Abstract
The recent outbreaks of cattle botulism in vaccinated Israeli dairy cattle prompted us to determine vaccine efficacy and reasons for vaccine failure. Analysis of clinical signs, feeding practice, vaccination history, and epidemic curves enabled us to define a study population in two outbreaks, where high doses of Clostridium botulinum neurotoxin type D (BoNT/D) were evenly consumed by the affected animal groups. Attack rates among unvaccinated 6- to 24-month-old heifers were 96% (55/57) and 85% (53/62). The attack rates in vaccinated parity 1, 2, and ≥3 cows were 40.4% (21/52), 14.3% (4/28), and 5.6% (3/54), respectively. Vaccine efficacies for these cow groups were 52.5%, 83.2%, and 93.4%, respectively. In younger, unvaccinated 2- to 6-month-old calves, presumably protected by maternal antibodies, the attack rate was 24% (17/71). These differences correlated with significant differences in levels of specific anti-BoNT/D antibody in serum by an enzyme-linked immunosorbent assay (ELISA). The ELISA performance for predicting protection was analyzed by receiver operating characteristic analysis and was found to be highly significant, with an area under the curve of 0.941 (standard error, 0.034; 95% confidence interval, 0.875 to 1.008; P < 0.000). No animals with serum ELISA unit levels above 0.33 were affected in these exposed groups. At this cutoff level, the specificity of the ELISA was 100%, sensitivity was 67%, and accuracy was 92%. We concluded that botulinum toxoids can confer adequate protection against natural exposure to lethal doses of BoNT/D; however, the vaccination protocols should be optimized. Our in-house ELISA system will enable us to optimize vaccination protocols in the animal population.
Botulism is a fatal disease characterized by muscular paralysis caused by neurotoxins produced by Clostridium botulinum (BoNTs), which affect mammals, birds, and fish. Cattle botulism is a worldwide problem that causes large economic losses. In recent years, sporadic cases and massive outbreaks of cattle botulism occurred in Europe, North America, South America, Australia, and Israel (2, 5, 6, 12, 14, 16, 18, 20, 23, 27, 35, 37, 38).
In Israel, a comprehensive vaccination program, initiated after a major disease outbreak in 1977 (10), was at first considered highly successful. Repeated botulism outbreaks in recent years, however, gave rise to concerns about improper or insufficient vaccination. In areas where cattle botulism is prevalent, an effective vaccination program is an important factor to reduce mortality. In Northern Australia, for example, about 500,000 cattle are inoculated each year against botulism (3). Better understanding of the reasons for the vaccination failure is crucial; therefore, the immune response in Israeli cattle should be evaluated. Protection against tetanus can be evaluated by determining the antibody titer and correlating it to a cutoff value that is considered protective (8); however, no similar value exists for botulism. Anti-BoNT antibody levels in cattle sera were measured by determining the enzyme-linked immunosorbent assay (ELISA) optical density (OD) and used to determine exposure to BoNT/C or BoNT/D (17, 22). Gregory and others (13) detected higher ELISA OD values in sera from naturally exposed, unvaccinated cattle that protected mice in a mouse neutralization bioassay, and this value was used later for vaccine evaluation (3). We have used epidemiological and serological data from natural outbreaks of cattle botulism to analyze the immune response and protection conferred by the currently used toxoids and vaccination protocol. Analyzing these data enabled us to determine a cutoff value above which no animal was affected. We believe that data collected in this study will enable us to optimize vaccination protocols in the animal population.
MATERIALS AND METHODS
Study population.
Between July 2002 and May 2005, we recorded 14 botulism outbreaks in Israeli dairy herds. We investigated the outbreaks while they were still ongoing, identified index cases, and recorded data on day-by-day time-space charts of cases. Feeding practices and ingredients were thoroughly investigated and recorded in an attempt to identify the sources of intoxication, time of exposure, and animal groups exposed to suspected feed.
On the farms, animal rations were formulated and designed by computer software, and total mixed rations (TMR) were accordingly prepared in a mixing wagon also used for distribution of the mixed feed to the appropriate animal groups. The date, time, and rations distributed to the various animals groups were recorded in the farm computer system, and information could be retrieved and analyzed.
Two-month-old calves were vaccinated by subcutaneous injection with one of the available commercial brands of type C and D bivalent toxoids (CSL Limited, Parkville, Victoria, Australia; Prondil S.A., Montevideo, Uruguay). The calves were vaccinated again 4 weeks after the first vaccine and once a year thereafter. Preliminary studies with both mice and cattle demonstrated that the two commercially available botulinum toxoids elicited similar immune responses (A. Steinman and N. Y. Shpigel, unpublished data). However, groups of unvaccinated calves and replacement heifers (animals before the first calving at the approximate age of 24 months) were present in the affected herds, as described in Results.
