Abstract
Antimicrobial host defense peptides are produced by all complex organisms as well as some microbes and have diverse and complex antimicrobial activities. Collectively these peptides demonstrate a broad range of antiviral and antibacterial activities and modes of action, and it is important to distinguish between direct microbicidal and indirect activities against such pathogens. The structural requirements of peptides for antiviral and antibacterial activities are evaluated in light of the diverse set of primary and secondary structures described for host defense peptides. Peptides with antifungal and antiparasitic activities are discussed in less detail, although the broad-spectrum activities of such peptides indicate that they are important host defense molecules. Knowledge regarding the relationship between peptide structure and function as well as their mechanism of action is being applied in the design of antimicrobial peptide variants as potential novel therapeutic agents.
INTRODUCTION
A wide variety of organisms produce antimicrobial peptides as part of their first line of defense (90). Antimicrobial peptides are typically relatively short (12 to 100 amino acids), are positively charged (net charge of +2 to +9), are amphiphilic, and have been isolated from single-celled microorganisms, insects and other invertebrates, plants, amphibians, birds, fish, and mammals, including humans (159, 257). To date, hundreds of such peptides have been identified (88), indicating their importance in the innate immune system (89). The expression of these antimicrobial peptides can be constitutive or can be inducible by infectious and/or inflammatory stimuli, such as proinflammatory cytokines, bacteria, or bacterial molecules that induce innate immunity, e.g., lipopolysaccharides (LPS) (44, 86). Some of these peptides are potent antimicrobials. In contrast, the direct antimicrobial activity of others is largely evident in dilute media, and direct microbe killing is almost certainly prevented by physiological conditions, including high monovalent or moderate divalent cation concentrations, host proteases, polyvalent anions such as glycosaminoglycans (e.g., heparan sulfate), and low local peptide concentrations. Conversely these peptides are important effector molecules of the innate immune system (24, 30). They are able to enhance phagocytosis, stimulate prostaglandin release, neutralize the septic effects of LPS, promote recruitment and accumulation of various immune cells at inflammatory sites (58, 266), promote angiogenesis (129), and induce wound repair (36). Peptides of mammalian origin have also been demonstrated to have an active role in the transition to the adaptive immune response by being chemotactic for human monocytes (246) and T cells (40) and by exhibiting adjuvant and polarizing effects in influencing dendritic cell development (47). Although such peptides may have a direct effect on the microbe, such as by damaging or destabilizing the bacterial, viral, or fungal membrane or acting on other targets, they appear to be broadly involved in the orchestration of the innate immune and inflammatory responses (89). Thus, they are increasingly being referred to as host defense peptides. For example α-defensins are almost certainly bactericidal at the high (mg/ml) concentrations found in neutrophil granules, but they probably act primarily as immunomodulators at the lower concentrations released by degranulation at inflammatory sites.
Despite their similar general physical properties, individual cationic peptides have very limited sequence homologies and a wide range of secondary structures with at least four major themes. The most prominent structures are amphiphilic peptides with two to four β-strands, amphipathic α-helices, loop structures, and extended structures (21, 87) (Fig. 1; Table 1). This review provides an overview of the (direct) antimicrobial functions of these peptides, with an emphasis on antiviral activity and an update on antibacterial, antifungal, and antiparasitic activities.
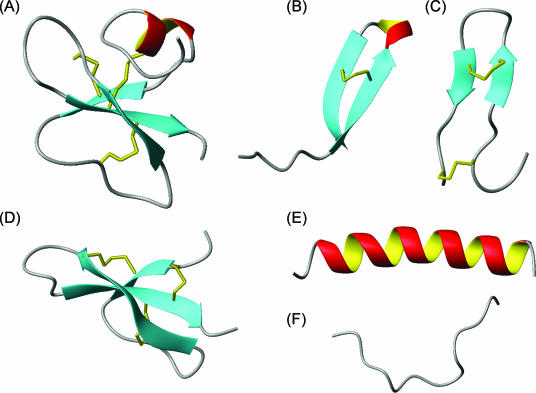
FIG. 1.
Structural classes of antimicrobial peptides. (A) Mixed structure of human β-defensin-2 (PDB code 1FQQ) (216); (B) looped thanatin (PDB code 8TFV) (156); (C) β-sheeted polyphemusin (PDB code 1RKK) (202); (D) rabbit kidney defensin-1 (PDB code 1EWS) (165); (E) α-helical magainin-2 (PDB code 2MAG) (76); (F) extended indolicidin (PDB code 1G89) (212). The disulfide bonds are indicated in yellow, and the illustrations have been prepared with use of the graphic program MolMol 2K.1 (132).
TABLE 1.
Some examples of the diverse primary sequence compositions of antimicrobial peptides
| Peptide | Primary amino acid sequencea | Reference(s) |
|---|---|---|
| Rabbit kidney defensin | MPC1SC2KKYC3DPWEVIDGSC2GLFNSKYIC3C1REK | 165 |
| Human β-defensin-2 | GIGDPVTC1LKSGAIC2HPVFC3PRRYKQIGTC2GLPGTKC1C3KKP | 216 |
| Magainin 2 | GIGKFLHSAKKFGKAFVGEIMNS | 76 |
| Indolicidin | ILPWKWPWWPWRR | 212 |
| Polyphemusin 1 | RRWC1FRVC2YRGFC2YRKC1R | 202 |
| Thanatin | GSKKPVPIIYC1NRRTGKC1QRM | 156 |
| Buforin II | TRSSRAGLQFPVGRVHRLLRK | 187 |
| Cecropin A1 | GWLKKIGKKIERVGQHTRDATIQGLGVAQQAANVAATAR | 205 |
| Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ | 84 |
| Human lactoferricin | GRRRRSVQWC1AVSQPEATKC2FQWQRNMRRVRGPPVSC2IKRDSPIQC1IQA | 17, 107, 118 |
| Bovine lactoferricin | FKC1RRWQWRMKKLGAPSITC1VRRAFA | 17, 107, 118 |
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | 81 |
Amino acid sequences are given in one-letter code. Cysteines forming disulfide bonds are numbered with subscripts to indicate their pairings. Boldface indicates cationic amino acid residues.
NATURAL DISTRIBUTION AND ACTIVITIES OF ANTIMICROBIAL HOST DEFENSE PEPTIDES
Antimicrobial peptides are a universal feature of the defense systems of virtually all forms of life, with representatives found in organisms ranging from bacteria to plants and invertebrate and vertebrate species, including mammals. They form part of the ancient, nonspecific innate immune system, which is the principal defense system for the majority of living organisms. In many cases, their primary role is in the killing of invading pathogenic organisms, and this is the focus of this review; however, it is increasingly recognized that they may also function as modulators of the innate immune response in higher organisms (23, 220, 265, 271). Collectively, they display direct microbicidal activities toward bacteria, fungi, and some parasites and viruses, although the importance of these activities in contributing to host defense may vary between different sites within a particular organism and also between different types of organisms. Antimicrobial peptides may be expressed constitutively in some cases or may be inducibly expressed in response to pathogenic challenge. In multicellular animals, they may be expressed systemically (for example, in insect hemolymph or vertebrate immune cells) and/or localized to specific cell or tissue types in the body most susceptible to infection, such as mucosal epithelia and the skin. The following is a brief overview of the distribution of antimicrobial peptides in nature and their roles in defense.
Antimicrobial peptides produced by bacteria were among the first to be isolated and characterized (163). While they do not protect against infection in the classical sense, they contribute to survival of individual bacterial cells by killing other bacteria that might compete for nutrients in the same environment. Bacterial antimicrobial peptides, also called bacteriocins, are thought to be produced by many or most bacteria (128, 206) and are generally extremely potent compared with most of their eukaryotic counterparts. Their activities may be either narrow or broad spectrum, capable of targeting bacteria within the same species or from different genera. The bacteriocins constitute a structurally diverse group of peptides, and it was recently proposed that they be classified into two broad categories: lanthionine containing (lantibiotics) and non-lanthionine containing (43). Lantibiotics are characterized by the inclusion of the unusual amino acid lanthionine and the necessity for posttranslational processing to acquire their active forms. The most extensively studied lantibiotic is nisin, produced by Lactococcus lactis, which has been commonly used for nearly 50 years as a food preservative without significant development of resistance. It is also extremely potent, displaying activity against a variety of gram-positive bacteria at MICs in the low nanomolar range. These properties have prompted intense study of the mechanism of action of nisin, which is discussed later in this review. Other lantibiotics have also received attention due to their possible applications in the treatment of bacterial species which have developed antibiotic resistance. Mersacidin, a tetracyclic peptide that is produced by Bacillus spp. (38, 39), displays bactericidal activity against methicillin-resistant Staphylococcus aureus that is comparable to that of vancomycin, but without the development of cross-resistance (135).
In plants, it is widely believed that antimicrobial peptides play an important and fundamental role in defense against infection by bacteria and fungi. Observations to support this role include the presence and expression of genes encoding antimicrobial peptides in a wide variety of plant species investigated thus far, demonstrations of their bactericidal and fungicidal activity in vitro, and correlations between expression levels of peptides and susceptibility to a given pathogen or the extent of resistance of a particular bacterium to plant-derived peptides and its virulence. So far, only peptides with a β-sheet globular structure have been identified in plants, with the two major and best-studied groups being thionins and defensins (reviewed in reference 72). Physiologically relevant concentrations of thionins are active against bacteria and fungi in vitro, and studies utilizing transgenic plants have shown that heterologous expression of thionins can confer protection against bacterial challenge (35, 59). Plant defensins display antibacterial and antifungal activities in vitro (245). Consistent with a defensive role, they are found in leaves, flowers, seeds, and tubers.
Since invertebrates lack the adaptive immune system found in vertebrate species, they are reliant solely upon their innate immune systems to counteract invading pathogens. Considering the extraordinary evolutionary success of this group of organisms, it is evident that invertebrate innate immune mechanisms are extremely effective. This has prompted intense studies of invertebrate species such as the arthropod fruit fly, Drosophila melanogaster, which has become a model system for the study of innate immunity and has led to the discovery of immune system strategies, such as pathogen recognition receptors (Toll-like receptors), that are conserved in higher organisms, including mammals. Numerous antimicrobial peptides have now been identified in invertebrates, and they are recognized as playing a key role in protection from pathogenic organisms. Indeed, the role of antimicrobial peptides and the regulation of their expression, including the signaling cascades involved, is well understood for Drosophila (111). Antimicrobial peptides are found in the hemolymph (plasma and hemocytes), in phagocytic cells, and in certain epithelial cells of invertebrates. They can be expressed constitutively, for example, in the hemocytes of marine arthropods such as shrimp, oyster, and horseshoe crab (11, 114), or induced in response to pathogen recognition, such as antifungal peptides in Drosophila (149). Among some of the prototypic invertebrate antimicrobial peptides are the α-helical cecropins (fly hemolymph) and melittin (bee venom) as well as the β-hairpin-like peptides tachyplesin and polyphemusin (horseshoe crab). The horseshoe crab-derived peptides possess some of the most potent antibacterial and antifungal activities observed, with MICs of <2 μg/ml (280). Interestingly, polyphemusin also displays activity against human immunodeficiency virus (HIV) (160). However, the most abundant group of antimicrobial peptides in invertebrates are the defensins, which are open-ended cyclic peptides with three or four disulfide bridges. The activities of invertebrate defensins can be divided according to whether their principal biological activity is directed toward bacteria or fungi (33).
Antimicrobial peptides have been isolated from a wide range of vertebrate species, including fish, amphibians, and mammals, indicating that, even in the presence of an adaptive immune response, these peptides have an important role in host defense. Direct microbicidal activity is associated with vertebrate antimicrobial peptides to various degrees under physiological conditions, and these activities likely contribute to the first line of defense, especially where they are found in very high concentrations, such as in the granules of phagocytic cells or the crypts of the small intestine (23, 25, 220, 265, 266). However, it is increasingly recognized that in addition to direct microbicidal activity, small cationic peptides perform critical immunomodulatory functions and may be involved in the control of inflammation, which serves to recruit a variety of other microbicidal mechanisms (24, 265, 266). Consistent with their role in direct and indirect antimicrobial defenses, antimicrobial peptides in vertebrates are found at sites that routinely encounter pathogens, such as mucosal surfaces and the skin, as well as within the granules of immune cells (24, 265, 266).
Amphibian skin glands have proven to be a rich source of antimicrobial peptides, with approximately 500 having been described to date as originating from this source. This represents a large proportion of the total number of reported antimicrobial peptides (207; http://www.bbcm.univ.trieste.it/∼tossi/pag1.html). The α-helical magainins (272) are the prototypic amphibian antimicrobial peptides, with strong membrane-permeabilizing activity towards gram-positive and -negative bacteria, fungi, yeasts, and viruses. Structure-function relationships and the mechanism of action of magainin have been extensively studied, and these peptides have subsequently served as the template for development of the first (although ultimately unsuccessful) clinical antibacterial peptide treatment (74, 137). The broad antibacterial and antifungal activities of dermaseptins, isolated from the skin of South American frogs, have also been widely studied. In addition to their presence in the skin, amphibian antimicrobial peptides are produced in the mucosa of the stomach, indicating a role in protection from ingested pathogens. The best-characterized examples are the Asian toad peptides buforin and buforin II, which are generated by cleavage of the nucleosome protein histone 2A. A number of excellent reviews have covered this large group of antimicrobial peptides (33, 207, 224).
Cathelicidins are a large and diverse group of vertebrate antimicrobial peptides. They are characterized by a well conserved N-terminal segment (the cathelin domain) that is proteolytically cleaved to generate the mature, active peptide contained within the C terminus. Hence, most cathelicidins are stored in an inactive propeptide state, mostly within granules of circulating immune cells. Neutrophil secretory granules are the predominant source of cathelicidins, but they may also be expressed in mucosal surfaces in the mouth, lung, and genitourinary tract and in skin keratinocytes in inflammatory disorders, as is the case with human cathelicidin LL-37 (hCAP18) (66). Beyond the common N terminus, the structure of mature cathelicidins is diverse, with α-helical, β-hairpin, and proline/arginine-rich peptides all represented. The structural diversity within the cathelicidin family is also indicative of their apparently distinct functions, and they exhibit a diverse spectrum of microbicidal and immunomodulatory activities. Cathelicidins have been isolated from many mammalian species, such as mice, rabbits, sheep, horses, and humans. In some mammals, such as cattle, multiple cathelicidins are found in the body, indicating that they likely perform varied biological roles in host defense. One of the best characterized bovine antimicrobial peptides is BMAP-28, an α-helical peptide which rapidly permeabilizes the membranes of a broad spectrum of bacteria and fungi at moderate concentrations in vitro (229), whereas the proline-rich bovine peptide Bac 5 shows selectivity for gram-negative bacteria under the same conditions (75). In contrast, only one cathelicidin is expressed in humans: LL-37 (hCAP18). Evidence in support of an early defensive role for LL-37 includes its upregulation in response to infection in the skin (54), as well as conditions in which a deficiency of LL-37 leads to chronic periodontal disease (204). In addition to direct microbicidal activity, LL-37 has important additional roles in host defense, including chemotactic properties and modulation of inflammatory responses (24, 271).
A second prominent group of mammalian antimicrobial peptides is the defensins (70, 221), cyclic peptides which are categorized into three subfamilies on the basis of the disulfide pairings between their six conserved cysteine residues (α- and β-defensins) or their macrocyclic nature (θ-defensins). As with cathelicidins, vertebrate defensins are synthesized as prepeptides which require proteolytic processing to their active peptide forms. The α- and β-defensins are widely distributed in vertebrate species, whereas θ-defensins have so far been identified only in Old World monkeys and apparently only in neutrophils and monocytes so far (244). Depending on the species, α- and β-defensins are found in the granules of neutrophils, macrophages, NK cells, intestinal Paneth cells and epithelial tissues, the skin, certain mucosal surfaces such as the respiratory passage and urinogenital tract, and many bodily fluids. Expression of the defensins may be constitutive, such as for human β-defensin-1 (hBD-1) in most tissues, or inducible, such as for hBD-2, the expression of which in monocytes is upregulated following exposure to bacteria or LPS (56, 62). In vitro studies demonstrate that collectively, defensins possess generally weak microbicidal activities towards bacteria, fungi, and some viruses. While the bactericidal activities of most α- and β-defensins are antagonized by increasing salt concentrations (e.g., 100 mM monovalent and/or 2 mM divalent cations, which are found at many body sites), the high concentrations of α-defensins that are found in some locations, particularly in the granules in phagocytic cells and in intestinal crypts, are thought to be sufficient to result in killing despite antagonism by salts (10, 71). θ-Defensins and hBD-3, on the other hand, retain their bactericidal activities in physiological salt conditions and also display antiviral activity in in vitro studies using HIV (42, 113). Transgenic and knockout experiments with mice have indicated a critical role for defensins in host defense. MMP-7-null mice, which lack all mature α-defensins due to the loss of the protease required for proteolytic cleavage, display a reduced clearance of Escherichia coli and higher mortality rates upon challenge with Salmonella enterica serovar Typhimurium, indicating a important role in intestinal immunity (258). Conversely, knock-in of human HD-5 defensin into mouse Paneth cells conferred immunity to oral challenge using Salmonella enterica serovar Typhimurium (214). Importantly, in each case a correlation was observed between the antibacterial activity of the altered Paneth cell products in vitro and their protective abilities in vivo.
ANTIVIRAL ACTIVITY
Representatives from all four structural classes of the cationic host defense peptides have shown the ability to inhibit viral infection. The spectra of viruses that are affected comprise primarily enveloped RNA and DNA viruses, with the exception of nonenveloped adenovirus (15, 102), feline calicivirus (164), and echovirus 6 (199). The antiviral activity of antimicrobial peptides often appears to be related to the viral adsorption and entry process (16) or is a result of a direct effect on the viral envelope (1, 208). However, it appears to be impossible to predict antiviral activity based primarily on secondary structures of peptides (Table 2). For example α-helical peptides such as cecropins, clavanins, and the cathelicidin LL-37 have been shown to cause minimal or no herpes simplex virus (HSV) inactivation (18, 185, 269), while α-helical magainins (Fig. 1), dermaseptin, and melittin have shown quite potent anti-HSV activity (Table 2) (1, 3, 16, 269). Conversely, β-sheet peptides such as defensins, tachyplesin, and protegrins as well as the β-turn peptide lactoferricin have all shown high activity towards HSV (Table 2) (4, 45, 120, 148, 225, 269, 270). It should be noted that within the different peptide subclasses, activity may vary considerably. For example, protegrin analogues lacking one or both disulfide bridges vary from highly active to inactive against HSV infections (269).
