Abstract
Bacterial infections following influenza are an important cause of morbidity and mortality worldwide. Based on the historical importance of pneumonia as a cause of death during pandemic influenza, the increasingly likely possibility that highly pathogenic avian influenza viruses will trigger the next worldwide pandemic underscores the need to understand the multiple mechanisms underlying the interaction between influenza virus and bacterial pathogens such as Streptococcus pneumoniae. There is ample evidence to support the historical view that influenza virus alters the lungs in a way that predisposes to adherence, invasion, and induction of disease by pneumococcus. Access to receptors is a key factor and may be facilitated by the virus through epithelial damage, by exposure or up-regulation of receptors, or by provoking the epithelial regeneration response to cytotoxic damage. More recent data indicate that alteration of the immune response by diminishing the ability of the host to clear pneumococcus or by amplification of the inflammatory cascade is another key factor. Identification and exploration of the underlying mechanisms responsible for this synergism will provide targets for prevention and treatment using drugs and vaccines.
INTRODUCTION
Influenza virus and Streptococcus pneumoniae rank as two of the most important pathogens affecting humans today. However, it may be their ability to work together that presents the greatest threat to world health. The catastrophic influenza A virus pandemic of 1918, which by conservative estimates killed 40 to 50 million persons worldwide (126), is an extreme example of the impact that this cooperative interaction can have. The pandemic that may be developing in Southeast Asia (159) is a warning that we need a better understanding of the basis of the synergism between these pathogens. Identification and exploration of the underlying mechanisms of viral-bacterial synergism will provide targets for prevention and treatment using drugs and vaccines. This review focuses primarily on the interaction between influenza virus and pneumococcus for two reasons: they are the pathogens most commonly associated with dual infections, and there is more known about the basis of their synergistic interaction than is known about any other pair of organisms.
BURDEN OF DISEASE
Evolution and Epidemiology of Influenza Viruses
Influenza viruses are members of the family Orthomyxoviridae and contain a single-stranded, negative-sense, segmented genome. Of the three types of influenza viruses, types A and B cause epidemic disease in humans on an annual basis, while type C causes only sporadic disease. Type A influenza viruses are further subtyped based on differences in the surface glycoproteins hemagglutinin (HA) and neuraminidase (NA). Sixteen subtypes of HA and nine of NA circulate in wild aquatic birds (48, 160). Viruses which express one of these antigenically novel HAs may periodically cross over into a variety of terrestrial animals, including humans, precipitating a global pandemic if they are able to transmit efficiently and cause mortality (85). Influenza B viruses do not have an animal reservoir or antigenically distinguishable subtypes and therefore do not represent a pandemic threat.
Protection against infection is mediated primarily by neutralizing antibodies directed against the HAs of influenza viruses. Seasonal variation in the surface of the HA through antigenic drift allows annual epidemics to occur. The infidelity of the viral RNA polymerase coupled with a lack of proofreading mechanisms creates a quasispecies pool of viruses in which many variants exist (100). When new variants have changes in the HA that abrogate binding by neutralizing antibodies, these nascent viruses are more likely to be successfully transmitted in an immune population and may emerge as an epidemic strain. Evolution occurs by negative selection as the new strains replace the old, infecting both naïve hosts and those with prior immunity against a different strain.
Because the genomes of influenza viruses are segmented, a second evolutionary process called reassortment can occur, where two viruses infecting one host exchange gene segments. This occurs commonly in humans with both influenza A and B viruses (87, 105). Because the avian reservoir provides a set of antigenically distinct HAs, however, influenza A viruses can undergo rapid, radical change by acquiring a new HA. This process, called antigenic shift, may lead to a pandemic, as occurred in 1957 and 1968 (160). Pandemics may also result from direct transfer to and adaptation in humans of a whole avian influenza virus, however, as is suspected to have occurred in or just before 1918 (148). It is feared that another such event may be occurring in Southeast Asia, where avian H5N1 influenza A viruses have become endemic in terrestrial poultry and are being transmitted to humans with increasing frequency (8).
Influenza and its complications are a leading cause of morbidity and mortality worldwide. Despite the availability of effective antiviral drugs and vaccines, the World Health Organization estimates that annual influenza epidemics cause 3 to 5 million severe illnesses and 250,000 to 500,000 deaths each year in the developed world alone (143). The impact in the developing world is unknown and is likely to be even higher. The potential toll from the next pandemic is staggering; the mortality from a mild pandemic has been conservatively estimated at 89,000 to 207,000 deaths in the United States alone (108), a figure that could be greatly exceeded if the H5N1 strains endemic in Southeast Asia retain their virulence after achieving widespread distribution.
Epidemiology of Streptococcus pneumoniae
The considerable morbidity and mortality for which S. pneumoniae is responsible make it one of the most important pathogens currently plaguing humans. The pneumococcus has historically been the most common etiologic agent of community-acquired pneumonia, as well as bacterial meningitis, otitis media, and sepsis (16, 44, 118, 168). Over 90 distinct serotypes exist based on the structural and chemical composition of its polysaccharide capsule. Multiple distinct genetic clones that share a common set of genes (clonotypes) can exist within and across serotypes (130, 166). Many factors contribute to the virulence of pneumococci. Animal challenge studies have implicated numerous cell wall-associated and secreted proteins, cell wall components such as teichoic acid and peptidoglycan, and the polysaccharide capsule that surrounds the organism (106). It is estimated that pneumococci possess over 100 surface proteins, many of which play a role in pathogenicity and virulence (166). Little is known about the expression patterns of these proteins in different clonotypes or how strain-dependent differences in structure or expression might account for differences in the invasiveness or virulence of particular bacteria.
Pneumococci are human commensals which colonize the nasopharynxes of 20 to 50% of healthy children and 8 to 30% of healthy adults (16, 66, 67, 71). Children typically acquire a succession of serotypes early in life and are the primary vector for transmission to vulnerable populations (56, 57, 71). A recently implemented conjugate vaccine is directed against the seven pneumococcal serotypes which most commonly cause invasive disease in the Western world (14). This vaccine has had a profound impact on invasive pneumococcal disease in children and a smaller but clinically significant effect on otitis media. While it has been suggested that a herd effect provides some protection to unvaccinated at-risk adults, significant morbidity and mortality still occur, with 22.8 to 27.3 cases of invasive pneumonia per 1,000 adults over the age of 50 years detectable by active surveillance since widespread implementation of the vaccine (84). The burden of pneumococcal disease in both adults and children in developing countries where the vaccine is not available is considerably higher than that in the United States (11, 18, 33, 44).
Synergism between Influenza Virus and Pneumococcus
It is well appreciated that upper respiratory tract viral infections are often complicated by more serious bacterial diseases. While influenza virus is most commonly thought of in this context, other respiratory viruses, including respiratory syncytial virus, measles virus, parainfluenza viruses, adenovirus, and rhinoviruses may also predispose to secondary infections. Several different bacteria have also been implicated, including Haemophilus influenzae, Staphylococcus aureus, Streptococcus pyogenes, Mycoplasma pneumoniae, and the pneumococcus. It is thought that certain pairings of organisms better complement each other than other potential pairings (7). However, support from epidemiologic studies for specific interactions other than that between influenza virus and pneumococcus is lacking (77), and the question of whether certain viral and bacterial organisms work poorly together or antagonize each other has not been addressed. The mechanisms that might underlie such selectivity are currently unclear.
In children, the most common secondary bacterial infection is acute otitis media (AOM), which affects 70% of all children by the age of 2 years. Respiratory viruses can be detected in the nasopharynxes of up to 90% of children with AOM and in the middle ear fluid of 20% (64). Pneumococcus is the most common bacterial cause of AOM, while multiple viruses, including influenza virus, can predispose to AOM. Sinusitis and community-acquired pneumonia (CAP) are also frequently of polymicrobial etiology in children. S. pneumoniae is the most commonly identified pathogen in hospitalized children with either primary or secondary CAP, accounting for 44% of all episodes and 54% of coinfections with a viral pathogen (109). Influenza viruses are the most common viral contributors, appearing in 22% of CAP cases overall and 39% of mixed infections. In adults, secondary bacterial pneumonia after influenza is an important cause of mortality, particularly in the elderly.
Strain Dependence of Disease
Influenza may cause death in one of three ways. The primary viral infection may be sufficiently virulent in itself to be fatal, death may result from a secondary bacterial infection, or the viral disease may increase the physiologic load in a person with chronic heart, lung, or metabolic disease such that he or she is unable to survive (111). In the majority of instances, the viral infection is not of sufficient virulence to cause death from pulmonary causes alone. Exceptions to this are infections caused by the 1918 pandemic influenza virus strain, which killed many of its victims within a few days of disease onset (1, 167), and the H5N1 avian influenza strains currently circulating in Southeast Asia, which cause a primary viral pneumonia with features characteristic of the acute respiratory distress syndrome that progresses to pulmonary failure (8). Although the primary viral pneumonia which killed many healthy, young persons in 1918 was the most striking clinical feature of this virus, it appears likely that secondary bacterial pneumonia was a more common cause of death during the pandemic. During typical epidemic years, both hospitalizations and deaths are most frequently due to secondary bacterial pneumonia or exacerbation of an underlying condition.
