Abstract
The survival in transport media of 30 strains of gonococci was determined. These strains comprised five distinguishable strains of each of six auxotypes. Survival of different auxotypes varied significantly, but this variability was reduced by charcoal. Delayed swab processing could yield isolates unrepresentative of those causing infection in a sampled patient population.
In vitro and clinical evaluations of transport medium for Neisseria gonorrhoeae reveal variation in survival of uncharacterized isolates within and between such studies (3, 7, 8). In the present study, we investigated whether auxotypes of N. gonorrhoeae differ in their abilities to survive in plain and charcoal-containing transport media.
Thirty clinical isolates of N. gonorrhoeae were studied, comprising five prototrophic strains (nonrequiring; NR) and five strains each of auxotypes requiring proline and arginine (Pro− Arg−), arginine and hypoxanthine (Arg− Hyp−), proline (Pro−), hypoxanthine (Hyp−), and arginine (Arg−). All were identified by standard methods—including the use of APINH galleries (BioMerieux, Marcy D'Etoile, France)—and were auxotyped by the method of Copley and Egglestone (2). All strains within an auxotype were distinguishable by serotyping with the Phadebact monoclonal panel (Boule Diagnostics AB, Huddinge, Sweden), epidemiologically unrelated, or both.
For this study, strains were recovered from storage at −70°C onto chocolate agar (Oxoid, Basingstoke, United Kingdom) and incubated at 37°C in 5% CO2 for 24 h. For each strain, two inocula were prepared. Initially a 2-ml suspension in phosphate-buffered saline (PBS; Sigma-Aldrich, Poole, United Kingdom) was prepared to a McFarland no. 1 standard; 0.5 ml of this suspension was added to 0.5 ml of PBS (PBS inoculum), and 0.5 ml was added to 0.5 ml of nutrient broth (PBS-broth inoculum; Public Health Laboratory Service, Colindale, United Kingdom). Rayon-tipped swabs with plastic shafts (Medical Wire & Equipment Co., Corsham, United Kingdom) were inoculated with 100 μl of each of these suspensions. For each strain, eight swabs were inoculated to allow comparison of all combinations of PBS and PBS-broth inocula, plain and charcoal-containing Amies transport media (both Transwab; Medical Wire), and 24 and 48 h of storage at room temperature.
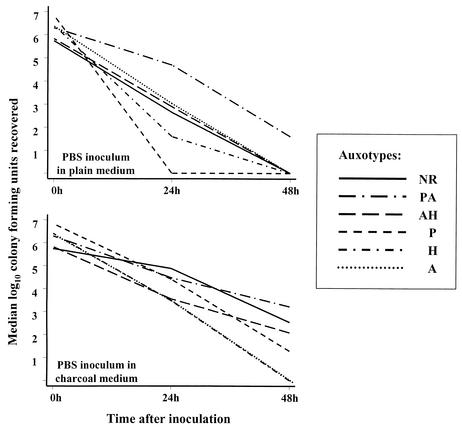
Gonococci were recovered from swabs by vortexing the tips in 1 ml of PBS for 30 s. Triplicate counts were performed with each washing on chocolate agar (Oxoid, Basingstoke, United Kingdom) by using a spiral plater (Don Whitley, Shipley, United Kingdom), and for each of the 30 strains, the median of the three counts was recorded for each of the eight possible inoculum-medium-time interval combinations. The results from the PBS experiments have been presented graphically by plotting the median of the five CFU values for an auxotype at each time point (Fig. 1).
FIG. 1.
Survival of 30 distinguishable isolates of N. gonorrhoeae in transport medium under different conditions. PA, Pro− Arg−; AH, Arg− Hyp−; P, Pro−; H, Hyp−; and A, Arg−. Lines connect median values for each auxotype.