TABLE 2.
Selected examples of antiviral peptides
| Peptide | Structure | Source(s) | Virus | Proposed antiviral mechanism | Reference(s) |
|---|---|---|---|---|---|
| Magainin | α-Helix | Frog | HSVa | Cellular target | 1, 3 |
| HIV | Suppresses viral gene expression | ||||
| Cecropin | α-Helix | Insect | Junin virus | Suppresses viral protein synthesis | 3, 255 |
| HSV | Cellular target | ||||
| HIV | Suppresses viral gene expression | ||||
| Mellitin | α-Helix | Bee | HSV | Cellular target | 3, 255, 269 |
| Junin virus | Cellular target | ||||
| LL-37 | α-Helix | Human | HSV | Weak viral inactivation | 269 |
| Brevinin-1 | α-Helix | Frog | HSV | Viral inactivation | 269 |
| θ-Defensin | Cyclic β-sheet | Primate, human | HIV | Binds glycosylated gp120 | 42, 270 |
| HSV | Binds gB and blocks viral attachment | ||||
| Defensin | β-Sheet | Human, rabbit | HSV | Interacts with HSV membrane/glycoprotein and cellular target but not heparan sulfate | 15, 45, 80, 178, 225, 270 |
| IAV | Inactivates viral particle | ||||
| HCMV | Inactivates viral particle | ||||
| VSV | Inactivates viral particle | ||||
| HIV | Cellular target | ||||
| Adenovirus | Unknown | ||||
| Dermaseptin | β-Sheet | Frog | HIV | Viral membrane disruption | 16, 153 |
| HSV | Activity at virus-cell interface | ||||
| Tachyplesin | β-Sheet | Horseshoe crab | HIV | Virus-cell fusion | 171, 174, 269 |
| HSV | Viral inactivation | ||||
| VSV | Viral envelope | ||||
| IAV | Viral envelope | ||||
| Protegrin | β-Sheet | Human, porcine | HIV | Unknown | 236, 269 |
| HSV | Viral inactivation | ||||
| Polyphemusin | β-Sheet | Horseshoe crab | HIV | Binds gp120 and CD4 | 177, 242 |
| Lactoferricin | β-Turn | Bovine, human | HCMV | Activity at virus-cell interface | 4, 6, 55, 120 |
| HIV | Unknown | ||||
| HSV | Blocks heparan sulfate, but a secondary effect has also been indicated | ||||
| Papillomavirus | Activity at virus-cell interface | ||||
| Indolicidin | Extended | Bovine | HIV | Inhibit integrase | 3, 134, 208 |
| HSV | Targets viral membrane/glycoprotein |
HSV indicates either HSV-1 or HSV-2 or both types.
Structural Requirements for Antiviral Peptides
Synthetic analogues of several naturally occurring antimicrobial peptides have been made in an attempt to identify important structural features contributing to the antiviral activity. Different strategies for design of such peptides have been pursued. Several groups have looked at the importance of charged and aromatic amino acids, since antiviral peptides are often highly cationic and amphiphilic (45, 119, 241, 269). The hydrophobic character of the peptides has been investigated for a hybrid peptide of cecropin A and magainin-2 (144), while the substitution of d- or l-amino acids has been studied on a set of θ-defensins (270).
The creation of a series of lactoferricin analogues and study of their activity towards HSV revealed a relationship between the peptide net charge and its antiviral activity (120, 121). However, the spatial positioning of the charged amino acids seemed to be more important for antiviral activity than the actual net charge (120). For lactoferricin the nature of the aromatic amino acid appeared to be of minor importance for the antiviral activity, although its contribution to the secondary structure and thereby presentation of the charged residues might be crucial (121). Detailed studies on the influence of secondary structure domains on anti-HSV activity illustrated that the α-helicity of a peptide could not explain its antiviral activity (122), thus implying that the presentation of the charged residues is of greatest importance with respect to anti-HSV activity (119). This is in accordance with results from Giansanti et al. (77) in a study on peptides derived from bovine lactoferrin and hen ovotransferrin. They concluded that the presence of hydrophobic and positively charged residues is critical but not sufficient for antiviral activity, and this may relate to different conformations adopted by these peptides in the context of the native protein (77).
θ-Defensins are rather rigid cyclic peptides from Old World monkeys. Analogues have been designed with a focus on Ile-to-Tyr or Arg-to-Tyr substitutions, in addition to the extensive use of d-amino acids, and have demonstrated the importance of the charge and spatial conformation of the peptides (270). Similarly, defined structures can provide effective antivirals in the case of other types of peptides. Lactoferricin and polyphemusin have β structures stabilized by one and two internal disulfide bridges, respectively. These disulfide bridges have been shown to be crucial for the antiviral activity of the peptides (6, 120, 241) (Fig. 1; Tables 1 and 2).
Despite their diverse structures, many peptides possess analogous antiviral modes of action (119, 120), indicating that these peptides are able to interact with their targets, despite large structural differences. A possible explanation would lie in the observation that antimicrobial host defense peptides are known to adopt amphipathic conformations that are intrinsic to antibacterial activity and, we propose here, to antiviral activity. Interestingly, although the viral target of these peptides appears to vary, the demonstrated antiviral effects are quite similar.
Mode of Action of Antiviral Peptides
Blocking of viral entry by heparan sulfate interaction.
Proteoglycans are found in all types of tissue, in intracellular granule secretions (130), in extracellular matrix (112), and on the cell surface (19). Proteoglycans consist of a core protein and one or more covalently attached glycosaminoglycan chains. The degree of sulfation in the glycosaminoglycan chains makes them among the most anionic compounds present on mammalian cell surfaces (249). This strong net negative charge permits glycosaminoglycans to bind to small cations (192), proteins (110), enzymes (198), growth factors (53, 127, 155), cytokines (34), chemokines (101), and lipoproteins (151, 182), in addition to a number of pathogens, including viruses (166, 234).
Heparan sulfate is the most important glycosaminoglycan molecule with respect to viral attachment (166, 234); consequently, blocking of heparan sulfate can reduce the viral infection (222, 260). The importance of heparan sulfate for different viral infections varies considerably. It has been demonstrated that recombinant cells lacking heparan sulfate and chondroitin sulfate expression demonstrate an 80% and 60% reduction, respectively, in susceptibility to HSV infection (158). Enzymatic removal of cellular heparan sulfate and chondroitin sulfate has led to the observation that these proteoglycan molecules have minor influences on HIV attachment to host cells. However, it has been demonstrated that they are of major importance for HIV entry and replication (8). In contrast, only highly sulfated heparan participates in the entry of hepatitis C virus (14). Inhibitors of heparin sulfate biosynthesis, such as heparin, heparinase I treatment, and sodium chlorate, all demonstrate the ability to inhibit human cytomegalovirus (HCMV) infection in a dose-dependent manner (231). It has even been indicated that naked coxsackievirus B3 makes use of a specific modified heparan sulfate molecule for viral entry (274).
One might hypothesize that antimicrobial peptides that interact with heparan sulfate should be able to block many different viral infections. The large number of positively charged residues in antimicrobial peptides enables them to interact electrostatically with negatively charged cell surface molecules, including heparan sulfate. Human α-defensin, LL-37, and magainin have all been shown to interact with different glycosaminoglycan molecules (116, 217, 218). Specific glycosaminoglycan binding domains have also been identified in bovine and human lactoferricin. These domains involve the sequence elements G1RRRRS6 and R28KVR31 in human lactoferricin (157) and the entire sequence of bovine lactoferricin (223). The sequence and structural diversity in these glycosaminoglycan binding peptides suggests that the critical factor driving interaction is how charged residues are presented in the secondary structure. Several lactoferricin analogues and synthetic α-helical peptides have been made in an attempt to better understand these interactions. The results illustrate that the affinity for heparan sulfate is only partly correlated with the net charge of the peptides (119, 120). For example, analogues with Arg residues appear to promote a higher glycosaminoglycan affinity than comparable analogues substituted with Lys (67, 99, 119, 237).
It has been demonstrated that lactoferricin and a set of short α-helical peptides are able to block HSV infection by binding to heparan sulfate in a way similar to that demonstrated by lactoferrin (5, 119, 120). This is supported by the fact that mixing the peptides with HSV prior to interaction did not increase antiviral activity (5). The peptides exhibited different antiviral effects for HSV type 1 (HSV-1) and HSV-2, an observation attributed to the combined effects of the amino acid content and the structures of the peptides (4, 119, 120, 122). Interestingly, differences in antiviral effects against HSV-1 and HSV-2 have also been reported for other polycationic and even polyanionic compounds (109, 138). These differences may reflect the viral specificity of particular receptor molecules and the differential ability of peptides to interact with the different viral receptors.
3-O-Heparan sulfate is a specific HSV entry receptor with structural similarities to the usual heparan sulfate, probably with increased affinity potential for cationic peptides. Peptide interaction with 3-O-heparan sulfate may result in interference or blocking of HSV glycoprotein D binding, resulting in specific inhibition of HSV entry. This might explain why several cationic peptides have demonstrated higher antiviral activity against HSV-1 than against HSV-2 (119, 120), since 3-O-heparan sulfate can serve as an entry receptor for HSV-1 and not for HSV-2 (261).
Bovine lactoferricin demonstrated an antiviral activity against human cytomegalovirus and human papillomavirus at the virus-cell interface (6, 55). Bovine lactoferricin also exhibited anti-HIV activity, and this might be related to heparan sulfate binding. Binding of HIV to the CD4 surface receptor is known to induce conformational changes in gp120 in the viral envelope, resulting in increased affinity for heparan sulfate. This finding implies that heparan sulfate is important at a later stage of the virus-cell attachment process (254).
(i) Blocking of cell-to-cell spread.
The effect of antiviral peptides is also related to their ability to inhibit the spread of virus from one cell to a neighboring cell across tight junctions (cell-to-cell spread) or inhibition of giant cell (syncytium) formation. This is a property of the α-helical alpha and gamma interferons, which reduce the cell-to-cell spread of HSV (167). Rabbit α-defensin NP-1 has also been reported to inhibit both primary entry and cell-to-cell spread of HSV (225). Similar anti-HSV activity has been indicated for bovine lactoferricin (5, 119), while the polyphemusin analogue T22 and tachyplesin I have been demonstrated to inhibit syncytium formation in cocultures of persistently HIV type 1 (HIV-1)-infected cells (171, 177).
Blocking of viral entry by interaction with specific cellular receptors.
Antimicrobial peptides might interact directly with specific viral receptors on the host cell (42, 240), influencing viral attachment, entry, or intracellular shuttling. The most obvious example of this is the known ability of the polyphemusin analogue T22 to bind to the chemokine receptor CXCR4, which serves as a coreceptor for HIV-1 entry into T cells (173, 243). Thus, it antagonizes that subgroup of HIV strains which use this chemokine receptor but not those that use CCR5 (263).
Recently a new HSV entry receptor was described (196); it is effectively blocked by binding of an α-helical peptide in a coiled-coil formation (197). Whether this receptor is specific for HSV-1 or also allows HSV-2 entry is unknown; therefore, this cannot definitively explain differences in the antiviral effects of peptides on the two viruses. However, there is certain evidence that this receptor may be a potential target for several α-helical cationic peptides (119). Similar coiled-coil domains (196) are found in fusion proteins such as the hemagglutinin of influenza virus and gp41 of HIV. Studies have illustrated that peptides mimicking these heptad repeat domains specifically interfere with membrane fusion and viral infection (115, 228).
Blocking of viral entry by interaction with viral glycoproteins.
Antimicrobial peptide interactions with glycoproteins in the viral envelope have been proposed to influence the viral entry process. θ-Defensin (retrocyclin 2) interacts with the HSV-2 glycoprotein B with high affinity, thus protecting the cells from HSV-2 infection (270). The closely related retrocyclin-1 binds HIV gp120 with high affinity, as long as the envelope protein is glycosylated, probably resulting in an anti-HIV activity. This makes the θ-defensin the first antimicrobial peptide isolated from vertebrates with a lectin-like character (256). The polyphemusin analogue T22 has been demonstrated to inhibit fusion between the HIV envelope and the host cell membrane (177) through specific binding of the viral envelope protein gp120 and the T-cell surface protein CD4 (242).
Membrane or viral envelope interaction. (i) Viral envelope interaction.
Antimicrobial peptides are known for their ability to interact with lipid membranes, resulting in destabilization, translocation, pore formation, or lysis (46, 226). This makes the viral envelope a potential target for direct interaction. Indolicidin causes a direct inactivation of the HIV-1 particle in a temperature-sensitive fashion, indicating a membrane-mediated antiviral mechanism (208). Dermaseptin also exerts an anti-HIV activity prior to viral entry by direct interaction with the viral particle, disturbing its organization and disrupting the viral membrane (153). In contrast, dermaseptin has no such direct effect on the HSV envelope. The anti-HSV activity of dermaseptin is proposed to result from blocking of viral entry by interaction with viral or cellular surface molecules involved in the attachment/adsorption/fusion phase of HSV (16). The antibacterial selectivity of dermaseptin depends in part on the lipid composition of the microbial membrane relative to that of the host cell (52). Similar requirements could also be hypothesized for the ability of antiviral peptides to interact with and destabilize viral membranes.
Human neutrophil peptide-1 (HNP-1) is an α-defensin that neutralizes HSV-1 in a time-, temperature-, and pH-dependent manner. Neutralization of HSV-1 is also antagonized by serum or serum albumin (45). Preincubation of HSV-2 and HNP-1 prior to infection has been shown to reduce infection by >98%. In contrast, pretreatment of host cells with HNP-1 had no obvious effect on the anti-HSV activity. Although the peptide did not compete with viral envelope proteins for binding to cellular heparan sulfate, it still prevented viral entry (225).
A concentration-, time-, and temperature-dependent inactivation of vesicular stomatitis virus (VSV) is observed when the virus is incubated with tachyplesin-1 or its isopeptides prior to infection. Electron micrographs of the tachyplesin-1-treated VSV particle showed naked and damaged virions, confirming the direct effect of peptides on the viral envelope. Similar but weaker inactivation has been observed for influenza A virus (IAV) (type H1N1), while HSV-1, HSV-2, adenovirus 1, reovirus 2, and poliovirus 1 were resistant (174).
Neither cecropin, magainin, nor bovine or human lactoferricin possesses the ability to directly inactivate HSV when mixed together prior to infection (3, 5). Using electron microscopy, it has been demonstrated that bovine lactoferricin did not interact directly with the HSV particle, indicating that interactions with HSV glycoproteins do not occur (H. Jenssen, unpublished results).
(ii) Cellular membrane interaction.
Host cell membranes are involved in several stages of viral interaction, and due to the ability of peptides to interact with and permeabilize membranes, this must be considered as a potential target. Similar permeabilization of the host cell membrane appears to occur (139), and the resulting alteration of host membranes could affect the efficiency of viral entry. An eight-residue cyclic dl-α-peptide has been shown to specifically prevent the lowering of pH in endocytic vesicles, thus arresting the entry of adenovirus particles by this pathway and abrogating the infection without having an apparent effect on host cell viability (102). This effect has been hypothesized to be a result of the peptide's membrane permeabilization properties. Similar effects could be anticipated for other membrane-acting antimicrobial peptides.
Intracellular targets and host cell stimulation.
It is known that antimicrobial host defense peptides such as PR39 and LL-37 are able to cross lipid membranes, including the plasma and nuclear membranes of host cells, while others are constitutively located as precursors inside host cell vacuoles (5, 93, 139). Cellular internalization of these antimicrobial peptides can result in gene/protein stimulation, influencing host cell antiviral mechanisms (26), or might block viral gene/protein expression (255).
The effect of antimicrobial peptides has also been demonstrated to be crucially dependent on the experimental conditions. Salt may influence the structure of the peptides and their association with anionic cell molecules, thus affecting their antimicrobial activity (117); e.g., both the antibacterial and antifungal activities of cathelicidin and the antibacterial activity of β-defensin are salt dependent (12, 230). Similarly, the antiviral mode of action of α-defensin has been demonstrated to be serum dependent (37). This indicates that the peptide's action in vivo may be dependent on the physiological milieu at the site of infection. Transmission electron microscopy studies have revealed that human and bovine lactoferricins can translocate intracellularly (5). Bovine lactoferricin was able to enter both chondroitin sulfate- and heparan sulfate-deficient cells in an energy-independent manner (5). This mechanism was described earlier (66, 67, 239), and it appears that the arginine content of the peptides is an important factor, as it is a known feature of nuclear localization signals. As human lactoferricin has multiple Arg residues (195), this probably contributes to the shuttling of the peptide into the nucleus, where it can bind DNA. Consistent with this, the antiviral peptides LL-37 and indolicidin both can act as nuclear localization signals to translocate antisense nucleic acids (215).
Because of the known ability of peptides to interact with DNA (104, 123, 187, 232), one might speculate that they can directly influence viral nucleic acid synthesis, as shown for polyphemusin T22 and lactoferrin. Conversely, peptides are known to have immunomodulatory activities which include the upregulation of interferons and chemokines (24, 27, 37, 219), and thus peptides might exert their antiviral activities in part by stimulating the antiviral immune system of the host cell.
Direct effects of peptides on the intracellular steps of viral infection have been demonstrated. For example, cecropin A has been shown to inhibit Junin virus multiplication by reduction of its protein synthesis under conditions where the synthesis of host cell proteins remains unaffected (3). Melittin and cecropin A are also able to inhibit cell-associated production of HIV-1 by suppressing HIV-1 gene expression (255). Early steps in the HIV-1 replication cycle are inhibited by protegrin-1 (236), while HIV-1 integrase is effectively inhibited by indolicidin (134). Retrocyclin has been demonstrated to inhibit proviral DNA formation and protect immortalized and primary human CD4+ lymphocytes from in vitro HIV-1 infection (42). Transport of HSV-2 tegument protein VP16 to the cell nucleus and expression of ICP4 are effectively blocked in the presence of rabbit α-defensin (NP-1) (225). In addition, the human α-defensin-1 has demonstrated a direct inactivation of HIV-1 in the absence of serum, an effect that is abolished by the presence of serum. Conversely, in the presence of serum the peptide inhibits HIV-1 replication, partly by interfering with host cell protein kinase C signaling (37).
