Abstract
Background
Marginal biotin deficiency may be a human teratogen. A biotin status indicator that is not dependent on renal function would be potentially useful in studies of biotin status during pregnancy. A previous study of experimental biotin deficiency suggested that propionyl-CoA carboxylase (PCC) activity in peripheral blood lymphocytes (PBL) is a sensitive indicator of biotin status.
Objective
We examined the utility of PCC and activation of PCC by biotin in detecting marginal biotin deficiency.
Design
Marginal biotin deficiency was induced in 7 adults (3 women) by egg-white feeding for 28 days. Blood and urine were obtained on days 0, 14, 28 (depletion phase), 44 and 65 (repletion phase). PBL were incubated with biotin (Activated) or without (Control) prior to PCC assay. Activation coefficient (AC) of PCC is the ratio of Activated to Control. Significance of differences for all measurements was tested by repeated measures ANOVA with Fisher’s post hoc test and Bonferroni correction.
Results
Changes in urinary biotin and 3-hydroxyisovaleric acid (3HIA) excretion confirmed that marginal biotin deficiency was successfully induced. By Day 14, PCC had decreased (P < 0.0001) and was abnormal in all subjects. By Day 28, AC of PCC had increased (P = 0.003) and was abnormal in 6 of 7 subjects.
Conclusion
PCC is the most sensitive indicator of biotin status tested to date. In future pregnancy studies, the use of lymphocyte PCC data should prove valuable in assessment of biotin status.
Keywords: Biotin, biotin deficiency, human, lymphocytes, propionyl-CoA carboxylase, activation coefficient
INTRODUCTION
Clinical studies from our group and others have shown that marginal biotin deficiency is common in certain circumstances including pregnancy and long-term anticonvulsant therapy (1–4). Because marginal biotin deficiency is clearly teratogenic in several animal species (5), recent reviews have expressed concern that maternal marginal biotin deficiency may be teratogenic in humans (5). Even more recently, we reported evidence of marginal biotin deficiency in women smokers (6), an observation that further emphasizes teratogenic concern, as well as raising the possibility that smoking and marginal biotin deficiency might have synergistic deleterious effects on the developing fetus. However, concerns about teratogenesis related to biotin deficiency must be evaluated in light of the following caveat. Most of the current conclusions concerning biotin status in pregnancy are based on urinary indicators of biotin status (2, 3), and renal function is affected by pregnancy per se. Accordingly, an indicator of biotin status that is largely independent of renal function would be useful, particularly in testing conclusions from previous studies.
We have recently published an initial evaluation of a blood-based indicator of biotin status (7). Our previous study of 11 subjects made biotin deficient by egg-white feeding indicated that that propionyl-CoA carboxylase activity (PCC) in peripheral blood lymphocytes (PBL) is an early and sensitive indicator of marginal biotin deficiency (7). In the current study, we sought to confirm or refute those findings in a new cohort of subjects and to examine the specificity of the PCC activity findings in these subjects. We also sought to test the validity of an additional blood-based indicator of biotin status - the biotin activation coefficient of PCC in PBL.
SUBJECTS AND METHODS
Subjects, study design, and diet
This study was approved by the University of Arkansas for Medical Sciences (UAMS) Institutional Review Board. Informed consent was obtained at enrollment and continued as a process throughout the study. Inclusion criteria included good health without a history of renal disease likely to lead to chronic impairment of renal function. Exclusion criteria included smoking and consuming either dietary supplements known to contain biotin or certain medications (e.g., certain anticonvulsants known to accelerate biotin catabolism (4)).
Eight healthy adult volunteers (3 women) completed the study. One man admitted that he did not comply with the study protocol; his data are excluded. The ages of seven remaining ranged from 19 to 43 years. BMI ranged from 22.2 to 28.4. Racial/ethnic demographics were three white, two Asian, one Hispanic, and one African American.