Diagnosis.
The tentative diagnosis of botulism was based on typical clinical signs of ascending flaccid paralysis and a lack of indicative macropathological changes in postmortem examinations. In all outbreaks, blood samples, rumen contents, internal organs, feces, and suspect feed materials were sent for toxicological and histopathological examination. Outbreaks were attributed to botulism when all tests were negative for commonly used pesticides, heavy metals, nitrates, and cyanides and no abnormal changes could be detected upon histopathological examination of internal organs and the central nervous system using standard techniques as previously described (11). Only outbreaks where C. botulinum type D organisms could be detected in material from at least one sample collected from affected animals were included in this study. Samples were processed and cultured as previously described (11), and the production of BoNT/D was verified by the mouse neutralization bioassay.
Fecal and serum samples were collected for PCR and serological analysis from affected and nonaffected animals during the outbreaks. Serum samples were also collected from vaccinated multiparous dairy cows, primiparous heifers, and unvaccinated, 4- to 17-week-old dairy heifers in nonaffected herds. Samples were kept at −80°C until analysis.
Fecal analysis by PCR.
Fecal samples (5 g) were inoculated into fortified egg meat medium (FEM) vials, which were then heated to 80°C for 20 min, sealed with sterile paraffin oil, and incubated at 33°C for 5 days (11). Afterwards, DNA was extracted from 2 ml of FEM using a G-Spin genomic DNA extraction kit for bacteria (iNtRON Biotechnology, Korea) according to the manufacturer's instructions.
Oligonucleotide primers were selected for detection of the BoNT/D light-chain gene (5′-TAAGTAAACCGCCCAGACC-3′ and 5′-TAGTATAGATAATGTTCCA-3′) as previously reported (33). PCR was performed with a 25-μl reaction mixture containing 1 μl of the template, 1 μl of each primer (Danyel Biotech Ltd., Rehovot, Israel), 9.5 μl double-distilled water, and 12.5 μl 2× ReddyMix PCR Master Mix (ABgene, Epsom, United Kingdom) with a programmable thermocycler (Biometra, Goettingen, Germany). Initially the samples were denatured for 5 min at 95°C. Each PCR cycle consisted of denaturation at 95°C for 60 s, annealing at 47°C for 60 s, and extension at 65°C for 120 s and was repeated 43 times. Final extension at 65°C for 10 min followed. The amplified PCR products were visualized in 1.5% agarose gels (Life Technologies, Paisley, Scotland).
Serological analysis.
The levels of specific anti-BoNT/D antibodies in the sera were determined using ELISA as described previously for humans, with minor modifications (24). Purified BoNT/D of >95% purity (1 μg/ml) (Metabiologics) was used as the capture antigen in the ELISA. The intra-assay (within-run) coefficient of variation (10 serum samples in five replicates) was less than 10%. Interassay (day-to-day) coefficients of variation (10 serum samples in five replicates for 5 days) were less than 15%. Wells of Nunc-Immuno ELISA plates (Nunc, Denmark) were filled with 100-μl volumes of antigen in coating carbonate-bicarbonate buffer (0.05 M; pH 9.6), sealed with Parafilm, and incubated overnight at 4°C. After three washes with phosphate-buffered saline (PBS) containing 0.05% Tween 20 and 0.02% NaN3 (250 μl per well), 200 μl of blocking solution (0.5 g I-Block in 90 ml washing buffer plus 10 ml inactivated goat serum) was inserted per well and incubated for 1 h at 37°C. After three washes with PBS (250 μl per well), 100 μl of blanks (blocking buffer plus inactivated goat serum) and plasma samples in duplicate, diluted to 1/100 in blocking buffer plus inactivated goat serum, were inserted per well and incubated for 1 h at 37°C. After three washes with PBS (250 μl per well), 100 μl of alkaline phosphatase-conjugated goat anti-bovine immunoglobulin G (IgG) (Kirkegaard & Perry Laboratories) with blocking buffer plus inactivated goat serum (1:1,000) was added and incubated again for 1 h at 37°C. After three washes with PBS (250 μl per well), 100 μl of a freshly prepared substrate solution was added per well (Sigma, Steinheim, Germany), and the optical density at 405 nm (OD405) and OD630 were read with an ELx808 ultra microplate reader (Bio-Tek Instruments, Vermont) after 30 min. Positive serum samples from heifers that had been vaccinated four times and had high neutralizing antibody titers (>1.0 IU/ml), as determined by the mouse inoculation bioassay (24), and negative serum samples collected from precolostrum calves were used on each plate.
Data and statistical analysis.