ANTIBACTERIAL ACTIVITY
By far the best-studied class of cationic antimicrobial peptides are those with antibacterial activity (60). It is well understood that regardless of their actual target of action, all antibacterial cationic peptides must interact with the bacterial cytoplasmic membrane (92). The driving physical forces behind antibacterial activity have been defined in detail (see references 46, 91, and 92 for overviews) and include net positive charge (enhancing interaction with anionic lipids and other bacterial targets), hydrophobicity (required for membrane insertion and often driven by this process), and flexibility (permitting the peptide to transition from its solution conformation to its membrane-interacting conformation). Each of these characteristics can vary substantially over a particular range but are essential for the function of the peptides as antimicrobial agents and allow them to interact with bacterial membranes, which is critical to their exertion of antimicrobial effects.
Structural Requirements for Antibacterial Peptides
As mentioned above, cationic antimicrobial peptides are generally categorized into four structural classes, i.e., α-helical, β-sheet, loop, or extended structures (21, 87); however, there are many peptides that do not fit into this simplified classification scheme (Fig. 1; Table 3). Many bacterially produced peptides, for instance, have two domains, one of which is α-helical in nature while the other has a β structure (251). For many peptides these secondary structures are observed only when the peptides interact with membranes; e.g., bovine neutrophil indolicidin is unstructured in an aqueous environment but adopts a boat-like conformation when interacting with membranes and membrane mimetics such as sodium dodecyl sulfate and dodecyl phosphocholine (212) (Fig. 1). The plasticity of the secondary structure of indolicidin has been suggested to permit different interactions with different molecules, including DNA and membranes (104).
TABLE 3.
Selected examples of natural antibacterial peptides
| Peptide | Structure | Source(s) | Proposed antibacterial mechanism | Reference(s) |
|---|---|---|---|---|
| Magainin | α-Helix | Frog | Permeabilizes bacterial membrane | 161, 273 |
| Cecropin A | α-Helix | Silk moth | Membrane destabilizing | 73, 106 |
| Mellitin | α-Helix | Bee | Membrane destabilizing | 32, 63 |
| LL-37 | α-Helix | Human | Membrane permeabilization; strongly salt antagonized | 12, 172, 176 |
| Buforin II | α-Helix/extended | Toad | Binding of nucleic acid | 187, 188 |
| α/β-Defensins | β-Sheet | Mammals, analogues in insects and fungi | Many are strongly salt antagonized; cell membrane and intracellular targets, inhibits macromolecular synthesis | 108, 147, 262 |
| Protegrin | β-Sheet | Human, porcine | Very potent, membrane permeabilization | 95 |
| Polyphemusin | β-Sheet | Horseshoe crab | Very potent, translocates into cells | 202, 280 |
| Indolicidin | Extended | Bovine | Inhibits macromolecular synthesis, Ca2+-calmodulin interaction | 61, 104, 227 |
| PR-39 | Extended | Porcine | Inhibits DNA/RNA/protein synthesis, no pore formation | 22 |
One approach to increase the antibacterial activity of cationic peptides has been to alter their flexible secondary structures. By changing the membrane-associated shape of indolicidin so that the N and C termini were drawn closer together, the activity against gram-negative bacteria was increased. This shape could also be stabilized by adding a cysteine residue to each end and creating a disulfide bridge (213). Conversely, a synthetic indolicidin analogue was made by introducing a covalent cross-link between Trp6 and Trp9 (183). Both changes resulted in decreased protease sensitivity, a strong potential asset in vivo, but did not inhibit antimicrobial activity. Similar attempts to stabilize specific structural elements have been made with a cecropin-melittin hybrid peptide, in which the α-helical structure in solution was stabilized by the introduction of a covalent lactam bond between two residues four amino acids apart, resulting in improved activity of some constructs while decreasing the activity of others (103). Stabilization of the helical structure in cecropin A has similarly demonstrated the importance of this structural domain in antibacterial activity against E. coli (68). Alternatively, introduction of a disulfide bond within the C-terminal α-helix of sakacin P to increase the amount of α-helical structure led to a broadening of the spectrum of activity (251). Thus, preconditioning peptides to adopt structures related to their final membrane-associated ones can occasionally be advantageous while giving rise to other assets such as protease stability.
The antibacterial activity of cationic peptides can also be modulated through alteration of the peptide's hydrophobicity or net charge. Studies have demonstrated that high levels of hydrophobicity can decrease selectivity between the desired bacterial targets and host cells (136, 275). Similarly, incorporation of charged residues above a certain maximum (varying with each peptide) does not lead to an increase in activity (46). Thus, this balance of charge and hydrophobicity can be delicate and must be empirically determined for each series of peptides.
Consequently, the inclusion of a particular peptide into a structural group does not give an indication as to its mode of action or its spectrum of activity. In fact, some peptides with very similar secondary structures have quite opposite characteristics with respect to antibacterial activity (Table 3). The α-helical melittin from bees, which is thought to perforate the membranes of both prokaryotic and eukaryotic organisms, falls within the α-helical structural class. Conversely, the α-helical toad peptide buforin translocates into cells and acts on macromolecular synthesis. Studies of α-helical analogues of a cecropin-melittin hybrid peptide have revealed that even peptides that have similar secondary structures and minimal differences in the primary sequence can possess quite different antibacterial activities (64). Indeed, different peptides may be membrane permeabilizing at their minimal effective concentrations or at concentrations well above or well below these concentrations. Nonetheless, antibacterial peptides seem largely able to effect their antimicrobial activity because of their amphipathicity or amphiphilicity and because of the presence of regions within the folded structure with high concentrations of positively charged residues (202).
Mode of Action of Antibacterial Peptides
It was originally proposed that permeabilization of the bacterial cell membrane was the sole mode of action of antibacterial peptides. There is an increasing body of evidence, however, that indicates that some antimicrobial peptides exert their effects through alternative modes of action or that they may in fact act upon multiple bacterial cell targets. Regardless of their precise mode of action, the activities of antibacterial peptides are almost universally dependent upon interaction with the bacterial cell membrane (92). The first step in this interaction is the initial attraction between the peptide and the target cell, which is thought to occur through electrostatic bonding between the cationic peptide and negatively charged components present on the outer bacterial envelope, such as phosphate groups within the lipopolysaccharides of gram-negative bacteria or lipoteichoic acids present on the surfaces of gram-positive bacteria. In the case of gram-negative bacteria, peptides are inserted into the outer membrane structure in a process driven by hydrophobic interactions and possibly involving prefolding of the peptides into a membrane-associated structure; this leads to a disturbance of the outer membrane structure and permeabilizes this membrane to other peptide molecules in a process termed self-promoted uptake. The net result is that the peptides arrive at the cytoplasmic membrane, where they enter the interfacial region of the membrane (the interface between the hydrophilic and hydrophobic portions of the membrane) in a process driven by electrostatic and hydrophobic interactions. The higher proportion of negatively charged lipids on the surface monolayer of the bacterial cytoplasmic membrane plays an important role in the selectivity of antimicrobial peptides for bacterial cells over eukaryotic cells, in which uncharged lipids predominate at the host cell surface. The events that occur at the membrane surface are the subject of considerable debate, and several prominent models (called variously the barrel-stave, carpet, detergent, toroidal pore, and aggregate models) have been proposed. Each of these indicates a different type of intermediate that can lead to one of three types of events: formation of a transient channel, micellarization or dissolution of the membrane, or translocation across the membrane. As a result, the peptide can permeabilize the membrane and/or translocate across the membrane and into the cytoplasm without causing major membrane disruption. Hence, the modes of action of antibacterial peptides can be broadly categorized as either membrane acting or non-membrane acting. While most cationic antibacterial peptides studied so far have been characterized as membrane permeabilizing, it should be noted that virtually any cationic amphiphilic peptide will cause membrane perturbation in model systems if a high enough concentration is applied, and there are few examples of studies with intact bacteria (193, 279). Thus, it is possible that such conclusions reflect the extensive studies of model membrane systems in which alternative mechanisms of action would not be detected and/or the use of media which do not reflect physiologic salt or pH conditions, leading to an overestimation of the permeabilizing activity of the peptide in question. Studies conducted with whole bacterial cells have revealed that several antibacterial peptides translocate into cells and do not cause membrane permeabilization but rather mediate bacterial cell death by targeting essential intracellular processes. Below is an overview of the current models for antibacterial peptide-mediated killing through either membrane-permeabilizing or non-membrane-permeabilizing mechanisms.
Membrane-permeabilizing peptides.
Several different models have been proposed to explain how, following initial attachment, antibacterial peptides insert into the bacterial membrane to form transmembrane pores which result in membrane permeabilization (Fig. 2). The amphipathic nature of antimicrobial peptides is a key characteristic for this process, as hydrophobic regions are necessary to interact directly with the lipid components of the membrane, while hydrophilic regions either interact with the phospholipid head groups or face the lumen of the pore. Generally, these models can explain the pore-forming ability of α-helical antibacterial peptides; however, the mechanisms utilized by β-sheet peptides such as defensins have not been as well studied. While β-sheet antimicrobial peptides can adopt amphipathic folds, there is little experimental evidence to indicate which of the following models is applicable to defensins.
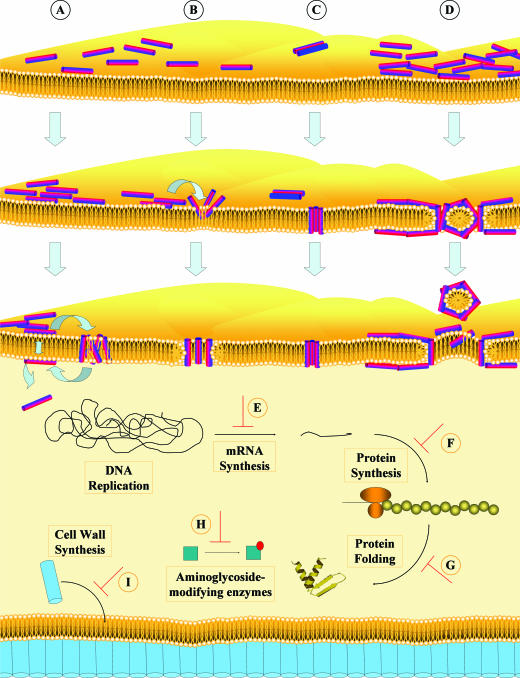
FIG. 2.
Mechanisms of action of antibacterial peptides. The bacterial membrane is represented as a yellow lipid bilayer with the peptides shown as cylinders, where the hydrophilic regions are colored red and the hydrophobic regions are blue. Cell wall-associated peptidoglycan molecules are depicted as purple cylinders. Models to explain mechanisms of membrane permeabilization are indicated (A to D). In the “aggregate” model (A), peptides reorient to span the membrane as an aggregate with micelle-like complexes of peptides and lipids, but without adopting any particular orientation. The “toroidal pore” model (B) proposes that peptides insert perpendicular to the plane of the bilayer, with the hydrophilic regions of the peptides associating with the phospholipid head groups while the hydrophobic regions associate with the lipid core. In this process, the membrane also curves inward such that the bilayer also lines the pore. In the “barrel-stave” model (C), the peptides insert in a perpendicular orientation to the plane of the bilayer, forming the “staves” in a “barrel”-shaped cluster, with the hydrophilic regions of the peptides facing the lumen of the pore and the hydrophobic regions interacting with the lipid bilayer. The “carpet” model (D) proposes that peptides aggregate parallel to the lipid bilayer, coating local areas in a “carpet”-like fashion. At a given threshold concentration, this is thought to result in a detergent-like activity, causing formation of micelles and membrane pores. The mechanisms of action of peptides which do not act by permeabilizing the bacterial membrane are depicted in panels E to I. The antimicrobial peptides buforin II, pleurocidin, and dermaseptin have all been shown to inhibit DNA and RNA synthesis at their MICs without destabilizing the membrane (E). Protein synthesis is another macromolecular target for antibacterial peptides such as indolicidin and PR-39, which have been shown to decrease the rate of protein synthesis in target bacterial cells (F). Several antibacterial peptides have been shown to act on other intracellular target processes, such as enzymatic activity. The ATPase activity of DnaK, an enzyme involved in chaperone-assisted protein folding, is targeted by pyrrhocidin (G), while inhibition of enzymes involved in the modification of aminoglycosides has also been demonstrated (H). Antimicrobial peptides can also target the formation of structural components, such as the cell wall (I). Lantibiotics such as nisin and mersacidin can bind to and inhibit, respectively, the transglycosylation of lipid II, which is necessary for the synthesis of peptidoglycan.
In all models peptides first interact preferentially with the negatively charged lipid head groups at the membrane surface, adopting an orientation parallel to (i.e., in the plane of) the membrane at the membrane interface. A mechanism, known as the aggregate model, with some similarity to the toroidal pore model, has been proposed by our laboratory (259) (Fig. 2A). This model has a less formal nature and can explain both membrane permeabilization, whereby informal channels with a variety of sizes and lifetimes form (259), and translocation across the bilayer, which is known to occur for several peptides (203). In this model peptides reorient to span the membrane as an aggregate with micelle-like complexes of peptides and lipids (as also suggested by Matsuzaki et al. [161, 162] and by the toroidal pore model), but in this model the peptides adopt no particular orientation. Predictions of this model are that the lack of a formal channel structure will lead to channels that vary dramatically in character, that the peptide has the capacity to translocate across the bilayer as the aggregates collapse, and that the membrane will undergo negative curvature strain, all of which have been shown for the horseshoe crab peptide polyphemusin. In the toroidal pore model (Fig. 2B), aggregates of peptides insert themselves in an orientation perpendicular to the membrane to form a pore, with the membrane also curving inward to form a hole with the head groups facing towards the center of the pore, and the peptides line this hole. One prediction of this model is that the membrane will exhibit positive curvature strain (due to the membrane bending around to form the toroidal hole with the peptides within), although the formation of a formal toroidal channel has not actually been demonstrated. Examples of antimicrobial peptides that are proposed to form this type of transmembrane pore include the magainins, melittin, and LL-37 (85, 98, 162, 267). In the barrel-stave model (57) (Fig. 2C), peptides reorient to become the “staves” in a “barrel”-shaped cluster which orients perpendicular to the plane of the membrane. The hydrophobic regions of each peptide in the cluster are associated with the lipid core, while the hydrophilic regions are facing the lumen of the newly formed transmembrane pore. This model predicts that there will be a consistent channel size (or sizes, also called substates), and this is not true for most peptides, although experimental evidence supports this mechanism of membrane permeabilization for the fungus-derived (noncationic) antimicrobial peptide alamethicin (94, 233) and for the cyclic decameric cationic peptide gramicidin S (279).
In contrast to the barrel-stave and toroidal pore models, the carpet model proposes that aggregates of peptide align parallel to the lipid bilayer, coating local areas in a carpet-like fashion (200) (Fig. 2D). At sufficiently high concentration, this is thought to have a detergent-like activity (causing patches of the membrane to break up into micelles), causing local disturbances in membrane stability which can lead to the formation of holes in the membrane. Many cationic antimicrobial peptides will do this at high enough concentrations due to their amphipathic character; however, there is very limited evidence demonstrating that most peptides cause membrane dissolution at the minimal effective concentrations in vivo (or, in many studied cases, in vitro; for example, Zhang et al. [279] examined nine representative peptides and demonstrated that most of them were able to cause membrane flip-flop at far lower concentrations than those at which they could cause calcein release). Based on mechanistic studies, however, this mechanism has been proposed for ovispirin, a rationally designed antibacterial peptide based on the sheep cathelicidin Smap29 (264). It is important to recognize that each of these models may have validity under different circumstances, and examination of a broad range of peptides with different sizes and structures has indicated that they leave quite different signatures of membrane interaction (280), while differential scanning calorimetry studies on LL-37 (98) and polyphemusin (203) have shown opposite effects on membrane curvature.
Peptides that do not act by membrane permeabilization.
All peptides must interact with the cytoplasmic membrane, if only to get to their site of action. It is now well established that several antimicrobial peptides do not cause membrane permeabilization at the minimal effective concentration yet still result in bacterial death. A growing number of peptides have been shown to translocate across the membrane and accumulate intracellularly, where they target a variety of essential cellular processes to mediate cell killing. Novel modes of action that have recently been demonstrated include inhibition of nucleic acid synthesis, protein synthesis, enzymatic activity, and cell wall synthesis (28) (Fig. 2).
The frog antimicrobial peptide buforin II translocates across the bacterial membrane without causing permeabilization and binds to both DNA and RNA within the cytoplasm of E. coli (187). Similarly, α-helical peptides such as derivatives of pleurocidin, a fish-derived antimicrobial peptide, and dermaseptin, isolated from frog skin, cause inhibition of DNA and RNA synthesis at their MICs without destabilizing the membrane of E. coli cells (193, 238) (Fig. 2E). Inhibition of nucleic acid synthesis has also been demonstrated for antimicrobial peptides from different structural classes, such as the β-sheet human defensin, HNP-1 (147), and the extended-structure bovine peptide indolicidin (238). Additionally, some of these peptides have been shown to interfere with protein synthesis. Pleurocidin and dermaseptin can block tritiated leucine uptake in E. coli, and PR-39- and indolicidin-treated cells also exhibit reduced rates of protein synthesis (22, 65, 193, 238) (Fig. 2F).
Inhibition of cellular enzymatic activity by proline-rich insect antimicrobial peptides has also been observed. Pyrrhocidin enters the target cell and binds to DnaK, a heat shock protein that is involved in chaperone-assisted protein folding. Specifically, the peptide inhibits the ATPase activity of DnaK, preventing protein folding, which results in the accumulation of misfolded proteins and cell death (133, 184) (Fig. 2G).
Antimicrobial peptides can also target the formation of structural components, such as the cell wall (Fig. 2I). The bacterially produced lantibiotic mersacidin interferes with transglycosylation of lipid II, a necessary step in the synthesis of peptidoglycan (29). Nisin, another lantibiotic, can also bind to lipid II, thus inhibiting cell wall synthesis in addition to its pore-forming activity. Interestingly, this is the same biosynthetic process that is targeted by the antibiotic vancomycin; however, mersacidin and nisin are thought to act by interacting with distinct molecular moieties within lipid II, explaining why these peptides are still active against vancomycin-resistant bacteria (31, 135).
It is likely that the mode of action of individual peptides may vary according to the particular bacterial target cell, the concentration at which they are assayed, and the physical properties of the interacting membrane. It is also likely that in the context of infection, antimicrobial peptides may use more than one mechanism of action, such as destabilization of the cell membrane combined with inhibition of one or more intracellular targets. We recently proposed a “multitarget” mechanism (201), which hypothesizes that highly charged cationic peptides can bind to anionic molecules within the cytoplasm, such as nucleic acids or enzymes with ionic surfaces, thus interfering with processes in which these molecules are involved. An example is that of aminoglycoside-modifying enzymes, which contain an anionic binding pocket. It was recently demonstrated that cationic peptides of various structural classes can bind and specifically inhibit the activity of these enzymes (20) (Fig. 2H). This high degree of complexity of the mechanism is almost certainly the cause of the observation that it is extremely difficult to select cationic-peptide-resistant mutants (235, 277).