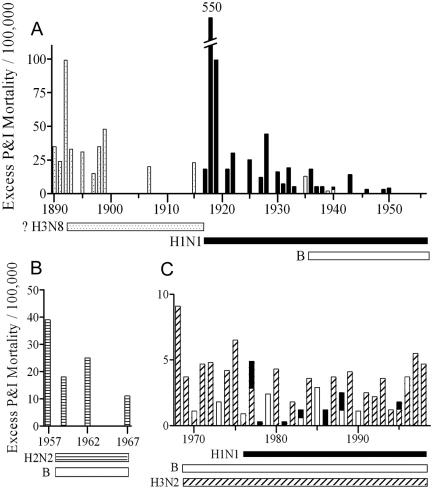
Pneumonia and influenza (P&I) mortality includes deaths from the primary viral infection as well as from secondary bacterial pneumonia, and these are considered together because they are difficult to distinguish using mortality data. This measure does not include deaths attributed to other causes, such as exacerbation of an underlying condition by the viral infection. The percentage of all influenza-associated deaths classified as P&I mortality appears to be strain dependent. For example, the all-cause excess death rate in the United States was 530 to 550 per 100,000 persons in 1918 to 1919, 64.9 to 99.7 per 100,000 in 1928 to 1929, and 44.2 to 49.8 per 100,000 in 1943 to 1944. However, P&I deaths accounted for 98% of all influenza-related deaths in 1918 to 1919 and 54% to 63% in 1928 to 1929 but for only 24% to 35% in 1943 to 1944 (27, 116). In the decade of the 1990s, P&I deaths accounted for only 15.8% of all influenza-related deaths (149). The number of all-cause deaths and the percentage that could be attributed to P&I varied from year to year during that decade, with more deaths and a higher rate of secondary pneumonia in years where an influenza A virus of the H3N2 subtype circulated (149). H3N2 subtype viruses were also associated with a higher rate of P&I hospitalization over 26 influenza seasons from 1970 to 1995 (138). Figure 1 illustrates the excess mortality differences seen the last 3 decades when H3N2 viruses are compared to either H1N1 or B viruses, but it also shows that higher mortality rates were seen with H1N1 viruses earlier in the century. We have proposed that strain-dependent differences in specific viral virulence factors account for these observed differences in excess mortality (122).
FIG. 1.
Excess mortality rates by year in selected cities in the United States. Excess mortality is defined as the number of deaths in excess of those that would be expected for a particular time of year in the absence of influenza; pictured is the rate of excess mortality attributable directly to pneumonia and influenza. Bars define the proportions attributable to different types and subtypes of influenza virus and are stacked together by year. Data are derived from those of Collins and Lehmann (25, 27, 28) (A), Housworth and Spoon (70) (B), and Reichert et al. and Simonsen et al. (128, 136) (C) and were calculated by different methods. Note the different scales. The first known epidemic of influenza B was in 1935, but the virus likely circulated before that time. H1N1 viruses circulated from 1917 to 1957 and then reentered humans in 1976 and have circulated since that time. It is postulated based on serology data that an H3N8 virus circulated prior to 1917.
HISTORICAL CONTEXT
Recognition of Dual Infections
The earliest suggestion that viral infections predispose to bacterial diseases has been attributed to R. T. H. Laennec, a French physician and the inventor of auscultation. He observed that the prevalence of pneumonia increased following an epidemic of influenza (“la grippe”) in 1803 (80). In 1847, the eminent London epidemiologist William Farr, remembered chiefly for his assistance to John Snow in determining that cholera was a waterborne disease, coined the term “excess mortality” to describe the increase in deaths that occurred during the influenza season and that were attributed to causes other than influenza itself. Using this concept, he developed in detail the methodology used today to quantitate mortality in influenza epidemics (43, 82). Selwyn Collins of the U.S. Public Health Service then further refined the terms by which we classify outcomes after influenza in a series of thorough and detailed reports on epidemic mortality in the early part of the 20th century (25-28).
The association between influenza and bacterial pneumonia came into particular focus following the 1918 pandemic, during which an estimated 40 to 50 million persons died (126), most of them from secondary bacterial pneumonia (1, 112, 144, 164). Reviews in the 1920s and 1930s of numerous reports from the 1889 and 1918 pandemics led to the conclusion that bacteria were secondary invaders and not the primary agents of disease (73; also reviewed in reference 88). The first case of secondary bacterial pneumonia proven to follow influenza infection was reported by Andrewes et al. in 1935. They described the recovery of virus from a febrile man who then developed pneumococcal pneumonia 7 days into his convalescence and died (6). Once the viral etiology of influenza was firmly established, a series of detailed studies on the pathology, bacteriology, and epidemiology of bacterial pneumonia during influenza followed over the next decade (47, 97, 120, 132, 145, 156). Influenza A and B viruses were both shown to predispose to bacterial infections, with S. pneumoniae and S. aureus as the most often cited invaders.
Modeling Viral-Bacterial Synergism in Animals
The earliest examples of modeling secondary bacterial infections in animals came during the 1918 pandemic. Wherry and Butterfield passed unfiltered sputum from ill patients by aerosol into a variety of animal species, including guinea pigs, mice, and rats (162). Approximately one-third of the exposed animals developed bacterial pneumonia and died. Although a number of attempts had been made to prove that influenza was caused by a filterable agent both before and after the 1918 pandemic, it was not until the demonstration in animals that canine distemper was caused by a virus that similar experiments were successfully conducted for influenza virus. The original breakthrough came in 1926, when Dunkin and Laidlaw published a series of experiments with dogs and ferrets demonstrating that Bordetella bronchiseptica was a secondary bacterial invader responsible for complications in dogs with a primary viral infection (37-39). Following this work, Shope isolated the agent of swine influenza and postulated that it was related to the virus causing human influenza. He then conducted a classic series of experiments demonstrating that intranasal inoculation of influenza virus or Haemophilus influenzae suis alone caused only mild disease in pigs, while inoculation of both together caused the characteristic severe illness (135).
The first well-controlled study of secondary bacterial pneumonia in mice was conducted by Thomas Francis and Mercedes V. de Torregosa in 1945 (49). They reported that H. influenzae, S. pneumoniae, and S. aureus could all cause a fatal pneumonia in an intranasal coinfection model with the mouse-adapted influenza virus A/Puerto Rico/8/34 (PR8), a strain that is still utilized in most laboratories studying influenza today. Those authors also demonstrated that the dose of infecting agents and the time between infections influenced the outcome. More recently, we have utilized a similar model to study the interaction between influenza viruses and S. pneumoniae (104). Because mouse models are limited to a select set of mouse-adapted viruses, however, we have also developed a ferret model of pneumococcal otitis media and sinusitis following infection with naturally occurring viruses (121, 123). This model is similar to an otitis media model in chinchillas in that it supports coinfections between influenza and pneumococcus (51, 150). However, use of certain strains of pneumococcus engineered to express luciferase has also allowed us to detect secondary bacterial pneumonia and meningitis in young ferrets, enhancing the utility of the model (unpublished data).
Mechanisms Underlying the Interaction
The earliest pathology reports from the 1918 pandemic were primarily descriptive, and it was assumed that since the lung disease was characteristic of bacterial pneumonia in most cases, a bacterial pathogen must be the underlying cause. After careful studies of large numbers of patients, primarily in army camps, the concept emerged that some entity, either a common bacterial pathogen such as H. influenzae or perhaps “an unknown associated virus” (144), “acts as a pioneer and prepares the way for the pneumococci, staphylococci, and streptococci, which are able to grow and multiply in the damaged mucous membrane of the bronchi and subsequently invade the lungs and even the blood” (164). Thus, the idea that a viral infection could damage the epithelial lining of the bronchi and lungs, allowing bacterial pathogens a foothold, was the first proposed mechanism to explain viral-bacterial synergism (112, 167). This explanation become dogma over the next 50 years and was strengthened by pathology studies during the “Asian flu” of 1957 to 1958. Death during that pandemic was frequently due to staphylococcal pneumonia, and autopsy findings suggested that S. aureus was able to adhere to portions of the trachea and bronchi damaged by viral infection (90, 94).
An alternative, or perhaps complementary, mechanism proposes that influenza virus-mediated dysfunction of immune effectors such as neutrophils might compromise local immunity at normally sterile sites such as the ear or alveolus, allowing pneumococcus to cause pyogenic infections (4). Virus-induced leukopenia does occur and has been associated with poor outcomes during secondary bacterial infections (90). In monkeys, influenza virus-induced leukopenia predisposed to secondary bacterial sepsis from a hemolytic group C Streptococcus (165). The leukopenia, granulocytopenia, and severity of viral disease were worsened by stressors such as cold and nutritional deficiencies (131). However, in most cases of secondary bacterial infection leukocytosis, not leukopenia, is a prominent clinical sign, with a preponderance of polymorphonuclear cells and immature forms (90, 94, 95, 156). Activated neutrophils and macrophages in the lung may contribute to the severity of infection by expression of inflammatory mediators, leading to tissue damage (161), particularly when combined with cytotoxins from the bacteria (24). Although these cells may be present in great numbers in the lung, various functional capabilities necessary for the clearance of bacteria may be altered, including chemotaxis, phagocytosis, and bacterial killing (30, 96). Thus, the host may suffer the damaging effects of the inflammatory response without the expected benefit of bacterial clearance. This depression of neutrophil function from influenza viruses has been shown in the chinchilla model to enhance the frequency and severity of secondary bacterial infections (2-4).
VIRAL EFFECTS ON THE HOST
Changes in the Respiratory Tract
The bulk of clinicopathological evidence suggests that virus-induced changes in the respiratory tract prime the upper airway and lung for subsequent bacterial infection. The classic dogma in the field has been that damage to the normally protective epithelial layer exposes extracellular matrix molecules and basement membrane elements to which bacteria can adhere. However, virus-induced changes in eustachian tube or pulmonary function may exacerbate bacterial superinfections, and alterations to the availability and permissivity of bacterial receptors may allow access of bacteria to normally sterile sites.
Epithelial damage.
The concept that influenza virus-induced epithelial damage provides increased numbers of attachment sites for bacteria is supported by mouse studies using the highly pathogenic, mouse-adapted virus PR8 (49, 61, 104, 107). This strain causes severe epithelial damage to the lungs, weight loss, and death in mice at relatively low doses (89). In mice secondarily infected with pneumococcus after infection with PR8, bacterial loads were increased in the lungs, and bacteria could be seen to adhere to areas of the respiratory tract damaged by the cytotoxicity of the virus (61, 125). In studies utilizing unadapted viruses of lower pathogenic potential, however, no pathological damage to the tracheo-bronchial tree was seen, and yet bacteria were able to persist in the lungs for prolonged periods of time (60, 134). We have attempted to distinguish between virus-induced cytotoxic epithelial damage and other effects of infection by using chemical-induced cytotoxicity as a surrogate. Lung epithelial and endothelial damage from 4-ipomeanol, an experimental cancer chemotherapeutic agent which has been shown to induce lung injury in mice similar to that seen with Sendai virus (40, 41), did not enhance secondary bacterial infection in our model (unpublished data). However, both Sendai virus and influenza virus, at low doses that induce minimal pathology, can support secondary invasion (5, 104). Therefore, while pathological studies suggest that epithelial damage is a major factor in human disease with highly virulent viruses such as the 1918 and 1957 pandemic strains, the relative contribution of this mechanism in years when less virulent viruses are circulating may be not be as robust.
