Abstract
Clinically important strains of Clostridium difficile that do not produce toxin A but produce toxin B and are cytotoxic (A−/B+) have been reported from multiple countries. In order to compare the relatedness of these strains, we typed 23 A−/B+ C. difficile isolates from the United Kingdom (6 isolates), Belgium (11 isolates), and the United States (6 isolates) by three well-described typing methods. Restriction endonuclease analysis (REA), PCR ribotyping, and serogrouping differentiated 11, 4, and 3 different strain types, respectively. Twenty-one of the 23 A−/B+ variants had a 1.8-kb truncation of the toxin A gene characteristic of toxinotype VIII strains; 20 of the 21 toxinotype VIII-like strains were PCR type 17. PCR type 17 isolates could be differentiated into two separate strain groups by serogrouping and by REA. REA further discriminated these isolates into eight subgroups (REA types). PCR type 17-serogroup F-REA group CF isolates were recovered from all three countries, and one specific REA type, CF4, was recovered from patients with C. difficile disease in the United Kingdom and the United States. C. difficile A−/B+ variants of apparent clonal origin are widely distributed in Europe and North America.
Pathogenic strains of Clostridium difficile typically produce two high-molecular-weight toxins that share a considerable degree of homology and function but vary in respect to target cell specificity. Toxin A, the enterotoxin, causes fluid secretion in the ileal loop assay, and toxin B is a potent cytotoxin to many different eukaryotic cell lines (15, 16). C. difficile strains that do not produce toxin A have been recovered from patients in countries on at least four continents (1, 2, 5, 9, 11-14, 19, 21, 25). Moreover, many clinical laboratories utilize an enzyme immunoassay for toxin A as the only test for the diagnosis of C. difficile disease and are not able to detect these strains (11).
Although initially thought to be nonpathogenic, toxin A-negative, toxin B-positive (A−/B+) strains have been recovered from numerous patients with classic manifestations of C. difficile disease (2, 11, 12, 14, 19, 25) and have been responsible for outbreaks of diarrhea and colitis in hospitals in Canada and The Netherlands (1, 13). Different typing systems have been used to characterize these A−/B+ C. difficile variants, and their relatedness is unclear. This study compares A−/B+ C. difficile strains from isolate collections in the United Kingdom, Belgium, and the United States by three well-described typing methods: PCR ribotyping, serogrouping, and restriction endonuclease analysis (REA).
MATERIALS AND METHODS
Bacterial isolates.
C. difficile isolates identified as A−/B+ from three large clinical-isolate collections were sent in a blinded fashion for typing analysis to laboratories in Cardiff, Wales, United Kingdom (PCR ribotyping analysis); Brussels, Belgium (serogrouping analysis); and Chicago, Ill. (REA typing analysis). All A−/B+ isolates from each collection that differed by internal typing methods or epidemiologic origin were included. All of the toxin variant C. difficile isolates (referred to as A−/B+) in this study tested negative in vitro by toxin A immunoassay and positive by cell cytotoxicity testing. Isolates 1 to 6 were submitted by the United Kingdom laboratory, isolates 7 to 17 were submitted by the Belgian laboratory, and isolates 18 to 23 were submitted by the U.S. laboratory. The origin and clinical data for each isolate are listed in Table 1.
TABLE 1.