ANTIFUNGAL ACTIVITY
Our knowledge of antifungal peptides has accelerated in recent years. Their mode of action was first described as involving either fungal cell lysis or interference with fungal cell wall synthesis (51). However, as the numbers of known antifungal peptides increase, new modes of action are being identified (Table 4). It is intriguing to note that peptides with primarily antifungal activity, such as many of those isolated from plants, tend to be relatively rich in polar and neutral amino acids, suggesting a unique structure-activity relationship (155).
TABLE 4.
Selected examples of antifungal peptides
| Peptide | Structure | Source(s) | Target organism(s) | Antifungal mode of action | Reference(s) |
|---|---|---|---|---|---|
| Melittin | α-Helix | Bee | C. albicans | Permeabilization | 141 |
| Magainin | α-Helix | Frog | C. albicans | Lysis | 250, 272 |
| Cecropin | α-Helix | Insect | Aspergillus fumigatus | Binds ergosterol/cholesterol in the membrane | 50, 51 |
| PMAP-23 | α-Helix | Porcine | C. albicans | Permeabilization | 141, 189 |
| Brevinin-1 | α-Helix | Frog | Batrachochytrium dendrobatidis | Lysis | 210 |
| Defensin | β-Sheet | Mammals | C. albicans | Membrane permeabilization and/or lysis | 148, 194 |
| Pn-AMP 1 | β-Sheet | Plant | C. albicans, S. cerevisiae | Depolymerase actin cytoskeleton | 83, 131 |
| Histatin | Histidine rich | Human, primate | C. albicans | Targets mitochondria | 96, 124 |
| Tenecin-3 | Extended turn | Insect | C. albicans, A. fumigatus | Unknown target with cytoplasmic localization | 125, 146 |
| Indolicidin | Extended | Bovine | T. beigelii | Disrupts structure of cell membrane | 142 |
Structural Requirements for Antifungal Peptides
Viejo-Diaz et al. (253) identified two novel human lactoferrin-derived peptides with different anti-Candida activities but quite high sequence homology (253). Alignments of one of these peptides with the sequence of brevinin-1Sa also indicated high homology (252). However, other studies have shown that antifungal peptides vary substantially in sequence and structure, and peptides as structurally diverse as eucommia (with a five-disulfide motif) (105), the α-helical P18 (140), and the extended peptide indolicidin (142), as well as plant defensins and a coleopteran β-sheet peptide from Acrocinus longimanus (13), have all shown antifungal activity. Thus, like for antibacterial peptides, there are no obvious conserved structural domains that give rise to antifungal activity.
Modification of ineffective antimicrobial peptides has revealed that relatively modest changes often result in antifungal activity. For example, conjugation of undecanoic acid or palmitic acid to magainin resulted in analogue peptides that had gained potent activity against both yeast and opportunistic fungal infections (9). The fusions of parts of magainin 2 and cecropin A to form the hybrid peptide P18 resulted in a peptide with potent fungicidal activity against pathogenic Candida albicans, Trichosporon beigelii, Aspergillus flavus, and Fusarium oxyspovrum (140). Studies with the 18-residue pig peptide protegrin, which has both antibacterial and antifungal activities, demonstrated that the antibacterial activity could be retained in a 12-residue deletion peptide but only two residues could be deleted for the potent antifungal properties of this peptide to be retained (41). Other studies with synthetic 12-mers based on the bovine peptide bactenecin indicate that different substitutions were required for optimizing antibacterial and antifungal properties (100).
Although no conserved sequences are evident for the antifungal peptides, several have been demonstrated to possess specific biochemical characteristics, such as chitin (69, 105) or heparin (7, 223) binding abilities. Structure-activity relationship studies on three synthetic bovine lactoferricin (amino acids 17 to 30)-derived peptides revealed a significant positive correlation between the pI values of peptides and their candidicidal activity (181). Another study also showed a direct correlation between the ability of peptides to form complexes with lipid mixtures and their antifungal activity (152).
Mode of Action of Antifungal Peptides
Lehrer et al. demonstrated quite early that the rabbit α-defensin NP-2 resulted in permeabilization of C. albicans (148, 194). Moerman et al. subsequently demonstrated that α-helical pore-forming peptides isolated from scorpion venom had antifungal activity (168). Using the organic compound SYTOX green, which penetrates only cells with leaky plasma membranes and fluoresces upon interaction with nucleic acids, it was demonstrated that the peptide permeabilizes fungi (211). Indeed, a good correlation could be demonstrated between the inhibition of growth of Neurospora crassa and enhancement of SYTOX green fluorescence (168). Lee et al. used scanning electron microscopy observations to demonstrate morphological changes in response to the potent permeabilizing peptides pig myeloid antimicrobial peptide (PMAP-23) and melittin (143). They also presented data indicating that indolicidin exerts its fungicidal activity by disrupting the structure of the fungal cell membrane, in a salt-dependent and energy-independent fashion, via direct interactions with the lipid bilayer (142). This contrasts to the situation for bacteria, in which indolicidin, although membrane active, appears to penetrate cells and act on macromolecular synthesis (61, 238). Bovine lactoferricin and the hybrid peptide of Helicobacter pylori ribosomal protein L1 and magainin-2, HP(2-9)-MA(1-12), have both been shown to result in profound ultrastructural damage to the cell wall of C. albicans (17, 191).
The mechanism of action of certain antifungal peptides is still a matter of debate. The formation of reactive oxygen species has been suggested to be the crucial step in the fungicidal mechanism of a number of antimicrobial peptides (179), including histatin 5 and lactoferrin-derived peptides (97, 154). Conversely, Veerman et al. have concluded that reactive oxygen species do not play a role in the histatin 5-mediated killing of C. albicans (252). Helmerhorst et al. (96) found that exposure to the cationic peptide histatin 5 caused a depletion of mitochondria in C. albicans. Histatins from saliva of humans and some other higher primates bind to a receptor on the fungal cell membrane and enter the cytoplasm, where they target the mitochondrion (124). Thus, it is reasonable to hypothesize that antimicrobial peptides may interact with mitochondria in a manner very similar to some of their actions on bacteria, as suggested by the studies of Helmerhorst et al. (96).
The glycine-rich peptide tenecin-3, both native and recombinant, has been demonstrated to possess candicidal activity (126). The peptide is able to quickly enter the cytoplasm of C. albicans in an energy-dependent mechanism influenced by the metabolic status of the target cells and the ionic environment (125). It does not result in membrane permeabilization or depolarization (125), although pegylated tenecin-3 peptide can result in K+ leakage from C. albicans (146), possibly explaining its improved antifungal activity. The antifungal mechanism of native tenecin-3 is unknown, but the loss of cell viability occurs after the peptide enters the host cell cytoplasm (125).
Pn-AMP1, is a small cysteine-rich plant peptide that causes depolymerization of the actin cytoskeleton in Saccharomyces cerevisiae and C. albicans. It was suggested that cell wall proteins worked as determinants of Pn-AMP1 resistance, while its ability to induce actin depolymerization was important for its antifungal activity (131).
ANTIPARASITIC ACTIVITY
Magainin 2 was one of the first antimicrobial host defense peptides demonstrated to display antiprotozoan activity, leading to swelling and eventual bursting of Paramecium caudatum (272). More recently an acylated synthetic antimicrobial peptide, Oct-CA(1-7)-M(2-9), has been shown to be both safe and effective for treating naturally acquired canine leishmaniasis (2), which is caused by the parasite Leishmania, which is also an important cause of morbidity and mortality in humans (48).
The antinematodal effect of the porcine cathelicidin PMAP-23 has been demonstrated against both the eggs and worms of Caenorhabditis elegans. Studies have indicated that this effect is exerted through disruption of the cell membrane via pore formation or via direct interaction with the lipid bilayers (190), resembling the antifungal mode of action for PMAP-23 (143).
Several antimicrobial peptides possess an antiprotozoan mode of action that indicates parallels with their antibacterial, antiviral, or antifungal modes of action. Analogues of mussel defensins have been demonstrated to efficiently kill Leishmania major and Trypanosoma brucei in a temperature-, time-, and dose-dependent manner. These peptides were found to interact with the external epithelium of T. brucei. However, structure/activity relationship studies indicated that the antiprotozoan and antiviral activities were mediated by different mechanisms (209). Analogous studies on magainin 2 analogues revealed that short stretches of hydrophobic amino acids were important for leishmanicidal activity (82). It seems likely that antiprotozoan activity may be dependent on peptide motifs fundamentally different from those required for bacterial, viral, and fungal activities.
DEVELOPMENT OF ANTIMICROBIAL PEPTIDES FOR CLINICAL APPLICATIONS
As the problem of emergence of bacterial resistance to current antibiotic drugs continues to grow, there has been considerable interest in the development of antimicrobial peptides as a novel therapeutic approach to treat infections. To date, several antimicrobial peptides have been developed and entered into clinical trials, and there are also peptides that are currently in the preclinical development stage, which are discussed later in this section. Antimicrobial-peptide-based therapies are attractive candidates as alternative antibiotic treatments, since they offer several potential advantages over currently used classes of drugs. First, they represent a naturally occurring means of combating pathogenic challenge by rapid microbicidal activity. In addition, since their mode of action, for the most part, exploits general but fundamental structural characteristics such as the bacterial cell membrane and in many cases they may have multiple targets within cells, the likelihood of the emergence of resistance is thought to be considerably reduced compared with that for many current antibiotics, which have more specific molecular targets. This prediction has been substantiated in several studies in which, despite serial passage in subinhibitory concentrations of peptide, resistant bacteria did not arise (74, 235, 277). The potential to develop antimicrobial peptides with broad-spectrum characteristics is also especially attractive in that multiple pathogens could potentially be targeted with one treatment possessing antibacterial, antiviral, or antifungal activity. Novel antimicrobial treatments could also potentially be used in conjunction with existing drugs as part of a “combination therapy” to create an additive or synergistic effect. The multidrug target approach has been successful in improving the efficacy of and reducing emergence of resistance to HIV therapies (49). Thus, in combination, peptides have the potential to ultimately reduce the rate of emergence of resistant microbes, since selective pressure is deviated away from one specific molecular target. The potential for additional antiseptic activity (79) also is a defined asset; for example, researchers at Migenix (Vancouver, British Columbia, Canada) have described in press releases that they observed anti-inflammatory activity in their phase II clinical trial of an indolicidin peptide against acne.
Current knowledge regarding the relationship between peptide structure and function as well as the mechanism of action can be applied in the semirational design of antimicrobial peptide variants with enhanced microbicidal activities or altered target pathogen specificities. However, progress has been somewhat slow using these methods, and possibly less than a few hundred peptides have been evaluated to date for clinical potential, a number that is quite small compared to those in many antibiotic development programs. Alternatively, we recently introduced a random screening method involving peptide arrays synthesized on cellulose sheets combined with a highly sensitive luminescence-based antimicrobial assay to permit hundreds of peptides to be synthesized and screened for activity (100).
Despite these promising attributes and recent successes in demonstrating the efficacy of antimicrobial peptides in animal models of disease which have spurred current development initiatives, there are considerable challenges in the clinical application of candidate peptide therapies. Among these are issues such as the susceptibility of peptides to proteolytic degradation in vivo, which would negatively affect pharmacokinetics and thus hinder the use of antimicrobial peptides for systemic applications; a lack of information regarding the potential toxicities of relatively large and highly charged peptides; doubts about the ability to achieve high microbicidal activity under physiologic salt, pH, and serum conditions; and the comparatively high costs associated with peptide development and manufacture.
There has been limited success with those antimicrobial peptides that have entered into clinical trials, and as yet, none have been granted FDA approval for clinical use. The most advanced are currently two indolicidin-based antimicrobial peptide variants, MBI-226 and MX-594AN, that have been developed by Migenix (formerly Micrologix) (Vancouver, British Columbia, Canada) for the treatment of catheter-related infections and acne, respectively. MBI-226 (Omiganin) showed promising results for secondary end points in phase IIIa trials, resulting in a 40% reduction of catheter colonization and a 50% decrease in tunnel infections, although its primary end point of a reduced rate of infections was not achieved (a 15% reduction was not statistically significant due to the small number of patients who contracted infections in this trial). After consultation with the FDA, Migenix was approved to repeat this phase III trial using the statistically significant effects as the primary end points. In collaboration with Cadence Pharmaceuticals Inc., phase IIIb trials were initiated in the United States in August 2005 and in Europe in February 2006, using the renamed CPI-226 as a gel-based treatment for catheter infections. MX-594AN significantly reduced acne lesions compared with vehicle controls in a phase IIb trial in 2003 and was licensed to Cutanea Life Sciences in late 2005.
AM-Pharma Holding BV have announced that they have completed phase I clinical trials with an 11-mer peptide from the N terminus of human lactoferrin, hLF1-11, that has proved efficacious in animal models of osteomyelitis (60) and other bacterial infections (180). AM-Pharma is apparently targeting the prevention of infections in patients undergoing stem cell, especially allogeneic, transplantation, who have high postoperative rates of severe infection and mortality. Subsequently, they have suggested that they will develop this compound as a systemic antifungal.
Other trials have not been as successful. Pexiganan, a broad-spectrum synthetic analogue of the African frog peptide magainin, was developed for the treatment of diabetic foot ulcers by Genaera (Plymouth, PA) (74, 272) after initially failing in a phase III trial for impetigo, in part due to the high rate (75%) of spontaneous resolution of disease in controls. In trials of efficacy against the polymicrobic burden associated with diabetic foot ulcers, Pexiganan resulted in either clearance of the pathogen or significant improvement in over 90% of patients, with a good toxicity profile. Despite these encouraging results, Pexiganan was denied approval for use in 1999 on the basis of insufficient evidence of efficacy, with the FDA requesting a placebo-controlled trial to establish that both Pexiganan and oral therapies such as ofloxacin conferred additional healing benefits over good wound care alone (137, 169). Iseganan was originally developed by Intrabiotics Pharmaceuticals Inc. (Mountain View, CA) as a mouth rinse to prevent polymicrobial infection associated with oral mucositis in patients receiving chemotherapy. Based on the pig peptide protegrin (186), Iseganan possesses broad-spectrum activity in vitro against bacteria and fungi; however, in clinical trials it failed to prevent or reduce oral mucositis compared with a placebo (78, 248). Intrabiotics subsequently withdrew Iseganan for this indication, but it was entered into trials as an aerosolized treatment for ventilator-associated pneumonia. Unfortunately, these trials were also halted after increased rates of pneumonia and mortality were observed in patients receiving the peptide. Another antimicrobial-peptide-containing mouth wash, indicated for the treatment of oral candidiasis, was developed using a variant of histatins, which are naturally occurring cationic peptides in saliva (124). This was recently licensed to the Vancouver, Canada, company Pacgen. P113 (Demegen, Pittsburgh, PA) has successfully completed phase II clinical trials in HIV patients, and another variant, P113D, was under consideration as an inhalation treatment for Pseudomonas aeruginosa infections in cystic fibrosis patients. However, we have been unable to confirm the antipseudomonal activity of P113D in 10% sputum from cystic fibrosis patients or in animal models of lung infection (277).
Although none of the peptides in the examples described above have been licensed for clinical use so far, the encouraging performance of certain antimicrobial peptide treatments indicates some cause for optimism, such that the development of antimicrobial peptides as a new class of drugs is still considered viable. As the field continues to mature, some of the challenges facing the development of peptide-based therapies that have been encountered so far may be addressed. Developing smaller peptides with the inclusion of protecting groups or unusual amino acids is a strategy being adopted by our laboratory (100) and by several companies to overcome the problems of costly manufacture and susceptibility to degradation (247, 268, 276). Also of note is the expansion of the field into the commercial development of peptides to treat viral infections, another area where there is presently an unmet clinical need; for example, Helix BioMedix (Bothell, WA) is currently developing broad-spectrum antiviral peptides indicated for the prevention of sexually transmitted diseases such as HIV and herpes simplex virus type 2 (278). Several antimicrobial peptides are currently undergoing preclinical development, which are described in detail by Zhang and Falla (276). Here, we highlight a small selection of particular interest.
Clearly the need for new treatments for multiresistant bacteria is urgent, and this is the focus for several companies. Ceragenix is targeting multidrug-resistant organisms such as Pseudomonas, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Staphylococcus aureus. Its squalamine-based cationic steroid antibiotics (170) share biophysical properties, such as net positive charge, amphipathicity, and selectivity for prokaryotic membranes, with human antimicrobial peptides and are effective against a broad spectrum of bacterial infections. The company plans to develop them for topical and systemic use. Helix BioMedix is developing a series of hexapeptides specifically designed to have activity in biological environments such as those high in lipids and serum. These peptides are currently in preclinical testing targeting bacterial and fungal infections for topical treatment of dermatological conditions. Novozymes (A/S Bagsvaerd, Denmark) has identified lead compounds based on Plectasin, the first defensin to be isolated from a fungus (Pseudoplectania nigrella) (175). This peptide is of particular interest since it is produced by recombinant methods and thus is relatively less expensive, has low toxicity in mice, and can be administered intravenously for the treatment of systemic infections. Efficacy has been demonstrated in both bacterial peritonitis and pneumonia models.
Some encouraging progress has been made in the field of peptide therapies in general, with the FDA approval in 2003 of enfuvirtide, a 36-amino-acid synthetic peptide homologous to the heptad repeat 2 region of the HIV envelope glycoprotein gp41. The peptide contains an acetylated N terminus and a carboxamide on the C terminus and targets multiple sites of HIV glycoproteins gp41 and gp120, thus inhibiting HIV entry (150). While it does not possess any of the typical features of an antimicrobial peptide (it is not cationic, hydrophobic, or amphipathic), having been designed on the basis of homology with the viral glycoprotein, the introduction of this new class of peptide HIV inhibitors demonstrates that problems of stability in vivo can be overcome.
Although they are not the subject of this review, the development of antimicrobial peptides with immunomodulatory properties should also be mentioned, as this is an important aspect of the development of new classes of peptide-based anti-infective drugs. As previously mentioned, many mammalian antimicrobial or host defense peptides aid in the clearance of invading pathogens not by direct killing but by stimulating the host's innate immune system. Currently, several peptides that display no direct microbicidal activity in vitro are being developed for their ability to either protect against bacterial infection or promote wound healing (24, 145).