Details of the experimental induction of biotin deficiency have been published previously (8). In order to normalize biotin status at the start of the depletion phase of the study (“Day 0”), subjects completed a biotin “loading and washout” phase before initiation of egg-white feeding. Subjects were housed as inpatients in the UAMS General Clinical Research Center (GCRC) from Day 0 to Day 28 and were provided a dietician-monitored, eucaloric diet. Subjects consumed an egg-white drink with each meal. The egg white contained sufficient avidin to bind 7 times the analyzed dietary biotin intake as described previously (9). Energy intake, biotin intake and egg-white drink consumption were monitored daily by the GCRC Research Dietician. On Day 28, subjects were discharged from the GCRC and consumed a self-selected mixed general diet thereafter. During this repletion phase, biotin status of the subjects was monitored for 38 days.
Despite the rigorous control of the GCRC environment, one subject admitted to being noncompliant with the dietary regimen; this subject both consumed foods obtained from outside the GCRC and failed to consume the majority of the egg-white beverage as instructed. As expected, the urinary excretion of biotin and 3HIA of this subject confirmed that marginal biotin deficiency was not induced. The data of that subject were excluded from statistical analyses.
Blood and Urine Sample Collection
Blood was collected in syringes (in which 1000 U/mL heparin was added to produce 15 U/mL whole blood to prevent coagulation) on Days 0, 14 and 28 during the depletion phase of the study and on Days 44 and 65 during the repletion phase. PBL were separated from whole blood by density gradient centrifugation and stored as previously described (7). Urine collections were obtained for the 24-h time period just prior to blood collections and processed as previously described (7).
Urinary Biotin and 3HIA
Urinary biotin was quantitated by HPLC separation followed by an avidin-binding assay performed as described previously (10). Urinary 3HIA was quantitated by gas chromatography-mass spectroscopy as described previously (9).
Determination of lymphocyte PCC activity and AC of PCC
The 14CO2 incorporation assay for determination of PCC activity in PBL was completed in triplicate for each blood sample as described previously (7, 11). Activity was normalized by protein concentration of the PBL preparation as determined by BOCA protein assay as previously described (7). For AC of PCC, the suspended PBL prepared as described above were incubated at 25°C for 30 min with (Activated) or without (Control) 10 no biotin before protein and PCC assay. Activation coefficients were the ratio of PCC of Activated to PCC of Control.
Statistical Analyses
Distributions of the normal population data for urinary biotin, urinary 3HIA, and PCC in PBL were not normally distributed. Accordingly, the upper and lower limits of these normal ranges were chosen as the 10th and 90th percentiles. For urinary excretion of biotin, the normal range was based on 54 subjects from several previous studies including Day 0 of three egg-white feeding studies including this study (2, 3, 6, 9, 12–15). For urinary excretion of 3HIA, the normal range was based on 68 subjects including Day 0 of the three egg-white feeding studies (2, 3, 6, 9, 15). For PCC, the normal range was based on values obtained on Day 0 for 18 subjects (11 women) including 7 subjects in this study and 11 from a previous study (7). The normal range for AC of PCC was defined as the range of the seven values from Day 0 of this study (before initiation of egg-white diet).
Significance of changes in urinary biotin, urinary 3HIA, PCC activity, and AC of PCC during the depletion phase (Day 0 to Day 28) was tested by one-way ANOVA with repeated measures. Significance of changes of each variable during the repletion phase (Day 28 to Day 65) was tested by one-way ANOVA with repeated measures. When ANOVA significance was P < 0.05, Fisher’s post hoc test was used to determine the significance of differences between time points. P values given are the least significant of the Fisher’s comparison P values after Bonferroni correction for multiple comparisons. For all analyses, Statview 5.01 (SASS Institute, Cary, NC) was used.