The numbers of affected animals were compared between parity (defined as the number of calvings a cow has undergone) groups using Pearson's chi-square tests. The risks (attack rates) for animals to be affected by botulism were calculated for parity groups 1, 2, and ≥3, and relative risks (RR) and their 95% confidence intervals (95% CI) were calculated for the parity 1 and 2 animal groups relative to the parity ≥3 animal group as previously described (29).
Vaccine efficacy, expressed as a percentage, was calculated as [ARU − (ARV/ARU)] × 100, as previously described (26), where ARU is the attack rate in the unvaccinated population and ARV is the attack rate in the vaccinated population.
Serological results are expressed in ELISA units and were calculated as (OD of the test sample − OD of the negative control)/(OD of the positive control − OD of the negative control) (9).
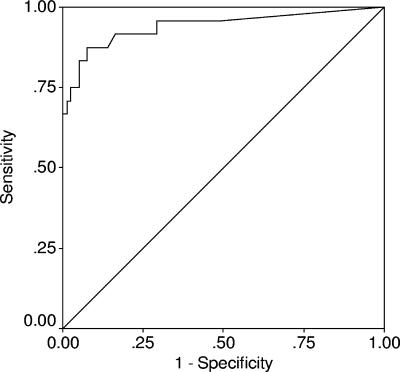
The performance of our in-house ELISA in quantifying the levels of the protective neutralizing serum antibodies in cows was analyzed using the nonparametric receiver operating characteristic (ROC) analysis (31). Based on the clinical signs described above, animals were categorized as nonaffected or affected. Analysis included only sera of animals from groups where clinical signs, attack rates, and feeding practice enabled us to assume that exposure was both to high levels of BoNT and homogenous (use of mixing wagon). We assumed that under these conditions, the probability of each animal in the group being exposed to a lethal dose of BoNT was the same. Serological results in ELISA units for each animal tested were compared with the definitive diagnosis (affected or nonaffected). For all possible cutoff values, the numbers of true-positive (a), false-positive (b), false-negative (c), and true-negative (d) results were determined, and the sensitivity, specificity, and accuracy were calculated as a/(a + c), d/(b + d), and (a + d)/(a + b + c + d), respectively. ROC curves were constructed by plotting sensitivity versus 1 minus the specificity at each possible cutoff point of serum ELISA units. The area under the curve (AUC) was quantified, and the diagnostic accuracy and optimal cutoff value were determined by the point on the ROC curve closest to the upper left-hand corner, which minimizes the sum of false-positive and false-negative results. Values for the AUC can fall between 0 and 1; a value of 0.5 means that the diagnostic test is no better than chance. Statistical analyses were performed using the SPSS program, version 10.0.1, 1999 (SPSS Inc.).
RESULTS
Clinical and epidemiological findings.
Three outbreaks involved large numbers of animals and are presented in detail, while in 11 outbreaks only one to nine animals were affected. Overall, a total of 210 animals were affected, all of which were Israeli Holstein dairy cows and calves. Of these, 52 were lactating cows, 129 were either young male calves or replacement heifers, and 29 were feedlot calves.
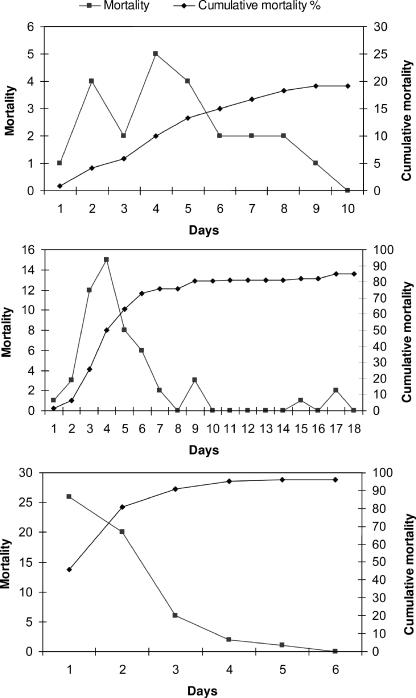
In most of the outbreaks, botulism was initially suspected when an animal was found either dead or recumbent (an index case). While animals were recumbent, several clinical signs were noted, which included reduced tongue tone, mild salivation, reduced tail and anal tone, increased respiratory rate, and paresis. A few of the animals exhibited an unsteady gait, which lasted several hours before they progressed to recumbency. In general, almost all animals showed no apparent clinical signs just before they were found recumbent, even days after the suspected exposure. None of the affected animals survived. In outbreak 1, a large batch of TMR which included wheat silage, grains, and fermented chicken manure was distributed on a Friday for the weekend. The first animal, the index case, was found dead 3 days later; within 9 days, a total of 23 feedlot calves, 4 to 14 months old, died out of 120 (19%), and most deaths occurred during days 2 to 5 (Fig. 1, top). All groups that were fed with the suspected TMR were intoxicated.