CONCLUSION
Antimicrobial cationic host defense peptides have been demonstrated to have activity towards a wide spectrum of infectious bacterial, viral, fungal, and parasitic pathogens. Their activities differ both within and between distinct structural peptide classes, although all the peptides appear to be able to adopt some sort of cationic and amphipathic structure. Their modes of action are strongly dependent on experimental conditions and demonstrate the tremendous ability of peptides to exert a diverse range of antimicrobial effects. Diverse immunomodulatory activities of such host defense peptides are the most recent characterized property and will provide an additional stimulus to consideration of these molecules as a new class of therapeutic agents.
Acknowledgments
Funding from the Canadian Institutes of Health Research and the Applied Food and Material Network for our antimicrobial peptide research is gratefully acknowledged. R.E.W.H. holds a Canada Research Chair.
REFERENCES
- 1.Aboudy, Y., E. Mendelson, I. Shalit, R. Bessalle, and M. Fridkin. 1994. Activity of two synthetic amphiphilic peptides and magainin-2 against herpes simplex virus types 1 and 2. Int. J. Pept. Protein Res. 43:573-582. [DOI] [PubMed] [Google Scholar]
- 2.Alberola, J., A. Rodriguez, O. Francino, X. Roura, L. Rivas, and D. Andreu. 2004. Safety and efficacy of antimicrobial peptides against naturally acquired leishmaniasis. Antimicrob. Agents Chemother. 48:641-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albiol Matanic, V. C., and V. Castilla. 2004. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int. J. Antimicrob. Agents 23:382-389. [DOI] [PubMed] [Google Scholar]
- 4.Andersen, J. H., H. Jenssen, and T. J. Gutteberg. 2003. Lactoferrin and lactoferricin inhibit herpes simplex 1 and 2 infection and exhibit synergy when combined with acyclovir. Antiviral Res. 58:209-215. [DOI] [PubMed] [Google Scholar]
- 5.Andersen, J. H., H. Jenssen, K. Sandvik, and T. J. Gutteberg. 2004. Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface. J. Med. Virol. 74:262-271. [DOI] [PubMed] [Google Scholar]
- 6.Andersen, J. H., S. A. Osbakk, L. H. Vorland, T. Traavik, and T. J. Gutteberg. 2001. Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts. Antiviral Res. 51:141-149. [DOI] [PubMed] [Google Scholar]
- 7.Andersson, E., V. Rydengard, A. Sonesson, M. Morgelin, L. Bjorck, and A. Schmidtchen. 2004. Antimicrobial activities of heparin-binding peptides. Eur. J. Biochem. 271:1219-1226. [DOI] [PubMed] [Google Scholar]
- 8.Argyris, E. G., E. Acheampong, G. Nunnari, M. Mukhtar, K. J. Williams, and R. J. Pomerantz. 2003. Human immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts. J. Virol. 77:12140-12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avrahami, D., and Y. Shai. 2003. Bestowing antifungal and antibacterial activities by lipophilic acid conjugation to d,l-amino acid-containing antimicrobial peptides: a plausible mode of action. Biochemistry 42:14946-14956. [DOI] [PubMed] [Google Scholar]
- 10.Ayabe, T., D. P. Satchell, C. L. Wilson, W. C. Parks, M. E. Selsted, and A. J. Ouellette. 2000. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1:113-118. [DOI] [PubMed] [Google Scholar]
- 11.Bachere, E., Y. Gueguen, M. Gonzalez, J. de Lorgeril, J. Garnier, and B. Romestand. 2004. Insights into the anti-microbial defense of marine invertebrates: the penaeid shrimps and the oyster Crassostrea gigas. Immunol. Rev. 198:149-168. [DOI] [PubMed] [Google Scholar]
- 12.Bals, R., X. Wang, Z. Wu, T. Freeman, V. Bafna, M. Zasloff, and J. M. Wilson. 1998. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J. Clin. Investig. 102:874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbault, F., C. Landon, M. Guenneugues, J. P. Meyer, V. Schott, J. L. Dimarcq, and F. Vovelle. 2003. Solution structure of Alo-3: a new knottin-type antifungal peptide from the insect Acrocinus longimanus. Biochemistry 42:14434-14442. [DOI] [PubMed] [Google Scholar]
- 14.Barth, H., C. Schafer, M. I. Adah, F. Zhang, R. J. Linhardt, H. Toyoda, A. Kinoshita-Toyoda, T. Toida, T. H. Van Kuppevelt, E. Depla, F. Von Weizsacker, H. E. Blum, and T. F. Baumert. 2003. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J. Biol. Chem. 278:41003-41012. [DOI] [PubMed] [Google Scholar]
- 15.Bastian, A., and H. Schafer. 2001. Human alpha-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul. Pept. 101:157-161. [DOI] [PubMed] [Google Scholar]
- 16.Belaid, A., M. Aouni, R. Khelifa, A. Trabelsi, M. Jemmali, and K. Hani. 2002. In vitro antiviral activity of dermaseptins against herpes simplex virus type 1. J. Med. Virol. 66:229-234. [DOI] [PubMed] [Google Scholar]
- 17.Bellamy, W., H. Wakabayashi, M. Takase, K. Kawase, S. Shimamura, and M. Tomita. 1993. Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med. Microbiol. Immunol. 182:97-105. [DOI] [PubMed] [Google Scholar]
- 18.Benincasa, M., B. Skerlavaj, R. Gennaro, A. Pellegrini, and M. Zanetti. 2003. In vitro and in vivo antimicrobial activity of two alpha-helical cathelicidin peptides and of their synthetic analogs. Peptides 24:1723-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernfield, M., R. Kokenyesi, M. Kato, M. T. Hinkes, J. Spring, R. L. Gallo, and E. J. Lose. 1992. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu. Rev. Cell Biol. 8:365-393. [DOI] [PubMed] [Google Scholar]
- 20.Boehr, D. D., K. A. Draker, K. Koteva, M. Bains, R. E. Hancock, and G. D. Wright. 2003. Broad-spectrum peptide inhibitors of aminoglycoside antibiotic resistance enzymes. Chem. Biol. 10:189-196. [DOI] [PubMed] [Google Scholar]
- 21.Boman, H. G. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13:61-92. [DOI] [PubMed] [Google Scholar]
- 22.Boman, H. G., B. Agerberth, and A. Boman. 1993. Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect. Immun. 61:2978-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowdish, D. M., D. J. Davidson, and R. E. Hancock. 2005. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr. Protein Pept. Sci. 6:35-51. [DOI] [PubMed] [Google Scholar]
- 24.Bowdish, D. M., D. J. Davidson, Y. E. Lau, K. Lee, M. G. Scott, and R. E. Hancock. 2005. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol. 77:451-459. [DOI] [PubMed] [Google Scholar]
- 25.Bowdish, D. M., D. J. Davidson, M. G. Scott, and R. E. Hancock. 2005. Immunomodulatory activities of small host defense peptides. Antimicrob. Agents Chemother. 49:1727-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowdish, D. M., D. J. Davidson, D. P. Speert, and R. E. Hancock. 2004. The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J. Immunol. 172:3758-3765. [DOI] [PubMed] [Google Scholar]
- 27.Bowdish, D. M., and R. E. Hancock. 2005. Anti-endotoxin properties of cationic host defence peptides and proteins. J. Endotoxin Res. 11:230-236. [DOI] [PubMed] [Google Scholar]
- 28.Brogden, K. A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238-250. [DOI] [PubMed] [Google Scholar]
- 29.Brotz, H., G. Bierbaum, P. E. Reynolds, and H. G. Sahl. 1997. The lantibiotic mersacidin inhibits peptidoglycan biosynthesis at the level of transglycosylation. Eur. J. Biochem. 246:193-199. [DOI] [PubMed] [Google Scholar]
- 30.Brown, K. L., and R. E. Hancock. 2006. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 18:24-30. [DOI] [PubMed] [Google Scholar]
- 31.Brumfitt, W., M. R. Salton, and J. M. Hamilton-Miller. 2002. Nisin, alone and combined with peptidoglycan-modulating antibiotics: activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. J. Antimicrob. Chemother. 50:731-734. [DOI] [PubMed] [Google Scholar]
- 32.Bucki, R., J. J. Pastore, P. Randhawa, R. Vegners, D. J. Weiner, and P. A. Janmey. 2004. Antibacterial activities of rhodamine B-conjugated gelsolin-derived peptides compared to those of the antimicrobial peptides cathelicidin LL37, magainin II, and melittin. Antimicrob. Agents Chemother. 48:1526-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bulet, P., R. Stocklin, and L. Menin. 2004. Anti-microbial peptides: from invertebrates to vertebrates. Immunol. Rev. 198:169-184. [DOI] [PubMed] [Google Scholar]
- 34.Camejo, E. H., B. Rosengren, G. Camejo, P. Sartipy, G. Fager, and G. Bondjers. 1995. Interferon gamma binds to extracellular matrix chondroitin-sulfate proteoglycans, thus enhancing its cellular response. Arterioscler. Thromb. Vasc. Biol. 15:1456-1465. [DOI] [PubMed] [Google Scholar]
- 35.Carmona, M. J., A. Molina, J. A. Fernandez, J. J. Lopez-Fando, and F. Garcia-Olmedo. 1993. Expression of the alpha-thionin gene from barley in tobacco confers enhanced resistance to bacterial pathogens. Plant J. 3:457-462. [DOI] [PubMed] [Google Scholar]
- 36.Chan, Y. R., and R. L. Gallo. 1998. PR-39, a syndecan-inducing antimicrobial peptide, binds and affects p130(Cas). J. Biol. Chem. 273:28978-28985. [DOI] [PubMed] [Google Scholar]
- 37.Chang, T. L., J. Vargas, Jr., A. DelPortillo, and M. E. Klotman. 2005. Dual role of alpha-defensin-1 in anti-HIV-1 innate immunity. J. Clin. Investig. 115:765-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatterjee, S., D. K. Chatterjee, R. H. Jani, J. Blumbach, B. N. Ganguli, N. Klesel, M. Limbert, and G. Seibert. 1992. Mersacidin, a new antibiotic from Bacillus. In vitro and in vivo antibacterial activity. J. Antibiot. (Tokyo) 45:839-845. [DOI] [PubMed] [Google Scholar]
- 39.Chatterjee, S., S. Chatterjee, S. J. Lad, M. S. Phansalkar, R. H. Rupp, B. N. Ganguli, H. W. Fehlhaber, and H. Kogler. 1992. Mersacidin, a new antibiotic from Bacillus. Fermentation, isolation, purification and chemical characterization. J. Antibiot. (Tokyo) 45:832-838. [DOI] [PubMed] [Google Scholar]
- 40.Chertov, O., D. F. Michiel, L. Xu, J. M. Wang, K. Tani, W. J. Murphy, D. L. Longo, D. D. Taub, and J. J. Oppenheim. 1996. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J. Biol. Chem. 271:2935-2940. [DOI] [PubMed] [Google Scholar]
- 41.Cho, Y., J. S. Turner, N. N. Dinh, and R. I. Lehrer. 1998. Activity of protegrins against yeast-phase Candida albicans. Infect. Immun. 66:2486-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cole, A. M., T. Hong, L. M. Boo, T. Nguyen, C. Zhao, G. Bristol, J. A. Zack, A. J. Waring, O. O. Yang, and R. I. Lehrer. 2002. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. USA 99:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777. [DOI] [PubMed] [Google Scholar]
- 44.Cunliffe, R. N., and Y. R. Mahida. 2004. Expression and regulation of antimicrobial peptides in the gastrointestinal tract. J. Leukoc. Biol. 75:49-58. [DOI] [PubMed] [Google Scholar]
- 45.Daher, K. A., M. E. Selsted, and R. I. Lehrer. 1986. Direct inactivation of viruses by human granulocyte defensins. J. Virol. 60:1068-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dathe, M., and T. Wieprecht. 1999. Structural features of helical antimicrobial peptides: their potential to modulate activity on model membranes and biological cells. Biochim. Biophys. Acta 1462:71-87. [DOI] [PubMed] [Google Scholar]
- 47.Davidson, D. J., A. J. Currie, G. S. Reid, D. M. Bowdish, K. L. MacDonald, R. C. Ma, R. E. Hancock, and D. P. Speert. 2004. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J. Immunol. 172:1146-1156. [DOI] [PubMed] [Google Scholar]
- 48.Davis, A. J., and L. Kedzierski. 2005. Recent advances in antileishmanial drug development. Curr. Opin. Investig. Drugs 6:163-169. [PubMed] [Google Scholar]
- 49.De Clercq, E. 2004. Antiviral drugs in current clinical use. J. Clin. Virol. 30:115-133. [DOI] [PubMed] [Google Scholar]
- 50.De Lucca, A. J., J. M. Bland, T. J. Jacks, C. Grimm, and T. J. Walsh. 1998. Fungicidal and binding properties of the natural peptides cecropin B and dermaseptin. Med. Mycol. 36:291-298. [PubMed] [Google Scholar]
- 51.De Lucca, A. J., and T. J. Walsh. 1999. Antifungal peptides: novel therapeutic compounds against emerging pathogens. Antimicrob. Agents Chemother. 43:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diaz-Achirica, P., J. Ubach, A. Guinea, D. Andreu, and L. Rivas. 1998. The plasma membrane of Leishmania donovani promastigotes is the main target for CA(1-8)M(1-18), a synthetic cecropin A-melittin hybrid peptide. Biochem. J. 330:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DiGabriele, A. D., I. Lax, D. I. Chen, C. M. Svahn, M. Jaye, J. Schlessinger, and W. A. Hendrickson. 1998. Structure of a heparin-linked biologically active dimer of fibroblast growth factor. Nature 393:812-817. [DOI] [PubMed] [Google Scholar]
- 54.Dorschner, R. A., V. K. Pestonjamasp, S. Tamakuwala, T. Ohtake, J. Rudisill, V. Nizet, B. Agerberth, G. H. Gudmundsson, and R. L. Gallo. 2001. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J. Investig. Dermatol. 117:91-97. [DOI] [PubMed] [Google Scholar]
- 55.Drobni, P. 2005. Papillomavirus binding and entry. The heparan sulfate receptor and inhibition by lactoferrin. Doctoral dissertation. Umea University, Umea, Sweden.