Clinical data and typing results for toxin variant isolates
| Study no. | Submitting laboratory | Isolate designation | Isolate origin | Patient diagnosis | Patient age | PCR type | Serogroup | REA type |
|---|---|---|---|---|---|---|---|---|
| 1 | United Kingdom | R10430 | Birmingham, United Kingdom | CDAD | Adult | 17 | F | CF4 |
| 2 | United Kingdom | R10525 | Dorchester, United Kingdom | CDAD | Adult | 17 | F | CF4 |
| 3 | United Kingdom | R7771 | Jersey, Channel Islands, United Kingdom | CDAD | Adult | 110 | X | DA1 |
| 4 | United Kingdom | CCUG 20309 | Birmingham, United Kingdom (strain 8864) | NAa | NA | 36 | A | CY1 |
| 5 | United Kingdom | R19541 | Birmingham, United Kingdom | CDAD | Adult | 47 | F | CF4 |
| 6 | United Kingdom | R7404 | Carlisle, United Kingdom | CDAD | Adult | 17 | F | CF4 |
| 7 | Belgium | 0002 | St. Luc's Hospital, Brussels, Belgium | Asymptomatic | Child | 17 | X | CG1 |
| 8 | Belgium | 0741 | St. Luc's Hospital, Brussels, Belgium | Asymptomatic | Child | 17 | F | CF1 |
| 9b | Belgium | 1470 | St. Luc's Hospital, Brussels, Belgium | Asymptomatic | Infant | 17 | F | CF1 |
| 10 | Belgium | 11723 | St. Luc's Hospital, Brussels, Belgium | Asymptomatic | Child | 17 | F | CF5 |
| 11 | Belgium | 12661 | St. Luc's Hospital, Brussels, Belgium | Asymptomatic | Infant | 17 | F | CF1 |
| 12 | Belgium | 13853 | St. Michel Hospital, Brussels, Belgium | Asymptomatic | Child | 17 | F | CF1 |
| 13 | Belgium | 20174 | Dinant, Belgium | Asymptomatic | Child | 17 | F | CF1 |
| 14 | Belgium | 20822 | St. Luc's Hospital, Brussels, Belgium | Asymptomatic | Child | 17 | X | CG1 |
| 15 | Belgium | 23682 | St. Luc's Hospital, Brussels, Belgium | Asymptomatic | Infant | 17 | X | CG3 |
| 16 | Belgium | 23741 | Villers, Belgium | Asymptomatic | Infant | 17 | X | CG1 |
| 17 | Belgium | 26766 | Mons, Belgium | Diarrhea | Infant | 17 | F | CF6 |
| 18 | United States | 5572 | Minneapolis, Minn. | CDAD | Adult | 17 | F | CF4 |
| 19 | United States | 4241 | Minneapolis, Minn. | CDAD | Adult | 17 | F | CF3 |
| 20 | United States | 5340 | Minneapolis, Minn. | CDAD | Adult | 17 | F | CF2 |
| 21 | United States | 3072 | Minneapolis, Minn. | CDAD | Adult | 17 | F | CF1p |
| 22c | United States | 5922 | Belgium | NA | NA | 17 | F | CF1 |
| 23b | United States | 6030 | ATCC 43598 (strain 1470) | Asymptomatic | Infant | 17 | F | CF1 |
PCR ribotyping method (United Kingdom laboratory).
DNA was isolated from C. difficile isolates as previously described (20). The template DNA was then amplified with primers complementary to the 3′ end of the 16S rRNA gene and the 5′ end of the 23S rRNA gene. The amplified products were separated by electrophoresis in 3% agarose gels and visualized by ethidium bromide staining. The patterns of the amplified 16S-23S rRNA spacer regions were analyzed with GelCompar software (version 4.1; Applied Maths, Kortrijk, Belgium).
Serogrouping method. (Belgian laboratory).
Serogrouping was performed by slide agglutination with rabbit antisera followed by polyacrylamide gel electrophoresis of whole-cell proteins (7).
REA typing method (U.S. laboratory).
Total cellular DNA was isolated from C. difficile isolates as previously described (6). The DNA was digested with HindIII restriction enzyme, and the fragments were separated on a 0.7% agarose gel. The patterns of each isolate were visually compared to the restriction patterns of the previously identified REA types in our clinical collection. New isolates which showed ≤6 band differences (similarity index, >90%) from an established REA type were placed within the same group (letter designation) as the REA type. An isolate restriction pattern was required to be identical to a pattern on file to be given the same type number; a single-band difference resulted in a different type designation.
PCR-restriction fragment length polymorphism (RFLP) analysis.