RESULTS
Induction of marginal biotin deficiency
Urinary excretion rates for biotin and for 3HIA have been previously validated as indicators of marginal biotin deficiency (9, 15). Increased excretion of 3HIA reflects decreased hepatic activity of the biotin-dependent enzyme MCC (16–20). As intended, none of the overt signs or symptoms of biotin deficiency developed in these subjects during the study. However, based on urinary biotin and 3HIA, marginal biotin deficiency was successfully induced in these seven subjects (Figure 1). By Day 28, urinary biotin excretion had decreased significantly (p =0.0002) and was less than the lower limit of normal in 7 of 7 subjects (Figure 1A). With repletion by consumption of a mixed general diet, biotin excretion rates increased significantly from Day 28 to Day 65 (P=0.011) and were greater than the lower limit of normal for 3 of 7 subjects. By Day 28, urinary 3HIA excretion had increased significantly (p = 0.0003) and was greater than the upper limit of normal in 6 of 7 subjects (Figure 1B). With repletion, 3HIA excretion decreased significantly from Day 28 to Day 65 (p < 0.0001) and was normal in 5 of 7 subjects.
Figure 1.
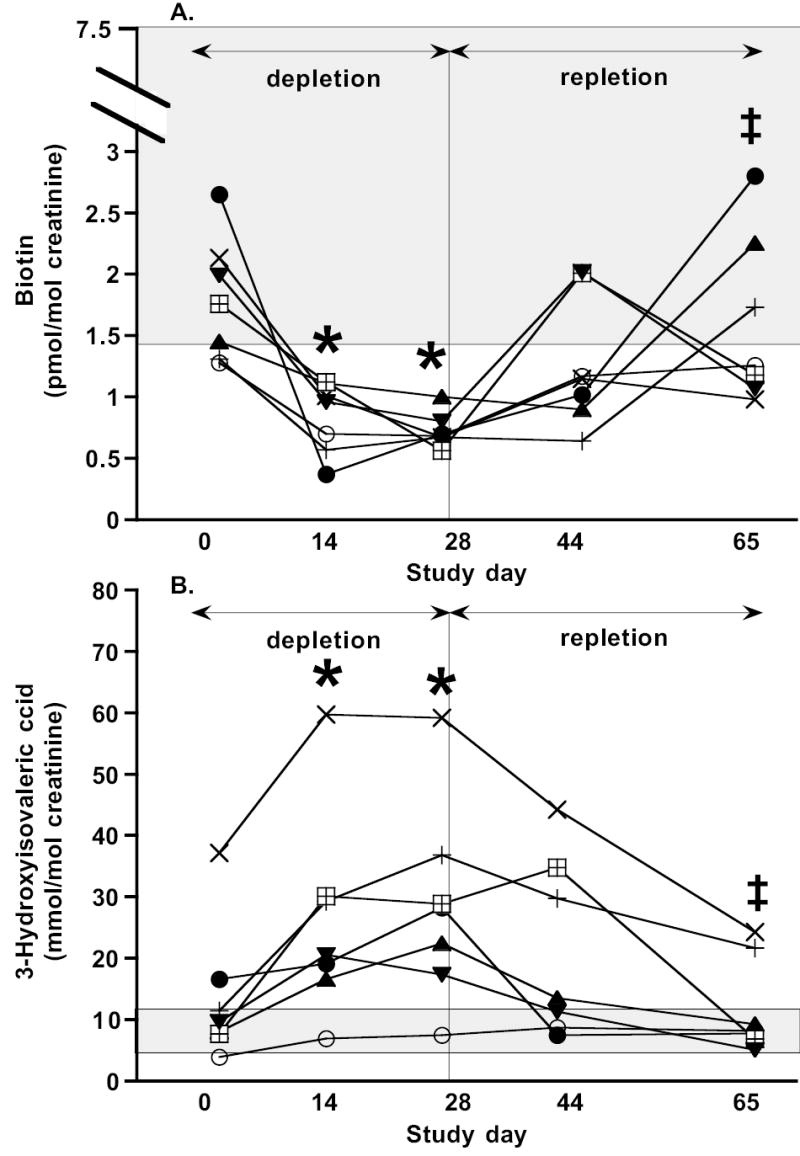
Evidence for induction of biotin deficiency. Urinary biotin (A) and 3-hydroxyisovalerice acid (B) excretion demonstrate successful induction of marginal biotin deficiency in 7 subjects fed the study diet. Individual subject data are shown as individual markers. Bars representing analytic variability for a single subject at a single time point are approximately the same size as symbols and are not depicted. The shaded rectangle denotes the normal range. Depletion ANOVA of Day 0, 14, and 28: * denotes value is different from Day 0 value at P < 0.002. Repletion ANOVA of Day 28, 44, and 65: ‡ denotes value is different from Day 28 at P < 0.008.
