Abstract
Bovine tuberculosis is a major cause of economic loss in countries where it is endemic, and in some countries, it may be a significant zoonotic disease problem. Therefore, new strategies for vaccine development are required, and among them, genetic immunization has potential value. The main goal of this study was to test the Mycobacterium bovis Ag85B gene as a DNA vaccine following challenge with an M. bovis virulent strain (ATCC 19274). Groups of BALB/c mice (n = 10) were immunized four times intramuscularly with the pCI-Ag85B construct or the pCI vector alone as the control. High titers of total immunoglobulin G (IgG), IgG1, and IgG2a anti-Ag85B were measured in pCI-Ag85B immunized mice when compared to the pCI control group. Regarding cellular immunity, significant levels of gamma interferon (IFN-γ) (1,100 ± 157 pg/ml) and tumor necrosis factor alpha (650 ± 42 pg/ml) but not interleukin-4 were detected in splenocyte culture supernatants of pCI-Ag85B-vaccinated mice following stimulation with recombinant Ag85B. Further, the main source of IFN-γ is CD8+ T cells, as demonstrated by intracellular cytokine staining. As far as protection, a significant reduction in bacterial load in spleens (P < 0.05) was detected in pCI-Ag85B-immunized mice compared to the pCI vector control group. The results obtained here suggest that use of the Ag85B DNA vaccine is a promising strategy to control M. bovis infection due to its ability to induce a Th1 type of immune response. However, protective efficacy needs to be improved, since partial protection was achieved in spleens but not in lungs of vaccinated mice.
Bovine tuberculosis, caused by Mycobacterium bovis, is a major economic problem around the world and represents a risk to public health in several countries. Although milk pasteurization has sharply reduced bovine tuberculosis transmission to human beings, this disease continues to bring about serious losses. The only controlling measure is based on a test-and-slaughter policy. This consists of carrying out the tuberculin test, and if the animal presents a strong reaction to the test, it is to be killed with no reutilization of its carcass (6). There is strong resistance by the farmers to adopt the test-and-slaughter program, especially in cases of disease with high prevalence, as there is no policy to make up for their loss.
In developing countries, there is a strong possibility of infection in human populations by M. bovis due to difficulties in adopting controlling measures, mainly because of social and economic problems and also due to health-related factors, such as malnutrition and human immunodeficiency virus infection, that can make people more prone to infection (24). Besides, the test-and-slaughter policy has been only partially effective in countries such as the United Kingdom, Ireland, and New Zealand, which have a reservoir of wild animals infected by M. bovis. Therefore, the use of an effective vaccine against bovine tuberculosis is desirable for both industrialized and developing countries (32).
M. bovis bacillus Calmette-Guérin (BCG) is an attenuated strain of M. bovis and is presently the only available vaccine that can be used against bovine tuberculosis. However, the reported protective efficacies of BCG vaccination in cattle over the last 70 years have differed greatly, ranging from none to about 70% protection (3). These different outcomes are similar to those observed for BCG vaccination in human populations (9). Moreover, BCG vaccination faces two additional problems: (i) BCG vaccination induces a delayed type hypersensitivity response to purified protein derivative (PPD) which cannot be distinguished from exposure to M. tuberculosis or M. bovis, and therefore it compromises the diagnostic efficacy of PPD; and (ii) sensitization of cattle to environmental mycobacteria adversely affected the subsequent protective efficacy of BCG. Therefore, alternatives to live vaccines, which do not interfere with the diagnosis of the disease, have been studied for the control of tuberculosis, among which are the subunit vaccines and the DNA vaccines (12, 21).
Since DNA vaccines can induce substantial cellular immunity and can evoke both CD4 and CD8 T-cell responses, DNA immunization has become a viable strategy in the development of new vaccines against intracellular pathogens (15). DNA vaccines expressing single Mycobacterium antigens have been shown to elicit protective immune responses against primary tuberculosis infections and to amplify BCG responses using prime/boost strategies (18, 27). Our data suggest that the DNA vaccine carrying the Ag85B gene induced high levels of anti-Ag85B antibodies, a Th1 type of immune response with significant production of gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) and low levels of interleukin-4 (IL-4) in BALB/c mice, and partial protection in immunized animals.
MATERIALS AND METHODS
Subcloning of the Ag85B gene.