- 56.Duits, L. A., B. Ravensbergen, M. Rademaker, P. S. Hiemstra, and P. H. Nibbering. 2002. Expression of beta-defensin 1 and 2 mRNA by human monocytes, macrophages and dendritic cells. Immunology 106:517-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ehrenstein, G., and H. Lecar. 1977. Electrically gated ionic channels in lipid bilayers. Q. Rev. Biophys. 10:1-34. [DOI] [PubMed] [Google Scholar]
- 58.Elsbach, P. 2003. What is the real role of antimicrobial polypeptides that can mediate several other inflammatory responses? J. Clin. Investig. 111:1643-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Epple, P., K. Apel, and H. Bohlmann. 1997. Overexpression of an endogenous thionin enhances resistance of Arabidopsis against Fusarium oxysporum. Plant Cell 9:509-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faber, C., H. P. Stallmann, D. M. Lyaruu, U. Joosten, C. von Eiff, A. van Nieuw Amerongen, and P. I. Wuisman. 2005. Comparable efficacies of the antimicrobial peptide human lactoferrin 1-11 and gentamicin in a chronic methicillin-resistant Staphylococcus aureus osteomyelitis model. Antimicrob. Agents Chemother. 49:2438-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Falla, T. J., D. N. Karunaratne, and R. E. Hancock. 1996. Mode of action of the antimicrobial peptide indolicidin. J. Biol. Chem. 271:19298-19303. [DOI] [PubMed] [Google Scholar]
- 62.Fang, X. M., Q. Shu, Q. X. Chen, M. Book, H. G. Sahl, A. Hoeft, and F. Stuber. 2003. Differential expression of alpha- and beta-defensins in human peripheral blood. Eur. J. Clin. Investig. 33:82-87. [DOI] [PubMed] [Google Scholar]
- 63.Fennell, J. F., W. H. Shipman, and L. J. Cole. 1968. Antibacterial action of melittin, a polypeptide from bee venom. Proc. Soc. Exp. Biol. Med. 127:707-710. [DOI] [PubMed] [Google Scholar]
- 64.Friedrich, C. L., D. Moyles, T. J. Beveridge, and R. E. Hancock. 2000. Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria. Antimicrob. Agents Chemother. 44:2086-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedrich, C. L., A. Rozek, A. Patrzykat, and R. E. Hancock. 2001. Structure and mechanism of action of an indolicidin peptide derivative with improved activity against gram-positive bacteria. J. Biol. Chem. 276:24015-24022. [DOI] [PubMed] [Google Scholar]
- 66.Frohm, M., B. Agerberth, G. Ahangari, M. Stahle-Backdahl, S. Liden, H. Wigzell, and G. H. Gudmundsson. 1997. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272:15258-15263. [DOI] [PubMed] [Google Scholar]
- 67.Fromm, J. R., R. E. Hileman, E. E. Caldwell, J. M. Weiler, and R. J. Linhardt. 1995. Differences in the interaction of heparin with arginine and lysine and the importance of these basic amino acids in the binding of heparin to acidic fibroblast growth factor. Arch. Biochem. Biophys. 323:279-287. [DOI] [PubMed] [Google Scholar]
- 68.Fu, H., A. Bjorstad, C. Dahlgren, and J. Bylund. 2004. A bactericidal cecropin-A peptide with a stabilized alpha-helical structure possess an increased killing capacity but no proinflammatory activity. Inflammation 28:337-343. [DOI] [PubMed] [Google Scholar]
- 69.Fujimura, M., M. Ideguchi, Y. Minami, K. Watanabe, and K. Tadera. 2004. Purification, characterization, and sequencing of novel antimicrobial peptides, Tu-AMP 1 and Tu-AMP 2, from bulbs of tulip (Tulipa gesneriana L.). Biosci. Biotechnol. Biochem. 68:571-577. [DOI] [PubMed] [Google Scholar]
- 70.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 71.Ganz, T., M. E. Selsted, and R. I. Lehrer. 1986. Antimicrobial activity of phagocyte granule proteins. Semin. Respir. Infect. 1:107-117. [PubMed] [Google Scholar]
- 72.Garcia-Olmedo, F., A. Molina, J. M. Alamillo, and P. Rodriguez-Palenzuela. 1998. Plant defense peptides. Biopolymers 47:479-491. [DOI] [PubMed] [Google Scholar]
- 73.Gazit, E., I. R. Miller, P. C. Biggin, M. S. Sansom, and Y. Shai. 1996. Structure and orientation of the mammalian antibacterial peptide cecropin P1 within phospholipid membranes. J. Mol. Biol. 258:860-870. [DOI] [PubMed] [Google Scholar]
- 74.Ge, Y., D. L. MacDonald, K. J. Holroyd, C. Thornsberry, H. Wexler, and M. Zasloff. 1999. In vitro antibacterial properties of pexiganan, an analog of magainin. Antimicrob. Agents Chemother. 43:782-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gennaro, R., B. Skerlavaj, and D. Romeo. 1989. Purification, composition, and activity of two bactenecins, antibacterial peptides of bovine neutrophils. Infect. Immun. 57:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gesell, J., M. Zasloff, and S. J. Opella. 1997. Two-dimensional 1H NMR experiments show that the 23-residue magainin antibiotic peptide is an alpha-helix in dodecylphosphocholine micelles, sodium dodecylsulfate micelles, and trifluoroethanol/water solution. J. Biomol. NMR 9:127-135. [DOI] [PubMed] [Google Scholar]
- 77.Giansanti, F., M. T. Massucci, M. F. Giardi, F. Nozza, E. Pulsinelli, C. Nicolini, D. Botti, and G. Antonini. 2005. Antiviral activity of ovotransferrin derived peptides. Biochem. Biophys. Res. Commun. 331:69-73. [DOI] [PubMed] [Google Scholar]
- 78.Giles, F. J., R. Rodriguez, D. Weisdorf, J. R. Wingard, P. J. Martin, T. R. Fleming, S. L. Goldberg, E. J. Anaissie, B. J. Bolwell, N. J. Chao, T. C. Shea, M. M. Brunvand, W. Vaughan, F. Petersen, M. Schubert, H. M. Lazarus, R. T. Maziarz, M. Silverman, R. A. Beveridge, R. Redman, J. G. Pulliam, P. Devitt-Risse, H. J. Fuchs, and D. D. Hurd. 2004. A phase III, randomized, double-blind, placebo-controlled, study of iseganan for the reduction of stomatitis in patients receiving stomatotoxic chemotherapy. Leukoc. Res. 28:559-565. [DOI] [PubMed] [Google Scholar]
- 79.Gough, M., R. E. Hancock, and N. M. Kelly. 1996. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect. Immun. 64:4922-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gropp, R., M. Frye, T. O. Wagner, and J. Bargon. 1999. Epithelial defensins impair adenoviral infection: implication for adenovirus-mediated gene therapy. Hum. Gene Ther. 10:957-964. [DOI] [PubMed] [Google Scholar]
- 81.Gudmundsson, G. H., B. Agerberth, J. Odeberg, T. Bergman, B. Olsson, and R. Salcedo. 1996. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 238:325-332. [DOI] [PubMed] [Google Scholar]
- 82.Guerrero, E., J. M. Saugar, K. Matsuzaki, and L. Rivas. 2004. Role of positional hydrophobicity in the leishmanicidal activity of magainin 2. Antimicrob. Agents Chemother. 48:2980-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ha, S. C., K. Min, J. C. Koo, Y. Kim, D. J. Yun, M. J. Cho, and K. K. Kim. 2001. Crystallization and preliminary crystallographic studies of an antimicrobial protein from Pharbitis nil. Acta Crystallogr. D 57:263-265. [DOI] [PubMed] [Google Scholar]
- 84.Habermann, E., and J. Jentsch. 1967. Sequence analysis of melittin from tryptic and peptic degradation products. Hoppe-Seyler's Z. Physiol. Chem. 348:37-50. [PubMed] [Google Scholar]
- 85.Hallock, K. J., D. K. Lee, and A. Ramamoorthy. 2003. MSI-78, an analogue of the magainin antimicrobial peptides, disrupts lipid bilayer structure via positive curvature strain. Biophys. J. 84:3052-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hancock, R. E. 2001. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 1:156-164. [DOI] [PubMed] [Google Scholar]
- 87.Hancock, R. E. 1997. Peptide antibiotics. Lancet 349:418-422. [DOI] [PubMed] [Google Scholar]
- 88.Hancock, R. E., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hancock, R. E., and G. Diamond. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8:402-410. [DOI] [PubMed] [Google Scholar]
- 90.Hancock, R. E., and R. Lehrer. 1998. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 16:82-88. [DOI] [PubMed] [Google Scholar]
- 91.Hancock, R. E., and A. Patrzykat. 2002. Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr. Drug Targets Infect. Disord. 2:79-83. [DOI] [PubMed] [Google Scholar]
- 92.Hancock, R. E., and A. Rozek. 2002. Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol. Lett. 206:143-149. [DOI] [PubMed] [Google Scholar]
- 93.Haukland, H. H., H. Ulvatne, K. Sandvik, and L. H. Vorland. 2001. The antimicrobial peptides lactoferricin B and magainin 2 cross over the bacterial cytoplasmic membrane and reside in the cytoplasm. FEBS Lett. 508:389-393. [DOI] [PubMed] [Google Scholar]
- 94.He, K., S. J. Ludtke, D. L. Worcester, and H. W. Huang. 1996. Neutron scattering in the plane of membranes: structure of alamethicin pores. Biophys. J. 70:2659-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heller, W. T., A. J. Waring, R. I. Lehrer, and H. W. Huang. 1998. Multiple states of beta-sheet peptide protegrin in lipid bilayers. Biochemistry 37:17331-17338. [DOI] [PubMed] [Google Scholar]
- 96.Helmerhorst, E. J., P. Breeuwer, W. van't Hof, E. Walgreen-Weterings, L. C. Oomen, E. C. Veerman, A. V. Amerongen, and T. Abee. 1999. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J. Biol. Chem. 274:7286-7291. [DOI] [PubMed] [Google Scholar]
- 97.Helmerhorst, E. J., R. F. Troxler, and F. G. Oppenheim. 2001. The human salivary peptide histatin 5 exerts its antifungal activity through the formation of reactive oxygen species. Proc. Natl. Acad. Sci. USA 98:14637-14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Henzler Wildman, K. A., D. K. Lee, and A. Ramamoorthy. 2003. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry 42:6545-6558. [DOI] [PubMed] [Google Scholar]
- 99.Hileman, R. E., J. R. Fromm, J. M. Weiler, and R. J. Linhardt. 1998. Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. Bioessays 20:156-167. [DOI] [PubMed] [Google Scholar]
- 100.Hilpert, K., R. Volkmer-Engert, T. Walter, and R. E. Hancock. 2005. High-throughput generation of small antibacterial peptides with improved activity. Nat. Biotechnol. 23:1008-1012. [DOI] [PubMed] [Google Scholar]
- 101.Hoogewerf, A. J., G. S. Kuschert, A. E. Proudfoot, F. Borlat, I. Clark-Lewis, C. A. Power, and T. N. Wells. 1997. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry 36:13570-13578. [DOI] [PubMed] [Google Scholar]
- 102.Horne, W. S., C. M. Wiethoff, C. Cui, K. M. Wilcoxen, M. Amorin, M. R. Ghadiri, and G. R. Nemerow. 2005. Antiviral cyclic d,l-alpha-peptides: targeting a general biochemical pathway in virus infections. Bioorg. Med. Chem. 13:5145-5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Houston, M. E., Jr., L. H. Kondejewski, D. N. Karunaratne, M. Gough, S. Fidai, R. S. Hodges, and R. E. Hancock. 1998. Influence of preformed alpha-helix and alpha-helix induction on the activity of cationic antimicrobial peptides. J. Pept. Res. 52:81-88. [DOI] [PubMed] [Google Scholar]
- 104.Hsu, C. H., C. Chen, M. L. Jou, A. Y. Lee, Y. C. Lin, Y. P. Yu, W. T. Huang, and S. H. Wu. 2005. Structural and DNA-binding studies on the bovine antimicrobial peptide, indolicidin: evidence for multiple conformations involved in binding to membranes and DNA. Nucleic Acids Res. 33:4053-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang, R. H., Y. Xiang, G. Z. Tu, Y. Zhang, and D. C. Wang. 2004. Solution structure of Eucommia antifungal peptide: a novel structural model distinct with a five-disulfide motif. Biochemistry 43:6005-6012. [DOI] [PubMed] [Google Scholar]
- 106.Hultmark, D., A. Engstrom, H. Bennich, R. Kapur, and H. G. Boman. 1982. Insect immunity: isolation and structure of cecropin D and four minor antibacterial components from Cecropia pupae. Eur. J. Biochem. 127:207-217. [DOI] [PubMed] [Google Scholar]
- 107.Hunter, H. N., A. R. Demcoe, H. Jenssen, T. J. Gutteberg, and H. J. Vogel. 2005. Human lactoferricin is partially folded in aqueous solution and is better stabilized in a membrane mimetic solvent. Antimicrob. Agents Chemother. 49:3387-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huttner, K. M., and C. L. Bevins. 1999. Antimicrobial peptides as mediators of epithelial host defense. Pediatr. Res. 45:785-794. [DOI] [PubMed] [Google Scholar]
- 109.Hutton, R. D., D. L. Ewert, and G. R. French. 1973. Differentiation of types 1 and 2 herpes simplex virus by plaque inhibition with sulfated polyanions. Proc. Soc. Exp. Biol. Med. 142:27-29. [DOI] [PubMed] [Google Scholar]
- 110.Iida, J., A. M. Meijne, T. R. Oegema, Jr., T. A. Yednock, N. L. Kovach, L. T. Furcht, and J. B. McCarthy. 1998. A role of chondroitin sulfate glycosaminoglycan binding site in alpha4beta1 integrin-mediated melanoma cell adhesion. J. Biol. Chem. 273:5955-5962. [DOI] [PubMed] [Google Scholar]
- 111.Imler, J. L., and P. Bulet. 2005. Antimicrobial peptides in Drosophila: structures, activities and gene regulation. Chem. Immunol. Allergy 86:1-21. [DOI] [PubMed] [Google Scholar]
- 112.Iozzo, R. V., and A. D. Murdoch. 1996. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 10:598-614. [PubMed] [Google Scholar]
- 113.Ishimoto, H., H. Mukae, Y. Date, T. Shimbara, M. S. Mondal, J. Ashitani, T. Hiratsuka, S. Kubo, S. Kohno, and M. Nakazato. 2006. Identification of hBD-3 in respiratory tract and serum: the increase in pneumonia. Eur. Respir. J. 27:253-260. [DOI] [PubMed] [Google Scholar]
- 114.Iwanaga, S., and S. Kawabata. 1998. Evolution and phylogeny of defense molecules associated with innate immunity in horseshoe crab. Front. Biosci. 3:D973-D984. [DOI] [PubMed] [Google Scholar]
- 115.Jahn, R., and T. C. Sudhof. 1999. Membrane fusion and exocytosis. Annu. Rev. Biochem. 68:863-911. [DOI] [PubMed] [Google Scholar]
- 116.James, S., B. F. Gibbs, K. Toney, and H. P. Bennett. 1994. Purification of antimicrobial peptides from an extract of the skin of Xenopus laevis using heparin-affinity HPLC: characterization by ion-spray mass spectrometry. Anal. Biochem. 217:84-90. [DOI] [PubMed] [Google Scholar]
- 117.Javadpour, M. M., and M. D. Barkley. 1997. Self-assembly of designed antimicrobial peptides in solution and micelles. Biochemistry 36:9540-9549. [DOI] [PubMed] [Google Scholar]
- 118.Jenssen, H. 2005. Anti herpes simplex virus activity of lactoferrin/lactoferricin—an example of antiviral activity of antimicrobial protein/peptide. Cell Mol. Life Sci. 62:3002-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jenssen, H., J. H. Andersen, D. Mantzilas, and T. J. Gutteberg. 2004. A wide range of medium-sized, highly cationic, alpha-helical peptides show antiviral activity against herpes simplex virus. Antiviral Res. 64:119-126. [DOI] [PubMed] [Google Scholar]
- 120.Jenssen, H., J. H. Andersen, L. Uhlin-Hansen, T. J. Gutteberg, and O. Rekdal. 2004. Anti-HSV activity of lactoferricin analogues is only partly related to their affinity for heparan sulfate. Antiviral Res. 61:101-109. [DOI] [PubMed] [Google Scholar]
- 121.Jenssen, H., T. J. Gutteberg, and T. Lejon. 2005. Modelling of anti-HSV activity of lactoferricin analogues using amino acid descriptors. J. Pept. Sci. 11:97-103. [DOI] [PubMed] [Google Scholar]
- 122.Jenssen, H., T. J. Gutteberg, and T. Lejon. 2006. Modelling the anti-herpes simplex virus activity of small cationic peptides using amino acid descriptors. J. Pept. Res. 66(Suppl. 1):48-56. [Google Scholar]
- 123.Kanyshkova, T. G., D. V. Semenov, V. N. Buneva, and G. A. Nevinsky. 1999. Human milk lactoferrin binds two DNA molecules with different affinities. FEBS Lett. 451:235-237. [DOI] [PubMed] [Google Scholar]
- 124.Kavanagh, K., and S. Dowd. 2004. Histatins: antimicrobial peptides with therapeutic potential. J. Pharm. Pharmacol. 56:285-289. [DOI] [PubMed] [Google Scholar]
- 125.Kim, D. H., D. G. Lee, K. L. Kim, and Y. Lee. 2001. Internalization of tenecin 3 by a fungal cellular process is essential for its fungicidal effect on Candida albicans. Eur. J. Biochem. 268:4449-4458. [DOI] [PubMed] [Google Scholar]
- 126.Kim, D. H., Y. T. Lee, Y. J. Lee, J. H. Chung, B. L. Lee, B. S. Choi, and Y. Lee. 1998. Bacterial expression of tenecin 3, an insect antifungal protein isolated from Tenebrio molitor, and its efficient purification. Mol. Cells 8:786-789. [PubMed] [Google Scholar]
- 127.Kjellen, L., and U. Lindahl. 1991. Proteoglycans: structures and interactions. Annu. Rev. Biochem. 60:443-475. [DOI] [PubMed] [Google Scholar]
- 128.Klaenhammer, T. R. 1988. Bacteriocins of lactic acid bacteria. Biochimie 70:337-349. [DOI] [PubMed] [Google Scholar]
- 129.Koczulla, R., G. von Degenfeld, C. Kupatt, F. Krotz, S. Zahler, T. Gloe, K. Issbrucker, P. Unterberger, M. Zaiou, C. Lebherz, A. Karl, P. Raake, A. Pfosser, P. Boekstegers, U. Welsch, P. S. Hiemstra, C. Vogelmeier, R. L. Gallo, M. Clauss, and R. Bals. 2003. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J. Clin. Investig. 111:1665-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kolset, S. O., and J. T. Gallagher. 1990. Proteoglycans in haemopoietic cells. Biochim. Biophys. Acta 1032:191-211. [DOI] [PubMed] [Google Scholar]
- 131.Koo, J. C., B. Lee, M. E. Young, S. C. Koo, J. A. Cooper, D. Baek, C. O. Lim, S. Y. Lee, D. J. Yun, and M. J. Cho. 2004. Pn-AMP1, a plant defense protein, induces actin depolarization in yeasts. Plant Cell Physiol. 45:1669-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Koradi, R., M. Billeter, and K. Wuthrich. 1996. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graph. 14:51-55, 29-32. [DOI] [PubMed] [Google Scholar]
- 133.Kragol, G., S. Lovas, G. Varadi, B. A. Condie, R. Hoffmann, and L. Otvos, Jr. 2001. The antibacterial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding. Biochemistry 40:3016-3026. [DOI] [PubMed] [Google Scholar]
- 134.Krajewski, K., C. Marchand, Y. Q. Long, Y. Pommier, and P. P. Roller. 2004. Synthesis and HIV-1 integrase inhibitory activity of dimeric and tetrameric analogs of indolicidin. Bioorg. Med. Chem. Lett. 14:5595-5598. [DOI] [PubMed] [Google Scholar]