Our results show marked differences in survival of auxotypes in plain transport medium and the value of charcoal both for reducing this variability (at least up to 24 h) and for improving recovery at 48 h (Fig. 1). In plain medium, auxotype Pro− Arg− has proven to be hardy, and auxotypes Pro− and Hyp− have proven to be less so; NR, Arg−, and Arg− Hyp− strains showed intermediate and comparable rates of survival. Although survival appears to be related to auxotype, any effect of specific nutritional requirements on survival in non-nutrient medium is difficult to explain. It is therefore more likely that auxotype is a marker for other factors affecting survival, such as differences in autolysis, envelope phenotype, or susceptibility to agar fatty acids, as described for auxotype Arg− Hyp− Ur− (6). Although fatty acids inhibit gonococci (4, 6), it is not clear whether those inferred by Ley and Mueller half a century ago to be present in “solid hydrated agar” and “dry, shredded agar” are present in modern agars (5).
For all auxotypes, survival at 24 h in plain medium was reduced when the inoculum was prepared in PBS-broth rather than PBS; this effect was corrected by charcoal, such that the rates of survival in charcoal-containing medium of auxotypes in PBS and PBS-broth were similar (results not shown).
Our observations may explain variations in viability among strains of N. gonorrhoeae described by others (3, 7, 8) and suggest that in vitro studies using broth suspensions to compare plain and charcoal media may have underestimated the performance of plain media (1).
Our finding that auxotype Pro− Arg− survives well in transport medium agrees with the results of a clinical study showing that, compared to immediate plating, a 24-h delay in processing swabs not only reduced the yield of N. gonorrhoeae, but also resulted in an artificially high prevalence of Pro− Arg− strains among those recovered (A. de Burgh-Thomas, M. Graver, M. Stevens, J. Taylor, J. Wade, and C. Wilkinson, Medical Society for the Study of Venereal Diseases/Scandinavian Society for Genitourinary Medicine Spring Meeting, Oslo, Norway, May 2002). These in vivo and in vitro observations have epidemiological implications, because auxotype can correlate with antimicrobial susceptibility and sexual practices. There are also practical implications. Because the normalizing effect of charcoal on survival of different auxotypes is less apparent at 48 h than at 24 h, swabs processed after 24 h are increasingly unlikely to yield isolates representative of those causing infection in the patient population sampled. Improved transport media capable of better preserving the less robust auxotypes of N. gonorrhoeae are required.
REFERENCES
- 1.Amies, C. R. 1967. A modified formula for the preparation of Stuart's transport medium. Can. J. Public Health 58:296-300. [PubMed] [Google Scholar]
- 2.Copley, C. G., and S. I. Egglestone. 1983. Auxotyping of Neisseria gonorrhoeae isolated in the United Kingdom. J. Med. Microbiol. 16:295-302. [DOI] [PubMed] [Google Scholar]
- 3.Farhat, S. E., M. Thibault, and R. Devlin. 2001. Efficacy of swab transport system in maintaining viability of Neisseria gonorrhoeae and Streptococcus pneumoniae. J. Clin. Microbiol. 39:2958-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knapp, H. R., and M. A. Melly. 1986. Bactericidal effects of polyunsaturated fatty acids. J. Infect. Dis. 154:84-94. [DOI] [PubMed] [Google Scholar]
- 5.Ley, H. L., Jr., and J. H. Mueller. 1946. On the isolation from agar of an inhibitor for Neisseria gonorrhoeae. J. Bacteriol. 52:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McFarland, L., T. A. Mietzner, J. S. Knapp, E. Sandstrom, K. K. Holmes, and S. A. Morse. 1983. Gonococcal sensitivity to fecal lipids can be mediated by an Mtr-independent mechanism. J. Clin. Microbiol. 18:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen, C. C., J. R. Schwebke, W. H. Benjamin, Jr., A. Beverly, and K. B. Waites. 1999. Comparison of direct inoculation and Copan transport systems for isolation of Neisseria gonorrhoeae from endocervical specimens. J. Clin. Microbiol. 37:3583-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson, D. S., and S. A. French. 1999. Comparison of commercial Amies transport systems with in-house medium for recovery of Neisseria gonorrhoeae. J. Clin. Microbiol. 37:3020-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]