The Ag85B gene of M. bovis was amplified by PCR and subcloned into the mammalian expression vector pCI (Promega Corp., Madison, WI). Primers containing one artificial restriction site at each end were constructed according to the Ag85B gene nucleotide sequence (GenBank accession number X62397). The primer sequences were (forward) 5′-GCGAATTCTTCTCCCGGCCGGGGCTG-3′ (EcoRI) and (reverse) 5′-GCTCTAGATCAGCCGGCGCCTAACGA-3′ (XbaI). PCR was performed in a 10-μl volume containing 1 ng DNA template (pMAL-Ag85B, previously constructed in our laboratory), 5 pmol of each primer, 10 mM deoxyribonucleoside triphosphate, 10× PCR buffer, and 5 U AmpliTaq polymerase. PCR amplification was conducted with a DNA thermal cycler under the following conditions: denaturation at 95°C for 3 min, annealing at 54°C for 45 s and extension at 72°C for 1 min (30 cycles). The amplified products were purified with the Quiaex II gel extraction kit (QIAGEN, Valencia, CA) and digested with EcoRI and XbaI restriction endonucleases (Invitrogen). After digestion, the PCR product was purified again by the same procedure and ligated to the predigested pCI vector. This construction was used to transform Escherichia coli DH5α, and a single recombinant clone was selected. Plasmid DNA was extracted with the Wizard Miniprep kit (Promega Corp, Madison, WI). The pCI-Ag85B construct was digested with endonuclease and DNA sequenced to confirm the presence and the orientation of Mycobacterium Ag85B gene.
DNA sequence analysis.
Double-stranded DNA sequencing of the M. bovis Ag85B gene inserted into pCI vector (Promega) was performed using the DYEnamic ET dye terminator cycle sequencing kit (MegaBACE) and the MegaBACE 1000 capillary sequencer (Amersham Biosciences, São Paulo, Brazil). The sequence data were compiled and analyzed with the sequence analysis program DNASIS V5.00 (Hitachi Software). Subsequent homology searches were performed with the BLAST programs available from the National Biotechnology Information Center (2).
Ag85B DNA vaccine.
DNA vaccine construct pCI-Ag85B was amplified in the E. coli DH5α strain, and DNA isolation was performed using the EndoFree Plasmid Giga kit (QIAGEN, Valencia, CA). DNA was resuspended in phosphate-buffered saline (PBS) at a final concentration of 1 mg/ml for further immunization. The plasmid preparation contained less than 0.05 endotoxin units per 100 μg of DNA, as assessed by the QCL-1000 Limulus amebocyte lysate analysis kit (BioWhittaker, Walkersville, MD). The relative amount (%) of supercoiled isoform in each vaccine sample was determined using densitometric analysis and reference plasmid preparations for calibration. The proportion of supercoiled isoform in the vaccinal solution was on average 70%.
Production of recombinant Ag85B protein.
Escherichia coli harboring the pMAL-Ag85B construct was cultured in LB medium, and expression of the fusion protein was induced by 0.4 mM IPTG (isopropyl-β-d-thiogalactopyranoside). After IPTG induction, bacterial cells were harvested by centrifugation at 4,000 × g for 20 min, and the supernatants were discarded. The pellets were resuspended in 100 ml of PBS (pH 8.4) containing 25 mg of lysozyme. The resuspended pellets were frozen and thawed three times at −70°C. The resulting suspensions were sonicated twice for 30 s and then centrifuged at 9,000 × g for 45 min. The protein present in the bacterial supernatant was located onto a polyacrylamide gel to confirm the presence of the recombinant protein by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). E. coli lysates containing the induced maltose binding protein (MBP)-Ag85B fusion protein were diluted 1:5 in PBS. Then, the suspensions were loaded into an amylose resin column (New England Biolabs) and washed 10 times with PBS. The fusion protein concentration was determined by a Bradford protein assay.
SDS-PAGE and immunoblotting.
