Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV)-induced pneumonia is a major problem, and vaccination is used to reduce losses associated with PRRSV. Porcine circovirus type 2 (PCV2) causes lymphoid depletion, and there is concern that this adversely affects the immune response. The objective of this study was to investigate the effect of PCV2 infection on the efficacy of modified live virus (MLV) PRRSV vaccine. Sixty-nine 2-week-old pigs were randomly assigned to one of seven groups of 9 to 10 pigs each. At 6 weeks of age, pigs in groups 4, 5, and 6 were inoculated intranasally with PCV2 ISU-40895. At 8 weeks of age, groups 3, 4, 6, and 7 were vaccinated with a PRRSV MLV vaccine. At 12 weeks of age, groups 2, 3, and 4 were challenged with PRRSV SDSU73. All pigs were necropsied 14 days after PRRSV challenge. PCV2-infected, PRRSV-vaccinated, and PRRSV-challenged pigs had significantly (P < 0.05) more-severe macroscopic lung lesions than did the PRRSV-vaccinated and PRRSV-challenged pigs that were not exposed to PCV2 prior to PRRSV vaccination. Nonvaccinated PRRSV-infected pigs had a significantly (P < 0.001) higher incidence of PRRSV antigen in lungs than did all other groups except the group infected with PCV2 prior to PRRSV vaccination and challenge. The nonvaccinated PRRSV-challenged group and the group challenged with PCV2 prior to PRRSV vaccination and challenge had significantly (P < 0.001) lower average daily weight gain than did the control and the vaccinated groups. This work suggests that PCV2 infection has an adverse effect on the development of protective immunity induced by PRRSV vaccine.
Porcine circovirus type 2 (PCV2), a small, nonenveloped, single-stranded DNA virus with a circular genome (48), is ubiquitous in the global swine population (2). The hallmark microscopic lesions of PCV2 infection are lymphoid depletion and histiocytic replacement of lymphoid follicles in the lymphoid tissues and variable degrees of lymphohistiocytic inflammation in a variety of organs (45). PCV2 is associated with postweaning multisystemic wasting syndrome (PMWS) (1, 12), which is characterized by moderate to excessive loss of body condition often accompanied by respiratory disease in nursery and grow-to-finish pigs (17). The incidence of PCV2-associated disease in affected herds generally varies from 4% to up to 30% in individual farms (2, 17, 23). On most farms, PCV2 infection is widespread and subclinical.
Studies on pigs with naturally acquired PMWS and PCV2 infection showed leukopenia characterized by decreased lymphocyte counts compared to those of healthy control pigs (10, 42, 43). Segalés et al. (42) found a significant decrease in the number of CD3+ and CD4+ cells in PMWS-affected pigs compared to numbers in clinically healthy pigs, whereas no difference was found in the numbers of CD8+ cells. Darwich et al. (10) compared clinically PMWS-affected pigs that were infected by PCV2, wasted pigs that were not infected by PCV2, and healthy control pigs and found that, regardless of PCV2 infection status, wasted pigs in general had decreased CD4+ cells. However, only PMWS-affected and PCV2-infected pigs had decreased numbers of CD8+ and double-positive cells. The amount of PCV2 in lymphoid tissues was correlated to the degree of lymphoid depletion and to the decrease in immunoglobulin M-positive and CD8+ cells in peripheral blood (10). Further detailed assessment of PCV2-induced alteration of cells of the immune system in pigs experimentally infected with PCV2 demonstrated a PCV2-induced lymphopenia present only in pigs that developed clinical PMWS but not in those that were subclinically infected (29). The mean lymphocyte levels of PCV2-infected pigs decreased below that of the control pigs, which was most evident at 10 days postinoculation (DPI). A prominent lymphopenia was present in PMWS-affected pigs starting from DPI 14 until the death of the pigs. All T-cell subpopulations were found to be susceptible to PCV2-induced lymphopenia (29).
Lymphoid depletion is a necessary and hallmark lesion of PCV2-associated PMWS (45); however, lymphoid depletion, to some degree, can also be observed in subclinically infected pigs (38). Studies on pigs with naturally acquired PMWS revealed that there was a reduction in the number of interfollicular dendritic cells and a reduction or absence of B cells and CD4+ cells (40). Chianini et al. (8) graded lymphoid lesions in pigs with natural PMWS as mild, moderate, and severe and showed a reduction or loss of B and T lymphocytes. It has been suggested that PCV2-associated lymphoid depletion compromises the immune system of the pig. This is supported by case reports describing PCV2 infection associated with coinfecting pathogens that are typically indicative of an immunosuppressed stage of the host, such as Pneumocystis carinii (9), Chlamydia spp. (6), pulmonary aspergillosis (44), and Cryptosporidium parvum (30).