Changes in airway function.
Secondary bacterial pneumonia is still a significant cause of morbidity and mortality in years where the viruses are not virulent enough to cause severe lung damage or death from the virus alone. The most likely explanation for this is that the virus can mediate effects on the lung that benefit bacteria other than simple cytotoxicity. Obstruction of the small airways due to disruption of surfactant, increased mucinous secretions combined with fibrin and edema fluid, and an influx of inflammatory cells create dead space and a culture medium for bacteria (60, 89). Pulmonary functional capacity and diffusion capacity are diminished (69, 72). Ciliary function is compromised both by a decreased beat frequency and by uncoordinated activity (68, 83, 119). Similar effects on ciliary activity are seen in the eustachian tube and contribute to otitis media (119). The net effect of these functional limitations in the lungs is decreased mechanical clearance of bacteria, increased airway hyperreactivity, and improved conditions for bacterial growth. In patients with preexisting lung disease such as chronic obstructive pulmonary disease, influenza predisposes to chronic bronchitis, exacerbations of chronic obstructive pulmonary disease, and pneumonia (20, 53, 140, 141).
Up-regulation and exposure of receptors.
The method by which the pneumococcus accesses the lower respiratory tract is not well understood. Furthermore, once there, the pneumococcus is unable to adhere without changes to the epithelium to expose or activate receptors. Aside from adhesins that the bacterium uses to bind to these putative receptors, pneumococcus expresses several virulence factors that may help the process (106). There are three methods by which adherence might be facilitated distinct from cytotoxicity. First, cryptic receptors may be exposed through the enzymatic activity of viral or bacterial NAs, which cleave terminal sialic acids away from cell surface glycoconjugates. Second, inflammation generated by the virus or bacteria may up-regulate inactive receptors. Third, fibrin and fibrinogen deposited during the regenerative process following viral infection may provide attachment sites.
Influenza virus possesses an NA which acts to cleave terminal sialic acid residues, allowing viral particles to be released from infected cells and to spread through mucinous secretions (58). Pneumococcus possesses two NAs (NanA and NanB) (17) and is thought to produce a third (12). The role of pneumococcal NA in the pathogenesis of bacterial infections has been investigated in animal models. Exogenously administered bacterial NA has been shown to increase adherence of pneumococcus in vitro to tracheal (152), eustachian tube (81), and middle ear (86) epithelia in an organ perfusion model using chinchilla tissues. NA is thought to contribute to adherence to epithelial tissues by exposing receptors for pneumococcal adherence and invasion (102, 151). In our mouse model, NA activity of the virus enhanced adherence of pneumococcus to epithelial cells in vitro and predisposed to fatal pneumonia (102). The secondary bacterial pneumonia could be prevented or treated using an inhibitor of the influenza virus NA, independent of the effect on viral lung load (101, 102). Pairs of otherwise isogenic influenza viruses engineered by reverse genetics to express different N2 NAs differentially contributed to both adherence and pneumonia at a rate proportional to their NA activity (122). Furthermore, deletion of the gene (nanA) coding for the major pneumococcal NA attenuates pneumococcus in an intranasal infection model (117), but the full virulence of the mutant can be restored by prior infection with influenza virus (unpublished data). Thus, the combined effect of the bacterial and viral NAs at the site of infection is an important mechanism in synergism between the organisms. Since NAs from N2 subtype influenza viruses have generally higher activity than recent N1 or influenza B virus NAs, this observation might partially explain why virulence and excess P&I mortality have been higher over the last 3 decades in years when H3N2 viruses circulate (52, 137, 149, 169).
The receptors that pneumococcus utilizes to adhere and invade in the lung are currently unknown. One proposed mediator of this process is the receptor for platelet activating factor (PAF-R), a ubiquitous G-coupled protein to which phosphorylcholine expressed on the surface of the pneumococcus can adhere (31, 129). The expression of PAF-R is up-regulated by inflammatory cytokines (32) and has been shown to be an important factor in the transition from the blood to the cerebrospinal fluid during the induction of meningitis (117). We had advanced the hypothesis that the inflammatory response to influenza virus infection up-regulates and activates PAF-R in the lung, providing sites for pneumococcal adherence (104). Preliminary studies using competitive inhibitors of PAF-R did not support this hypothesis, as secondary bacterial pneumonia occurred at the same rate in the presence or absence of inhibition. In knockout mice which lack the PAF-R entirely, ongoing studies support the use of PAF-R by pneumococcus in the transition from the lung compartment into the blood but suggest that the receptor is not necessary for the induction of pneumonia (unpublished data). However, it is still possible that the mechanism is utilized by pneumococcus with another, as-yet-unidentified receptor.
Following the initial insult and the inflammatory changes associated with influenza virus infection, the airway undergoes a regeneration and remodeling phase (89). Although pneumococcus binds poorly to ciliated epithelium, it may adhere more readily to nonciliated, differentiating cells involved in the proliferation and regeneration response (35, 124). Deposition of fibronectin, collagen, and other matrix elements during this process provides further attachment sites for bacteria such as S. pneumoniae and S. aureus. In part, this regeneration process in response to inflammatory damage is driven by the immunoregulatory molecule transforming growth factor β (TGF-β) (99). An intriguing link to the problem of viral-bacterial synergism is the finding that the influenza virus NA can activate latent TGF-β to its active form (133). Thus, the NA activity of the virus may initiate or accelerate the regeneration response, benefiting bacterial bystanders. Using isogenic influenza viruses differing only in their NAs (122), we have found that the magnitude of induction of TGF-β is directly related to the level of NA-specific activity of the virus (unpublished data). One could speculate that differential effects on the postinjury healing response may occur with different strains of the virus.
Alteration of the Innate Immune Response
The host defense against pathogenic microorganisms involves both innate and acquired immunity. The acquired immune responses to influenza virus and pneumococcus are quite different, as CD8+ cell-mediated killing of influenza virus-infected cells facilitates clearance of primary viral infections, while antibody-dependent opsonization and neutrophil-mediated phagocytosis account for the clearance of bacteria from the lung during pneumonia. However, there is considerable overlap in the innate immune responses to the two pathogens. Physical barriers, viscous secretions imbued with nonspecific binding molecules, and ciliary beat to clear this mucus conspire to keep the organisms out of the lungs. Recognition of pathogen-associated molecular patterns by pattern recognition receptors such as the Toll-like receptors (TLRs) leads to activation of signaling cascades and the generation of an inflammatory response (75). The effect of influenza virus on physical barriers has been considered above; here I consider the combined effect of influenza virus and pneumococcus on the inflammatory response.
Elucidation of the pathways involved in generation of inflammation and immunity to pathogens has progressed rapidly in recent years. Both pneumococcus and influenza virus are recognized by TLRs, generating a cytokine response and triggering an influx of immune effector cells. Lipoteichoic acid from the cell wall of S. pneumoniae is recognized by TLR2, resulting in NF-κB activation through pathways involving MyD88 and other cofactors and intermediates (42, 78, 79, 170). Pneumolysin is recognized by TLR4 (15, 92), leading to a MyD88-independent signaling cascade mediated by IRF3 and STAT1 but also leading to NF-κB activation through TRAF6 (13). The combined effect of activation of these pathways is the production of proinflammatory cytokines and chemokines such as interleukin-1 (IL-1), IL-6, tumor necrosis factor alpha (TNF-α), MIP-1-alpha, KC, and gamma interferon, as well as the anti-inflammatory cytokine IL-10 (10, 34). The single-stranded RNA of influenza virus is recognized through TLR7 (91) and perhaps through TLR8 in humans, while double-stranded RNA intermediates formed during influenza virus replication are recognized through TLR3 (59). TLR7 and TLR8 signal through pathways similar to those stimulated by TLR2, requiring MyD88 and leading to NF-κB activation, while TLR3 utilizes the same MyD88-independent pathway as TLR4, leading to IRF3 and STAT1 activation (13). Because the pathways and intermediate signaling molecules are similar, it is not surprising that the proinflammatory response to influenza virus mirrors that to pneumococcus, with induction of IL-1, IL-6, TNF-α, RANTES, MIP-1-alpha, IL-8, and gamma interferon (62, 74). IL-10 is also elevated following influenza virus infection, but this occurs late in the course of illness during the induction of memory and the conversion from TH1 to TH2.
The use of the same pathways, cofactors, and intermediates, and the overlap in the inflammatory mediators produced, creates an opportunity for either interference with or augmentation of the immune response during dual or sequential infection. In a coinfection model using H. influenzae and S. pneumoniae, Ratner et al. demonstrated synergistic activation of proinflammatory cytokines with resulting neutrophil accumulation, albeit in a TLR-independent manner (127). We have attempted to study this possibility in our model by looking at the cytokine response to single infection compared to a synergistic infection. We have found that certain cytokines, including TNF-α, IL-1, IL-6, and IL-10, as well as the neutrophil- and macrophage-chemoattractant chemokines KC and MIP-1α, are strikingly elevated in mice with secondary pneumococcal pneumonia in our model (141a). We hypothesized that this balanced elevation of both proinflammatory and anti-inflammatory cytokines and chemokines was dysfunctional. Large numbers of neutrophils and macrophages were invading the lung, amplifying the immune response and causing inflammatory damage, but were not effectively clearing the bacteria. In a similar model, van der Sluijs et al. proposed that IL-10 was the key mediator of this dysfunctional process, since it has an inhibitory effect on the function of neutrophils during pneumococcal pneumonia (157, 158). Inhibition of IL-10 in mice improved survival from bacterial pneumonia late after influenza virus infection. Thus, the enhanced anti-inflammatory response when influenza virus infection precedes pneumococcal infection may sabotage the ability of the host to successfully eliminate the bacteria before they can do lethal damage.