In order to characterize the genetic variations of these isolates and to relate these variations to previously described variants, the genes for toxin A and toxin B were amplified and the resultant products were digested with restriction enzymes. The standard toxigenic C. difficile strain VPI 10463 and the nontoxigenic REA type M3 (isolate 1413) were chosen as the positive and negative control, respectively. Oligonucleotide primers were designed based on the published sequence of the VPI 10463 pathogenicity region (EMBL database accession no. X92982). Primers B-u1-b and 3pB-D bracketed the entire toxin B gene (tcdB), and primers ACU-2 and PL-d bracketed the region from tcdA through tcdC. The sequences and locations of primers B-u1-b, 3pB-D, and ACU-2 have been previously described (25). PL-d was designed as a downstream primer for the tcdA-tcdC amplicon and starts at base pair position 22,121 of the pathogenicity region as defined by Hundsberger et al. (10) with the following DNA sequence: CCACTTTAATTTTTCTGGTTCTG. DNA was isolated for PCR as previously described (6) and then amplified in the eLONGase system (Gibco-BRL Life Technologies, Grand Island, N.Y.) according to the manufacturer's directions. Each PCR included 1 U of eLONGase enzyme; deoxynucleoside triphosphates, each at a concentration of 200 μM; reaction buffer B at a final concentration of 20% and MgSO4 at 2.0 mM; 63 ng of each primer; and 200 ng of template DNA. The amplification cycles included denaturation at 94°C for 20 s, annealing at 52°C for 50 s, and extension at 68°C for 1 min per kb of amplicon for a total of 30 cycles. The amplified products were separated by electrophoresis on a 0.8% agarose gel and visualized by ethidium bromide staining. The amplified products were also analyzed by restriction enzyme gel electrophoresis using PstI and HincII, respectively, for tcdA and tcdB amplified products.
RESULTS
Typing comparison.
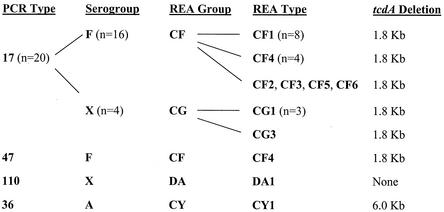
REA was the most discriminatory typing method for the A−/B+ C. difficile variants (Table 1 and Fig. 1). Among the 23 isolates, REA distinguished 11 REA types, whereas PCR ribotyping and serogrouping distinguished 4 and 3 unique types, respectively. PCR ribotyping analysis correlated most closely with the toxin gene analysis. Twenty of the 21 isolates with the 1.8-kb tcdA deletion (see below) were PCR type 17. Two main groupings of PCR type 17 isolates were distinguished by serogrouping and by REA; serogroup F-REA group CF and serogroup X-REA group CG. PCR type 17-serogroup F-REA group CF isolates were recovered from all three countries, whereas the PCR type 17-serogroup X-REA group CG isolates were only found in the Belgian collection.
FIG. 1.
Relationships of PCR ribotype (PCR Type), serogroup, REA group, and REA type analyses for 23 A−/B+ C.difficile toxin variants. The type designations for individual isolates are shown on the same horizontal lines. Isolates with the same designation were grouped, and the numbers of isolates are indicated in parentheses. The toxin A gene (tcdA) deletions determined by PCR amplification are shown for comparison with the typing results. One serogroup F-REA group CF isolate was differentiated from the top cluster by PCR ribotype and included separately (PCR type 47-serogroup F-REA type CF4).
One isolate (study isolate 5) was distinguished by PCR ribotyping as PCR type 47 yet was also identified as serogroup F and REA type CF4. Study isolate 3 was identified as PCR type 110 and serogroup X but had an REA profile unrelated to any previously characterized REA group in the U.S. collection, and it was subsequently placed in a new REA group and called REA type DA1. Study isolate 4 is the previously characterized toxin variant strain 8864 (3, 17, 27), and this isolate was unique by all three typing methods; it is now designated PCR type 36-serogroup A-REA type CY1.
Serogroups and REA groups correlated closely. All serogroup F strains were REA group CF, and all serogroup X strains except one (study isolate 3) were REA group CG. REA, however, was able to distinguish unique strain patterns (REA types) within each of these groups: six REA CF types and two CG types (Fig. 2). REA type CF4 isolates were recovered from patients in the United Kingdom and the United States. Three previously unrecognized REA type patterns were identified and designated REA types CF5, CF6, and CG3.
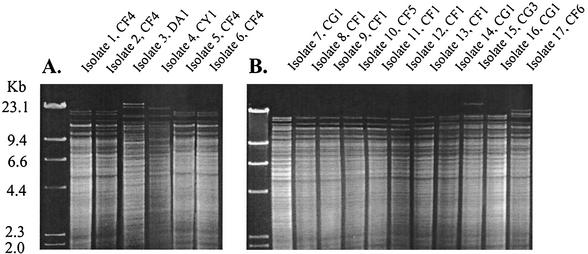
FIG. 2.