Porcine reproductive and respiratory syndrome virus (PRRSV) is the major contributor to the porcine respiratory disease syndrome complex (PRDC) in the United States (18) and is estimated to cost the U.S. swine industry $560 million annually (28). PRRSV is an enveloped, positive-sense, single-stranded RNA virus classified in the order Nidovirales, family Arteriviridae, genus Arterivirus (7). Clinical signs of PRRSV infection in growing pigs include fever, sneezing, dyspnea, tachypnea, anorexia, and decreased weight gain. Vaccination is a common procedure to minimize economic losses associated with this pathogen, and vaccines have proven to be effective in experimental trials (33) and field studies (24, 47). However, PRRSV vaccine failures are not uncommon in the field, and PRRSV continues to be the most common primary pathogen in cases of PRDC (18). PRRSV vaccine failure may be due to lack of cross-protection between the vaccine and field strains or failure of the pig to mount an effective response to PRRSV vaccine.
The effect of PCV2 infection on PRRSV vaccine efficacy has, to our knowledge, not been reported in the literature, and the objective of this study was to investigate the effects of PCV2 infection on the efficacy of a PRRSV modified live virus (MLV) vaccine.
MATERIALS AND METHODS
Animals.
Sixty-nine segregated, early weaned, specific-pathogen-free (SPF), crossbred pigs were brought to the research facility at Iowa State University at approximately 12 to 14 days of age. The pigs were purchased from a herd determined to be free of PRRSV based on regular serological testing. PCV2-associated disease was not observed in the source herd or the offspring of this herd.
Housing and feeding.
On the day of arrival at the research facility, the pigs were randomly assigned to seven groups and rooms with 9 to 10 pigs in each room. The pen size was 3 by 3.6 m, and each pen contained a nipple waterer and one self-feeder. The rooms were identical in size and environmental controls. The pigs were fed a complete, phased, corn- and soybean-based ration.
Experimental design, inoculations, and vaccination.
The experimental design is summarized in Table 1. Pigs were vaccinated with PRRSV MLV vaccine at 14 days after PCV2 infection when PCV2-associated lymphoid depletion is typically most severe and the amount of PCV2 genomic copy numbers in serum is typically highest in this experimental SPF pig model (32, 34, 36).
TABLE 1.
Experimental design to determine the effect of PCV2 infection on the efficacy of PRRSV vaccine
| Group no. (n) | Designationa | Treatment at age (wk):
|
||
|---|---|---|---|---|
| 6 | 8 | 12 | ||
| 1 (10) | Control | |||
| 2 (9) | NV-PRRSV | PRRSV | ||
| 3 (10) | V-PRRSV | Vaccinationb | PRRSV | |
| 4 (10) | PCV2-V-PRRSV | PCV2 | Vaccination | PRRSV |
| 5 (10) | PCV2 | PCV2 | ||
| 6 (10) | PCV2-V | PCV2 | Vaccination | |
| 7 (10) | V | Vaccination | ||
NV, nonvaccinated; V, vaccinated.
Ingelvac PRRS ATP MLV (Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO).
The experimental protocols were approved by the Iowa State University Committee on Animal Care. After waning of the passively acquired antibodies to PCV2 at 6 weeks of age, pigs in groups 4, 5, and 6 were inoculated intranasally with 3 ml of the PCV2 ISU-40895. At 8 weeks of age, pigs in groups 3, 4, 6, and 7 were vaccinated intramuscularly in the right side of the neck with 2 ml of the Ingelvac PRRS ATP MLV vaccine (Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO) according to the manufacturer's recommendations. At 12 weeks of age, each pig in groups 2, 3, and 4 received 2 ml of PRRSV SDSU73 intranasally.
PCV2 inoculum.
The PCV2 virus stock was generated through direct transfection of PK-15 cells with an infectious DNA clone of PCV2 ISU-40895 as previously described (13). PCV2 isolate ISU-40895 was isolated in 1998 from a wasting pig with respiratory disease in Iowa. The pig came from a group of 300 pigs with a history of increased respiratory disease and lethargy, with approximately 50% of the pigs affected (14). Inoculation was done with the PCV2 virus stock at a dose of 105.2 50% tissue culture infective doses per ml.
PRRSV inoculum.
Highly virulent PRRSV isolate SDSU73 was isolated from a sow herd with a high prevalence of abortions and higher-than-usual sow mortality in 1996 (26). Passage 2 of PRRSV SDSU73 (Courtesy of M. Roof, Boehringer Ingelheim Vetmedica, Inc.) at a titer of 105 50% tissue culture infective doses per ml was used for inoculation of the pigs.
Serology.
Blood samples were collected on arrival of the pigs, followed by weekly blood collections. Sera collected at 2 (arrival), 8 (PRRSV vaccination), 9, 10, 11, 12 (PRRSV inoculation), 13, and 14 (during necropsy) weeks of age were tested for the presence of PRRSV-specific antibodies by a commercial PRRSV enzyme-linked immunosorbent assay (ELISA) (HerdChek PRRSV antibody test kit; Idexx Laboratories Inc., Westbrook, MA). An optical density at 405 nm greater than or equal to 0.4 was set as the ELISA cutoff value. A fluorescent focus neutralization assay to determine the amount of PRRSV SDSU73 neutralizing antibodies was done on sera from all PRRSV-vaccinated groups (3, 4, 6, and 7) collected on the day of vaccination and at 7, 14, 21, and 28 days postvaccination according to protocols used at the Iowa State University Veterinary Diagnostic Laboratory.