Alternatively, the proinflammatory response may be the most important factor in poor outcomes from secondary bacterial pneumonia. Recent work from Tumpey et al. suggests that the severe lung inflammation seen with the 1918 pandemic strain (154) may be due to increased levels of proinflammatory cytokines and a massive influx of neutrophils into the lung (155). Similar findings for humans with highly pathogenic H5N1 avian influenza viruses provide further support for this concept (8, 21). Data from our lab suggest that PB1-F2, a recently discovered proapoptotic protein encoded by most human influenza A viruses (19, 50), contributes to this inflammatory response (J. L. McAuley, F. Hornung, K. L. Boyd, R. McKeon, R. Salomon, E. Hoffmann, J. Bennink, J. W. Yewdell, and J. A. McCullers, submitted for publication). In mice, a virus expressing PB1-F2 enhanced secondary bacterial pneumonia, while an isogenic virus unable to express this protein did not (McAuley et al., submitted). The sequences of the PB1 present in H1N1 viruses isolated since 1956 predict that these viruses produce a truncated PB1-F2 protein of 67 amino acids instead of the 87 amino acids found in most other human influenza viruses, due to the introduction of a stop codon. The resulting PB1-F2 protein, if it is expressed, would lack the C-terminal sequences that are required for induction of apoptosis (50). Such a truncated PB1-F2 might not be able to prime the host for secondary bacterial infections as efficiently as viruses expressing a full-length PB1-F2. Thus, differences in the PB1-F2 may contribute to the differences in excess mortality exhibited by H1N1 and H3N2 strains. Further work is needed to establish the precise interactions between the pathways, mediators, and effector cells of the complex cytokine network and inflammatory cascade activated during lung infection with more than one pathogen.
CONTRIBUTION FROM THE BACTERIA
Effects on the Virus
Tashiro et al. were the first to suggest that the synergistic effect might not be unidirectional. Because cleavage of the nascent HA0 glycoprotein to its active components HA1 and HA2 is required for infectivity, and because the virus must utilize tissue-specific proteases to replicate, it was suggested that bacterium-derived proteases might complement this need (146). Proteases derived from S. aureus and concentrated in vitro are able to cleave the HA, supporting the hypothesis (147). Thus, some of the enhanced pathogenicity seen during secondary bacterial pneumonia might be a function of increased viral virulence in concert with the bacterial infection. Louria et al. reviewed 33 cases of severe influenza infection in 1957 and 1958 and found that bacterial infections that occurred coincident with the primary influenza virus infection were more severe and more difficult to treat than bacterial infections that followed influenza with a distinct period of initial recovery between (90). They argued that this represented the effect of the bacteria on the virus, enhancing the lethality of the primary viral disease and explaining why antibiotic therapy was less successful.
In our mouse model of secondary bacterial pneumonia, the viral lung load is increased following bacterial challenge and presumably contributes to the severe lung damage and death of superinfected animals (104). It is unclear at present what mechanism accounts for this increased viral titer. Pneumococcus does possess several putative proteases, so cleavage activation of the HA is one potential answer. To this point we have examined only one of these candidates, choline binding protein G (CbpG), a multifunctional protein that shares sequence homology with serine proteases (55, 93). In our model the absence of CbpG did not affect viral lung load or the induction of secondary pneumonia (J. A. McCullers, A. R. Iverson, K. L. Boyd, and C. J. Orihuela, submitted for publication). Another potential candidate, the pneumococcal NA NanA, also does not enhance viral titers in the lung and did not have an additive effect on the incidence or severity of secondary bacterial pneumonia (unpublished data). Examination of other pneumococcal virulence factors in the context of dual-infection models is necessary to answer this important question.
Enhancement of Inflammation
Several components of the pneumococcus contribute to the induction of inflammation, including the cell wall, the cytotoxin pneumolysin, and the pyruvate oxidase SpxB (24, 46, 54, 75, 106, 142). SpxB is responsible for endogenous H2O2 production by pneumococcus and contributes to cell damage and to inflammation during infection. Studies using a deletion mutant suggest that SpxB is involved in the pathogenesis of lung and systemic infections in mice (117). In our mouse model of secondary bacterial pneumonia following influenza, we found SpxB to be an indispensable virulence factor during pneumococcal superinfection (McCullers et al., submitted). The incidence of secondary pneumococcal pneumonia was decreased with a mutant lacking SpxB, and the inflammatory response to the coinfection was greatly diminished in those mice that did develop pneumonia. In combination with the knockout virus lacking PB1-F2, the incidence of pneumonia was further decreased. These data demonstrate the impact that a single virulence factor from the virus or the bacterium can have on the synergistic inflammatory response. It is likely that multiple factors contribute to the interaction and account for the intense reaction to dual infections.
TREATMENT AND PREVENTION
Given the prominent role that influenza plays in the development of secondary bacterial infections, it would seem logical that prevention or treatment of the predisposing viral illness would ameliorate subsequent bacterial complications. We have addressed the treatment issue in our mouse model of secondary bacterial pneumonia. The NA inhibitor oseltamivir could prevent or treat pneumonia alone or in combination with an antibacterial compound (101, 102). The importance of the NA as a mediator of the synergistic interaction was emphasized in the latter study, as inhibition of the virus with rimantadine, an antiviral compound that targets the influenza A virus M2 ion channel, did not have a similar effect on bacterial outcomes. The timing of administration of oseltamivir was important, as the drug had to be given before challenge with bacteria, but it could mediate an effect up to 5 days after the primary viral infection (101). Vaccination against the influenza virus HA or NA or both is effective at preventing secondary bacterial pneumonia in this model (unpublished data).
Studies with humans support the preclinical data from mice. Oseltamivir treatment of influenza virus-infected children reduced both the development of acute otitis media and the need for antibiotics (163). In healthy adults aged 18 to 65 years, oseltamivir treatment of influenza had similar effects, reducing the occurrence of secondary complications, as well as antibiotic use (153). Analyses of multiple trials studying treatment of influenza with NA inhibitors concluded that lower respiratory tract complications, antibiotic use, and hospitalizations could be prevented both in healthy adults under 65 years old and in those who were 65 years or older or had chronic illnesses (29, 76). Similar effects on secondary complications such as bacterial pneumonia have not been seen with the M2 inhibitors amantadine and rimantadine (36, 110).
Influenza vaccination in humans appears to confer a substantial benefit in the reduction of secondary complications as well as P&I mortality. In children, inactivated influenza vaccine reduced the incidence of acute otitis media during influenza outbreaks by 36% and 32% in two different studies designed to evaluate effects on otitis media (23, 65). Similarly, a live, attenuated intranasal influenza vaccine reduced acute otitis media with fever by 30% (9). In elderly adults, vaccination against influenza prevented pneumonia hospitalizations (45, 113, 114) and, in one study, had a substantial effect on all-cause mortality (115). Vaccination with both influenza vaccine and 23-valent pneumococcal polysaccharide vaccine reduced hospitalizations for pneumonia, invasive pneumococcal infections, and mortality (22, 63). Although the overall impact on mortality was likely overestimated in these observational studies (139), the trend in reduction of serious wintertime illness in high-risk groups is clear.
SUMMARY OF A MULTIFACTORIAL PROCESS
The full details of the mechanisms of synergism between influenza virus and pneumococcus remain to be elucidated. There is ample evidence that influenza virus alters the host in a way that predisposes to adherence, invasion, and induction of disease by pneumococcus. Access to receptors is a key factor and may be facilitated by the virus through epithelial damage, by exposure or up-regulation of existing receptors, or by provoking the regeneration response of epithelia following cytotoxic damage. Alteration of the immune response either by diminishing the ability of the host to clear pneumococcus or by amplification of the inflammatory cascade likely contributes to the severity of the resulting infection. Although the precise contributions of the many virulence factors expressed by these important pathogens are not completely understood, it is clear that the process is multifactorial and complex. It is likely that differences in these virulence factors between strains of the virus or the bacterium account in part for differences in the spectrum and severity of disease in humans. The insight gained into the pathogenesis of the interaction between influenza virus and pneumococcus may serve as a starting point towards the elucidation of the mechanisms underlying the many other polymicrobial interactions that contribute to disease in humans and animals.
CONCLUDING REMARKS
The repeated incursions of highly pathogenic H5N1 avian influenza viruses into humans in Southeast Asia are a warning that we are overdue for the next influenza pandemic. Based on history, it can be predicted that much of the morbidity and mortality from this pandemic will stem from bacterial complications. It is therefore imperative that we have a firm understanding of the molecular basis of the interaction between influenza and the epidemiologically important bacteria that follow it—S. pneumoniae and S. aureus. Effective vaccination and timely use of the NA inhibitor class of drugs will likely have an impact on the incidence and severity of secondary bacterial complications during a pandemic. However, the availability of the drugs in this class is limited, and ongoing hurdles to developing an effective vaccine exist. Targeting viral or bacterial virulence factors that participate in the interaction between these organisms by using novel vaccine or anti-infective approaches should be a priority for the scientific and pharmaceutical communities.
Acknowledgments
I am indebted to Elaine Tuomanen, Robert Webster, Lone Simonsen, Stacey Schultz-Cherry, and Jeffrey Taubenberger for numerous helpful conversations and insight on the subject of this review.
Much of the work described here was supported by NIH (grants AI-49178, AI-54802, and AI-66349) and by the American Lebanese Syrian Associated Charities (ALSAC).