E. coli extracts expressing the MBP-Ag85B fusion protein and purified MBP-Ag85B protein were separated on a 10% denaturing polyacrylamide gel by electrophoresis. From the gel, the proteins were transferred to nitrocellulose membranes (Bio-Rad) by Western blotting as previously described (20). The nitrocellulose membranes were blocked with 5% skim milk at 4°C overnight and reacted with rabbit anti-MBP serum (1:10,000) for 2 h at room temperature. After a reaction with the primary antibody, the blots were washed three times with TBST (0.5 M NaCl-0.02 M Tris [pH 7.5], 0.05% Tween 20) and incubated for 1 h at room temperature with anti-rabbit immunoglobulin (IgG)-alkaline phosphatase conjugate (1:10,000) (Promega). After three washes with TBST, the reaction mixture was developed following incubation at room temperature with nitroblue tetrazolium chloride and BCIP (5-bromo-4-chloro-3-indolyl-1-phosphate) for alkaline phosphatase.
Animals and immunization protocol.
Forty BALB/c mice, on average 8 weeks old, were divided into four groups of 10 mice each. For intramuscular (i.m.) DNA immunization, mice were preinjected with 10 mM cardiotoxin (Sigma) 5 days before vaccination. The cardiotoxin was given to optimize the immune response by inducing regeneration in muscle cells and DNA uptake as previously described (13). At days 0, 15, 30, and 45, the construct pCI-Ag85B was administered by i.m. injection. Each quadriceps was inoculated with 50 μl of DNA at a concentration of 1 μg/μl in PBS such that each animal received a total of 100 μg of plasmid DNA. As a negative control, one group of mice was injected with pCI plasmid without an insert. Another mouse group was vaccinated with 106 CFU of BCG obtained from Fundação Ataulpho de Paiva (São Paulo, Brazil) and injected subcutaneously. As a negative control of BCG immunization, PBS was administered subcutaneously to another mouse group.
Analysis of antibody response.
Mice were bled 15 days after each immunization, and individual mouse sera were tested for antibody responses by enzyme-linked immunosorbent assay (ELISA). Plates (96-well) (Maxisorp; Nunc) were incubated overnight with the recombinant Ag85B protein (5 μg/ml) in carbonate-bicarbonate buffer, pH 9.6, at 4°C. Then, blocking solution (PBS containing Tween 20 [0.05%] plus 10% fetal bovine serum) was added for 2 h at 37°C. Sera from immunized mice were diluted 1:100 in PBS-Tween 20, added to the plates and incubated for 2 h at 37°C. To determine total serum IgG, IgG1, or IgG2a, plates were treated with peroxidase-conjugated goat anti-mouse IgG whole molecule (1:10,000; Sigma) or peroxidase-conjugated goat anti-mouse IgG1 or IgG2a (1:6,000; Sigma), respectively. The reaction mixture was developed by the addition of 200 μmol of o-phenylenediamine (Sigma) and 0.04% H2O2. The reaction was stopped by the addition of 5% H2SO4, and plates were then read at 492 nm in an ELISA reader (Bio-Rad).
Cytokine assay.
Spleens of mice 2 weeks after the last immunization were passed through steel mesh to obtain single-cell suspensions. Splenocytes were then isolated by density gradient centrifugation with Ficoll (Sigma). Cells were washed twice with sterile PBS then cultured in RPMI 1640 medium supplemented with penicillin G-sodium (100 U/ml), streptomycin sulfate (100 μg/ml), amphotericin B (250 ng/ml), and 10% fetal bovine serum and then placed at 1 × 106 cells/well in 96-well tissue culture plates. Murine splenocytes from vaccinated animals were stimulated with recombinant Ag85B protein at 25 μg/ml. Nonstimulated splenocytes were used as a negative control, and splenocytes stimulated with concanavalin A (5 μg/ml) were used as a T-cell-activating control. After 72 h at 37°C in air with 5% CO2, splenocyte culture supernatants were tested for the presence of cytokines with the ELISA kit DuoSet (R&D Systems, MN) (mouse TNF-α, IL-4, and IFN-γ).
Intracellular cytokine staining.