Sera collected at 2 (arrival of the pigs), 6 (PCV2 inoculation), and 14 (necropsy) weeks of age were tested for the presence of PCV2-specific antibodies by a PCV2 ELISA based on the recombinant ORF2 capsid protein of PCV2 (27). Samples were considered positive if the calculated sample-to-positive (S/P) ratio was 0.2 or greater.
Clinical evaluation.
The pigs were monitored daily and scored for severity of clinical respiratory disease ranging from 0 (normal) to 6 (severe dyspnea) and abdominal breathing (16). In addition, pigs were evaluated daily for clinical signs, including sneezing and jaundice. Rectal temperatures, wasting, and behavioral changes such as lethargy were recorded daily. The pigs were weighed at weekly intervals.
PCV2 DNA quantification.
DNA extraction on sera collected at the day of PRRSV challenge and at 14 days after PRRSV challenge, and on bronchoalveolar lavage (BAL) fluid collected during necropsy, was performed using a QIAamp DNA mini kit (QIAGEN, Valencia, CA). DNA extracts were used for quantification of the amount of PCV2 genomic DNA by real-time PCR as previously described (36).
PRRSV RNA detection and quantification.
RNA extraction on sera collected 7 and 14 days after PRRSV challenge and on bronchoalveolar lung lavage fluid collected during necropsy was performed using a QIAamp viral RNA mini kit (QIAGEN). Primer-probe combinations specific for the North American PRRSV (PRRSORF7F [TGTCAGATTCAGGGAGRATAAGTTAC], PRRSORF7R [ATCARGCGCACAGTRTGATGC], and PRRSORF7P [6FAM-TGTGGAGTTYAGTYTGCC]) and the European PRRSV (LELYRTF [GCTGAAGATGACRTYCGGCA], LELYRTR [GCAGTYCCTGCGCCTTGAT], and LELYRTP [VIC-TGCAATCGATYCAGAC]) were used for the real-time, reverse transcriptase PCR (RT-PCR). Each PCR consisted of 2.5 μl of the RNA template and 22.5 μl of PCR master mix. The PCR master mix contained 0.35 μl of RT mix from a QuantiTect probe RT-PCR kit (QIAGEN) with the magnesium chloride concentration adjusted to 6 mM. Forward and reverse primers and detection probes were used at concentrations of 800, 800, and 275 nM and 400, 400, and 100 nM for the North American and European PRRSV, respectively. An additional 0.25 μl (1.25 U) of HotStarTaq (QIAGEN) was added to each reaction. Each reaction included five progressive 1:10 dilutions of a known copy number of PRRSV that served to generate a standard curve. Each plate was run in a sequence detection system (GeneAmp 7900; Applied Biosystems) under company-specific conditions (30 min at 50°C, 15 min at 95°C, followed by 35 cycles of 15 seconds at 94°C and 60 seconds at 60°C).
Necropsy.
Necropsy was performed at 14 days after PRRSV challenge when the pigs were 14 weeks old. Pathologists were blinded to the treatment group for evaluation of gross lesions. The total amount of lung affected by pneumonia (0 to 100% of the lung affected by grossly visible pneumonia) was recorded for each pig at necropsy in a blinded fashion as described previously (16). The scoring system is based on the approximate volume that each lung lobe contributes to the entire lung: the right cranial lobe, right middle lobe, cranial part of the left cranial lobe, and the caudal part of the left cranial lobe contribute 10% each to the total lung volume, the accessory lobe contributes 5%, and the right and left caudal lobes contribute 27.5% each (16). The size of lymph nodes (score range from 0 to 3: 0, normal; 1, two times the normal size; 2, three times the normal size; and 3, four times the normal size) was estimated and recorded for each pig (36). BAL fluid for bacterial and virologic examinations was collected as previously described using 50 milliliters of sterile phosphate-buffered saline for each lung (25). Sections of lungs, lymph nodes (superficial inguinal, mediastinal, tracheobronchial, and mesenteric), tonsil, thymus, ileum, kidney, colon, spleen, and liver were collected at necropsy and fixed in 10% neutral-buffered formalin and routinely processed for histological examination.
Histopathology.
Microscopic lesions were evaluated in a blinded fashion. Lung sections were scored for the presence and severity of interstitial pneumonia ranging from 0 to 6 (0, normal; 1, mild multifocal; 2, mild diffuse; 3, moderate multifocal; 4, moderate diffuse; 5, severe multifocal; and 6, severe diffuse) (16). Sections of heart, liver, kidney, ileum, and colon were evaluated for the presence of lymphohistiocytic inflammation and scored from 0 (none) to 3 (severe) (32). Lymphoid tissues, including lymph nodes, tonsil, and spleen, were evaluated for the presence of lymphoid depletion ranging from 0 (normal) to 3 (severe) and histiocytic inflammation and replacement of follicles ranging from 0 (normal) to 3 (severe) (32).
IHC for detection of PRRSV and PCV2 antigens.
Immunohistochemistry (IHC) for detection of PRRSV-specific antigen was performed on formalin-fixed and paraffin-embedded lung tissue sections as previously described (15). Similarly, IHC for detection of PCV2-specific antigen was performed on formalin-fixed and paraffin-embedded lung tissue sections using a rabbit polyclonal antiserum as previously described (46).