REFERENCES
- 1.Abrahams, A., N. Hallows, and H. French. 1919. A further investigation into influenzo-pneumococcal and influenzo-streptococcal septicaemia. Lancet 1919:1-11. [Google Scholar]
- 2.Abramson, J. S., G. S. Giebink, E. L. Mills, and P. G. Quie. 1981. Polymorphonuclear leukocyte dysfunction during influenza virus infection in chinchillas. J. Infect. Dis. 143:836-845. [DOI] [PubMed] [Google Scholar]
- 3.Abramson, J. S., G. S. Giebink, and P. G. Quie. 1982. Influenza A virus-induced polymorphonuclear leukocyte dysfunction in the pathogenesis of experimental pneumococcal otitis media. Infect. Immun. 36:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abramson, J. S., E. L. Mills, G. S. Giebink, and P. G. Quie. 1982. Depression of monocyte and polymorphonuclear leukocyte oxidative metabolism and bactericidal capacity by influenza A virus. Infect. Immun. 35:350-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alymova, I. V., A. Portner, T. Takimoto, K. L. Boyd, Y. S. Babu, and J. A. McCullers. 2005. The novel parainfluenza virus hemagglutinin-neuraminidase inhibitor BCX 2798 prevents lethal synergism between a paramyxovirus and Streptococcus pneumoniae. Antimicrob. Agents Chemother. 49:398-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrewes, C. H., P. P. Laidlaw, and W. Smith. 1935. Influenza: observations on the recovery of virus from man and on the antibody content of human sera. Br. J. Exp. Pathol. 16:566-582. [Google Scholar]
- 7.Bakaletz, L. O. 2004. Developing animal models for polymicrobial diseases. Nat. Rev. Microbiol. 2:552-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beigel, J. H., J. Farrar, A. M. Han, F. G. Hayden, R. Hyer, J. de, S. Lochindarat, T. K. Nguyen, T. H. Nguyen, T. H. Tran, A. Nicoll, S. Touch, and K. Y. Yuen. 2005. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 353:1374-1385. [DOI] [PubMed] [Google Scholar]
- 9.Belshe, R. B., P. M. Mendelman, J. Treanor, J. King, W. C. Gruber, P. Piedra, D. I. Bernstein, F. G. Hayden, K. Kotloff, K. Zangwill, D. Iacuzio, and M. Wolff. 1998. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N. Engl. J. Med. 338:1405-1412. [DOI] [PubMed] [Google Scholar]
- 10.Bergeron, Y., N. Ouellet, A. M. Deslauriers, M. Simard, M. Olivier, and M. G. Bergeron. 1998. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect. Immun. 66:912-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berkley, J. A., B. S. Lowe, I. Mwangi, T. Williams, E. Bauni, S. Mwarumba, C. Ngetsa, M. P. Slack, S. Njenga, C. A. Hart, K. Maitland, M. English, K. Marsh, and J. A. Scott. 2005. Bacteremia among children admitted to a rural hospital in Kenya. N. Engl. J. Med. 352:39-47. [DOI] [PubMed] [Google Scholar]
- 12.Berry, A. M., and J. C. Paton. 2000. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect. Immun. 68:133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beutler, B. 2004. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430:257-263. [DOI] [PubMed] [Google Scholar]
- 14.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 15.Branger, J., S. Knapp, S. Weijer, J. C. Leemans, J. M. Pater, P. Speelman, S. Florquin, and T. van der Poll. 2004. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect. Immun. 72:788-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridy-Pappas, A. E., M. B. Margolis, K. J. Center, and D. J. Isaacman. 2005. Streptococcus pneumoniae: description of the pathogen, disease epidemiology, treatment, and prevention. Pharmacotherapy 25:1193-1212. [DOI] [PubMed] [Google Scholar]
- 17.Camara, M., T. J. Mitchell, P. W. Andrew, and G. J. Boulnois. 1991. Streptococcus pneumoniae produces at least two distinct enzymes with neuraminidase activity: cloning and expression of a second neuraminidase gene in Escherichia coli. Infect. Immun. 59:2856-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell, J. D., K. L. Kotloff, S. O. Sow, M. Tapia, M. M. Keita, T. Keita, S. Diallo, J. C. Hormazabal, P. Murray, and M. M. Levine. 2004. Invasive pneumococcal infections among hospitalized children in Bamako, Mali. Pediatr. Infect. Dis. J. 23:642-649. [DOI] [PubMed] [Google Scholar]
- 19.Chen, W., P. A. Calvo, D. Malide, J. Gibbs, U. Schubert, I. Bacik, S. Basta, R. O'Neill, J. Schickli, P. Palese, P. Henklein, J. R. Bennink, and J. W. Yewdell. 2001. A novel influenza A virus mitochondrial protein that induces cell death. Nat. Med. 7:1306-1312. [DOI] [PubMed] [Google Scholar]
- 20.Chen, Y., P. Stewart, R. Dales, H. Johansen, S. Bryan, and G. Taylor. 2005. In a retrospective study of chronic obstructive pulmonary disease inpatients, respiratory comorbidities were significantly associated with prognosis. J. Clin. Epidemiol. 58:1199-1205. [DOI] [PubMed] [Google Scholar]
- 21.Cheung, C. Y., L. L. Poon, A. S. Lau, W. Luk, Y. L. Lau, K. F. Shortridge, S. Gordon, Y. Guan, and J. S. Peiris. 2002. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 360:1831-1837. [DOI] [PubMed] [Google Scholar]
- 22.Christenson, B., P. Lundbergh, J. Hedlund, and A. Ortqvist. 2001. Effects of a large-scale intervention with influenza and 23-valent pneumococcal vaccines in adults aged 65 years or older: a prospective study. Lancet 357:1008-1011. [DOI] [PubMed] [Google Scholar]
- 23.Clements, D. A., L. Langdon, C. Bland, and E. Walter. 1995. Influenza A vaccine decreases the incidence of otitis media in 6- to 30-month-old children in day care. Arch. Pediatr. Adolesc. Med. 149:1113-1117. [DOI] [PubMed] [Google Scholar]
- 24.Cockeran, R., A. J. Theron, H. C. Steel, N. M. Matlola, T. J. Mitchell, C. Feldman, and R. Anderson. 2001. Proinflammatory interactions of pneumolysin with human neutrophils. J. Infect. Dis. 183:604-611. [DOI] [PubMed] [Google Scholar]
- 25.Collins, S. D. 1930. Influenza-pneumonia mortality in a group of about 95 cities in the United States, 1920-1929. Public Health Rep. 45:361-406. [Google Scholar]
- 26.Collins, S. D. 1952. Excess mortality from causes other than influenza and pneumonia during influenza epidemics. Public Health Rep. 47:2159-2179. [Google Scholar]
- 27.Collins, S. D. 1945. Influenza and pneumonia excess mortality at specific ages in the epidemic of 1943-44, with comparative data for preceding epidemics. Public Health Rep. 60:821-835. [Google Scholar]
- 28.Collins, S. D., and J. Lehmann. 1951. Trends and epidemics of influenza and pneumonia: 1918-1951. Public Health Rep. 66:1487-1516. [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper, N. J., A. J. Sutton, K. R. Abrams, A. Wailoo, D. Turner, and K. G. Nicholson. 2003. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. Br. Med. J. 326:1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craft, A. W., M. M. Reid, and W. T. Low. 1976. Effect of virus infections on polymorph function in children. Br. Med. J. 1:1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cundell, D. R., C. Gerard, I. Idanpaan-Heikkila, E. I. Tuomanen, and N. P. Gerard. 1996. PAf receptor anchors Streptococcus pneumoniae to activated human endothelial cells. Adv. Exp. Med. Biol. 416:89-94. [DOI] [PubMed] [Google Scholar]
- 32.Cundell, D. R., N. P. Gerard, C. Gerard, I. Idanpaan-Heikkila, and E. I. Tuomanen. 1995. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377:435-438. [DOI] [PubMed] [Google Scholar]
- 33.Cutts, F. T., S. M. Zaman, G. Enwere, S. Jaffar, O. S. Levine, J. B. Okoko, C. Oluwalana, A. Vaughan, S. K. Obaro, A. Leach, K. P. McAdam, E. Biney, M. Saaka, U. Onwuchekwa, F. Yallop, N. F. Pierce, B. M. Greenwood, and R. A. Adegbola. 2005. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 365:1139-1146. [DOI] [PubMed] [Google Scholar]
- 34.Dallaire, F., N. Ouellet, Y. Bergeron, V. Turmel, M. C. Gauthier, M. Simard, and M. G. Bergeron. 2001. Microbiological and inflammatory factors associated with the development of pneumococcal pneumonia. J. Infect. Dis. 184:292-300. [DOI] [PubMed] [Google Scholar]
- 35.de Bentzmann, S., C. Plotkowski, and E. Puchelle. 1996. Receptors in the Pseudomonas aeruginosa adherence to injured and repairing airway epithelium. Am. J. Respir. Crit. Care Med. 154:S155-S162. [DOI] [PubMed] [Google Scholar]
- 36.Doyle, W. J., D. P. Skoner, C. M. Alper, G. Allen, S. A. Moody, J. T. Seroky, and F. G. Hayden. 1998. Effect of rimantadine treatment on clinical manifestations and otologic complications in adults experimentally infected with influenza A (H1N1) virus. J. Infect. Dis. 177:1260-1265. [DOI] [PubMed] [Google Scholar]
- 37.Dunkin, G. W., and P. P. Laidlaw. 1926. Studies in dog-distemper. III. The nature of the virus. J. Comp. Pathol. 39:222-230. [Google Scholar]
- 38.Dunkin, G. W., and P. P. Laidlaw. 1926. Studies in dog-distemper. II. Experimental distemper in the dog. J. Comp. Pathol. 39:213-221. [Google Scholar]
- 39.Dunkin, G. W., and P. P. Laidlaw. 1926. Studies in dog distemper. I. Dog-distemper in the ferret. J. Comp. Pathol. 39:201-212. [Google Scholar]
- 40.Durham, S. K., J. G. Babish, and W. L. Castleman. 1987. 4-Ipomeanol-induced effects on Sendai viral pneumonia in mice. Am. J. Pathol. 126:364-375. [PMC free article] [PubMed] [Google Scholar]
- 41.Durham, S. K., M. R. Boyd, and W. L. Castleman. 1985. Pulmonary endothelial and bronchiolar epithelial lesions induced by 4-ipomeanol in mice. Am. J. Pathol. 118:66-75. [PMC free article] [PubMed] [Google Scholar]
- 42.Echchannaoui, H., K. Frei, C. Schnell, S. L. Leib, W. Zimmerli, and R. Landmann. 2002. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J. Infect. Dis. 186:798-806. [DOI] [PubMed] [Google Scholar]
- 43.Farr, W. 1885. In N. A. Humphreys (ed.), Vital statistics: a memorial volume of selections from the reports and writings of William Farr. p. 330. Office of the Sanitary Institute, London, United Kingdom.