For intracytoplasmatic cytokine staining, splenocytes from five mice/group were pooled and adjusted to 2 × 105 cells per well. Splenocytes were maintained in culture at 37°C in 5% CO2 in medium alone or stimulated with rAg85B (50 μg/ml), or ConA (5 μg/ml). After 16 h of culture, 1 μl/ml of brefeldin A (1-mg/ml stock) (Sigma Chemical Co., St. Louis, MO) was added to impair cytokine secretion. After 4 h of incubation, these cells were stained for surface markers and intracellular cytokines as described by Bottrel et al. (4). Briefly, cultured cells were stained for surface markers using fluorescein isothiocyanate-labeled anti-CD4 (Serotec, Düsseldorf, Germany) and anti-CD8 (Serotec) monoclonal antibodies by incubation for 20 min with antibody solution (0.15 M PBS, 0.5% bovine serum albumin, 2 mM NaN3), followed by washes and fixation using 2% formaldehyde solution. These cells were permeabilized and further stained with phycoerythrin-labeled anti-IFN-γ (Serotec) and anti-TNF-α (Serotec) monoclonal antibodies in a 0.5% saponin solution in PBS. After 15 min, cells were washed with permeabilization solution and resuspended in PBS. A minimum of 30,000 splenocyte-gated events were acquired in list mode and analyzed using a lymphocyte gate determined based on size and granularity profiles. All quadrants were set according to the labeled isotype controls and analyzed using the CELLquest software (Becton Dickinson, San Jose, CA).
M. bovis challenge.
Two weeks after the fourth DNA immunization, mice were infected intravenously with 1 × 106 CFU of virulent M. bovis (ATCC 19274). Animals were then killed 2 weeks after challenge infection. Spleens and lungs from infected and noninfected control mice were homogenized in PBS, 10-fold serially diluted, and plated on Lowenstein-Jensen medium. Plates were incubated at 37°C in air with 5% CO2, and the number of CFU was counted visually after 21 days.
Statistical analysis.
Statistical analysis was performed with Student's t test using the MINITAB software package (Minitab, State College, PA). For challenge studies, a mean value for each spleen and lung count was obtained after log conversion. Log units of protection were obtained by subtracting the mean CFU for BCG- or DNA-immunized mouse groups from the mean CFU for the corresponding control groups that received PBS or plasmid control alone, respectively.
RESULTS
Mycobacterium Ag85B protein production.
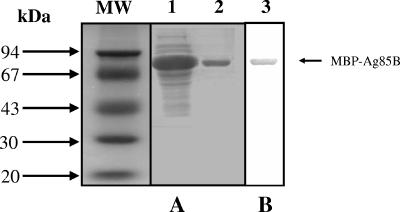
The heterologous expression of M. bovis Ag85B protein was performed in E. coli as an MBP fusion. Recombinant Ag85B was purified by affinity chromatography on an amylose resin column (Fig. 1A). Purified rAg85B was analyzed by immunoblotting using rabbit antiserum to MBP (Fig. 1B). The observed molecular mass of the recombinant protein was in accordance with the estimated molecular mass of the MBP fusion, 72 kDa (30 kDa for Ag85B and 42 kDa for MBP). The immunoblotting analysis confirmed the identity of the MBP-Ag85B fusion protein produced through rabbit anti-MBP antibody recognition.
FIG. 1.
(A) Coomassie blue-stained SDS-10% PAGE profile of E. coli extracts expressing the MBP-Ag85B fusion protein (lane 1), or purified MBP-Ag85B fusion protein (lane 2). (B) Western blot analysis showing MBP-Ag85B recognition by rabbit anti-MBP antibodies (lane 3). The arrow indicates the MBP-Ag85B band. The numbers at the left indicate the molecular mass standard (MW) in kilodaltons (Amersham Biosciences).
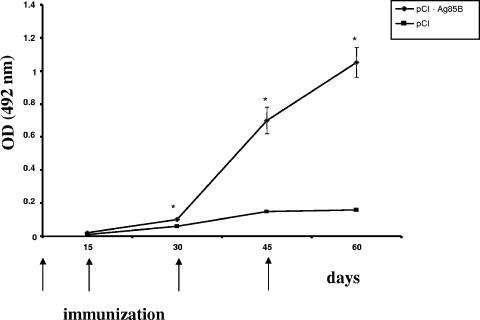
Kinetics of total IgG anti-Ag85B production.
In order to determine the level of total IgG induced after i.m. gene immunization by the pCI-Ag85B plasmid, blood samples were collected from the retro-orbital plexus of BALB/c mice 15 days after each immunization. In Fig. 2, the kinetics of total IgG produced by vaccinated mice was analyzed. The group immunized with the pCI-Ag85B plasmid generated very high levels of specific anti-Ag85B IgG antibodies after the third immunization compared to the group immunized with pCI plasmid alone.
FIG. 2.