FIG. 1.
Epidemic curves of three outbreaks of Clostridium botulinum type D intoxications in three groups of unvaccinated dairy heifers and calves. The numbers of affected animals (mortality) and cumulative mortality rates (expressed as percentages) are presented day by day, commencing with the index cases and continuing to the last day of mortality. Outbreak 1 (top), outbreak 2 (center), and outbreak 3 (bottom) are described in the text.
In outbreak 2, a batch of 2 days' worth of TMR consisting of sorghum silage, concentrated grains, and fermented chicken manure was mixed in a mixing wagon and distributed to five groups of replacement heifers, 6 to 24 months old. The same mixing wagon was used the next morning for the preparation and distribution of the lactating cows' TMR. Two days thereafter, a group of pregnant heifers, the biggest and oldest of the lot, which were in the last feeding group, were found either dead or recumbent (index cases). Within 5 days, 55 of 57 (96%) replacement heifers (Fig. 1, center) were dead. Three days after the first case was detected in the replacement heifer group, one lactating cow was found recumbent and died. Two additional lactating cows were found recumbent on the fifth day of the outbreak (all together, 3% of the herd of cows). These animals were probably intoxicated by the contaminated mixing wagon used for the preparation of the lactating cows' TMR. Although the sorghum silage was a common feed ingredient for both groups, other animals fed were not affected.
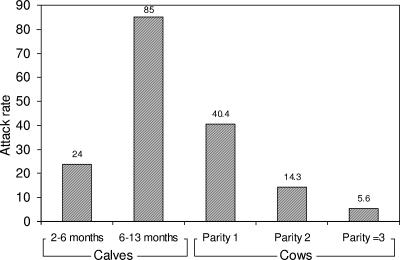
In outbreak 3, the suspected source of intoxication was a batch of lactating cows' TMR that was fed only to the four affected animal groups. The feeding order of the mixing wagon was first two lactating cow groups (out of four in the herd), calves 2 to 6 months old, and finally replacement heifers, 6 to 10 months old. Other animal groups fed with the same feed ingredients—lactating cows, dry cows, pregnant replacement heifers, and inseminated heifers—were not affected in this outbreak. This batch of TMR consisted of sorghum silage, wheat hay and straw, and dairy concentrates. A dead heifer (the index case) was found in the replacement heifer group. Within 9 days, 99 animals, belonging to the four different groups in the herd mentioned above, were affected. The attack rates of botulism in the affected lactating-cow groups were 40.4% (21/52), 14.3% (4/28), and 5.6% (3/54) for parities 1, 2, and ≥3, respectively (Fig. 2). The RR for parity 1 cows to be affected by botulism compared to parity 2 cows was 2.8 (95% CI, 1.1 to 7.4; χ2 = 5.77; P = 0.0163). The RR for parity 1 and 2 cows to be affected by botulism compared to parity ≥3 cows were 7.3 (95% CI, 2.3 to 22.9) and 2.6 (95% CI, 0.6 to 10.7), respectively. The attack rates of botulism among the 2- to 6-month-old calves and the 6- to 9-month-old replacement heifers were 24% (17/71) and 85% (53/62), respectively (Fig. 2). The vaccine efficacies for parity 1, 2, and ≥3 cows were 52.5%, 83.2%, and 93.4%, respectively (by using the older calf group for the ARU value).
FIG. 2.
Attack rates (expressed as percentages) among calves of different age groups and cows of different parity, all fed with the same batch of total mixed rations leading to botulinum type D intoxication (outbreak 3).
Serological and bacteriological findings.
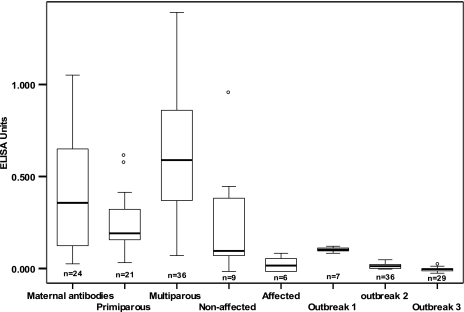
In all three outbreaks, the levels of specific anti-BoNT/D antibodies found during the time of the outbreak in calves and nulliparous heifers were very low, indicating a lack of immunization (Fig. 3, outbreaks 1 to 3). Higher levels were detected in nonaffected cows than in affected cows in outbreak 3 (Fig. 3). In nonaffected herds, levels of antibodies were statistically significantly higher in vaccinated multiparous cows than in vaccinated primiparous cows (comparison of means by one-way analysis of variance; P < 0.05). Marked variations in maternally derived anti-BoNT/D antibodies were detected among unvaccinated 1- to 5-month-old calves (Fig. 3). The serology test results were used to analyze the ELISA performance and protective antibody levels. The ELISA performance for all samples is presented in the ROC curve (Fig. 4). The AUC for this test, 0.941 (standard error, 0.034; 95% CI, 0.875 to 1.008), was significantly (P < 0.001) better than that for a random test with no discriminatory ability (line of no information; AUC, 0.5). No animals with serum ELISA unit levels above 0.33 were affected in these exposed groups. At this cutoff level, the specificity of the ELISA was 100%, sensitivity was 67%, and accuracy was 92%.