Statistical analysis.
Prior to data analysis, descriptive statistics were performed to assess the overall quality of the data. Continuous data (weight, rectal temperature, macroscopic lung lesions, and S/P ratios) were analyzed with a one-way analysis of variance (ANOVA). If a one-way ANOVA was significant (P < 0.05), pairwise testing using Tukey's adjustment was performed. Discrete data (microscopic lesions and clinical observations) were analyzed by the nonparametric Kruskal-Wallis one-way ANOVA. If the nonparametric ANOVA was significant (P < 0.05), Wilcoxon tests were used for pairwise testing. Response feature analysis was performed to account for clinical observations. The observations obtained after PRRSV challenge were combined, and averages for each pig and differences among groups were compared by using a nonparametric Kruskal-Wallis ANOVA. Fisher's exact test was used to evaluate differences in incidences.
RESULTS
Clinical disease.
Clinical disease was characterized by increased rectal temperatures, by respiratory disease with labored breathing and sneezing, and by reduced average daily weight gain after PRRSV challenge. The NV-PRRSV, PCV2-V-PRRSV, and V-PRRSV groups had increased rectal temperatures after PRRSV challenge. The temperatures ranged from 40.1 to 41.8°C with peak temperatures at 5 to 7 days after PRRSV challenge, with 80% of the pigs being febrile. After challenge, there were significant (P < 0.001) differences in mean rectal temperatures between groups. The NV-PRRSV pigs had the highest rectal temperatures, and all PRRSV-challenged groups had significantly higher rectal temperatures than did non-PRRSV-inoculated groups.
After PRRSV challenge, the highest average group respiratory scores were observed for the NV-PRRSV pigs (mean score, 1.6), the PCV2-V-PRRSV pigs (mean score, 1.2), and the V-PRRSV pigs (mean score, 1.1), which were all significantly (P < 0.0001) higher than the scores for the other groups but not different from each other (data not shown).
The average daily weight gain is summarized in Table 2. From the day of PRRSV vaccination to the day of PRRSV challenge, the average daily gains were not significantly (P = 0.24) different between the groups. From the day of PRRSV challenge to the day of necropsy, NV-PRRSV pigs had significantly (P < 0.0001) lower average daily gains than did all other groups. The average weight gains for the V-PRRSV and the PCV2-V-PRRSV pigs were not different from each other but were significantly (P < 0.001) lower than for the control, PCV2, PCV2-V, and V groups. When the total average weight gains for the period from the day of PRRSV vaccination until necropsy were evaluated, the NV-PRRSV and PCV2-V-PRRSV pigs had significantly (P < 0.001) lower average daily gains than did the control and V groups.
TABLE 2.
Average daily weight gains for the period from vaccination to prior to inoculation with PRRSV, the period after PRRSV challenge, and the entire period from vaccination to necropsy
| Group | ADG fora:
|
||
|---|---|---|---|
| ADG 1 | ADG 2 | ADG 3 | |
| Control | 774.9 ± 28.8 | 1,277.4 ± 33.3A | 875.4 ± 29.1A |
| NV-PRRSV | 793.2 ± 36.1 | 388.2 ± 108.0B | 694.9 ± 36.1B |
| V-PRRSVb | 813.5 ± 23.6 | 783.3 ± 47.9C | 807.5 ± 20.8AB |
| PCV2-V-PRRSVb | 715.0 ± 34.0 | 711.0 ± 37.2C | 714.2 ± 31.0B |
| PCV2 | 732.2 ± 38.8 | 1,099.3 ± 32.3AD | 805.6 ± 33.1AB |
| PCV2-Vb | 739.6 ± 26.8 | 1,044.3 ± 41.1D | 800.6 ± 22.5AB |
| Vb | 797.3 ± 36.5 | 1,138.2 ± 46.8AD | 865.5 ± 35.2A |
ADG, average daily weight gain (group means [g] ± standard errors); ADG 1, period from vaccination to prior to inoculation with PRRSV; ADG 2, period after PRRSV challenge; ADG 3, the entire period from vaccination to necropsy. Values within a column with no common superscript are significantly (P < 0.0001) different.
Vaccinated with Ingelvac PRRS ATP MLV vaccine (Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO).
Antibody response.
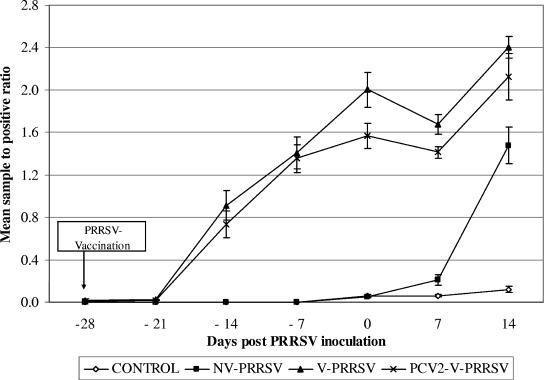
All pigs were free of PRRSV-specific antibodies at arrival in the research facility. The V-PRRSV, PCV2-V-PRRSV, and PCV2-V pigs seroconverted to PRRSV between 7 and 21 days postvaccination. The mean group S/P ratios were numerically but not significantly lower in the PCV2-V-PRRSV group (Fig. 1) and in the V-PCV2 group than they were in the V-PRRSV group. The nonvaccinated and non-PRRSV-challenged groups (control and PCV2) remained seronegative until the termination of the experiment. The NV-PRRSV pigs seroconverted within 2 weeks after PRRSV challenge. Neutralizing antibodies to SDSU73 were not detected in any of the vaccinated pigs prior to PRRSV challenge.