Total anti-Ag85B-specific IgG responses of mice immunized with DNA vaccine. Animals (n = 10) were injected i.m. with DNA vaccine construct pCI-Ag85B, or control plasmid pCI. DNA immunization was performed at days 0, 15, 30, and 45, and specific IgG levels to Ag85B were assayed by ELISA at days 15, 30, 45, and 60 after each vaccination in a working dilution of 1:100. Results are expressed as means of each group immunized. Error bars indicate standard deviations of the means. Statistically significant differences to pCI control group are denoted by an asterisk (P < 0.05). OD, optical density.
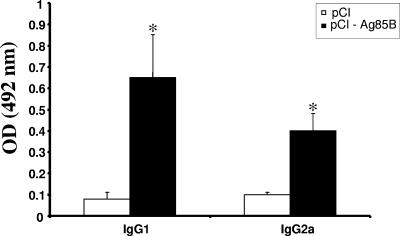
Levels of IgG1 and IgG2a produced in BALB/c mice vaccinated with pCI-Ag85B.
In order to determine the type of immune response induced, the subclasses IgG1 and IgG2a were also analyzed. Both isotypes IgG1 and IgG2a anti-Ag85B were produced at significantly higher levels in pCI-Ag85B-vaccinated mice than in pCI-vaccinated mice after the third immunization (Fig. 3).
FIG. 3.
Antibody subclass response in mice following i.m. immunization with the Ag85B gene. IgG1 and IgG2a subclass levels in sera of mice (n = 10) immunized with DNA vaccine pCI-Ag85B or control plasmid pCI were determined after the third immunization (day 45). Results are expressed as means of each group immunized. Error bars indicate standard deviations of the means. Statistically significant differences to pCI control group are denoted by an asterisk (P < 0.05). OD, optical density.
Induction of Th1 cytokine profile following Ag85B DNA immunization.
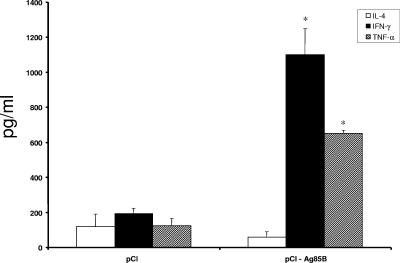
To further determine the cytokine profile after DNA vaccination, the levels of IFN-γ, TNF-α, and IL-4 in immunized mice were measured 2 weeks after the last immunization. Figure 4 shows that high levels of IFN-γ and TNF-α were detected in cell supernatants of pCI-Ag85B-vaccinated mice in vitro stimulated with rAg85B compared to pCI-vaccinated animals. Regarding IL-4, no significant levels were detected when cells from pCI-Ag85B and control groups were specifically activated. For a negative control, MBP alone was used to stimulate splenocytes, and no significant T-cell activation was detected in either mouse group (data not shown). In contrast, ConA significantly stimulated the cytokine production by splenocytes from all vaccinated mouse groups (data not shown).
FIG. 4.
Production of IL-4, IFN-γ, and TNF-α from mouse splenocytes (n = 5) immunized with the pCI-Ag85B DNA vaccine i.m. following 72 h of rAg85B stimulation. Bars indicate the mean values of cytokines in pg/ml ± standard deviations. Statistically significant differences compared to pCI control group are denoted by an asterisk (P < 0.05).
CD8+ T cells are the main source of IFN-γ.
To determine which cell phenotype was responsible for cytokine production, splenocytes were stained for CD4 and CD8 surface markers and for cytoplasmatic IFN-γ and TNF-α. CD8+ T cells were the major source of IFN-γ in splenocyte cultures of mice immunized with pCI/Ag85B compared to pCI empty plasmid-injected animals. (Table 1).
TABLE 1.
T-cell subpopulation producing IFN-γ or TNF-α following DNA immunization with pCI-Ag85B
| Cell subpopulation | Cytokine | % Cytokine-producing cells
|
|
|---|---|---|---|
| Nonstimulated | rAg85Ba | ||
| CD4+ | IFN-γ | 0.50 ± 0.16 | 1.0 ± 0.18* |
| TNF-α | 0.14 ± 0.05 | 0.84 ± 0.10* | |
| CD8+ | IFN-γ | 0.22 ± 0.08 | 2.2 ± 0.35*# |
| TNF-α | 0.10 ± 0.06 | 0.40 ± 0.1 | |
*, statistically significant difference compared to nonstimulated cells; #, statistically significant difference compared to CD4+ T cells producing IFN-γ.
