study. Howell et al. [6] considered that this continued spinal growth might be the cause of continued progression of scoliosis among young adults. The present study indicates that the spinal growth occurring during progression of adolescent scoliosis occurs almost exclusively in the vertebrae, although it is possible that discal wedging can progress in the absence of growth in height of the discs.
Key Points
Growth in the spine between the ages of 10 and 20 years occurs almost exclusively by height increases in the vertebrae, not the discs.
The absence of disc growth implies that the mechanism of progressive wedging during adolescent growth in scoliosis differs between vertebrae and discs.
Acknowledgments
Supported in part by NIH R01 AR 049370. Participation of LW was supported by the ’MENTOR-CIHR program for research into spinal deformities’.
References
- 1.Aldegheri R, Agostini S. A chart of anthropometric values. J Bone Joint Surg Br. 1993;75(1):86–88. doi: 10.1302/0301-620X.75B1.8421044. [DOI] [PubMed] [Google Scholar]
- 2.Nicolopoulos KS, Burwell RG, Webb JK. Stature and its components in healthy children, sexual dimorphism and age related changes. J Anat. 1985;141:105–14. [PMC free article] [PubMed] [Google Scholar]
- 3.Lee TS, Chao T, Tang RB, Hsieh CC, Chen SJ, Ho LT. A longitudinal study of growth patterns in schoolchildren in one Taipei District. II: Sitting height, arm span, body mass index and skinfold thickness. J Chin Med Assoc. 2005;68(1):16–20. doi: 10.1016/S1726-4901(09)70126-1. [DOI] [PubMed] [Google Scholar]
- 4.Butler GE, McKie M, Ratcliffe SG. The cyclical nature of prepubertal growth. Ann Hum Biol. 1990;17(3):177–98. doi: 10.1080/03014469000000952. [DOI] [PubMed] [Google Scholar]
- 5.Diméglio A. Growth in Paediatric Orthopaedics. In: Morrissy RT and Weinstein SL (Eds). Lovell and Winter’s Pediatric Orthopaedics 5th Ed. Lippincott Williams and Wilkins, Philadelphia, 2001. pp 33–98.
- 6.Howell FR, Mahood JK, Dickson RA. Growth beyond skeletal maturity. Spine. 1992;17(4):437–40. doi: 10.1097/00007632-199204000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Dickson RA, Deacon P. Annotation: Spinal growth. J Bone Joint Surg (Br) 1987;69:690–692. doi: 10.1302/0301-620X.69B5.3680325. [DOI] [PubMed] [Google Scholar]
- 8.Tulsi RS. Growth of the human vertebral column. An osteological study. Acta Anat. 1971;79:570–580. doi: 10.1159/000143664. [DOI] [PubMed] [Google Scholar]
- 9.Stokes IAF, Spence H, Aronsson DD, Kilmer N. Mechanical modulation of vertebral body growth: implications for scoliosis progression. Spine. 1996;21(10):1162–1167. doi: 10.1097/00007632-199605150-00007. [DOI] [PubMed] [Google Scholar]
- 10.Urban JPG, Roberts S. Development and degeneration of the intervertebral discs. Molecular Medicine Today. 1995;1:329–335. doi: 10.1016/s1357-4310(95)80032-8. [DOI] [PubMed] [Google Scholar]
- 11.Hulse Neufeld J, Haghighi P, Machado T. Growth related increase in rat intervertebral disc size: a quantitative radiographic and histologic comparison. Lab Anim Sci. 1990;40:303–7. [PubMed] [Google Scholar]
- 12.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98(4):996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Little DG, Song KM, Katz D, Herring JA. Relationship of peak height velocity to other maturity indicators in idiopathic scoliosis in girls. J Bone Joint Surg Am. 2000;82(5):685–693. doi: 10.2106/00004623-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Lonstein JE, Carlson JE. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg [Am] 1984;66:1061–1071. [PubMed] [Google Scholar]
- 15.Hagglund G, Karlberg J, Willner S. Growth in girls with adolescent idiopathic scoliosis. Spine. 1992;17(1):108–111. doi: 10.1097/00007632-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Roaf R. Vertebral growth and its mechanical control. J Bone Joint Surg [Br] 1960;42:343–349. doi: 10.1302/0301-620X.42B1.40. [DOI] [PubMed] [Google Scholar]
- 17.Risser JC. Scoliosis: Past and present. J Bone Joint Surg (Am) 1964;46:167–199. [PubMed] [Google Scholar]
- 18.Stokes IAF, Bigalow LC, Moreland MS. Three-dimensional spinal curvature in idiopathic scoliosis. J Orthop Res. 1987;5:102–113. doi: 10.1002/jor.1100050113. [DOI] [PubMed] [Google Scholar]
- 19.Tanner JM, Whitehouse RH, Marubini E, Resele LF. The adolescent growth spurt of boys and girls of the Harpenden growth study. Ann Hum Biol. 1976;3(2):109–26. doi: 10.1080/03014467600001231. [DOI] [PubMed] [Google Scholar]
- 20.Willner S. The proportion of legs to trunk in girls with idiopathic structural scoliosis. Acta Orthop Scand. 1975;46:84–89. doi: 10.3109/17453677508989195. [DOI] [PubMed] [Google Scholar]