FIG. 3.
Levels of anti-botulinum neurotoxin type D IgG in serum (expressed in ELISA units) in various animal groups. The boxplot shows, from left to right, levels in preweaning calves (maternal antibodies), in primiparous and multiparous cows, in nonaffected and affected cows in outbreak 3, and in calves in outbreaks 1 through 3.
FIG. 4.
ROC curve of in-house ELISA measuring serum anti-botulinum type D IgG levels for animals sampled in groups affected by botulism. The AUC is 0.941 (standard error, 0.034; 95% CI, 0.875 to 1.008), significantly better than that for the diagonal line (AUC = 0.5) (P < 0.000).
C. botulinum type D DNA was found by PCR in enriched fecal samples from 5 out of 5 affected animals and from 23 out of 36 (64%) nonaffected animals in the same herds.
DISCUSSION
The Israeli cattle herd has been routinely vaccinated against type C and type D botulism since the late 1970s. In June 2002, a large type D botulism outbreak occurred in southern Israel, involving 28 dairy farms (S. Bruckstein, personal communication). All animals were vaccinated with a bivalent toxoid, using the currently recommended vaccination protocol: two doses 4 weeks apart at the age of 2 months followed by an annual booster. The reasons for the vaccine failure were unknown, and the following hypotheses were raised: (i) inadequate vaccine quality, (ii) inadequate vaccination protocols, (iii) exposure to very high levels of BoNTs against which the vaccine is not effective, and (iv) intoxication with BoNT types not included in the vaccine. For this reason, thorough investigations of all outbreaks of type D botulism that affected the Israeli cattle herd during the years 2002 to 2005 were carried out. Sporadic cases of type B botulism, which is not included in the vaccine and results in different clinical signs (38), and of type C botulism, affecting a limited number of animals, were reported in Israeli cattle herds during those years. However, no cases of either type B or type C cattle botulism, toxin, or organisms were observed in the herds participating in the present study.
In order to determine the levels of specific anti-BoNT/D antibodies, an in-house ELISA was used. ELISA systems are considered an effective and suitable means to analyze immunological responses to botulinum toxins (19). Previously described systems were based on purified toxins, recombinant peptides, or vaccine antigens (19, 24, 30). Using ELISA, an antibody response to BoNT was described in two cases of infant botulism as early as 25 years ago (30). Since then, ELISA systems have been used for diagnosis of botulism in various species, including wild birds (28), cattle (17, 22), and recently a dog (4). ELISA systems, which offer greater flexibility and economy than other immunoassay techniques, were also used for the evaluation of the immune response to various botulinum vaccines in mice (21), primates (19), humans (32), and cattle (3). Previously, the ELISA positive/negative cutoff values used for diagnosis of botulism in cattle were defined as the upper 99% confidence limit of the mean of a population of cattle not exposed to BoNTs plus 3 sample standard deviations (13). Higher ELISA OD values were detected in serum samples from four naturally exposed unvaccinated heifers, which protected mice in a mouse neutralization bioassay (13), and were used for vaccine evaluation in cattle (3). The threshold of a positive response in vaccinated humans was defined as the mean OD plus 5 standard deviations at a 1:80 dilution of 150 nonvaccinated serum samples (24). The performance of our in-house ELISA for predicting protection was analyzed by ROC analysis and found to be highly significant, and a cutoff level was determined such that no animals with serum ELISA unit levels above the cutoff were affected. At this cutoff level, the specificity of the ELISA was 100%, sensitivity was 67%, and accuracy was 92%. The lower sensitivity indicates that a few animals with serum ELISA unit levels below the cutoff were not affected. This most probably should be explained by incomplete exposure of some animals to a lethal dose of BoNT or, less likely, by the inability of the ELISA system to detect some protective neutralizing serum antibodies.