Level of protection induced by vaccination with the Ag85B gene.
Regarding protective immunity, mice immunized with BCG had a significant reduction in CFU numbers of M. bovis in both spleens (log 0.87) and lungs (log 1.56) compared to the control mice that received PBS. Moreover, Ag85B DNA immunization induced a statistically significant reduction in M. bovis CFU numbers in spleens (log 0.56) compared to what was seen for mice that received the plasmid pCI alone (P < 0.05) (Table 2). However, there was no significant reduction in bacterial load in lungs of pCI-Ag85B-immunized mice compared to the pCI control group.
TABLE 2.
Protection level induced by immunization with the Ag85B DNA vaccine compared to BCG
| Treatment | Log value
|
|||
|---|---|---|---|---|
| Lung
|
Spleen
|
|||
| CFUa | Protection | CFUa | Protection | |
| PBS | 8.12 ± 0.34 | 8.53 ± 0.15 | ||
| pCI | 7.86 ± 0.37 | 8.56 ± 0.17 | ||
| pCI-Ag85B | 7.83 ± 0.25 | 0.03 | 8.00 ± 0.05 | 0.56c |
| BCG | 6.53 ± 0.14 | 1.56b | 7.66 ± 0.06 | 0.87b |
Mean representative ± standard deviation of one experiment that was performed twice with similar results.
P < 0.05, significant compared to the PBS control.
P < 0.05, significant compared to the pCI control group.
DISCUSSION
Protective immunity against mycobacterial infection is mediated by interactions between specifically primed CD4+ and CD8+ T cells and activated macrophage effector cells harboring the intracellular pathogen (14). IFN-γ is a critical cytokine that activates macrophages and plays a pivotal role in antimicrobial protection, as demonstrated with gene knockout mice (10). Gene vaccines have been employed successfully in animal studies to induce protective immunity against a variety of bacteria, viruses, and parasites (1). This type of vaccine is capable of eliciting a strong cell-mediated immunity that is required to control infection by many intracellular agents, such as Mycobacterium spp. (23). Here, we demonstrated that DNA vaccination using a plasmid encoding Mycobacterium bovis Ag85B is a potent strategy to generate a specific Th1 type of immune response and partial protection against M. bovis infection.
More than 50 million cattle are infected with M. bovis worldwide yearly, and the resulting economic losses are estimated to be around US$3 billion per year (28). BCG, the attenuated vaccine strain of M. bovis, has been used as a vaccine against human tuberculosis (11). However, primarily due to its interference with the intradermal tuberculin test as well as lack of protective immunity, the BCG vaccine is not used for bovine tuberculosis prevention (29). Immunization with genes encoding mycobacterial antigens such as hsp65, Ag85A, Ag85B, MPT-64, and ESAT-6 have been reported to be effective in experimental models of human tuberculosis (16, 18, 22, 30). However, few reports dealt with experimental models for bovine tuberculosis using DNA vaccines. Vordermeier et al. (31), using MPT70 and MPT83 DNA vaccines in cattle, induced specific antibody and IFN-γ responses but failed to engender protective immunity against M. bovis challenge. On the other hand, DNA immunization encoding the combination of M. tuberculosis antigens Ag85B, MPT64, and MPT83 induced partial protection in cattle against M. bovis challenge (7). The Ag85 antigen complex, including Ag85A, Ag85B, and Ag85C, is recognized by T cells isolated from M. bovis-infected animals, and this complex constitutes 20 to 30% of all proteins present in supernatant of Mycobacterium short-term culture.