- 135.Kruszewska, D., H. G. Sahl, G. Bierbaum, U. Pag, S. O. Hynes, and A. Ljungh. 2004. Mersacidin eradicates methicillin-resistant Staphylococcus aureus (MRSA) in a mouse rhinitis model. J. Antimicrob. Chemother. 54:648-653. [DOI] [PubMed] [Google Scholar]
- 136.Kustanovich, I., D. E. Shalev, M. Mikhlin, L. Gaidukov, and A. Mor. 2002. Structural requirements for potent versus selective cytotoxicity for antimicrobial dermaseptin S4 derivatives. J. Biol. Chem. 277:16941-16951. [DOI] [PubMed] [Google Scholar]
- 137.Lamb, H. M., and L. R. Wiseman. 1998. Pexiganan acetate. Drugs 56:1047-1054. [DOI] [PubMed] [Google Scholar]
- 138.Langeland, N., L. J. Moore, H. Holmsen, and L. Haarr. 1988. Interaction of polylysine with the cellular receptor for herpes simplex virus type 1. J. Gen. Virol. 69:1137-1145. [DOI] [PubMed] [Google Scholar]
- 139.Lau, Y. E., A. Rozek, M. G. Scott, D. L. Goosney, D. J. Davidson, and R. E. Hancock. 2005. Interaction and cellular localization of the human host defense peptide LL-37 with lung epithelial cells. Infect. Immun. 73:583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee, D. G., K. S. Hahm, and S. Y. Shin. 2004. Structure and fungicidal activity of a synthetic antimicrobial peptide, P18, and its truncated peptides. Biotechnol. Lett. 26:337-341. [DOI] [PubMed] [Google Scholar]
- 141.Lee, D. G., D. H. Kim, Y. Park, H. K. Kim, H. N. Kim, Y. K. Shin, C. H. Choi, and K. S. Hahm. 2001. Fungicidal effect of antimicrobial peptide, PMAP-23, isolated from porcine myeloid against Candida albicans. Biochem. Biophys. Res. Commun. 282:570-574. [DOI] [PubMed] [Google Scholar]
- 142.Lee, D. G., H. K. Kim, S. A. Kim, Y. Park, S. C. Park, S. H. Jang, and K. S. Hahm. 2003. Fungicidal effect of indolicidin and its interaction with phospholipid membranes. Biochem. Biophys. Res. Commun. 305:305-310. [DOI] [PubMed] [Google Scholar]
- 143.Lee, D. G., P. I. Kim, Y. Park, E. R. Woo, J. S. Choi, C. H. Choi, and K. S. Hahm. 2002. Design of novel peptide analogs with potent fungicidal activity, based on PMAP-23 antimicrobial peptide isolated from porcine myeloid. Biochem. Biophys. Res. Commun. 293:231-238. [DOI] [PubMed] [Google Scholar]
- 144.Lee, D. G., Y. Park, I. Jin, K. S. Hahm, H. H. Lee, Y. H. Moon, and E. R. Woo. 2004. Structure-antiviral activity relationships of cecropin A-magainin 2 hybrid peptide and its analogues. J. Pept. Sci. 10:298-303. [DOI] [PubMed] [Google Scholar]
- 145.Lee, P. H., J. A. Rudisill, K. H. Lin, L. Zhang, S. M. Harris, T. J. Falla, and R. L. Gallo. 2004. HB-107, a nonbacteriostatic fragment of the antimicrobial peptide cecropin B, accelerates murine wound repair. Wound Repair Regen. 12:351-358. [DOI] [PubMed] [Google Scholar]
- 146.Lee, Y. T., D. H. Kim, J. Y. Suh, J. H. Chung, B. L. Lee, Y. Lee, and B. S. Choi. 1999. Structural characteristics of tenecin 3, an insect antifungal protein. Biochem. Mol. Biol. Int. 47:369-376. [DOI] [PubMed] [Google Scholar]
- 147.Lehrer, R. I., A. Barton, K. A. Daher, S. S. Harwig, T. Ganz, and M. E. Selsted. 1989. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Investig. 84:553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lehrer, R. I., D. Szklarek, T. Ganz, and M. E. Selsted. 1985. Correlation of binding of rabbit granulocyte peptides to Candida albicans with candidacidal activity. Infect. Immun. 49:207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 150.Liu, S., H. Lu, J. Niu, Y. Xu, S. Wu, and S. Jiang. 2005. Different from the HIV fusion inhibitor C34, the anti-HIV drug fuzeon (T-20) inhibits HIV-1 entry by targeting multiple sites in gp41 and gp120. J. Biol. Chem. 280:11259-11273. [DOI] [PubMed] [Google Scholar]
- 151.Lookene, A., R. Savonen, and G. Olivecrona. 1997. Interaction of lipoproteins with heparan sulfate proteoglycans and with lipoprotein lipase. Studies by surface plasmon resonance technique. Biochemistry 36:5267-5275. [DOI] [PubMed] [Google Scholar]
- 152.Lopez-Garcia, B., J. F. Marcos, C. Abad, and E. Perez-Paya. 2004. Stabilisation of mixed peptide/lipid complexes in selective antifungal hexapeptides. Biochim. Biophys. Acta 1660:131-137. [DOI] [PubMed] [Google Scholar]
- 153.Lorin, C., H. Saidi, A. Belaid, A. Zairi, F. Baleux, H. Hocini, L. Belec, K. Hani, and F. Tangy. 2005. The antimicrobial peptide dermaseptin S4 inhibits HIV-1 infectivity in vitro. Virology 334:264-275. [DOI] [PubMed] [Google Scholar]
- 154.Lupetti, A., A. Paulusma-Annema, S. Senesi, M. Campa, J. T. Van Dissel, and P. H. Nibbering. 2002. Internal thiols and reactive oxygen species in candidacidal activity exerted by an N-terminal peptide of human lactoferrin. Antimicrob. Agents Chemother. 46:1634-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lustig, F., J. Hoebeke, G. Ostergren-Lunden, F. Velge-Roussel, G. Bondjers, U. Olsson, U. Ruetschi, and G. Fager. 1996. Alternative splicing determines the binding of platelet-derived growth factor (PDGF-AA) to glycosaminoglycans. Biochemistry 35:12077-12085. [DOI] [PubMed] [Google Scholar]
- 156.Mandard, N., P. Sodano, H. Labbe, J. M. Bonmatin, P. Bulet, C. Hetru, M. Ptak, and F. Vovelle. 1998. Solution structure of thanatin, a potent bactericidal and fungicidal insect peptide, determined from proton two-dimensional nuclear magnetic resonance data. Eur. J. Biochem. 256:404-410. [DOI] [PubMed] [Google Scholar]
- 157.Mann, D. M., E. Romm, and M. Migliorini. 1994. Delineation of the glycosaminoglycan-binding site in the human inflammatory response protein lactoferrin. J. Biol. Chem. 269:23661-23667. [PubMed] [Google Scholar]
- 158.Mardberg, K., E. Trybala, F. Tufaro, and T. Bergstrom. 2002. Herpes simplex virus type 1 glycoprotein C is necessary for efficient infection of chondroitin sulfate-expressing gro2C cells. J. Gen. Virol. 83:291-300. [DOI] [PubMed] [Google Scholar]
- 159.Martin, E., T. Ganz, and R. I. Lehrer. 1995. Defensins and other endogenous peptide antibiotics of vertebrates. J. Leukoc. Biol. 58:128-136. [DOI] [PubMed] [Google Scholar]
- 160.Masuda, M., H. Nakashima, T. Ueda, H. Naba, R. Ikoma, A. Otaka, Y. Terakawa, H. Tamamura, T. Ibuka, T. Murakami, et al. 1992. A novel anti-HIV synthetic peptide, T-22 ([Tyr5,12,Lys7]-polyphemusin II). Biochem. Biophys. Res. Commun. 189:845-850. [DOI] [PubMed] [Google Scholar]
- 161.Matsuzaki, K. 1998. Magainins as paradigm for the mode of action of pore forming polypeptides. Biochim. Biophys. Acta 1376:391-400. [DOI] [PubMed] [Google Scholar]
- 162.Matsuzaki, K., O. Murase, N. Fujii, and K. Miyajima. 1996. An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry 35:11361-11368. [DOI] [PubMed] [Google Scholar]
- 163.Mattick, A. T. R., and A. Hirsch. 1947. Further observations on an inhibitory substance (nisin) from lactic streptococci. Lancet ii:5-7. [DOI] [PubMed] [Google Scholar]
- 164.McCann, K. B., A. Lee, J. Wan, H. Roginski, and M. J. Coventry. 2003. The effect of bovine lactoferrin and lactoferricin B on the ability of feline calicivirus (a norovirus surrogate) and poliovirus to infect cell cultures. J. Appl. Microbiol. 95:1026-1033. [DOI] [PubMed] [Google Scholar]
- 165.McManus, A. M., N. F. Dawson, J. D. Wade, L. E. Carrington, D. J. Winzor, and D. J. Craik. 2000. Three-dimensional structure of RK-1: a novel alpha-defensin peptide. Biochemistry 39:15757-15764. [DOI] [PubMed] [Google Scholar]
- 166.Mettenleiter, T. C. 2002. Brief overview on cellular virus receptors. Virus Res. 82:3-8. [DOI] [PubMed] [Google Scholar]
- 167.Mikloska, Z., and A. L. Cunningham. 2001. Alpha and gamma interferons inhibit herpes simplex virus type 1 infection and spread in epidermal cells after axonal transmission. J. Virol. 75:11821-11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Moerman, L., S. Bosteels, W. Noppe, J. Willems, E. Clynen, L. Schoofs, K. Thevissen, J. Tytgat, J. Van Eldere, J. Van Der Walt, and F. Verdonck. 2002. Antibacterial and antifungal properties of alpha-helical, cationic peptides in the venom of scorpions from southern Africa. Eur. J. Biochem. 269:4799-4810. [DOI] [PubMed] [Google Scholar]
- 169.Moore, A. 2003. The big and small of drug discovery. Biotech versus pharma: advantages and drawbacks in drug development. EMBO Rep. 4:114-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Moore, K. S., S. Wehrli, H. Roder, M. Rogers, J. N. Forrest, Jr., D. McCrimmon, and M. Zasloff. 1993. Squalamine: an aminosterol antibiotic from the shark. Proc. Natl. Acad. Sci. USA 90:1354-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Morimoto, M., H. Mori, T. Otake, N. Ueba, N. Kunita, M. Niwa, T. Murakami, and S. Iwanaga. 1991. Inhibitory effect of tachyplesin I on the proliferation of human immunodeficiency virus in vitro. Chemotherapy 37:206-211. [DOI] [PubMed] [Google Scholar]
- 172.Murakami, M., T. Ohtake, R. A. Dorschner, B. Schittek, C. Garbe, and R. L. Gallo. 2002. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J. Investig. Dermatol. 119:1090-1095. [DOI] [PubMed] [Google Scholar]
- 173.Murakami, T., T. Nakajima, Y. Koyanagi, K. Tachibana, N. Fujii, H. Tamamura, N. Yoshida, M. Waki, A. Matsumoto, O. Yoshie, T. Kishimoto, N. Yamamoto, and T. Nagasawa. 1997. A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. J. Exp. Med. 186:1389-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Murakami, T., M. Niwa, F. Tokunaga, T. Miyata, and S. Iwanaga. 1991. Direct virus inactivation of tachyplesin I and its isopeptides from horseshoe crab hemocytes. Chemotherapy 37:327-334. [DOI] [PubMed] [Google Scholar]
- 175.Mygind, P. H., R. L. Fischer, K. M. Schnorr, M. T. Hansen, C. P. Sonksen, S. Ludvigsen, D. Raventos, S. Buskov, B. Christensen, L. De Maria, O. Taboureau, D. Yaver, S. G. Elvig-Jorgensen, M. V. Sorensen, B. E. Christensen, S. Kjaerulff, N. Frimodt-Moller, R. I. Lehrer, M. Zasloff, and H. H. Kristensen. 2005. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 437:975-980. [DOI] [PubMed] [Google Scholar]
- 176.Nagaoka, I., K. Kuwahara-Arai, H. Tamura, K. Hiramatsu, and M. Hirata. 2005. Augmentation of the bactericidal activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by amino acid substitutions. Inflamm. Res. 54:66-73. [DOI] [PubMed] [Google Scholar]
- 177.Nakashima, H., M. Masuda, T. Murakami, Y. Koyanagi, A. Matsumoto, N. Fujii, and N. Yamamoto. 1992. Anti-human immunodeficiency virus activity of a novel synthetic peptide, T22 ([Tyr-5,12, Lys-7]polyphemusin II): a possible inhibitor of virus-cell fusion. Antimicrob. Agents Chemother. 36:1249-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nakashima, H., N. Yamamoto, M. Masuda, and N. Fujii. 1993. Defensins inhibit HIV replication in vitro. AIDS 7:1129. [DOI] [PubMed] [Google Scholar]
- 179.Narasimhan, M. L., B. Damsz, M. A. Coca, J. I. Ibeas, D. J. Yun, J. M. Pardo, P. M. Hasegawa, and R. A. Bressan. 2001. A plant defense response effector induces microbial apoptosis. Mol. Cell 8:921-930. [DOI] [PubMed] [Google Scholar]
- 180.Nibbering, P. H., E. Ravensbergen, M. M. Welling, L. A. van Berkel, P. H. van Berkel, E. K. Pauwels, and J. H. Nuijens. 2001. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect. Immun. 69:1469-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nikawa, H., H. Fukushima, S. Makihira, T. Hamada, and L. P. Samaranayake. 2004. Fungicidal effect of three new synthetic cationic peptides against Candida albicans. Oral Dis. 10:221-228. [DOI] [PubMed] [Google Scholar]
- 182.Olsson, U., G. Camejo, E. Hurt-Camejo, K. Elfsber, O. Wiklund, and G. Bondjers. 1997. Possible functional interactions of apolipoprotein B-100 segments that associate with cell proteoglycans and the ApoB/E receptor. Arterioscler. Thromb. Vasc. Biol. 17:149-155. [DOI] [PubMed] [Google Scholar]
- 183.Osapay, K., D. Tran, A. S. Ladokhin, S. H. White, A. H. Henschen, and M. E. Selsted. 2000. Formation and characterization of a single Trp-Trp cross-link in indolicidin that confers protease stability without altering antimicrobial activity. J. Biol. Chem. 275:12017-12022. [DOI] [PubMed] [Google Scholar]
- 184.Otvos, L., Jr., I. O., M. E. Rogers, P. J. Consolvo, B. A. Condie, S. Lovas, P. Bulet, and M. Blaszczyk-Thurin. 2000. Interaction between heat shock proteins and antimicrobial peptides. Biochemistry 39:14150-14159. [DOI] [PubMed] [Google Scholar]
- 185.Ourth, D. D., T. D. Lockey, and H. E. Renis. 1994. Induction of cecropin-like and attacin-like antibacterial but not antiviral activity in Heliothis virescens larvae. Biochem. Biophys. Res. Commun. 200:35-44. [DOI] [PubMed] [Google Scholar]
- 186.Panyutich, A., J. Shi, P. L. Boutz, C. Zhao, and T. Ganz. 1997. Porcine polymorphonuclear leukocytes generate extracellular microbicidal activity by elastase-mediated activation of secreted proprotegrins. Infect. Immun. 65:978-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Park, C. B., H. S. Kim, and S. C. Kim. 1998. Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem. Biophys. Res. Commun. 244:253-257. [DOI] [PubMed] [Google Scholar]
- 188.Park, C. B., K. S. Yi, K. Matsuzaki, M. S. Kim, and S. C. Kim. 2000. Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: the proline hinge is responsible for the cell-penetrating ability of buforin II. Proc. Natl. Acad. Sci. USA 97:8245-8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Park, K., D. Oh, S. Y. Shin, K. S. Hahm, and Y. Kim. 2002. Structural studies of porcine myeloid antibacterial peptide PMAP-23 and its analogues in DPC micelles by NMR spectroscopy. Biochem. Biophys. Res. Commun. 290:204-212. [DOI] [PubMed] [Google Scholar]
- 190.Park, Y., S. H. Jang, D. G. Lee, and K. S. Hahm. 2004. Antinematodal effect of antimicrobial peptide, PMAP-23, isolated from porcine myeloid against Caenorhabditis elegans. J. Pept. Sci. 10:304-311. [DOI] [PubMed] [Google Scholar]
- 191.Park, Y., D. G. Lee, and K. S. Hahm. 2004. HP(2-9)-magainin 2(1-12), a synthetic hybrid peptide, exerts its antifungal effect on Candida albicans by damaging the plasma membrane. J. Pept. Sci. 10:204-209. [DOI] [PubMed] [Google Scholar]
- 192.Parker, K. H., C. P. Winlove, and A. Maroudas. 1988. The theoretical distributions and diffusivities of small ions in chondroitin sulphate and hyaluronate. Biophys. Chem. 32:271-282. [DOI] [PubMed] [Google Scholar]
- 193.Patrzykat, A., C. L. Friedrich, L. Zhang, V. Mendoza, and R. E. W. Hancock. 2002. Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli. Antimicrob. Agents Chemother. 46:605-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Patterson-Delafield, J., R. J. Martinez, and R. I. Lehrer. 1980. Microbicidal cationic proteins in rabbit alveolar macrophages: a potential host defense mechanism. Infect. Immun. 30:180-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Penco, S., S. Scarfi, M. Giovine, G. Damonte, E. Millo, B. Villaggio, M. Passalacqua, M. Pozzolini, C. Garre, and U. Benatti. 2001. Identification of an import signal for, and the nuclear localization of, human lactoferrin. Biotechnol. Appl. Biochem. 34:151-159. [DOI] [PubMed] [Google Scholar]
- 196.Perez, A., Q. X. Li, P. Perez-Romero, G. Delassus, S. R. Lopez, S. Sutter, N. McLaren, and A. O. Fuller. 2005. A new class of receptor for herpes simplex virus has heptad repeat motifs that are common to membrane fusion proteins. J. Virol. 79:7419-7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Perez-Romero, P., and A. O. Fuller. 2005. The C terminus of the B5 receptor for herpes simplex virus contains a functional region important for infection. J. Virol. 79:7431-7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pettersson, I., M. Kusche, E. Unger, H. Wlad, L. Nylund, U. Lindahl, and L. Kjellen. 1991. Biosynthesis of heparin. Purification of a 110-kDa mouse mastocytoma protein required for both glucosaminyl N-deacetylation and N-sulfation. J. Biol. Chem. 266:8044-8049. [PubMed] [Google Scholar]
- 199.Pietrantoni, A., M. G. Ammendolia, A. Tinari, R. Siciliano, P. Valenti, and F. Superti. 2006. Bovine lactoferrin peptidic fragments involved in inhibition of Echovirus 6 in vitro infection. Antiviral Res. 69:98-106. [DOI] [PubMed] [Google Scholar]
- 200.Pouny, Y., D. Rapaport, A. Mor, P. Nicolas, and Y. Shai. 1992. Interaction of antimicrobial dermaseptin and its fluorescently labeled analogues with phospholipid membranes. Biochemistry 31:12416-12423. [DOI] [PubMed] [Google Scholar]
- 201.Powers, J. P., and R. E. Hancock. 2003. The relationship between peptide structure and antibacterial activity. Peptides 24:1681-1691. [DOI] [PubMed] [Google Scholar]
- 202.Powers, J. P., A. Rozek, and R. E. Hancock. 2004. Structure-activity relationships for the beta-hairpin cationic antimicrobial peptide polyphemusin I. Biochim. Biophys. Acta 1698:239-250. [DOI] [PubMed] [Google Scholar]
- 203.Powers, J. P., A. Tan, A. Ramamoorthy, and R. E. Hancock. 2005. Solution structure and interaction of the antimicrobial polyphemusins with lipid membranes. Biochemistry 44:15504-15513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Putsep, K., G. Carlsson, H. G. Boman, and M. Andersson. 2002. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet 360:1144-1149. [DOI] [PubMed] [Google Scholar]
- 205.Ramos-Onsins, S., and M. Aguade. 1998. Molecular evolution of the Cecropin multigene family in Drosophila. Functional genes vs. pseudogenes. Genetics 150:157-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Riley, M. A. 1998. Molecular mechanisms of bacteriocin evolution. Annu. Rev. Genet. 32:255-278. [DOI] [PubMed] [Google Scholar]