Analysis of sera collected from animals during those outbreaks demonstrated that calves and nulliparous heifers which were most severely affected had low levels of specific anti-BoNT/D antibodies, in accordance with the lack of immunization. Higher antibody levels were detected in multiparous cows than in primiparous heifers in both affected and nonaffected herds. In groups where clinical signs, attack rates, and feeding practice enabled us to assume that exposure was both to high levels of BoNT and homogenous (use of mixing wagon), the relative risk of being affected could be determined. The relative risk for primiparous heifers to be affected compared to parity ≥3 cows was 7.3, and the attack rate was 40.4% compared to 5.6%, respectively. Similar results were also seen during the 2002 outbreak in Israel, where the mortality rate among heifers was 64%, compared to 3% for cows from the fourth lactation onward (S. Bruckstein, personal communication). Based on the above results, we concluded that the botulism toxoids can confer adequate protection against natural exposure to lethal doses of BoNT/D but that vaccination protocols used on dairy farms were inadequate and should be optimized. However, lower anti-BoNT/D IgG levels in younger animals might be explained by repeated natural exposure to subclinical toxin levels of older animals, as previously demonstrated in Australia (13). The results of our study differ from those of previously reported vaccination studies of cattle (15, 34), which demonstrated better protection after fewer inoculations; this difference might be explained by exposure to higher levels of toxin or botulinum strain differences. Additional inoculations, as currently suggested by the Centers for Disease Control and Prevention for the pentavalent (ABCDE) botulinum toxoid, aluminum phosphate absorbed (PBT), used to vaccinate at-risk human populations, might result in desired levels of antibodies. We are currently conducting a vaccination study to evaluate the effect of additional doses on the levels of specific anti-BoNT antibodies in calves.
Severe clinical signs characterized the outbreaks described. Cattle had dramatically deteriorated to recumbency without any prior signs noticed. Later, while recumbent, animals demonstrated classical clinical signs of botulism and eventually died without notable agonal movements. A few animals showed an unsteady gait which progressed to recumbency within hours, but in general, subclinical cases were not observed. In these outbreaks, all affected animals died or were euthanized due to the poor prognosis for recovery after recumbency (1, 2). These findings differ from those for previously reported cases of type D cattle botulism, which were usually milder and resulted in lower mortality rates (1, 2, 10, 14).
C. botulinum type D DNA was found in many fecal samples collected from nonaffected cattle in affected herds. It is currently accepted that although C. botulinum can be a normal inhabitant of the gastrointestinal tract, it is rarely isolated from healthy cattle (36). C. botulinum type D DNA was found in fecal samples from animals that were housed in the same groups as the affected animals; therefore, the existence of bacterial DNA might indicate that these animals ingested bacteria, further indicating that C. botulinum and probably BoNTs were evenly distributed and consumed in the affected groups. On the other hand, it is possible that this is a random finding and that the prevalence of the bacteria in healthy animals is higher than previously reported. To the best of our knowledge, the prevalence of C. botulinum type D in fecal samples of healthy cattle has not been reported. In a preliminary study conducted by us, BoNT-producing organisms could not be detected by the mouse neutralization bioassay after culture of fecal samples collected from healthy cattle in five herds in Israel, two of which had experienced a type D botulism outbreak 3 years earlier (M. Chaffer, unpublished results). In contrast, C. botulinum type B was detected in 73% of fecal samples that were collected from cows at slaughterhouses in Sweden, but no samples were positive for type E and F neurotoxin genes (7). C. botulinum type B was also found in 60 to 100% of the fecal samples obtained from cows in herds that suffered from a botulism outbreak a year earlier (25).
In conclusion, analyzing serological and epidemiological data enabled us to better understand the recent outbreaks in Israel and evaluate the different hypotheses for vaccine failure. We concluded that botulinum toxoids can confer adequate protection against natural exposure to lethal doses of BoNT/D; however, vaccination protocols used on dairy farms are inadequate and should be optimized. Our in-house ELISA system will enable us to optimize vaccination protocols in the animal population.
Acknowledgments
This study was funded by the Israeli Milk Board, grant 668-0035.