PCC activity
In the depletion phase of the study, the activity of PCC in PBL decreased progressively in each compliant subject (Figure 2). By Day 14, PCC activity was less than the lower limit of normal for all subjects, and the mean PCC activity had decreased to 46% of the mean Day 0 value (p < 0.0002). Moreover, by Day 28, mean PCC activity had decreased to only 23% of Day 0 (p < 0.0002). The diagnostic sensitivity was 100% both at Day 14 and at Day 28. PCC activities slowly but significantly recovered with repletion (p <0.0001). After 15 days of repletion, only 1 of 7 subjects’ PCC activity had returned to the normal range (p=0.07). After 36 days of repletion, 5 of 7 subjects’ PCC activities had returned to the normal range (p < 0.0002).
Figure 2.
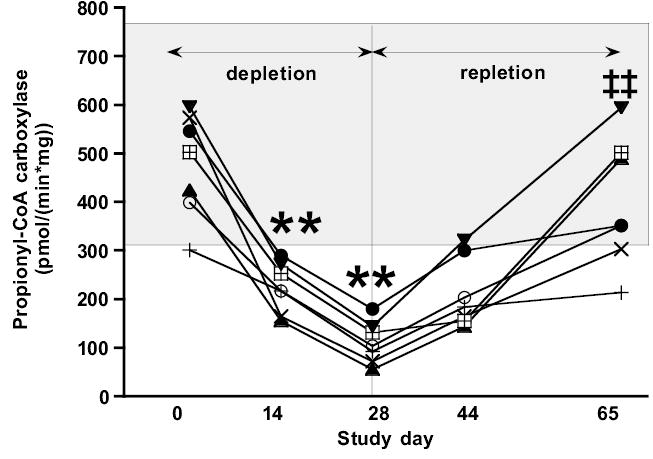
Effect of biotin depletion and repletion on lymphocyte propionyl-CoA carboxylase (PCC) activity. Individual representations, analytic variability, normal range, and statistical analysis are as per Figure 1. ** denotes value is different from Day 0 value at P < 0.0002; ‡‡ denotes value is different from Day 28 value at P < 0.0002.
AC of PCC
AC of PCC (Figure 3) did not increase significantly until Day 28 (p = 0.0034). At Day 28, AC of PCC values for 6 of 7 subjects had increased to greater than the upper limit of normal; thus the diagnostic sensitivity was 86%. After 12 days of repletion, AC of PCC had decreased significantly (p < 0.002) and was within the normal range in 7 of 7 subjects. AC of PCC remained normal for the duration of the repletion phase (p < 0.002).
Figure 3.
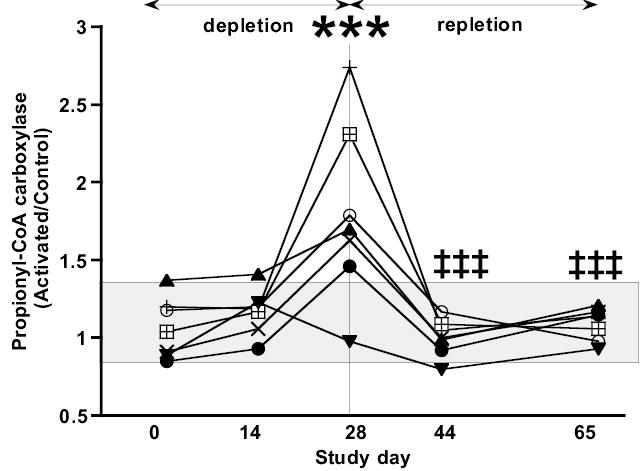
Effect of biotin depletion and repletion on the activation coefficient (AC) of PCC in lymphocytes. The AC is defined as the ratio of the PCC activity in viable lymphocytes incubated with biotin to the PCC activity of lymphocytes without biotin. Individual representations, analytic variability, and statistical analysis are as per Figure 1. The shaded rectangle represents the range of the AC at Day 0 for the 7 subjects. *** denotes value is different from Day 0 value at P < 0.004 and ‡‡‡ denotes value is different from Day 28 value at P < 0.002.