- 44.Fedson, D. S., J. Anthony, and G. Scott. 1999. The burden of pneumococcal disease among adults in developed and developing countries: what is and is not known. Vaccine 17(Suppl. 1):S11-S18. [DOI] [PubMed] [Google Scholar]
- 45.Fedson, D. S., A. Wajda, J. P. Nicol, G. W. Hammond, D. L. Kaiser, and L. L. Roos. 1993. Clinical effectiveness of influenza vaccination in Manitoba. JAMA 270:1956-1961. [PubMed] [Google Scholar]
- 46.Feldman, C., T. J. Mitchell, P. W. Andrew, G. J. Boulnois, R. C. Read, H. C. Todd, P. J. Cole, and R. Wilson. 1990. The effect of Streptococcus pneumoniae pneumolysin on human respiratory epithelium in vitro. Microb. Pathog. 9:275-284. [DOI] [PubMed] [Google Scholar]
- 47.Finland, M., M. W. Barnes, and B. A. Samper. 1945. Influenza virus isolations and serological studies made in Boston during the winter of 1943-1944. J. Clin. Investig. 24:192-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fouchier, R. A., V. Munster, A. Wallensten, T. M. Bestebroer, S. Herfst, D. Smith, G. F. Rimmelzwaan, B. Olsen, and A. D. Osterhaus. 2005. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 79:2814-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francis, T. E., and M. V. de Torregrosa. 1945. Combined infection of mice with H. influenzae and influenza virus by the intranasal route. J. Infect. Dis. 76:70-77. [Google Scholar]
- 50.Gibbs, J. S., D. Malide, F. Hornung, J. R. Bennink, and J. W. Yewdell. 2003. The influenza A virus PB1-F2 protein targets the inner mitochondrial membrane via a predicted basic amphipathic helix that disrupts mitochondrial function. J. Virol. 77:7214-7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giebink, G. S. 1999. Otitis media: the chinchilla model. Microb. Drug Resist. 5:57-72. [DOI] [PubMed] [Google Scholar]
- 52.Glezen, W. P. 1982. Serious morbidity and mortality associated with influenza epidemics. Epidemiol. Rev. 4:25-44. [DOI] [PubMed] [Google Scholar]
- 53.Glezen, W. P., S. B. Greenberg, R. L. Atmar, P. A. Piedra, and R. B. Couch. 2000. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA 283:499-505. [DOI] [PubMed] [Google Scholar]
- 54.Gosink, K., and E. Tuomanen. 2000. Streptococcus pneumoniae: invasion and inflammation, p. 214-224. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 55.Gosink, K. K., E. R. Mann, C. Guglielmo, E. I. Tuomanen, and H. R. Masure. 2000. Role of novel choline binding proteins in virulence of Streptococcus pneumoniae. Infect. Immun. 68:5690-5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gray, B. M., G. M. Converse III, and H. C. Dillon, Jr. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142:923-933. [DOI] [PubMed] [Google Scholar]
- 57.Gray, B. M., and H. C. J. Dillon. 1986. Clinical and epidemiologic studies of pneumococcal infection in children. Pediatr. Infect. Dis. J. 5:201-207. [DOI] [PubMed] [Google Scholar]
- 58.Gubareva, L. V., L. Kaiser, and F. G. Hayden. 2000. Influenza virus neuraminidase inhibitors. Lancet 355:827-835. [DOI] [PubMed] [Google Scholar]
- 59.Guillot, L., G. R. Le, S. Bloch, N. Escriou, S. Akira, M. Chignard, and M. Si-Tahar. 2005. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280:5571-5580. [DOI] [PubMed] [Google Scholar]
- 60.Harford, C. G., and M. Hara. 1950. Pulmonary edema in influenzal pneumonia of the mouse and the relation of fluid in the lung to the inception of pneumococcal pneumonia. J. Exp. Med. 91:245-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harford, C. G., V. Leidler, and M. Hara. 1949. Effect of the lesion due to influenza virus on the resistance of mice to inhaled pneumococci. J. Exp. Med. 89:53-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayden, F. G., R. Fritz, M. C. Lobo, W. Alvord, W. Strober, and S. E. Straus. 1998. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J. Clin. Investig. 101:643-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hedlund, J., B. Christenson, P. Lundbergh, and A. Ortqvist. 2003. Effects of a large-scale intervention with influenza and 23-valent pneumococcal vaccines in elderly people: a 1-year follow-up. Vaccine 21:3906-3911. [DOI] [PubMed] [Google Scholar]
- 64.Heikkinen, T., and T. Chonmaitree. 2003. Importance of respiratory viruses in acute otitis media. Clin. Microbiol. Rev. 16:230-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heikkinen, T., O. Ruuskanen, M. Waris, T. Ziegler, M. Arola, and P. Halonen. 1991. Influenza vaccination in the prevention of acute otitis media in children. Am. J. Dis. Child. 145:445-448. [DOI] [PubMed] [Google Scholar]
- 66.Hendley, J. O., M. A. Sande, P. M. Stewart, and J. M. J. Gwaltney. 1975. Spread of Streptococcus pneumoniae in families. I. Carriage rates and distribution of types. J. Infect. Dis. 132:55-61. [DOI] [PubMed] [Google Scholar]
- 67.Hodges, R. G., C. M. MacLeod, and W. G. Bernhard. 1946. Epidemic pneumococcal pneumonia. III. Pneumococcal carrier studies. Am. J. Hygiene. 44:207-212. [DOI] [PubMed] [Google Scholar]
- 68.Hoke, C. H., Jr., J. A. Hopkins, G. Meiklejohn, and S. R. Mostow. 1979. Comparison of several wild-type influenza viruses in the ferret tracheal organ culture system. Rev. Infect. Dis. 1:946-954. [DOI] [PubMed] [Google Scholar]
- 69.Horner, G. J. and F. D. Gray, Jr. 1973. Effect of uncomplicated, presumptive influenza on the diffusing capacity of the lung. Am. Rev. Respir. Dis. 108:866-869. [DOI] [PubMed] [Google Scholar]
- 70.Housworth, W. J., and M. M. Spoon. 1971. The age distribution of excess mortality during A2 Hong Kong influenza epidemics compared with earlier A2 outbreaks. Am. J. Epidemiol. 94:348-350. [DOI] [PubMed] [Google Scholar]
- 71.Hussain, M., A. Melegaro, R. G. Pebody, R. George, W. J. Edmunds, R. Talukdar, S. A. Martin, A. Efstratiou, and E. Miller. 2005. A longitudinal household study of Streptococcus pneumoniae nasopharyngeal carriage in a UK setting. Epidemiol. Infect. 133:891-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johanson, W. G., Jr., A. K. Pierce, and J. P. Sanford. 1969. Pulmonary function in uncomplicated influenza. Am. Rev. Respir. Dis. 100:141-146. [DOI] [PubMed] [Google Scholar]
- 73.Jordan, E. O. 1927. Epidemic influenza, p. 356-438. American Medical Association, Chicago, Ill.
- 74.Julkunen, I., T. Sareneva, J. Pirhonen, T. Ronni, K. Melen, and S. Matikainen. 2001. Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 12:171-180. [DOI] [PubMed] [Google Scholar]
- 75.Kadioglu, A., and P. W. Andrew. 2004. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol. 25:143-149. [DOI] [PubMed] [Google Scholar]
- 76.Kaiser, L., C. Wat, T. Mills, P. Mahoney, P. Ward, and F. Hayden. 2003. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch. Intern. Med. 163:1667-1672. [DOI] [PubMed] [Google Scholar]
- 77.Kleemola, M., J. Nokso-Koivisto, E. Herva, R. Syrjanen, M. Lahdenkari, T. Kilpi, and T. Hovi. 2005. Is there any specific association between respiratory viruses and bacteria in acute otitis media of young children? J. Infect. 52:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koedel, U., B. Angele, T. Rupprecht, H. Wagner, A. Roggenkamp, H. W. Pfister, and C. J. Kirschning. 2003. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J. Immunol. 170:438-444. [DOI] [PubMed] [Google Scholar]
- 79.Koedel, U., T. Rupprecht, B. Angele, J. Heesemann, H. Wagner, H. W. Pfister, and C. J. Kirschning. 2004. MyD88 is required for mounting a robust host immune response to Streptococcus pneumoniae in the CNS. Brain 127:1437-1445. [DOI] [PubMed] [Google Scholar]
- 80.Laennec, R. T. H. 1923. Translation of selected passages from De l'Auscultation Mediate, p. 88-95. Williams Wood & Co., New York, N.Y.