FIG. 1.
Mean group anti-PRRSV-specific antibodies as determined by ELISA. A sample-to-positive ratio of equal to or greater than 0.4 is considered to be positive. Error bars represent standard errors.
At arrival, the majority of the pigs had maternal antibodies to PCV2, which waned over the following weeks. On the day of PCV2 inoculation, all pigs were negative for PCV2-specific antibodies. Seroconversion to PCV2 was observed at the termination of the study in 10 of 10 PCV2-V-PRRSV pigs, 9 of 10 PCV2-V pigs, and 7 of 10 PCV2 pigs. The mean S/P ratios were significantly (P < 0.001) higher in the PCV2-V-PRRSV (0.87 ± 0.08) group than in the PCV2 (0.41 ± 0.09) and PCV2-V (0.49 ± 0.05) groups.
Amount and incidence of PRRSV RNA detection.
The control and PCV2 groups were negative for PRRSV RNA in serum and lung lavage fluid (Table 3). The incidences of PRRSV-viremic pigs were 9 of 9 for NV-PRRSV, 9 of 10 for V-PRRSV, 10 of 10 for PCV2-V-PRRSV, 3 of 10 for PCV2-V, and 2 of 10 for the V group on DPI 7. The incidences of PRRSV RNA-positive pigs were 8 of 9 for NV-PRRSV, 6 of 10 for V-PRRSV, 5 of 10 for PCV2-V-PRRSV, 0 of 10 for PCV2-V, and 0 of 10 for V group on DPI 14. The amount of PRRSV RNA in serum was significantly (P < 0.001) higher in the NV-PRRSV group than in the V-PRRSV and PCV2-V-PRRSV groups at 7 and at 14 days after PRRSV infection (Fig. 2). The incidence of PRRSV RNA-positive lavage fluids is summarized in Table 3.
TABLE 3.
Incidence and severity of macroscopic and microscopic lung lesions and presence of PRRSV and PCV2 nucleic acids and antigens in lungsa
| Group | Result for indicated lung lesionb:
|
Detected presence of:
|
||||
|---|---|---|---|---|---|---|
| PRRSV
|
PCV2
|
|||||
| Macroscopic | Microscopic | BAL fluid PCR | IHC | BAL fluid PCR | IHC | |
| Control | 1/10 (0.3 ± 0.3)A | 10/10 (1.2 ± 0.1)A | 0/10 | NAc | NA | NA |
| NV-PRRSV | 9/9 (50.1 ± 3.1)B | 9/9 (4.6 ± 0.4)B | 9/9 | 8/9 | NA | NA |
| V-PRRSVd | 10/10 (13.2 ± 2.6)C | 10/10 (2.5 ± 0.3)AC | 10/10 | 0/10 | NA | NA |
| PCV2-V-PRRSVd | 10/10 (31.7 ± 5.0)D | 10/10 (3.3 ± 0.5)BC | 10/10 | 4/10 | 10/10 | 0/10 |
| PCV2 | 4/10 (1.0 ± 0.6)A | 10/10 (1.2 ± 0.1)A | 0/10 | NA | 10/10 | 0/10 |
| PCV2-Vd | 3/10 (2.2 ± 1.3)AC | 10/10 (1.8 ± 0.2)A | 8/10 | 0/10 | 10/10 | 0/10 |
| Vd | 0/10 (0.0 ± 0.0)A | 8/10 (1.2 ± 0.3)A | 2/10 | 0/10 | NA | NA |
Detected by PCR on BAL fluid and IHC at 14 days after PRRSV challenge.
Data presented as incidences/total and group means ± standard errors (for macroscopic lesions, mean of the percentage of lung grossly affected by lesions ranging from 0 to 100%; for microscopic lesions, mean of the interstitial pneumonia score ranging from 0 [normal] to 6 [severe, diffuse]). Values within a column with no common superscript represent significantly (P < 0.05) different group means.
NA, not applicable.
Vaccinated with Ingelvac PRRS ATP MLV vaccine (Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO).
FIG. 2.
Mean log-transformed group PRRSV genomic copies. Error bars represent standard errors. *, significant (P < 0.001) differences between groups.
Amount and incidence of PCV2 DNA detection.
At the day of PRRSV challenge, PCV2 DNA was detected in 8 of 10 PCV2-V-PRRSV pigs, in 7 of 10 PCV2-V pigs, and in 6 of 10 PCV2 pigs. At the day of necropsy, PCV2 DNA was detected in 9 of 10 PCV2-V-PRRSV pigs, in 7 of 10 PCV2-V pigs, and in 5 of 10 PCV2 pigs. All PCV2-infected pigs were positive for PCV2 DNA on BAL fluid (Table 3). The amounts of PCV2 antigen were not different (P > 0.05) among the three groups.