DISCUSSION
In this study, we observed a highly significant decrease in the propionyl-CoA carboxylase activity in the peripheral blood lymphocytes of healthy adults who were experimentally made marginally biotin deficient by feeding a diet high in egg white. The findings from this study confirm the high diagnostic sensitivity of PCC activity in PBL observed in a previous study (7) In addition, we found that the activation coefficient of PCC in PBL is also a sensitive indicator of marginal biotin deficiency in humans. However, PCC activity decreased more quickly than AC of PCC activity increased. The diagnostic sensitivity of PCC was 100% at Day 14, while that of AC of PCC was only 14%. This observation suggests that PCC is an earlier marker of biotin depletion. Consistent with this interpretation is the observation that PCC activity returned to the normal range more slowly than AC of PCC; after 14 days of repletion, 86% of AC of PCC values were within the normal range, but none of the PCC values had returned to normal. Based on this analysis, we conclude that AC of PCC activity as measured here is not as sensitive an indicator of marginal biotin deficiency as PCC activity.
However, confidence in this conclusion concerning relative sensitivity of AC of PCC is limited by our arbitrary choice of conditions for incubation of the PBL with biotin. The generally accepted mechanism for activation is accumulation of apoenzyme intracellularly during deficiency. The apoenzymes are thought to be activated when the deficient vitamin is provided in vitro. For this study, we propose that apopropionyl-CoA carboxylase α chains accumulate in PBL and are biotinylated by holocarboxylase synthetase when biotin is transported into the PBL during in vitro incubation with biotin. The observed activation also implies that adequate amounts of apopropionyl-CoA carboxylase β chain and holocarboxylase synthetase are present intracellularly. We did not conduct optimization experiments for time, temperature, or extracellular concentration of biotin because there were insufficient PBL available under our IRB limitations. Further, Baez-Saldana et al. (21) recently reported results of in vivo biotin supplementation on carboxylase activities. Individuals with and without type-2 diabetes who were not biotin deficient were supplemented with 5 mg of biotin three times a day for 28 days. Activities of PCC, PC, and ACC in PBL increased approximately three fold. This observation indicates that the activity of biotin-dependent carboxylases will increase substantially more with chronic in vivo supplementation than was observed here. Accordingly, there is a distinct possibility that the transport into the lymphocytes was not optimal in restoring biotin status and that sufficient time and temperature were not allowed for maximal carboxylation of the accumulated apoPCC. We speculate that AC of PCC might exhibit a different pattern and sensitivity with an optimized in vitro biotin incubation. Nevertheless, the incubation of concentration for biotin was 20 times the physiologic concentration, and the absolute magnitudes of AC of PCC activity we observed were similar to activation coefficients for other vitamins (22) suggesting that adequate biotin was transported and incorporated.
Comparison of the PBL PCC data to 3HIA excretion provides preliminary evidence that PBL PCC activity is a better indicator of marginal biotin deficiency. Based on PBL PCC activity, 7 of 7 subjects were marginally biotin deficient by Day 14. In contrast, one subject never excreted abnormal amounts of 3HIA. This observation is consistent with our previous studies of 3HIA in which marginal biotin deficiency was induced experimentally (9, 15). In those studies, about 10% of subjects did not demonstrate increased 3HIA excretion. We speculate that the 3HIA pathway for the metabolic disposition of intracellular accumulation of 3-methylcrotonyl CoA is not active in those particular individuals. We speculate that the direct quantitation of PCC activity may well prove to be both more sensitive and more specific for detecting marginal biotin deficiency.