- 81.LaMarco, K. L., W. F. Diven, and R. H. Glew. 1986. Experimental alteration of chinchilla middle ear mucosae by bacterial neuraminidase. Ann. Otol. Rhinol. Laryngol. 95:304-308. [DOI] [PubMed] [Google Scholar]
- 82.Langmuir, A. D. 1976. William Farr: founder of modern concepts of surveillance. Int. J. Epidemiol. 5:13-18. [DOI] [PubMed] [Google Scholar]
- 83.Levandowski, R. A., T. R. Gerrity, and C. S. Garrard. 1985. Modifications of lung clearance mechanisms by acute influenza A infection. J. Lab. Clin. Med. 106:428-432. [PubMed] [Google Scholar]
- 84.Lexau, C. A., R. Lynfield, R. Danila, T. Pilishvili, R. Facklam, M. M. Farley, L. H. Harrison, W. Schaffner, A. Reingold, N. M. Bennett, J. Hadler, P. R. Cieslak, and C. G. Whitney. 2005. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 294:2043-2051. [DOI] [PubMed] [Google Scholar]
- 85.Li, K. S., Y. Guan, J. Wang, G. J. Smith, K. M. Xu, L. Duan, A. P. Rahardjo, P. Puthavathana, C. Buranathai, T. D. Nguyen, A. T. Estoepangestie, A. Chaisingh, P. Auewarakul, H. T. Long, N. T. Hanh, R. J. Webby, L. L. Poon, H. Chen, K. F. Shortridge, K. Y. Yuen, R. G. Webster, and J. S. Peiris. 2004. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 430:209-213. [DOI] [PubMed] [Google Scholar]
- 86.Linder, T. E., D. J. Lim, and T. F. DeMaria. 1992. Changes in the structure of the cell surface carbohydrates of the chinchilla tubotympanum following Streptococcus pneumoniae-induced otitis media. Microb. Pathog. 13:293-303. [DOI] [PubMed] [Google Scholar]
- 87.Lindstrom, S. E., Y. Hiromoto, R. Nerome, K. Omoe, S. Sugita, Y. Yamazaki, T. Takahashi, and K. Nerome. 1998. Phylogenetic analysis of the entire genome of influenza A (H3N2) viruses from Japan: evidence for genetic reassortment of the six internal genes. J. Virol. 72:8021-8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loosli, C. G. 1968. Synergism between respiratory viruses and bacteria. Yale J. Biol. Med. 40:522-540. [PMC free article] [PubMed] [Google Scholar]
- 89.Loosli, C. G., S. F. Stinson, D. P. Ryan, M. S. Hertweck, J. D. Hardy, and R. Serebrin. 1975. The destruction of type 2 pneumocytes by airborne influenza PR8-A virus; its effect on surfactant and lecithin content of the pneumonic lesions of mice. Chest 67:7S-14S. [DOI] [PubMed] [Google Scholar]
- 90.Louria, D., H. Blumenfeld, J. Ellis, E. D. Kilbourne, and D. Rogers. 1959. Studies on influenza in the pandemic of 1957-58. II. Pulmonary complications of influenza. J. Clin. Investig. 38:213-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Malley, R., P. Henneke, S. C. Morse, M. J. Cieslewicz, M. Lipsitch, C. M. Thompson, E. Kurt-Jones, J. C. Paton, M. R. Wessels, and D. T. Golenbock. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 100:1966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mann, B., C. J. Orihuela, J. Antikainen, G. Gao, J. E. Sublett, T. K. Korhonen, and E. Tuomanen. 2006. Multifunctional role of choline binding protein G in pneumococcal pathogenesis. Infect. Immun. 74:821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martin, C. M., C. M. Kunin, L. S. Gottlieb, M. W. Barnes, C. Liu, and M. Finland. 1959. Asian influenza A in Boston, 1957-1958. I. Observations in thirty-two influenza-associated fatal cases. AMA Arch. Intern. Med. 103:515-531. [DOI] [PubMed] [Google Scholar]
- 95.Martin, C. M., C. M. Kunin, L. S. Gottlieb, and M. Finland. 1959. Asian influenza A in Boston, 1957-1958. II. Severe staphylococcal pneumonia complicating influenza. AMA Arch. Intern. Med. 103:532-542. [DOI] [PubMed] [Google Scholar]
- 96.Martin, R. R., R. B. Couch, S. B. Greenberg, T. R. Cate, and G. A. Warr. 1981. Effects of infection with influenza virus on the function of polymorphonuclear leukocytes. J. Infect. Dis. 144:279-280. [DOI] [PubMed] [Google Scholar]
- 97.Maxwell, E. S., T. G. Ward, and T. E. Van Metre. 1949. The relation of influenza virus and bacteria in the etiology of pneumonia. J. Clin. Investig. 28:307-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reference deleted.
- 99.McCartney-Francis, N. L., and S. M. Wahl. 1994. Transforming growth factor beta: a matter of life and death. J. Leukoc. Biol. 55:401-409. [DOI] [PubMed] [Google Scholar]
- 100.McCullers, J. A. 2006.. Influenza mutations: trying to hit a moving target. Fut. Virol. 1:255-258. [Google Scholar]
- 101.McCullers, J. A. 2004. Effect of antiviral treatment on the outcome of secondary bacterial pneumonia after influenza. J. Infect. Dis. 190:519-526. [DOI] [PubMed] [Google Scholar]
- 102.McCullers, J. A., and K. C. Bartmess. 2003. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J. Infect. Dis. 187:1000-1009. [DOI] [PubMed] [Google Scholar]
- 103.Reference deleted.
- 104.McCullers, J. A., and J. E. Rehg. 2002. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J. Infect. Dis. 186:341-350. [DOI] [PubMed] [Google Scholar]
- 105.McCullers, J. A., T. Saito, and A. R. Iverson. 2004. Multiple genotypes of influenza B virus circulated between 1979 and 2003. J. Virol. 78:12817-12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McCullers, J. A., and E. I. Tuomanen. 2001. Molecular pathogenesis of pneumococcal pneumonia. Front. Biosci. 6:D877-D889. [DOI] [PubMed] [Google Scholar]
- 107.McCullers, J. A., and R. G. Webster. 2001. A mouse model of dual infection with influenza virus and Streptococcus pneumoniae, p. 601-607. In A. D. M. E. Osterhaus, N. Cox, and A. W. Hampson (ed.), Options for the control of influenza IV. Elsevier Science B.V., Amsterdam, The Netherlands.
- 108.Meltzer, M. I., N. J. Cox, and K. Fukuda. 1999. The economic impact of pandemic influenza in the United States: priorities for intervention. Emerg. Infect. Dis. 5:659-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Michelow, I. C., K. Olsen, J. Lozano, N. K. Rollins, L. B. Duffy, T. Ziegler, J. Kauppila, M. Leinonen, and G. H. McCracken, Jr. 2004. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics 113:701-707. [DOI] [PubMed] [Google Scholar]
- 110.Monto, A. S. 2003. The role of antivirals in the control of influenza. Vaccine 21:1796-1800. [DOI] [PubMed] [Google Scholar]
- 111.Mote, J. R. 1940. Virus and rickettsial diseases, p. 429-516. Harvard University Press, Cambridge, Mass.
- 112.Muir, R., and G. H. Wilson. 1919. Influenza and its complications. Br. Med. J. i:3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nichol, K. L., K. L. Margolis, J. Wuorenma, and T. von Sternberg. 1994. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N. Engl. J. Med. 331:778-784. [DOI] [PubMed] [Google Scholar]
- 114.Nichol, K. L., J. Wuorenma, and T. von Sternberg. 1998. Benefits of influenza vaccination for low-, intermediate-, and high-risk senior citizens. Arch. Intern. Med. 158:1769-1776. [DOI] [PubMed] [Google Scholar]
- 115.Nordin, J., J. Mullooly, S. Poblete, R. Strikas, R. Petrucci, F. Wei, B. Rush, B. Safirstein, D. Wheeler, and K. L. Nichol. 2001. Influenza vaccine effectiveness in preventing hospitalizations and deaths in persons 65 years or older in Minnesota, New York, and Oregon: data from 3 health plans. J. Infect. Dis. 184:665-670. [DOI] [PubMed] [Google Scholar]
- 116.Olson, D. R., L. Simonsen, P. J. Edelson, and S. S. Morse. 2005. Epidemiological evidence of an early wave of the 1918 influenza pandemic in New York City. Proc. Natl. Acad. Sci. USA 102:11059-11063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Orihuela, C. J., G. Gao, K. P. Francis, J. Yu, and E. I. Tuomanen. 2004. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J. Infect. Dis. 190:1661-1669. [DOI] [PubMed] [Google Scholar]
- 118.Ostroff, S. M., and J. W. Leduc. 2000. Global epidemiology of infectious diseases, p. 167-169. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Mandell, Douglas, and Bennett's principles and practices of infectious diseases. Churchill Livingstone, Philadelphia, Pa.
- 119.Park, K., L. O. Bakaletz, J. M. Coticchia, and D. J. Lim. 1993. Effect of influenza A virus on ciliary activity and dye transport function in the chinchilla eustachian tube. Ann. Otol. Rhinol. Laryngol. 102:551-558. [DOI] [PubMed] [Google Scholar]
- 120.Pearson, H. E., E. C. Eppinger, J. H. Dingle, and J. F. Enders. 1941. A study of influenza in Boston during the winter of 1940-1941. N. Engl. J. Med. 225:763-770. [Google Scholar]
- 121.Peltola, V. T., J. McAuley, J. E. Rehg, K. L. Boyd, and J. A. McCullers. 2006. Bacterial otitis media and sinusitis in ferrets following influenza. Infect. Immun. 74:2562-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peltola, V. T., K. G. Murti, and J. A. McCullers. 2005. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J. Infect. Dis. 192:249-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peltola, V. T., J. E. Rehg, and J. A. McCullers. 2004. A ferret model of synergism between influenza virus and Streptococcus pneumoniae. Int. Congr. Ser. 1263C:486-490. [Google Scholar]
- 124.Plotkowski, M. C., O. Bajolet-Laudinat, and E. Puchelle. 1993. Cellular and molecular mechanisms of bacterial adhesion to respiratory mucosa. Eur. Respir. J. 6:903-916. [PubMed] [Google Scholar]
- 125.Plotkowski, M. C., E. Puchelle, G. Beck, J. Jacquot, and C. Hannoun. 1986. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. Am. Rev. Respir. Dis. 134:1040-1044. [DOI] [PubMed] [Google Scholar]
- 126.Potter, C. W. 1998. Chronicle of influenza pandemics, p. 3-18. In K. G. Nicholson, R. G. Webster, and A. J. Hay (ed.), Textbook of influenza. Blackwell Scientific Publications, London, United Kingdom.