Macroscopic lesions.
PRRSV-induced macroscopic lung lesions were characterized by failure of the lungs to collapse and by focal-to-diffuse, mottled-tan, well-to-poorly demarcated areas of pneumonia. There were significant differences among groups in the mean percentages of lung macroscopically affected by pneumonia, as summarized in Table 3.
Microscopic lesions.
PRRSV challenge induced moderate-to-severe lung lesions characterized by type 2 pneumocyte hypertrophy and hyperplasia, septal infiltration with mononuclear cells, and increased amounts of necrotic alveolar exudate. No lesions or mild lung lesions characterized by septal infiltration with mononuclear cells and mild peribronchiolar fibroplasia and histiocytic infiltrates were observed for singular PCV2-inoculated pigs in this study, consistent with our previous studies (32, 36).
PRRSV and PCV2 antigen in lungs.
The incidence of PRRSV and PCV2 antigen detection in lungs as determined by IHC stains is summarized in Table 3. NV-PRRSV pigs had a significantly (P < 0.001) higher incidence of PRRSV antigen detection than did all other groups except PCV2-V-PRRSV.
DISCUSSION
PCV2 infection is widespread, and PCV2 is now commonly associated with various conditions in pigs, such as PMWS, enteritis, abortion, and respiratory disease. Another important impact of PCV2 may actually be its adverse effect on the immune system in the form of compromising the development of a protective immune response to commercial vaccines commonly used on pigs around the time of PCV2 infection. PCV2 infection commonly occurs in pigs at 6 to 12 weeks of age in the United States (37). Vaccines commonly used at that time include vaccines for Mycoplasma hyopneumoniae, swine influenza virus, and PRRSV. PRDC is a major problem in the pig industry, and PRRSV is the most commonly associated primary pathogen in PRDC. Coinfections with PRRSV and PCV2 are commonly observed in the field (37, 49).
The overall goal of this study was to investigate the effect of PCV2 infection on the efficacy of the PRRSV MLV vaccine for protecting pigs against PRRSV-induced clinical disease and lesions. The vaccination protocol used in this study has previously been shown to be effective in reducing clinical disease and lesions associated with PRRSV SDSU73 infection (33).
The effects of other immunomodulating pathogens, such as PRRSV (11, 22) or Trypanosoma evansi (20), on vaccine efficacy have been investigated. Pigs were inoculated with a European PRRSV strain, vaccinated with a pseudorabies virus vaccine 2 weeks later, and challenged with pseudorabies virus 10 weeks after PRRSV inoculation. The results of that study indicated that the lymphoproliferative response was quicker and longer in pigs that were not previously infected by PRRSV. However, PRRSV infection did not inhibit the vaccine efficacy (11). Another study investigated the effect of PRRSV infection on the antibody response to classical swine fever virus (CSFV) vaccination (22). In that study, pigs were vaccinated 2 days after PRRSV inoculation and it was found that the PRRSV-inoculated pigs had significantly lower levels of anti-CSFV antibodies than did non-PRRSV-inoculated pigs within 3 to 5 weeks after vaccination. Pigs infected with T. evansi and vaccinated 4 weeks later against CSFV and challenged 12 weeks after T. evansi infection with CSFV had significantly reduced antibody response compared to noninfected control pigs. However, there was no effect on growth performance or feed conversion (20).
In this study, differences in immunoglobulin G antibody response or in the presence of neutralizing antibodies against PRRSV were not observed between V-PRRSV and PCV2-V-PRRSV pigs. However, we did observe a significant (P < 0.05) increase in PRRSV-associated macroscopic lung lesions, a higher incidence of PRRSV antigen in lung sections, and more-severe microscopic PRRSV-associated lung lesions in pigs that were subclinically infected by PCV2 at the time of PRRSV vaccination compared to those for pigs that were not infected with PCV2.
PRRSV vaccination typically does not prevent PRRSV infection in vaccinated pigs. Moreover, in this study we used a quantitative RT-PCR method, which is more sensitive than a regular RT-PCR assay, and detection of small amounts of PRRSV RNA in pigs exposed to PRRSV at some time in their life is expected. Comparison of the amounts of PRRSV nucleic acids in BAL fluid was not attempted since we did not use an internal control and the collection of lavage fluid strongly depends on the technique (i.e., the amount of fluid put into the lungs, the lung lobes lavaged, and the amount of fluid recovered). To better characterize the amount of PRRSV present in the lungs, we did IHC stains for PRRSV and found positive animals only in the NV-PRRSV group and in the PCV2-V-PRRSV group.