REA of A−/B+ C.difficile toxin variants from the United Kingdom and Belgium. HindIII REA patterns are shown for study isolates 1 to 6 from the United Kingdom (A) and study isolates 7 to 17 from Belgium (B). The HindIII-restricted lambda phage molecular size marker is shown in the far left lane of each gel. The study isolate numbers and the REA type designations are shown above the gel lanes.
The clinical characteristics of the patients infected with A−/B+ strains were not similar among the three isolate collections. Nine of the 10 isolates in the United Kingdom and the United States collections for which clinical information was available were recovered from adults with C. difficile-associated disease (CDAD). In contrast, 10 of the 11 isolates from the Belgian collection were recovered from asymptomatic children or infants.
PCR-RFLP analysis.
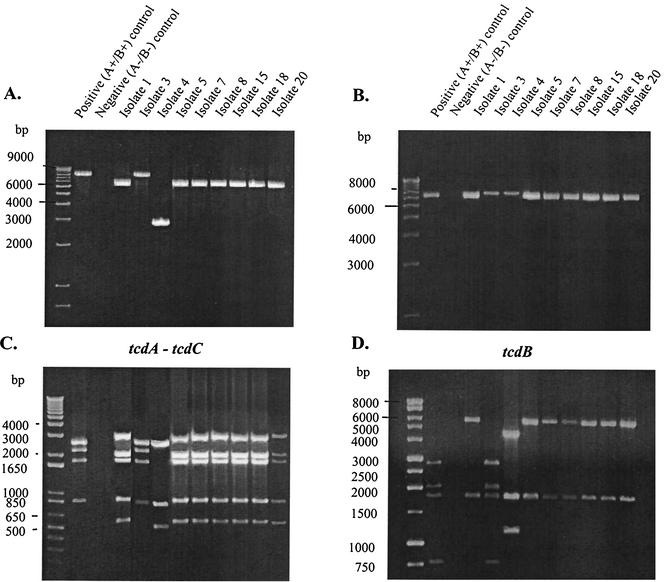
Amplification of the tcdA-tcdC region of all the variant isolates, with one exception (22 of 23), showed tcdA-tcdC amplified products smaller than the tcdA-tcdC amplified product from the standard toxigenic strain VPI 10463 (Fig. 3A). Twenty-one of the 23 study isolates exhibited a tcdA-tcdC amplified product which was ∼1.8 kb smaller. One of these isolates (study isolate 20; strain 5340) was previously shown to have a 1,821-bp truncation of the tcdA gene (25), which is characteristic of toxinotype VIII variants (23). Study isolate 4 (strain 8864; PCR type 36; REA type CY1) had an even smaller tcdA-tcdC amplified product (Fig. 3A), consistent with the previous characterization of this strain, which has a larger, 6.0-kb tcdA deletion (3, 17, 26). Study isolate 3 (PCR type 110; serogroup X; REA type DA1), however, had a tcdA-tcdC gene region of the same size as that of the standard toxigenic strain VPI 10463 (Fig. 3A). The restriction pattern of the tcdA-tcdC product after digestion with PstI offered no further discrimination among the variant strains (Fig. 3C).
FIG. 3.
PCR amplification and RFLP analysis of selected A−/B+ C.difficile toxin variants. Results of PCR amplification of the tcdA-tcdC gene (A) and the tcdB gene (B) are shown, with the corresponding restriction enzyme digestion patterns of the amplified products below: Pst-I-restricted tcdA-tcdC amplicons (C) and HincII-restricted tcdB amplicons (D). The 1-kb Plus DNA ladder molecular size marker is shown in the far left lane of each gel. The study isolate numbers are shown above the gel lanes. The standard toxigenic strain VPI 10463 and the nontoxigenic REA type M3 isolate 1413 are also indicated above the lanes as the positive (A+/B+) and negative (A−/B−) control, respectively.
Amplification of the tcdB gene in all variant isolates showed no size difference compared with the tcdB gene of the standard toxigenic strain VPI 10463 (Fig. 3B). However, digestion of the tcdB product with HincII revealed different restriction fragment patterns. Whereas HincII digestion of the tcdB product from strain VPI 10463 produced four fragment bands after gel electrophoresis, only two fragment bands were produced in the 21 strains that were also shown to have the 1.8-kb tcdA deletion (Fig. 3D). The HincII restriction fragment pattern of the tcdB product from study isolate 4 (strain 8864) produced three fragment bands, whereas the pattern from study isolate 3 (PCR type 110-serogroup X-REA type DA1) was identical to that of the standard toxigenic strain VPI 10463 (Fig. 3D).