Available data indicate that reduced expression of the genes for the alpha and beta chain of PCC contribute little if any to the dramatic reduction in PCC activity. In a previously published study in these same 7 subjects (8), we observed that the mRNA in PBL for the alpha chain of PCC decreased to 75% of the value at Day 0 by Day 28 (p = 0.027); mRNA for the beta chain of PCC decreased to 86% of the Day 0 value (p = 0.067). Thus, decreased gene expression could explain at most only one-fourth of the decreased PCC activity.
A related study in our pregnant mouse model of biotin deficiency (23) provides additional data consistent with the proposed mechanisms for decreased PCC activity and increased AC of PCC. In this study, mouse dams and fetuses were rendered biotin deficient by egg-white feeding. We found that decreased carboxylase activity was mediated by decreased abundance of the biotinylated carboxylases rather than decreased expression of their mRNA. The abundance of biotinylated carboxylases decreased in both maternal liver (to approximately 48% of control) and fetal liver (to approximately 6% of control) in parallel with decreased PCC activity. In contrast, mRNA for the alpha chain of PCC did not change significantly. Moreover, the less metabolically active dam exhibited a smaller decrease than the more metabolically active fetus.
This study is limited in two additional ways. First, the number of individuals who successfully completed this rigorous inpatient study is small; thus, extrapolation to larger populations must be done with caution. Notwithstanding, the results of this study concerning the utility of PCC activity in PBL are entirely consistent with the results of a previous study of 11 subjects (7). Second, the normal range for activity AC for PCC in PBL was determined on only 7 subjects; no other reports of this index have been published. Although this represents a careful initial observation, inferences concerning diagnostic sensitivity must be made with caution.
The in vitro increase of vitamin-dependent enzyme activity with vitamin incubation is frequently used as a measure of vitamin status for several water-soluble vitamins including B1, B2, B6, and B12 (22, 24–26). These activation coefficients are generally accepted as valid measures of vitamin status (27, 28); however, the functional implications of identified deficiencies remains controversial in most cases. In lymphoid tissue, the turnover of biotin as judged by 14C-biotin degradation (29) is slow compared to liver. Thus, the PCC decrease and the increase of AC of PCC activity in lymphocytes may lag behind biotin depletion in more metabolically active tissues such as hepatocytes, and the deleterious effects of deficiency might even precede these early indicators.
Previous studies utilizing urinary excretion of 3HIA as the primary indicator of biotin status have indicated that marginal biotin deficiency occurs commonly during normal human gestation. Despite the limitations discussed above, we conclude that the study presented here provides evidence that PCC activity of PBL will be useful in future studies that attempt to further assess biotin status in normal human gestation. An important subsequent step would then be to assess the functional implications of the identified biotin deficiency.
Acknowledgments
With appreciation to Cecil Bogy who served as Study Coordinator with assistance from Shawna L. Stratton and Nell I. Mock
Shawna L. Stratton isolated the lymphocytes for PCC and AC of PCC assays with assistance from Anna Bogusiewicz, performed the statistical analyses, drafted the manuscript and prepared the figures for the manuscript.
Anna Bogusiewicz measured creatinine concentrations on all urine samples and assisted Matthew M. Mock and Nell I. Mock in measurement of biotin via the HPLC and avidin-binding assay.
Matthew M. Mock, performed all of the PCC and AC of PCC assays on lymphocytes, determined biotin with assistance from Nell I. Mock and Anna Bogusiewicz, and performed all protein determinations for lymphocytes.
Nell I. Mock reviewed all measurements of urine volume collections and times, and using creatinine concentrations and total creatinine excretion calculated actual 24-h urine collections, reviewed and compiled the results of the PCC and biotin assays, extracted the 3HIA from urine which was measured at the GC/MS Core facility of Vanderbilt University under the direction of David Hachey, Ph.D. (5M01RR00095) to quantitate 3HIA excretion, and assisted in the writing of the manuscript.