Previous studies have investigated the interaction of PCV2 and PRRSV in experimental models using colostrum-deprived pigs, cesarean-derived/colostrum-deprived (CDCD) pigs, or conventional pigs. In all these models, PCV2 and PRRSV inoculations were done within a narrow time window (same day, or within one week). Allan et al. (4) inoculated 1- to 2-day-old colostrum-deprived pigs with PCV2 and PRRSV and observed upregulation of PCV2 in coinfected pigs. The replication and distribution of PRRSV in concurrently infected pigs were not enhanced compared to those for single PRRSV-infected pigs (4). Harms et al. (19) coinfected 3-week-old CDCD pigs with PCV2 and PRRSV and showed that PCV2 infection increased the severity of PRRSV-induced interstitial pneumonia in CDCD pigs. Rovira et al. (39) inoculated 5-week-old conventional pigs with PRRSV and 7 days later with PCV2 and confirmed that PRRSV infection enhances PCV2 replication. A longer duration of PRRSV viremia and a higher proportion of viremic pigs were observed in the coinfected pigs than in singular PRRSV-infected pigs (39). In the current study, PCV2 infection and PRRSV challenge were done 6 weeks apart, since we were most interested in the PCV2-PRRSV MLV vaccine interaction. We did not expect to observe a PRRSV-PCV2 interaction since we know from past PCV2 studies that PCV2-associated lesions typically peak at 2 to 3 weeks postinoculation and usually become minimal or resolved by 4 to 6 weeks postinoculation (34, 35). Low levels of PCV2 DNA were present at the time of PRRSV challenge as determined by real-time PCR. However, all lung sections were negative for PCV2 antigen, as determined by IHC staining, suggesting that the lung lesions were not due to PCV2.
The interval of 2 weeks between PCV2 inoculation and PRRSV MLV vaccination was chosen because at this time we frequently observe large amounts of PCV2 genomic copies in serum in our pig model. A relation between PCV2 DNA load and amount of PCV2 antigen in lymphoid lesions has been demonstrated previously (31). In addition, Nielsen et al. (29) found that the white blood cell counts and the mean lymphocyte counts were significantly decreased at 10 DPI following experimental PCV2 infection compared to those of uninfected controls. Rovira et al. (39) found maximal PCV2 DNA loads in the serum of experimentally PCV2-infected pigs at 21 DPI. In contrast, Allan et al. (3) observed minimal amounts of PCV2 antigen in mesenteric lymph nodes starting at 10 DPI with increasing density and distribution of PCV2 antigen at 14, 17, 21, and 26 DPI.
Krakowka et al. (21) reported evidence that strong immunostimulation can upregulate PCV2 infection towards manifestation of clinical PMWS in the gnotobiotic pig model. Similar results were observed in the conventional pig model using commercially available adjuvanted, killed vaccines (5, 36). To our knowledge, PRRSV MLV vaccines have not yet been tested for possible interaction with PCV2 infection. In the current study, we found no evidence of vaccine-induced enhancement of PCV2-associated disease. None of the PCV2-V pigs developed clinical disease, and lung sections were negative for the presence of PCV2 antigen by IHC. Mild interstitial pneumonia lesions were present in a portion of the PCV2-V pigs; however, this was not different from control, PCV2, or V pigs. Interestingly, there was a significantly (P < 0.05) higher incidence of PRRSV RNA-positive bronchoalveolar lavage samples in the PCV2-V group than in the V group. However, PRRSV antigen as determined by IHC was not detected in any of the PCV2-V or V pigs. We concluded that the use of PRRSV MLV vaccines in subclinically PCV2-infected pigs appeared to be safe under the conditions of this study. This is in contrast to the findings that PRRSV MLV vaccine predisposed pigs to disease associated with Streptococcus suis infection (41).
In this study, we found that subclinical infection of SPF pigs with PCV2 prior to PRRSV MLV vaccination and subsequent PRRSV challenge resulted in increased PRRSV-induced macroscopic and microscopic lung lesions. Future studies should investigate the effect of PCV2 on cellular immunity. The adverse effect of PCV2 infection on the development of protective immunity against PRRSV and other respiratory pathogen vaccines may be an important factor in controlling PRDC and other diseases in growing pigs.
Acknowledgments
This study was funded in part by a grant from the Healthy Livestock Initiative and by Fort Dodge Animal Health, Inc.
The vaccine and the PRRSV inoculum for this study were kindly provided by Boehringer Ingelheim Vetmedica, Inc.