REFERENCES
- 1.Abbitt, B., M. J. Murphy, A. C. Ray, J. C. Reagor, A. K. Eugster, L. G. Gayle, H. W. Whitford, R. J. Sutherland, R. A. Fiske, and J. Pusok. 1984. Catastrophic death losses in a dairy herd attributed to type D botulism. J. Am. Vet. Med. Assoc. 185:798-801. [PubMed] [Google Scholar]
- 2.Braun, U., K. Feige, G. Schweizer, and A. Pospischil. 2005. Clinical findings and treatment of 30 cattle with botulism. Vet. Rec. 156:438-441. [DOI] [PubMed] [Google Scholar]
- 3.Brown, A. T., A. R. Gregory, T. M. Ellis, and M. N. Hearnden. 1999. Comparative immunogenicity of two bivalent botulinum vaccines. Aust. Vet. J. 77:388-391. [DOI] [PubMed] [Google Scholar]
- 4.Bruchim, Y., A. Steinman, M. Markovitz, G. Baneth, D. Elad, and N. Y. Shpigel. 2006. Toxicological, bacteriological and serological diagnosis of botulism in a dog. Vet. Rec. 158:768-769. [DOI] [PubMed] [Google Scholar]
- 5.Bruckstein, S., and A. M. Tromp. 2001. Food poisoning in three family dairy herds associated with Clostridium botulinum type B. Isr. J. Vet. Med. 56:95-98. [Google Scholar]
- 6.Cobb, S. P., R. A. Hogg, D. J. Challoner, M. M. Brett, C. T. Livesey, R. T. Sharpe, and T. O. Jones. 2002. Suspected botulism in dairy cows and its implications for the safety of human food. Vet. Rec. 150:5-8. [DOI] [PubMed] [Google Scholar]
- 7.Dahlenborg, M., E. Borch, and P. Radstrom. 2003. Prevalence of Clostridium botulinum types B, E and F in faecal samples from Swedish cattle. Int. J. Food Microbiol. 82:105-110. [DOI] [PubMed] [Google Scholar]
- 8.Danilova, E., A. Shiryayev, E. K. Kristoffersen, and H. Sjursen. 2005. Attenuated immune response to tetanus toxoid in young healthy men protected against tetanus. Vaccine 23:4980-4983. [DOI] [PubMed] [Google Scholar]
- 9.Dijkstra, T., H. W. Barkema, M. Eysker, M. L. Beiboer, and W. Wouda. 2003. Evaluation of a single serological screening of dairy herds for Neospora caninum antibodies. Vet. Parasitol. 110:161-169. [DOI] [PubMed] [Google Scholar]
- 10.Egyed, M. N., A. Shlosberg, U. Klopfer, T. A. Nobel, and E. Mayer. 1978. Mass outbreaks of botulism in ruminants associated with ingestion of feed containing poultry waste. Refuah. Vet. 35:93-99. [Google Scholar]
- 11.Elad, D., E. Yas-Natan, I. Aroch, M. H. Shamir, S. Kleinbart, D. Hadash, M. Chaffer, K. Greenberg, and A. Shlosberg. 2004. Natural Clostridium botulinum type C toxicosis in a group of cats. J. Clin. Microbiol. 42:5406-5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galey, F. D., R. Terra, R. Walker, J. Adaska, M. A. Etchebarne, B. Puschner, E. Fisher, R. H. Whitlock, T. Rocke, D. Willoughby, and E. Tor. 2000. Type C botulism in dairy cattle from feed contaminated with a dead cat. J. Vet. Diagn. Investig. 12:204-209. [DOI] [PubMed] [Google Scholar]
- 13.Gregory, A. R., T. M. Ellis, T. F. Jubb, R. J. Nickels, and D. V. Cousins. 1996. Use of enzyme-linked immunoassays for antibody to types C and D botulinum toxins for investigations of botulism in cattle. Aust. Vet. J. 73:55-61. [DOI] [PubMed] [Google Scholar]
- 14.Heider, L. C., J. T. McClure, and E. R. Leger. 2001. Presumptive diagnosis of Clostridium botulinum type D intoxication in a herd of feedlot cattle. Can. Vet. J. 42:210-212. [PMC free article] [PubMed] [Google Scholar]
- 15.Jansen, B. C., P. C. Knoetze, and F. Visser. 1976. The antibody response of cattle to Clostridium botulinum types C and D toxoids. Onderstepoort J. Vet. Res. 43:165-173. [PubMed] [Google Scholar]
- 16.Jean, D., G. Fecteau, D. Scott, R. Higgins, and S. Quessy. 1995. Clostridium botulinum type C intoxication in feedlot steers being fed ensiled poultry litter. Can. Vet. J. 36:626-628. [PMC free article] [PubMed] [Google Scholar]
- 17.Jubb, T. F., T. M. Ellis, and A. R. Gregory. 1993. Diagnosis of botulism in cattle using ELISA to detect antibody to botulinum toxins. Aust. Vet. J. 70:226-227. [DOI] [PubMed] [Google Scholar]
- 18.Kelch, W. J., L. A. Kerr, J. K. Pringle, B. W. Rohrbach, and R. H. Whitlock. 2000. Fatal Clostridium botulinum toxicosis in eleven Holstein cattle fed round bale barley haylage. J. Vet. Diagn. Investig. 12:453-455. [DOI] [PubMed] [Google Scholar]