Because study isolate 3 was identical to the standard toxigenic strain VPI 10463 by PCR-RFLP analysis, the isolate was retested for toxin A production and cytotoxicity in vitro. Supernatant from a trypticase soy broth medium culture of study isolate 3 was negative by toxin A enzyme immunoassay (Tox-A Test; TechLab, Blacksburg, Va.), with an optical density of 0.084 compared to optical densities of 1.512 and 0.069 for the positive and negative controls, respectively. The supernatant from this isolate was cytotoxic in a human fibroblast cell culture assay (C. difficile cytotoxicity assay kit; Bartels, Inc., Issaquah, Wash.), with a typical spindle-shaped cytopathic effect.
DISCUSSION
A−/B+ C. difficile variant isolates have now been reported from countries in Europe (2, 5, 9, 13, 21), Asia (12), North America (1, 11, 14, 19, 25), and South America (M. C. Legaria, G. Lumelsky, S. Rosetti, J. S. Brazier, and M. Gal, Abstr. Anaerobe Olympiad 2002: Anaerobe Society of the Americas, abstr. PII-35, 2002). This comparison, using three of the most established typing methods for C. difficile, documents a high degree of similarity among A−/B+ isolates from Europe and the United States. The majority of variant isolates were PCR type 17, serogroup F, and REA group CF, and these strains were found in the United States, Belgium, and the United Kingdom. The toxin gene variations of these strains were characterized by a 1.8-kb truncation in the 3′ end of the toxin A gene (tcdA) and an altered PstI restriction pattern of the tcdA amplified product compared with the standard toxigenic strain VPI 10463. Although the size of the toxin B gene (tcdB) was unaltered, the HincII restriction pattern of the tcdB amplified product differed from that of the standard toxigenic strain. These toxin gene variations are consistent with the toxinotype VIII pattern described by Rupnik et al. (23).
All but one of the 21 toxinotype VIII pattern variants were PCR ribotype 17, confirming the findings of a previous study comparing PCR ribotyping with toxinotyping (24). Serogrouping and REA typing analyses were able to discriminate two separate groups of strains within this toxinotype pattern: serogroup F-REA group CF and serogroup X-REA group CG. Using different typing methods, Wozniak et al. also demonstrated the genetic relatedness of serogroups F and X and suggested that strains belonging to these two groups represented two evolutionary branches from a common ancestor with the same toxinotype VIII variation (28).
REA was able to further discriminate subgroups (REA types) within both of these groupings. PCR 17-serogroup F-REA group CF isolates could be differentiated into six different REA types, CF1 to CF6. One specific REA type, CF4, was recovered from patients with C. difficile disease in the United States and the United Kingdom. Within North America, REA type CF4 isolates have been recovered from patients in Minnesota (this study), Chicago, Ill. (11), and California and Winnipeg, Canada (unpublished data). The wide geographic distribution of A−/B+ C. difficile variants in general, and REA type CF4 strains in particular, suggests that the evolutionary changes resulting in these variants were not recent events.
Two additional A−/B+ genetic variants were characterized in this study. Strain 8864 is a unique toxin-negative variant with a larger tcdA deletion of 6.0 kb, but it has been recovered from only one clinical specimen and its clinical significance is unclear (3, 17, 27). The typing pattern for this isolate, PCR type 36-serogroup A-REA type CY1, is also unique by all three typing methods without similar strains in any of the isolate collections surveyed in this study. The other variant, identified as PCR type 110-serogroup X-REA type DA1, appeared to have the same toxin genotype as the standard toxigenic strain VPI 10463 yet did not produce toxin A in vitro. Further characterization of this strain is being conducted to explain the lack of toxin A production.
The prevalences of A−/B+ C. difficile variants in various studies and isolate collections have ranged from 0.2 to 12% (2, 5, 12, 18, 21). The potential clinical importance of these variants is emphasized by two recent reports of hospital outbreaks due to A−/B+ toxinotype VIII pattern variants in Canada and The Netherlands (1, 13). Preliminary REA typing results in our laboratory indicate that an REA group CF strain was involved in both of these outbreaks (unpublished data). Clinicians and laboratories should be aware of the widespread distribution of the toxin variant strain PCR type 17-serogroup F-REA type CF4 and similar strains that are not detected by toxin A immunoassays yet cause typical CDAD syndromes, including pseudomembranous colitis.