Amanda Wells calculated the low biotin diet for each subject and determined the quantity of egg-white beverage required to produce biotin deficiency. She monitored each subject’s daily diet, caloric intake, and weight to maintain constant body weight during the 28 day depletion phase. She calculated total caloric intake, biotin intake, and other significant nutrient intakes to ensure that the diet was complete except for the presence of the biotin binding egg white.
Donald M. Mock served as the principal investigator and was responsible overseeing the study coordinators, the research technicians, and the research dietician. He is responsible for the final version of this manuscript, the statistics, the figures, and the figure legends.
Footnotes
Address reprint requests to corresponding author.
None of the authors has any conflicts of interest concerning financial or personal interests with the sponsors of this work.
Sources of Support: National Institutes of Health RO1DDK36823 and UAMS GCRC M01RR14288
References
- 1.Velazquez A, Martin-del-Campo C, Baez A, et al. Biotin deficiency in protein-energy malnutrition. Eur J Clin Nutr. 1988;43:169–173. [PubMed] [Google Scholar]
- 2.Mock DM, Stadler DD, Stratton SL, Mock NI. Biotin status assessed longitudinally in pregnant women. J Nutr. 1997;127:710–716. doi: 10.1093/jn/127.5.710. [DOI] [PubMed] [Google Scholar]
- 3.Mock DM, Quirk JG, Mock NI. Marginal biotin deficiency during normal pregnancy. Am J Clin Nutr. 2002;75:295–299. doi: 10.1093/ajcn/75.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krause K-H, Berlit P, Bonjour J-P. Impaired biotin status in anticonvulsant therapy. Ann Neurol. 1982;12:485–486. doi: 10.1002/ana.410120513. [DOI] [PubMed] [Google Scholar]
- 5.Zempleni J, Mock DM. Marginal biotin deficiency is teratogenic. Proc Soc Exp Biol Med. 2000;223:14–21. doi: 10.1046/j.1525-1373.2000.22303.x. [DOI] [PubMed] [Google Scholar]
- 6.Sealey WM, Teague AM, Stratton SL, Mock DM. Smoking accelerates biotin catabolism in women. Am J Clin Nutr. 2004;80:932–5. doi: 10.1093/ajcn/80.4.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mock DM, Henrich CL, Carnell N, Mock NI, Swift L. Lymphocyte propionyl-CoA carboxylase and accumulation of odd-chain fatty acid in plasma and erythrocytes are useful indicators of marginal biotin deficiency. J Nutr Biochem. 2002;13:462–470. doi: 10.1016/s0955-2863(02)00192-4. [DOI] [PubMed] [Google Scholar]
- 8.Vlasova TI, Stratton SL, Wells AM, Mock NI, Mock DM. Biotin deficiency reduces expression of SLC19A3, a potential biotin transporter, in leukocytes from human blood. J Nutr. 2005;135:42–7. doi: 10.1093/jn/135.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mock NI, Malik MI, Stumbo PJ, Bishop WP, Mock DM. Increased urinary excretion of 3-hydroxyisovaleric acid and decreased urinary excretion of biotin are sensitive early indicators of decreased status in experimental biotin deficiency. Am J Clin Nutr. 1997;65:951–958. doi: 10.1093/ajcn/65.4.951. [DOI] [PubMed] [Google Scholar]
- 10.Mock DM. Determinations of biotin in biological fluids. In: McCormick DB, Suttie JW, Wagner C, eds. Methods in Enzymology. New York, NY: Academic Press, 1997:265–275. [DOI] [PubMed]
- 11.Zempleni J, Trusty TA, Mock DM. Lipoic acid reduces the activities of biotin-dependent carboxylases in rat liver. J Nutr. 1997;127:1776–1781. doi: 10.1093/jn/127.9.1776. [DOI] [PubMed] [Google Scholar]
- 12.Mock DM, Lankford GL, Cazin J., Jr Biotin and biotin analogs in human urine: Biotin accounts for only half of the total. J Nutr. 1993;123:1844–1851. doi: 10.1093/jn/123.11.1844. [DOI] [PubMed] [Google Scholar]