- 19.Lindsey, C. Y., L. A. Smith, M. W. West, J. W. Boles, and J. E. Brown. 2003. Evaluation of a botulinum fragment C-based ELISA for measuring the humoral immune response in primates. Biologicals 31:17-24. [DOI] [PubMed] [Google Scholar]
- 20.Livesey, C. T., R. T. Sharpe, and R. A. Hogg.2004. Recent association of cattle botulism with poultry litter. Vet. Rec. 154:734-735. [PubMed] [Google Scholar]
- 21.Mahmut, N., K. Inoue, Y. Fujinaga, H. Arimitsu, Y. Sakaguchi, L. Hughes, R. Hirst, T. Murphy, T. Tsuji, T. Watanabe, T. Ohyama, T. Karasawa, S. Nakamura, K. Yokota, and K. Oguma. 2002. Mucosal immunisation with Clostridium botulinum type C 16 S toxoid and its non-toxic component. J. Med. Microbiol. 51:813-820. [DOI] [PubMed] [Google Scholar]
- 22.Main, D. C., and A. R. Gregory. 1996. Serological diagnosis of botulism in dairy cattle. Aust. Vet. J. 73:77-78. [DOI] [PubMed] [Google Scholar]
- 23.Martin, S. 2003. Clostridium botulinum type D intoxication in a dairy herd in Ontario. Can. Vet. J. 44:493-495. [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery, V. A., R. S. Makuch, J. E. Brown, and D. C. Hack. 1999. The immunogenicity in humans of a botulinum type F vaccine. Vaccine 18:728-735. [DOI] [PubMed] [Google Scholar]
- 25.Notermans, S., J. Dufrenne, and J. Oosterom. 1981. Persistence of Clostridium botulinum type B on a cattle farm after an outbreak of botulism. Appl. Environ. Microbiol. 41:179-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orenstein, W. A., R. H. Bernier, T. J. Dondero, A. R. Hinman, J. S. Marks, K. J. Bart, and B. Sirotkin. 1985. Field evaluation of vaccine efficacy. Bull. W. H. O. 63:1055-1068. [PMC free article] [PubMed] [Google Scholar]
- 27.Ortolani, E. L., L. A. Brito, C. S. Mori, U. Schalch, J. Pacheco, and L. Baldacci. 1997. Botulism outbreak associated with poultry litter consumption in three Brazilian cattle herds. Vet. Hum. Toxicol. 39:89-92. [PubMed] [Google Scholar]
- 28.Rocke, T. E., S. R. Smith, and S. W. Nashold. 1998. Preliminary evaluation of a simple in vitro test for the diagnosis of type C botulism in wild birds. J. Wildl. Dis. 34:744-751. [DOI] [PubMed] [Google Scholar]
- 29.Rothman, K. J., and S. Greenland (ed.). 1998. Modern epidemiology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 30.Rubin, L. G., M. Dezfulian, and R. H. Yolken. 1982. Serum antibody response to Clostridium botulinum toxin in infant botulism. J. Clin. Microbiol. 16:770-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shpigel, N. Y., Y. Avidar, and E. Bogin. 2003. Value of measurements of the serum activities of creatine phosphokinase, aspartate aminotransferase and lactate dehydrogenase for predicting whether recumbent dairy cows will recover. Vet. Rec. 152:773-776. [DOI] [PubMed] [Google Scholar]
- 32.Siegel, L. S. 1988. Human immune response to botulinum pentavalent (ABCDE) toxoid determined by a neutralization test and by an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 26:2351-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szabo, E. A., J. M. Pemberton, and P. M. Desmarchelier. 1993. Detection of the genes encoding botulinum neurotoxin types A to E by the polymerase chain reaction. Appl. Environ. Microbiol. 59:3011-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tammemagi, L., and K. M. Grant. 1967. Vaccination in the control of bovine botulism in Queensland. Aust. Vet. J. 43:368-372. [DOI] [PubMed] [Google Scholar]
- 35.Trueman, K. F., R. E. Bock, R. J. Thomas, J. D. Taylor, P. A. Green, H. M. Roeger, and P. J. Ketterer. 1992. Suspected botulism in three intensively managed Australian cattle herds. Vet. Rec. 130:398-400. [DOI] [PubMed] [Google Scholar]
- 36.Whitlock, R. H., and J. M. Williams. 1999. Botulism toxicosis of cattle, p. 45-53. In R. A. Smith (ed.), Proceedings of the 32nd annual convention of the American Association of Bovine Practitioners. American Association of Bovine Practitioners, Nashville, Tenn.
- 37.Wilson, R. B., M. T. Boley, and B. Corwin. 1995. Presumptive botulism in cattle associated with plastic-packaged hay. J. Vet. Diagn. Investig. 7:167-169. [DOI] [PubMed] [Google Scholar]
- 38.Yeruham, I., D. Elad, Y. Avidar, K. Grinberg, D. Tiomkin, and A. Monbaz. 2003. Outbreak of botulism type B in a dairy cattle herd: clinical and epidemiological aspects. Vet. Rec. 153:270-272. [DOI] [PubMed] [Google Scholar]