Acknowledgments
This study was supported by grants from the U.S. Department of Veterans Affairs Research Service and Pfizer, Inc.
REFERENCES
- 1.Alfa, M. J., A. Kabani, D. Lyerly, S. Moncrief, L. M. Neville, A. Al-Barrak, G. K. H. Harding, B. Dyck, K. Olekson, and J. M. Embil. 2000. Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile responsible for a nosocomial outbreak of Clostridium difficile-associated diarrhea. J. Clin. Microbiol. 38:2706-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbut, F., V. Lalande, B. Burghoffer, H. V. Thien, E. Grimprel, and J.-C. Petit. 2002. Prevalence and genetic characterization of toxin A variant strains of Clostridium difficile among adults and children with diarrhea in France. J. Clin. Microbiol. 40:2079-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borriello, S. P., B. W. Wren, S. Hyde, S. V. Seddon, P. Sibbons, M. M. Krishna, S. Tabaqchali, S. Manek, and A. B. Price. 1992. Molecular, immunological, and biological characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile. Infect. Immun. 60:1492-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brazier, J. S., M. E. Mulligan, M. Delmée, S. Tabaqchali, and the International Clostridium difficile Study Group. 1997. Preliminary findings of the international typing study on Clostridium difficile. Clin. Infect. Dis. 25(Suppl. 2):S199-S201. [DOI] [PubMed] [Google Scholar]
- 5.Brazier, J. S., S. L. Stubbs, and B. I. Duerden. 1999. Prevalence of toxin A negative/B positive Clostridium difficile strains. J. Hosp. Infect. 42:248-249. [PubMed] [Google Scholar]
- 6.Clabots, C. R., S. Johnson, K. M. Bettin, P. A. Mathie, M. E. Mulligan, D. R. Schaberg, L. R. Peterson, and D. N. Gerding. 1993. Development of a rapid and efficient restriction endonuclease analysis typing system for Clostridium difficile and correlation with other typing systems. J. Clin. Microbiol. 31:1870-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delmée, M., Y. Laroche, V. Avesani, and G. Cornelis. 1986. Comparison of serogrouping and polyacrylamide gel electrophoresis for typing of Clostridium difficile. J. Clin. Microbiol. 24:991-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delmée, M., and V. Avesani. 1990. Virulence of ten serogroups of Clostridium difficile in hamsters. J. Med. Microbiol. 33:85-90. [DOI] [PubMed] [Google Scholar]
- 9.Depitre, C., M. Delmée, V. Avesani, R. L'Haridon, A. Roels, M. Popoff, and G. Cothier. 1993. Serogroup F strains of Clostridium difficile produce toxin B but not toxin A. J. Med. Microbiol. 38:434-441. [DOI] [PubMed] [Google Scholar]
- 10.Hundsberger, T., V. Braun, M. Weidmann, P. Leukel, M. Sauerborn, and C. von Eichel-Streiber. 1997. Transcription analysis of the genes tcdA-E of the pathogenicity locus of Clostridium difficile. Eur. J. Biochem. 244:735-742. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, S., S. A. Kent, K. J. O'leary, M. M. Merrigan, S. P. Sambol, L. R. Peterson, and D. N. Gerding. 2001. Fatal pseudomembranous colitis associated with a variant Clostridium difficile strain not detected by toxin A immunoassay. Ann. Intern. Med. 135:434-438. [DOI] [PubMed] [Google Scholar]
- 12.Kato, H., N. Kato, K. Watanabe, N. Iwai, H. Nakamura, T. Yamamoto, K. Suzuki, S. M. Kim, Y. Chong, and E. B. Wasito. 1998. Identification of toxin A-negative, toxin B-positive Clostridium difficile by PCR. J. Clin. Microbiol. 36:2178-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuijper, E. J., J. de Weerdt, H. Kato, N. Kato, A. P. van Dam, E. R. van der Vorm, J. Weel, C. van Rheenen, and J. Dankert. 2001. Nosocomial outbreak of Clostridium difficile-associated diarrhea due to a clindamycin-resistant enterotoxin A-negative strain. Eur. J. Clin. Microbiol. Infect. Dis. 20:528-534. [DOI] [PubMed] [Google Scholar]