- 207.Rinaldi, A. C. 2002. Antimicrobial peptides from amphibian skin: an expanding scenario. Curr. Opin. Chem. Biol. 6:799-804. [DOI] [PubMed] [Google Scholar]
- 208.Robinson, W. E., Jr., B. McDougall, D. Tran, and M. E. Selsted. 1998. Anti-HIV-1 activity of indolicidin, an antimicrobial peptide from neutrophils. J. Leukoc. Biol. 63:94-100. [DOI] [PubMed] [Google Scholar]
- 209.Roch, P., A. Beschin, and E. Bernard. 2004. Antiprotozoan and antiviral activities of non-cytotoxic truncated and variant analogues of mussel defensin. Evid Based Complement Alternat. Med. 1:167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rollins-Smith, L. A., C. Carey, J. Longcore, J. K. Doersam, A. Boutte, J. E. Bruzgal, and J. M. Conlon. 2002. Activity of antimicrobial skin peptides from ranid frogs against Batrachochytrium dendrobatidis, the chytrid fungus associated with global amphibian declines. Dev. Comp. Immunol. 26:471-479. [DOI] [PubMed] [Google Scholar]
- 211.Roth, B. L., M. Poot, S. T. Yue, and P. J. Millard. 1997. Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl. Environ. Microbiol. 63:2421-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rozek, A., C. L. Friedrich, and R. E. Hancock. 2000. Structure of the bovine antimicrobial peptide indolicidin bound to dodecylphosphocholine and sodium dodecyl sulfate micelles. Biochemistry 39:15765-15774. [PubMed] [Google Scholar]
- 213.Rozek, A., J. P. Powers, C. L. Friedrich, and R. E. Hancock. 2003. Structure-based design of an indolicidin peptide analogue with increased protease stability. Biochemistry 42:14130-14138. [DOI] [PubMed] [Google Scholar]
- 214.Salzman, N. H., D. Ghosh, K. M. Huttner, Y. Paterson, and C. L. Bevins. 2003. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422:522-526. [DOI] [PubMed] [Google Scholar]
- 215.Sandgren, S., A. Wittrup, F. Cheng, M. Jonsson, E. Eklund, S. Busch, and M. Belting. 2004. The human antimicrobial peptide LL-37 transfers extracellular DNA plasmid to the nuclear compartment of mammalian cells via lipid rafts and proteoglycan-dependent endocytosis. J. Biol. Chem. 279:17951-17956. [DOI] [PubMed] [Google Scholar]
- 216.Sawai, M. V., H. P. Jia, L. Liu, V. Aseyev, J. M. Wiencek, P. B. McCray, Jr., T. Ganz, W. R. Kearney, and B. F. Tack. 2001. The NMR structure of human beta-defensin-2 reveals a novel alpha-helical segment. Biochemistry 40:3810-3816. [DOI] [PubMed] [Google Scholar]
- 217.Schmidtchen, A., I. M. Frick, E. Andersson, H. Tapper, and L. Bjorck. 2002. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 46:157-168. [DOI] [PubMed] [Google Scholar]
- 218.Schmidtchen, A., I. M. Frick, and L. Bjorck. 2001. Dermatan sulphate is released by proteinases of common pathogenic bacteria and inactivates antibacterial alpha-defensin. Mol. Microbiol. 39:708-713. [DOI] [PubMed] [Google Scholar]
- 219.Scott, M. G., D. J. Davidson, M. R. Gold, D. Bowdish, and R. E. Hancock. 2002. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 169:3883-3891. [DOI] [PubMed] [Google Scholar]
- 220.Scott, M. G., and R. E. Hancock. 2000. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit. Rev. Immunol. 20:407-431. [PubMed] [Google Scholar]
- 221.Selsted, M. E., and A. J. Ouellette. 2005. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6:551-557. [DOI] [PubMed] [Google Scholar]
- 222.Shieh, M. T., D. WuDunn, R. I. Montgomery, J. D. Esko, and P. G. Spear. 1992. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J. Cell Biol. 116:1273-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Shimazaki, K., T. Tazume, K. Uji, M. Tanaka, H. Kumura, K. Mikawa, and T. Shimo-Oka. 1998. Properties of a heparin-binding peptide derived from bovine lactoferrin. J. Dairy Sci. 81:2841-2849. [DOI] [PubMed] [Google Scholar]
- 224.Simmaco, M., G. Mignogna, and D. Barra. 1998. Antimicrobial peptides from amphibian skin: what do they tell us? Biopolymers 47:435-450. [DOI] [PubMed] [Google Scholar]
- 225.Sinha, S., N. Cheshenko, R. I. Lehrer, and B. C. Herold. 2003. NP-1, a rabbit alpha-defensin, prevents the entry and intercellular spread of herpes simplex virus type 2. Antimicrob. Agents Chemother. 47:494-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sitaram, N., and R. Nagaraj. 1999. Interaction of antimicrobial peptides with biological and model membranes: structural and charge requirements for activity. Biochim. Biophys. Acta 1462:29-54. [DOI] [PubMed] [Google Scholar]
- 227.Sitaram, N., C. Subbalakshmi, and R. Nagaraj. 2003. Indolicidin, a 13-residue basic antimicrobial peptide rich in tryptophan and proline, interacts with Ca(2+)-calmodulin. Biochem. Biophys. Res. Commun. 309:879-884. [DOI] [PubMed] [Google Scholar]
- 228.Skehel, J. J., and D. C. Wiley. 1998. Coiled coils in both intracellular vesicle and viral membrane fusion. Cell 95:871-874. [DOI] [PubMed] [Google Scholar]
- 229.Skerlavaj, B., R. Gennaro, L. Bagella, L. Merluzzi, A. Risso, and M. Zanetti. 1996. Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J. Biol. Chem. 271:28375-28381. [DOI] [PubMed] [Google Scholar]
- 230.Skerlavaj, B., M. Scocchi, R. Gennaro, A. Risso, and M. Zanetti. 2001. Structural and functional analysis of horse cathelicidin peptides. Antimicrob. Agents Chemother. 45:715-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Song, B. H., G. C. Lee, M. S. Moon, Y. H. Cho, and C. H. Lee. 2001. Human cytomegalovirus binding to heparan sulfate proteoglycans on the cell surface and/or entry stimulates the expression of human leukocyte antigen class I. J. Gen. Virol. 82:2405-2413. [DOI] [PubMed] [Google Scholar]
- 232.Song, Y. M., Y. Park, S. S. Lim, S. T. Yang, E. R. Woo, I. S. Park, J. S. Lee, J. I. Kim, K. S. Hahm, Y. Kim, and S. Y. Shin. 2005. Cell selectivity and mechanism of action of antimicrobial model peptides containing peptoid residues. Biochemistry 44:12094-12106. [DOI] [PubMed] [Google Scholar]
- 233.Spaar, A., C. Munster, and T. Salditt. 2004. Conformation of peptides in lipid membranes studied by x-ray grazing incidence scattering. Biophys. J. 87:396-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Spillmann, D. 2001. Heparan sulfate: anchor for viral intruders? Biochimie 83:811-817. [DOI] [PubMed] [Google Scholar]
- 235.Steinberg, D. A., M. A. Hurst, C. A. Fujii, A. H. Kung, J. F. Ho, F. C. Cheng, D. J. Loury, and J. C. Fiddes. 1997. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 41:1738-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Steinstraesser, L., B. Tippler, J. Mertens, E. Lamme, H. H. Homann, M. Lehnhardt, O. Wildner, H. U. Steinau, and K. Uberla. 2005. Inhibition of early steps in the lentiviral replication cycle by cathelicidin host defense peptides. Retrovirology 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Stenlund, P., M. J. Lindberg, and L. A. Tibell. 2002. Structural requirements for high-affinity heparin binding: alanine scanning analysis of charged residues in the C-terminal domain of human extracellular superoxide dismutase. Biochemistry 41:3168-3175. [DOI] [PubMed] [Google Scholar]
- 238.Subbalakshmi, C., and N. Sitaram. 1998. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol. Lett. 160:91-96. [DOI] [PubMed] [Google Scholar]
- 239.Suzuki, T., S. Futaki, M. Niwa, S. Tanaka, K. Ueda, and Y. Sugiura. 2002. Possible existence of common internalization mechanisms among arginine-rich peptides. J. Biol. Chem. 277:2437-2443. [DOI] [PubMed] [Google Scholar]
- 240.Tamamura, H., T. Ishihara, A. Otaka, T. Murakami, T. Ibuka, M. Waki, A. Matsumoto, N. Yamamoto, and N. Fujii. 1996. Analysis of the interaction of an anti-HIV peptide, T22 ([Tyr5, 12, Lys7]-polyphemusin II), with gp120 and CD4 by surface plasmon resonance. Biochim. Biophys. Acta 1298:37-44. [DOI] [PubMed] [Google Scholar]
- 241.Tamamura, H., T. Murakami, M. Masuda, A. Otaka, W. Takada, T. Ibuka, H. Nakashima, M. Waki, A. Matsumoto, N. Yamamoto, et al. 1994. Structure-activity relationships of an anti-HIV peptide, T22. Biochem. Biophys. Res. Commun. 205:1729-1735. [DOI] [PubMed] [Google Scholar]
- 242.Tamamura, H., A. Otaka, T. Murakami, T. Ishihara, T. Ibuka, M. Waki, A. Matsumoto, N. Yamamoto, and N. Fujii. 1996. Interaction of an anti-HIV peptide, T22, with gp120 and CD4. Biochem. Biophys. Res. Commun. 219:555-559. [DOI] [PubMed] [Google Scholar]
- 243.Tamamura, H., Y. Xu, T. Hattori, X. Zhang, R. Arakaki, K. Kanbara, A. Omagari, A. Otaka, T. Ibuka, N. Yamamoto, H. Nakashima, and N. Fujii. 1998. A low-molecular-weight inhibitor against the chemokine receptor CXCR4: a strong anti-HIV peptide T140. Biochem. Biophys. Res. Commun. 253:877-882. [DOI] [PubMed] [Google Scholar]
- 244.Tang, Y. Q., J. Yuan, G. Osapay, K. Osapay, D. Tran, C. J. Miller, A. J. Ouellette, and M. E. Selsted. 1999. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science 286:498-502. [DOI] [PubMed] [Google Scholar]
- 245.Terras, F. R., H. M. Schoofs, M. F. De Bolle, F. Van Leuven, S. B. Rees, J. Vanderleyden, B. P. Cammue, and W. F. Broekaert. 1992. Analysis of two novel classes of plant antifungal proteins from radish (Raphanus sativus L.) seeds. J. Biol. Chem. 267:15301-15309. [PubMed] [Google Scholar]
- 246.Territo, M. C., T. Ganz, M. E. Selsted, and R. Lehrer. 1989. Monocyte-chemotactic activity of defensins from human neutrophils. J. Clin. Investig. 84:2017-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tew, G. N., D. Liu, B. Chen, R. J. Doerksen, J. Kaplan, P. J. Carroll, M. L. Klein, and W. F. DeGrado. 2002. De novo design of biomimetic antimicrobial polymers. Proc. Natl. Acad. Sci. USA 99:5110-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Trotti, A., A. Garden, P. Warde, P. Symonds, C. Langer, R. Redman, T. F. Pajak, T. R. Fleming, M. Henke, J. Bourhis, D. I. Rosenthal, E. Junor, A. Cmelak, F. Sheehan, J. Pulliam, P. Devitt-Risse, H. Fuchs, M. Chambers, B. O'Sullivan, and K. K. Ang. 2004. A multinational, randomized phase III trial of iseganan HCl oral solution for reducing the severity of oral mucositis in patients receiving radiotherapy for head-and-neck malignancy. Int. J. Radiat. Oncol. Biol. Phys. 58:674-681. [DOI] [PubMed] [Google Scholar]
- 249.Trybala, E., S. Olofsson, K. Mardberg, B. Svennerholm, K. Umemoto, J. C. Glorioso, and T. Bergstrom. 2004. Structural and functional features of the polycationic peptide required for inhibition of herpes simplex virus invasion of cells. Antiviral Res. 62:125-134. [DOI] [PubMed] [Google Scholar]
- 250.Tytler, E. M., G. M. Anantharamaiah, D. E. Walker, V. K. Mishra, M. N. Palgunachari, and J. P. Segrest. 1995. Molecular basis for prokaryotic specificity of magainin-induced lysis. Biochemistry 34:4393-4401. [DOI] [PubMed] [Google Scholar]
- 251.Uteng, M., H. H. Hauge, P. R. Markwick, G. Fimland, D. Mantzilas, J. Nissen-Meyer, and C. Muhle-Goll. 2003. Three-dimensional structure in lipid micelles of the pediocin-like antimicrobial peptide sakacin P and a sakacin P variant that is structurally stabilized by an inserted C-terminal disulfide bridge. Biochemistry 42:11417-11426. [DOI] [PubMed] [Google Scholar]
- 252.Veerman, E. C., K. Nazmi, W. Van't Hof, J. G. Bolscher, A. L. Den Hertog, and A. V. Nieuw Amerongen. 2004. Reactive oxygen species play no role in the candidacidal activity of the salivary antimicrobial peptide histatin 5. Biochem. J. 381:447-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Viejo-Diaz, M., M. T. Andres, and J. F. Fierro. 2005. Different anti-Candida activities of two human lactoferrin-derived peptides, Lfpep and kaliocin-1. Antimicrob. Agents Chemother. 49:2583-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Vives, R. R., A. Imberty, Q. J. Sattentau, and H. Lortat-Jacob. 2005. Heparan sulfate targets the HIV-1 envelope glycoprotein gp120 coreceptor binding site. J. Biol. Chem. 280:21353-21357. [DOI] [PubMed] [Google Scholar]
- 255.Wachinger, M., A. Kleinschmidt, D. Winder, N. von Pechmann, A. Ludvigsen, M. Neumann, R. Holle, B. Salmons, V. Erfle, and R. Brack-Werner. 1998. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J. Gen. Virol. 79:731-740. [DOI] [PubMed] [Google Scholar]
- 256.Wang, W., A. M. Cole, T. Hong, A. J. Waring, and R. I. Lehrer. 2003. Retrocyclin, an antiretroviral theta-defensin, is a lectin. J. Immunol. 170:4708-4716. [DOI] [PubMed] [Google Scholar]
- 257.Wang, Z., and G. Wang. 2004. APD: the Antimicrobial Peptide Database. Nucleic Acids Res. 32:D590-D592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wilson, C. L., A. J. Ouellette, D. P. Satchell, T. Ayabe, Y. S. Lopez-Boado, J. L. Stratman, S. J. Hultgren, L. M. Matrisian, and W. C. Parks. 1999. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286:113-117. [DOI] [PubMed] [Google Scholar]
- 259.Wu, M., E. Maier, R. Benz, and R. E. Hancock. 1999. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 38:7235-7242. [DOI] [PubMed] [Google Scholar]
- 260.WuDunn, D., and P. G. Spear. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 63:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Xia, G., J. Chen, V. Tiwari, W. Ju, J. P. Li, A. Malmstrom, D. Shukla, and J. Liu. 2002. Heparan sulfate 3-O-sulfotransferase isoform 5 generates both an antithrombin-binding site and an entry receptor for herpes simplex virus, type 1. J. Biol. Chem. 277:37912-37919. [DOI] [PubMed] [Google Scholar]
- 262.Xiong, Y. Q., M. R. Yeaman, and A. S. Bayer. 1999. In vitro antibacterial activities of platelet microbicidal protein and neutrophil defensin against Staphylococcus aureus are influenced by antibiotics differing in mechanism of action. Antimicrob. Agents Chemother. 43:1111-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Xu, Y., H. Tamamura, R. Arakaki, H. Nakashima, X. Zhang, N. Fujii, T. Uchiyama, and T. Hattori. 1999. Marked increase in anti-HIV activity, as well as inhibitory activity against HIV entry mediated by CXCR4, linked to enhancement of the binding ability of tachyplesin analogs to CXCR4. AIDS Res. Hum. Retroviruses. 15:419-427. [DOI] [PubMed] [Google Scholar]
- 264.Yamaguchi, S., D. Huster, A. Waring, R. I. Lehrer, W. Kearney, B. F. Tack, and M. Hong. 2001. Orientation and dynamics of an antimicrobial peptide in the lipid bilayer by solid-state NMR spectroscopy. Biophys. J. 81:2203-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Yang, D., A. Biragyn, D. M. Hoover, J. Lubkowski, and J. J. Oppenheim. 2004. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu. Rev. Immunol. 22:181-215. [DOI] [PubMed] [Google Scholar]
- 266.Yang, D., A. Biragyn, L. W. Kwak, and J. J. Oppenheim. 2002. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23:291-296. [DOI] [PubMed] [Google Scholar]
- 267.Yang, L., T. A. Harroun, T. M. Weiss, L. Ding, and H. W. Huang. 2001. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 81:1475-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yang, N., M. B. Strom, S. M. Mekonnen, J. S. Svendsen, and O. Rekdal. 2004. The effects of shortening lactoferrin derived peptides against tumour cells, bacteria and normal human cells. J. Pept. Sci. 10:37-46. [DOI] [PubMed] [Google Scholar]
- 269.Yasin, B., M. Pang, J. S. Turner, Y. Cho, N. N. Dinh, A. J. Waring, R. I. Lehrer, and E. A. Wagar. 2000. Evaluation of the inactivation of infectious herpes simplex virus by host-defense peptides. Eur. J. Clin. Microbiol. Infect. Dis. 19:187-194. [DOI] [PubMed] [Google Scholar]
- 270.Yasin, B., W. Wang, M. Pang, N. Cheshenko, T. Hong, A. J. Waring, B. C. Herold, E. A. Wagar, and R. I. Lehrer. 2004. Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 78:5147-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zanetti, M. 2004. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75:39-48. [DOI] [PubMed] [Google Scholar]
- 272.Zasloff, M. 1987. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 84:5449-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zasloff, M., B. Martin, and H. C. Chen. 1988. Antimicrobial activity of synthetic magainin peptides and several analogues. Proc. Natl. Acad. Sci. USA 85:910-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zautner, A. E., U. Korner, A. Henke, C. Badorff, and M. Schmidtke. 2003. Heparan sulfates and coxsackievirus-adenovirus receptor: each one mediates coxsackievirus B3 PD infection. J. Virol. 77:10071-10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zelezetsky, I., U. Pag, H. G. Sahl, and A. Tossi. 2005. Tuning the biological properties of amphipathic alpha-helical antimicrobial peptides: rational use of minimal amino acid substitutions. Peptides 26:2368-2376. [DOI] [PubMed] [Google Scholar]
- 276.Zhang, L., and T. J. Falla. 2006. Antimicrobial peptides—therapeutic potential. Expert Opin. Pharmacother. 7:653-663. [DOI] [PubMed] [Google Scholar]
- 277.Zhang, L., J. Parente, S. M. Harris, D. E. Woods, R. E. W. Hancock, and T. J. Falla. 2005. Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrob. Agents Chemother. 49:2921-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zhang, L., S. Parente, S. M. Harris, and T. J. Falla. 2004. Presented at the 24th General Meeting of the American Society of Microbiology, New Orleans, La.
- 279.Zhang, L., A. Rozek, and R. E. Hancock. 2001. Interaction of cationic antimicrobial peptides with model membranes. J. Biol. Chem. 276:35714-35722. [DOI] [PubMed] [Google Scholar]
- 280.Zhang, L., M. G. Scott, H. Yan, L. D. Mayer, and R. E. Hancock. 2000. Interaction of polyphemusin I and structural analogs with bacterial membranes, lipopolysaccharide, and lipid monolayers. Biochemistry 39:14504-14514. [DOI] [PubMed] [Google Scholar]