In this study, we analyzed both the humoral and the cell-mediated immune responses induced by the M. bovis Ag85B DNA vaccine. Mice immunized with the M. bovis Ag85B DNA vaccine mounted a strong anti-Ag85B IgG response after the third immunization compared to animals that received pCI vector alone. Regarding IgG isotypes, both IgG1 and IgG2a to Ag85B were produced in DNA-immunized mice compared to control group. As demonstrated here, Huygen et al. (16) reported that mice vaccinated with a DNA vaccine encoding Ag85A produced high levels of IgG1 and IgG2a. The protective immunity against tuberculosis depends on the recruitment of antigen-specific T cells, especially CD4+, and the release of cytokines, particularly IFN-γ, for the activation of microbicidal mechanisms of macrophages (19). Therefore, we measured IL-4, IFN-γ, and TNF-α levels from splenocyte supernatants of DNA-vaccinated mice. The results clearly indicated that the Ag85B DNA vaccine induced a Th1 type of immune response in mice, with higher levels of IFN-γ and TNF-α and no IL-4. Several studies have implicated, besides the role of IFN-γ, the role of TNF-α in the control of infections caused by Mycobacterium spp. (5, 25). Moreover, TNF-α in synergy with IFN-γ induces expression of NOS2 (14). Therefore, proinflammatory cytokine synthesis and production of nitric oxide are the main effective mechanisms in fighting mycobacteria (26). Regarding the cell phenotype responsible for IFN-γ production, intracellular cytokine staining was performed, and we observed that the main source of IFN-γ is CD8+ T lymphocytes. As demonstrated by Caruso et al. (8), early production of IFN-γ by CD4+ T cells is essential to control M. tuberculosis infection, and IFN-γ production by other cells cannot substitute for the CD4 T-cell contribution. Therefore, the low efficacy of the Ag85B DNA vaccine may be due to the lower production of IFN-γ by CD4+ T cells induced by this immunogen.
Regarding protective immunity, M. bovis Ag85 DNA immunization induced a significant reduction in bacterial burden in spleens but not in lungs of vaccinated mice (Table 2). Pulmonary immunity requires activation of memory T cells producing IFN-γ with homing properties to the lung (17). Systemic immunizations are not particularly effective for inducing such pulmonary effector T cells, but combinations of intramuscular injections with mucosal plasmid instillations may overcome this problem. The major difficulty to mucosal plasmid DNA delivery is the necessity of protecting the DNA against nuclease degradation. Experiments are under way in our laboratory combining systemic vaccination with intranasal boosting with the Ag85B DNA vaccine in order to enhance protective immunity. Finally, DNA vaccination may open new horizons for vaccination against animal tuberculosis.
Acknowledgments
This work was supported by CNPq, CAPES-PQI, and FAPEMIG.
We thank Gracia M. S. Rosinha and Cristina T. Fonseca for technical assistance.
REFERENCES
- 1.Ada, G., and I. Ramshaw. 2003. DNA vaccination. Expert Opin. Emerg. Drugs 8:27-35. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Behr, M. A. 2002. BCG—different strains, different vaccines? Lancet Infect. Dis. 2:86-92. [DOI] [PubMed] [Google Scholar]
- 4.Bottrel, R. L., W. O. Dutra, F. A. Martins, B. Gontijo, E. Carvalho, M. Barral-Netto, A. Barral, R. P. Almeida, W. Mayrink, R. Locksley, and K. J. Gollob. 2001. Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis. Infect. Immun. 69:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botha, T., and B. Ryffel. 2003. Reactivation of latent tuberculosis infection in TNF-deficient mice. J. Immunol. 171:3110-3118. [DOI] [PubMed] [Google Scholar]
- 6.Buddle, B. M. 2001. Vaccination of cattle against Mycobacterium bovis. Tuberculosis 81:125-132. [DOI] [PubMed] [Google Scholar]
- 7.Cai, H., X. Tian, X. D. Hu, S. X. Li, D. H. Yu, and Y. X. Zhu. 2005. Combined DNA vaccines formulated either in DDA or in saline protect cattle from Mycobacterium bovis infection. Vaccine 23:3887-3895. [DOI] [PubMed] [Google Scholar]
- 8.Caruso, A. M., N. Serbina, E. Klein, K. Triebold, B. R. Bloom, and J. L. Flynn. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J. Immunol. 162:5407-5416. [PubMed] [Google Scholar]