- 14.Limaye, A. P., D. K. Turgeon, B. T. Cookson, and T. R. Fritsche. 2000. Pseudomembranous colitis caused by a toxin A− B+ strain of Clostridium difficile. J. Clin. Microbiol. 38:1696-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyerly, D. M., D. E. Lockwood, S. H. Richardson, and T. D. Wilkins. 1982. Biological activities of toxins A and B of Clostridium difficile. Infect. Immun. 35:1147-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyerly, D. M., K. E. Saum, D. K. MacDonald, and T. D. Wilkins. 1985. Effects of Clostridium difficile toxins given intragastrically to animals. Infect. Immun. 47:349-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyerly, D. M., L. A. Barroso, T. D. Wilkins, C. Depitre, and G. Corthier. 1992. Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile. Infect. Immun. 60:4633-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyerly, D. M., L. M. Neville, D. T. Evans, J. Fill, S. Allen, W. Greene, R. Sautter, P. Hnatuck, D. J. Torpey, and R. Schwalbe. 1998. Multicenter evaluation of the Clostridium difficile TOX A/B TEST. J. Clin. Microbiol. 36:184-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moncrief, J. S., L. Zheng, L. M. Neville, and D. M. Lyerly. 2000. Genetic characterization of toxin A-negative, toxin B-positive Clostridium difficile isolates by PCR. J. Clin. Microbiol. 38:3072-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Neill, G. L., F. T. Ogunsola, J. S. Brazier, and B. I. Duerden. 1996. Modification of a PCR ribotyping method for application as a routine typing scheme for Clostridium difficile. Anaerobe 2:205-209. [Google Scholar]
- 21.Pituch, H., N. van den Braak, W. van Leeuwen, A. van Belkum, G. Martirosian, P. Obuch-Woszczatynski, M. Luczak, and F. Meisel-Mikolajczyk. 2001. Clonal dissemination of a toxin-A-negative/toxin-B-positive Clostridium difficile strain from patients with antibiotic-associated diarrhea in Poland. Clin. Microbiol. Infect. 7:442-446. [DOI] [PubMed] [Google Scholar]
- 22.Rupnik, M., V. Braun, F. Soehn, M. Janc, M. Hoffstetter, R. Laufenberg-Feldmann, and C. von Eichel-Streiber. 1997. Characterization of polymorphisms in the toxin A and B genes of Clostridium difficile. FEMS Microbiol. Lett. 148:197-202. [DOI] [PubMed] [Google Scholar]
- 23.Rupnik, M., V. Avesani, M. Janc, C. von Eichel-Streiber, and M. Delmée. 1998. A novel toxinotyping scheme and correlation of toxinotypes with serogroups of Clostridium difficile isolates. J. Clin. Microbiol. 36:2240-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rupnik, M., J. S. Brazier, B. I. Duerden, M. Grabnar, and S. L. J. Stubbs. 2001. Comparison of toxinotyping and PCR ribotyping of Clostridium difficile strains and description of novel toxinotypes. Microbiology 147:439-447. [DOI] [PubMed] [Google Scholar]
- 25.Sambol, S. P., M. M. Merrigan, D. Lyerly, D. N. Gerding, and S. Johnson. 2000. Toxin gene analysis of a variant strain of Clostridium difficile that causes human disease. Infect. Immun. 68:5480-5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soehn, F., A. Wagenknecht-Weisner, P. Leukel, M. Kohl, M. Weidmann, C. von Eichel-Streiber, and V. Braun. 1998. Genetic rearrangements in the pathogenicity locus of Clostridium difficile strain 8864—implications for transcription, expression and enzymatic activity of toxins A and B. Mol. Gen. Genet. 258:222-232. [DOI] [PubMed] [Google Scholar]
- 27.Torres, J. F. 1991. Purification and characterization of toxin B from a strain of Clostridium difficile that does not produce toxin A. J. Med. Microbiol. 35:40-44. [DOI] [PubMed] [Google Scholar]
- 28.Wozniak, G., P. Trontelj, and M. Rupnik. 2000. Genomic relatedness of Clostridium difficile strains from different toxinotypes and serogroups. Anaerobe 6:261-267. [Google Scholar]