- 127.Ratner, A. J., E. S. Lysenko, M. N. Paul, and J. N. Weiser. 2005. Synergistic proinflammatory responses induced by polymicrobial colonization of epithelial surfaces. Proc. Natl. Acad. Sci. USA 102:3429-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reichert, T. A., L. Simonsen, A. Sharma, S. A. Pardo, D. S. Fedson, and M. A. Miller. 2004. Influenza and the winter increase in mortality in the United States, 1959-1999. Am. J. Epidemiol. 160:492-502. [DOI] [PubMed] [Google Scholar]
- 129.Rosenow, C., P. Ryan, J. N. Weiser, S. Johnson, P. Fontan, A. Ortqvist, and H. R. Masure. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25:819-829. [DOI] [PubMed] [Google Scholar]
- 130.Sandgren, A., K. Sjostrom, B. Olsson-Liljequist, B. Christensson, A. Samuelsson, G. Kronvall, and N. B. Henriques. 2004. Effect of clonal and serotype-specific properties on the invasive capacity of Streptococcus pneumoniae. J. Infect. Dis. 189:785-796. [DOI] [PubMed] [Google Scholar]
- 131.Saslaw, S., H. E. Wilson, C. A. Doan, O. C. Woolpert, and J. L. Schwab. 1946. Reactions of monkeys to experimentally induced influenza A virus infection. J. Exp. Med. 84:113-125. [PMC free article] [PubMed] [Google Scholar]
- 132.Scadding, J. G. 1937. Lung changes in influenza. Q. J. Med. 6:425-465. [Google Scholar]
- 133.Schultz-Cherry, S., and V. S. Hinshaw. 1996. Influenza virus neuraminidase activates latent transforming growth factor β. J. Virol. 70:8624-8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sellers, T. F., Jr., J. Schulman, C. Bouvier, R. McCune, and E. D. Kilbourne. 1961. The influence of influenza virus infection on exogenous staphylococcal and endogenous murine bacterial infection of the bronchopulmonary tissues of mice. J. Exp. Med. 114:237-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shope, R. E. 1931. Swine influenza. I. Experimental trasmission and pathology. II. A hemophilic bacillus from the respiratory tract of infected swine. III. Filtration experiments and etiology. J. Exp. Med. 54:349-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Simonsen, L., M. J. Clarke, L. B. Schonberger, N. H. Arden, N. J. Cox, and K. Fukuda. 1998. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J. Infect. Dis. 178:53-60. [DOI] [PubMed] [Google Scholar]
- 137.Simonsen, L., M. J. Clarke, G. D. Williamson, D. F. Stroup, N. H. Arden, and L. B. Schonberger. 1997. The impact of influenza epidemics on mortality: introducing a severity index. Am. J. Public Health 87:1944-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Simonsen, L., K. Fukuda, L. B. Schonberger, and N. J. Cox. 2000. The impact of influenza epidemics on hospitalizations. J. Infect. Dis. 181:831-837. [DOI] [PubMed] [Google Scholar]
- 139.Simonsen, L., T. A. Reichert, C. Viboud, W. C. Blackwelder, R. J. Taylor, and M. A. Miller. 2005. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch. Intern. Med. 165:265-272. [DOI] [PubMed] [Google Scholar]
- 140.Smith, C. B., C. Golden, M. R. Klauber, R. Kanner, and A. Renzetti. 1976. Interactions between viruses and bacteria in patients with chronic bronchitis. J. Infect. Dis. 134:552-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Smith, C. B., R. E. Kanner, C. A. Golden, M. R. Klauber, and A. D. Renzetti, Jr. 1980. Effect of viral infections on pulmonary function in patients with chronic obstructive pulmonary diseases. J. Infect. Dis. 141:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141a.Smith, M. W., J. E. Schmidt, J. E. Rehg, C. J. Oriheula, and J. A. Mcullers. Induction of pro- and anti-inflammatory molecules in a mouse model of pneumococcal pneumonia following influenza. Comp. Med., in press. [PMC free article] [PubMed]
- 142.Spellerberg, B., D. R. Cundell, J. Sandros, B. J. Pearce, I. Idanpaan-Heikkila, C. Rosenow, and H. R. Masure. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 19:803-813. [DOI] [PubMed] [Google Scholar]
- 143.Stohr, K. 2003. Preventing and treating influenza. Br. Med. J. 326:1223-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stone, W. J., and G. W. Swift. 1919. Influenza and influenzal pneumonia at Fort Riley, Kansas. JAMA 72:487-493. [Google Scholar]
- 145.Stuart-Harris, C. H., J. Laird, D. A. Tyrrell, M. H. Kelsall, and Z. C. Franks. 1949. The relationship between influenza and pneumonia. J. Hyg. 47:434-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tashiro, M., P. Ciborowski, H. D. Klenk, G. Pulverer, and R. Rott. 1987. Role of Staphylococcus protease in the development of influenza pneumonia. Nature 325:536-537. [DOI] [PubMed] [Google Scholar]
- 147.Tashiro, M., P. Ciborowski, M. Reinacher, G. Pulverer, H. D. Klenk, and R. Rott. 1987. Synergistic role of staphylococcal proteases in the induction of influenza virus pathogenicity. Virology 157:421-430. [DOI] [PubMed] [Google Scholar]
- 148.Taubenberger, J. K., A. H. Reid, R. M. Lourens, R. Wang, G. Jin, and T. G. Fanning. 2005. Characterization of the 1918 influenza virus polymerase genes. Nature 437:889-893. [DOI] [PubMed] [Google Scholar]
- 149.Thompson, W. W., D. K. Shay, E. Weintraub, L. Brammer, N. Cox, L. J. Anderson, and K. Fukuda. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179-186. [DOI] [PubMed] [Google Scholar]
- 150.Tong, H. H., L. M. Fisher, G. M. Kosunick, and T. F. DeMaria. 2000. Effect of adenovirus type 1 and influenza A virus on Streptococcus pneumoniae nasopharyngeal colonization and otitis media in the chinchilla. Ann. Otol. Rhinol. Laryngol. 109:1021-1027. [DOI] [PubMed] [Google Scholar]
- 151.Tong, H. H., M. James, I. Grants, X. Liu, G. Shi, and T. F. DeMaria. 2001. Comparison of structural changes of cell surface carbohydrates in the eustachian tube epithelium of chinchillas infected with a Streptococcus pneumoniae neuraminidase-deficient mutant or its isogenic parent strain. Microb. Pathog. 31:309-317. [DOI] [PubMed] [Google Scholar]
- 152.Tong, H. H., M. A. McIver, L. M. Fisher, and T. F. DeMaria. 1999. Effect of lacto-N-neotetraose, asialoganglioside-GM1 and neuraminidase on adherence of otitis media-associated serotypes of Streptococcus pneumoniae to chinchilla tracheal epithelium. Microb. Pathog. 26:111-119. [DOI] [PubMed] [Google Scholar]
- 153.Treanor, J. J., F. G. Hayden, P. S. Vrooman, R. Barbarash, R. Bettis, D. Riff, S. Singh, N. Kinnersley, P. Ward, R. G. Mills, et al. 2000. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. JAMA 283:1016-1024. [DOI] [PubMed] [Google Scholar]
- 154.Tumpey, T. M., C. F. Basler, P. V. Aguilar, H. Zeng, A. Solorzano, D. E. Swayne, N. J. Cox, J. M. Katz, J. K. Taubenberger, P. Palese, and A. Garcia-Sastre. 2005. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 310:77-80. [DOI] [PubMed] [Google Scholar]
- 155.Tumpey, T. M., A. Garcia-Sastre, J. K. Taubenberger, P. Palese, D. E. Swayne, M. J. Pantin-Jackwood, S. Schultz-Cherry, A. Solorzano, N. van Rooijen, J. M. Katz, and C. F. Basler. 2005. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J. Virol. 79:14933-14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tyrrell, D. A. J. 1952. The pulmonary complications of influenza as seen in Sheffield in 1949. Q. J. Med. 21:291-306. [PubMed] [Google Scholar]
- 157.van der Poll, T., A. Marchant, C. V. Keogh, M. Goldman, and S. F. Lowry. 1996. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J. Infect. Dis. 174:994-1000. [DOI] [PubMed] [Google Scholar]
- 158.van der Sluijs, K. F., L. J. van Elden, M. Nijhuis, R. Schuurman, J. M. Pater, S. Florquin, M. Goldman, H. M. Jansen, R. Lutter, and T. van der Poll. 2004. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J. Immunol. 172:7603-7609. [DOI] [PubMed] [Google Scholar]
- 159.Webby, R. J., and R. G. Webster. 2003. Are we ready for pandemic influenza? Science 302:1519-1522. [DOI] [PubMed] [Google Scholar]
- 160.Webster, R. G., W. J. Bean, O. T. Gorman, T. M. Chambers, and Y. Kawaoka. 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56:152-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Weiss, S. J. 1989. Tissue destruction by neutrophils. N. Engl. J. Med. 320:365-376. [DOI] [PubMed] [Google Scholar]
- 162.Wherry, W. B., and C. T. Butterfield. 1920. Inhalation experiments on influenza and pneumonia, and on the importance of spray-borne bacteria in respiratory infections. J. Infect. Dis. 27:315-326. [Google Scholar]
- 163.Whitley, R. J., F. G. Hayden, K. S. Reisinger, N. Young, R. Dutkowski, D. Ipe, R. G. Mills, and P. Ward. 2001. Oral oseltamivir treatment of influenza in children. Pediatr. Infect. Dis. J. 20:127-133. [DOI] [PubMed] [Google Scholar]
- 164.Wilson, C. B., and P. Steer. 1919. Bacteriological and pathological observations on influenza as seen in France during 1918. Br. Med. J. 1919:634-635. [Google Scholar]
- 165.Wilson, H. E., S. Saslaw, C. A. Doan, O. C. Woolpert, and J. L. Schwab. 1947. Reactions of monkeys to experimental mixed influenza and streptococcus infections. J. Exp. Med. 85:199-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wizemann, T. M., J. H. Heinrichs, J. E. Adamou, A. L. Erwin, C. Kunsch, G. H. Choi, S. C. Barash, C. A. Rosen, H. R. Masure, E. Tuomanen, A. Gayle, Y. A. Brewah, W. Walsh, P. Barren, R. Lathigra, M. Hanson, S. Langermann, S. Johnson, and S. Koenig. 2001. Use of a whole-genome approach to identify vaccine molecules affording protection against Streptococcus pneumoniae infection. Infect. Immun. 69:1593-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wolbach, S. B. 1919. Comments on the pathology and bacteriology of fatal influenza cases, as observed at Camp Devens, Mass. Bull. J. Hopkins Hosp. 1:104-109. [Google Scholar]
- 168.World Health Organization Young Infants Study Group. 1999. Bacterial etiology of serious infections in young infants in developing countries: results of a multicenter study. Pediatr. Infect. Dis. J. 18:S17-S22. [DOI] [PubMed] [Google Scholar]
- 169.Wright, P. F., J. Thompson, and D. T. Karzon. 1980. Differing virulence of H1N1 and H3N2 influenza strains. Am. J. Epidemiol. 112:814-819. [DOI] [PubMed] [Google Scholar]
- 170.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golenbock. 1999. Cutting edge: recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163:1-5. [PubMed] [Google Scholar]