- 13.Mock DM, Heird GM. Urinary biotin analogs increase in humans during chronic supplementation: The analogs are biotin metabolites. Am J Physiol Endocrinol Metab. 1997;272:83–87. doi: 10.1152/ajpendo.1997.272.1.E83. [DOI] [PubMed] [Google Scholar]
- 14.Mock DM, Stadler DD. Conflicting indicators of biotin status from a cross-sectional study of normal pregnancy. J Am Coll Nutr. 1997;16:252–257. doi: 10.1080/07315724.1997.10718682. [DOI] [PubMed] [Google Scholar]
- 15.Mock DM, Henrich CL, Carnell N, Mock NI. Indicators of marginal biotin deficiency and repletion in humans: validation of 3-hydroxyisovaleric acid excretion and a leucine challenge. Am JClin Nutr. 2002;76:1061–8. doi: 10.1093/ajcn/76.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mock DM, Mock NI, Stewart CW, LaBorde JB, Hansen DK. Marginal biotin deficiency is teratogenic in ICR mice. J Nutr. 2003;133:2519–2525. doi: 10.1093/jn/133.8.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mock NI, Mock DM. Biotin deficiency in rats: Disturbances of leucine metabolism are detectable early. J Nutr. 1992;122:1493–1499. doi: 10.1093/jn/122.7.1493. [DOI] [PubMed] [Google Scholar]
- 18.Bonjour J-P. Biotin-dependent enzymes in inborn errors of metabolism in human. World Rev Nutr Diet. 1981;38:1–88. [Google Scholar]
- 19.Wolf B. Disorders of biotin metabolism: Treatable neurological syndromes. In: Rosenberg RN, Prusiner BS, Mauro SD, Barchi RL, Kunkel LM, eds. The Molecular and Genetic Basis of Neurological Disease. Stoneham, MA: Butterworth, 1992:569–581.
- 20.Sweetman L, Williams JC. Branched chain organic acidurias. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease. 7 ed. New York, NY: McGraw-Hill Inc, 1995:1387–1422.
- 21.Baez-Saldana A, Zendejas-Ruiz I, Revilla-Monsalve C, et al. Effects of biotin on pyruvate carboxylase, acetyl-CoA carboxylase, propionyl-CoA carboxylase, and markers for glucose and lipid homeostasis in type 2 diabetic patients and nondiabetic subjects. Am J Clinical Nutrition. 2004;79:238–243. doi: 10.1093/ajcn/79.2.238. [DOI] [PubMed] [Google Scholar]
- 22.McCormick D. Riboflavin. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 10 ed. Baltimore, MD: Lippincott Williams & Wilkins, 2006:434–441.
- 23.Sealey W, Stratton SL, Hansen DK, Mock DM. Marginal maternal biotin deficiency in CD-1 mice reduces fetal mass of biotin-dependent carboxylases. J Nutr. 2005;135:973–977. doi: 10.1093/jn/135.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butterworth RF. Thiamin. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 10 ed. Baltimore, MD: Lippincott Williams & Wilkins, 2006:426–433.
- 25.Mackey AD, Davis SR, Gregory III JF. Vitamin B6 In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 10 ed. Baltimore, MD: Lippincott Williams & Wilkins, 2006:452–461.
- 26.Carmel R. Cobalamin (Vitamin B12). In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 10 ed. Baltimore, MD: Lippincott Williams & Wilkins, 2006:482–497.
- 27.Rucker RB, Suttie JW, McCormick DB, Machlin LJ, eds. Handbook of Vitamins. 3rd ed. New York: Marcel Dekker, Inc., 2001.
- 28.Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 10 ed. Baltimore, MD: Lippincott Williams & Wilkins, 2006.
- 29.Zempleni J, Mock DM. Uptake and metabolism of biotin by human peripheral blood mononuclear cells. Am J Physiol Cell Physiol. 1998;275:C382–C388. doi: 10.1152/ajpcell.1998.275.2.C382. [DOI] [PubMed] [Google Scholar]