- 9.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 10.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich, G., J. F. Viret, and J. Hess. 2003. Mycobacterium bovis BCG-based vaccines against tuberculosis: novel developments. Vaccine 21:667-670. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly, J. J., J. B. Ulmer, and M. A. Liu. 1994. Immunization with DNA. J. Immunol. Methods 176:145-152. [DOI] [PubMed] [Google Scholar]
- 14.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 15.Gurunathan, S., C. Y. Wu, B. L. Freidag, and R. A. Seder. 2000. DNA vaccines: a key for inducing long-term cellular immunity. Curr. Opin. Immunol. 12:442-447. [DOI] [PubMed] [Google Scholar]
- 16.Huygen, K., J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, R. R. Deck, et al. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 17.Huygen, K. 2005. Plasmid DNA vaccination. Microbes Infect. 7:932-938. [DOI] [PubMed] [Google Scholar]
- 18.Kamath, A. T., C. G. Feng, M. Macdonald, H. Briscoe, and W. J. Britton. 1999. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 67:1702-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamath, A. T., N. L. Groat, A. G. Bean, and W. J. Britton. 2000. Protective effect of DNA immunization against mycobacterial infection is associated with the early emergence of interferon-gamma (IFN-gamma)-secreting lymphocytes. Clin. Exp. Immunol. 120:476-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leclercq, S., J. S. Harms, G. M. S. Rosinha, V. Azevedo, and S. C. Oliveira. 2002. Induction of a Th1-type of immune response but not protective immunity by intramuscular DNA immunization with Brucella abortus GroEL heat-shock gene. J. Med. Microbiol. 51:20-26. [DOI] [PubMed] [Google Scholar]
- 21.Lowrie, D. B., R. E. Tascon, and C. L. Silva. 1995. Vaccination against tuberculosis. Int. Arch. Allergy Immunol. 108:309-312. [DOI] [PubMed] [Google Scholar]
- 22.Lowrie, D. B., R. E. Tascon, V. L. Bonato, V. M. F. Lima, L. H. Faccioli, E. Stavropoulos, et al. 1999. Therapy of tuberculosis in mice by DNA vaccination. Nature 400:269-271. [DOI] [PubMed] [Google Scholar]
- 23.Nagata, T., T. Aoshi, M. Uchijima, M. Suzuki, and Y. Koide. 2004. Cytotoxic T-lymphocyte-, and helper T-lymphocyte-oriented DNA vaccination. DNA Cell Biol. 23:93-106. [DOI] [PubMed] [Google Scholar]
- 24.Narain, J. P., M. C. Raviglione, and A. Kochi. 1992. HIV-associated tuberculosis in developing countries: epidemiology and strategies for prevention. Tuberc. Lung Dis. 73:311-321. [DOI] [PubMed] [Google Scholar]
- 25.Roach, D. R., A. G. Bean, C. Demangel, M. P. France, H. Briscoe, and W. J. Britton. 2002. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J. Immunol. 168:4620-4627. [DOI] [PubMed] [Google Scholar]
- 26.Scanga, C. A., A. Bafica, C. G. Feng, A. W. Cheever, S. Hieny, and A. Sher. 2004. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect. Immun. 72:2400-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skinner, M. A., B. M. Buddle, D. N. Wedlock, D. Keen, G. W. Lisle, R. E. Tascon, J. C. Ferraz, D. B. Lowrie, P. J. Cockle, H. M. Vordermeier, and R. G. Hewinson. 2003. A DNA prime-Mycobacterium bovis BCG boost vaccination strategy for cattle induces protection against bovine tuberculosis. Infect. Immun. 71:4901-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steele, J. H. 1995. Regional and country status reports, p. 47-61. In C. O. Thoen and J. H. Steele (ed.), Mycobacterium bovis infection in animals and humans. Iowa State University Press, Ames, Iowa.
- 29.Suazo, F. M., A. M. A. Escalera, and R. M. G. Torres. 2003. A review of M. bovis BCG protection against TB in cattle and other animal species. Prevent. Vet. Med. 58:1-13. [DOI] [PubMed] [Google Scholar]
- 30.Tanghe, A., O. Denis, B. Lambrecht, V. Motte, T. van den Berg, and K. Huygen. 2000. Tuberculosis DNA vaccine encoding Ag85A is immunogenic and protective when administered by intramuscular needle injection but not by epidermal gene gun bombardment. Infect. Immun. 68:3854-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vordermeier, H. M., P. J. Cockle, A. O. Whelan, S. Rhodes, M. A. Chambers, D. Clifford, et al. 2001. Effective DNA vaccination of cattle with the mycobacterial antigens MPT83 and MPT70 does not compromise the specificity of the comparative intradermal tuberculin skin test. Vaccine 19:246-1225. [DOI] [PubMed] [Google Scholar]
- 32.Wedlock, D. N., M. A. Skinner, G. W. Lisle, and B. M. Buddle. 2002. Control of Mycobacterium bovis infections and the risk to human populations. Microbes Infect. 4:471-480. [DOI] [PubMed] [Google Scholar]