REFERENCES
- 1.Allan, G., B. Meehan, D. Todd, S. Kennedy, F. McNeilly, J. Ellis, E. G. Clark, J. Harding, E. Espuna, A. Botner, and C. Charreyre. 1998. Novel porcine circoviruses from pigs with wasting disease syndromes. Vet. Rec. 142:467-468. [PubMed] [Google Scholar]
- 2.Allan, G. M., and J. A. Elli. 2000. Porcine circoviruses: a review. J. Vet. Diagn. Investig. 12:3-14. [DOI] [PubMed] [Google Scholar]
- 3.Allan, G. M., F. McNeilly, B. M. Meehan, J. A. Ellis, T. J. Connor, I. McNair, S. Krakowka, and S. Kennedy. 2000. A sequential study of experimental infection of pigs with porcine circovirus and porcine parvovirus: immunostaining of cryostat sections and virus isolation. J. Vet. Med. B 47:81-94. [DOI] [PubMed] [Google Scholar]
- 4.Allan, G. M., F. McNeilly, J. Ellis, S. Krakowka, B. Meehan, I. McNair, I. Walker, and S. Kennedy. 2000. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch. Virol. 145:2421-2429. [DOI] [PubMed] [Google Scholar]
- 5.Allan, G. M., F. McNeilly, S. Kennedy, B. Meehan, J. Ellis, and S. Krakowka. 2000. Immunostimulation, PCV-2 and PMWS. Vet. Rec. 147:170-171. [PubMed] [Google Scholar]
- 6.Carrasco, L., J. Segalés, M. J. Bautista, J. C. Gómez-Villamandos, C. Rosell, E. Ruiz-Villamor, and M. A. Sierra. 2000. Intestinal chlamydial infection concurrent with postweaning multisystemic wasting syndrome in pigs. Vet. Rec. 146:21-23. [DOI] [PubMed] [Google Scholar]
- 7.Cavanaugh, D. 1997. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 142:629-633. [PubMed] [Google Scholar]
- 8.Chianini, F., N. Majo, J. Segales, J. Dominguez, and M. Domingo. 2003. Immunohistochemical characterisation of PCV2 associate lesions in lymphoid and non-lymphoid tissues of pigs with natural postweaning multisystemic wasting syndrome (PMWS). Vet. Immunol. Immunopathol. 94:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark, E. G. 1997. Post-weaning multisystemic wasting syndrome. Proc. Am. Assoc. Swine Pract. (Quebec) 28:499-501. [Google Scholar]
- 10.Darwich, L., J. Segalés, M. Domingo, and F. Mateu. 2002. Changes in CD4+, CD8+, CD4+ CD8+, and immunoglobulin M-positive peripheral blood mononuclear cells of postweaning multisystemic wasting syndrome-affected pigs and age-matched uninfected wasted and healthy pigs correlate with lesions and porcine circovirus type 2 load in lymphoid tissues. Clin. Diagn. Lab. Immunol. 9:236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Bruin, M. G., J. N. Samsom, J. J. Voermans, E. M. van Rooij, Y. E. De Visser, and A. T. Bianchi. 2000. Effects of a porcine reproductive and respiratory syndrome virus infection on the development of the immune response against pseudorabies virus. Vet. Immunol. Immunopathol. 76:125-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis, J., L. Hassard, E. Clark, J. Harding, G. Allan, P. Willson, J. Strokappe, K. Martin, F. McNeilly, B. Meehan, D. Todd, and D. Haines. 1998. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can. Vet. J. 39:44-51. [PMC free article] [PubMed] [Google Scholar]
- 13.Fenaux, M., P. G. Halbur, G. Haqshenas, R. Royer, P. Thomas, P. Nawagitgul, M. Gill, T. E. Toth, and X. J. Meng. 2002. Cloned genomic DNA of type 2 porcine circovirus is infectious when injected directly into the liver and lymph nodes of pigs: characterization of clinical disease, virus distribution, and pathologic lesions. J. Virol. 76:541-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenaux, M., P. G. Halbur, M. Gill, T. E. Toth, and X. J. Meng. 2000. Genetic characterization of type 2 porcine circovirus (PCV-2) from pigs with postweaning multisystemic wasting syndrome in different geographic regions of North America and development of a differential PCR-restriction fragment length polymorphism assay to detect and differentiate between infections with PCV-1 and PCV-2. J. Clin. Microbiol. 38:2494-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halbur, P. G., J. J. Andrews, E. L. Hoffman, P. S. Paul, X. J. Meng, and Y. Niyo. 1994. Development of a streptavidin-biotin immunoperoxidase procedure for the detection of porcine reproductive and respiratory syndrome virus antigen in porcine lung. J. Vet. Diagn. Investig. 6:254-257. [DOI] [PubMed] [Google Scholar]
- 16.Halbur, P. G., P. S. Paul, M. L. Frey, J. Landgraf, K. Eernisse, X. J. Meng, M. A. Lum, J. J. Andrews, and J. A. Rathje. 1995. Comparison of the pathogenicity of two U.S. porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 32:648-660. [DOI] [PubMed] [Google Scholar]
- 17.Harding, J. C. S., and E. G. Clark. 1997. Recognizing and diagnosing postweaning multisystemic wasting syndrome (PMWS). Swine Health Prod. 5:201-203. [Google Scholar]
- 18.Harms, P. A., P. G. Halbur, and S. D. Sorden. 2002. Three cases of porcine respiratory disease complex associated with porcine circovirus type 2 infection. J. Swine Health Prod. 10:27-30. [Google Scholar]
- 19.Harms, P. A., S. D. Sorden, P. G. Halbur, S. R. Bolin, K. M. Lager, I. Morozov, and P. S. Paul. 2001. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet. Pathol. 38:528-539. [DOI] [PubMed] [Google Scholar]
- 20.Holland, W. G., T. T. Do, N. T. Huong, N. T. Dung, N. G. Thanh, J. Vercruysse, and B. M. Goddeeris. 2003. The effect of Trypanosoma evansi infection on pig performance and vaccination against classical swine fever. Vet. Parasitol. 111:115-123. [DOI] [PubMed] [Google Scholar]
- 21.Krakowka, S., J. A. Ellis, F. McNeilly, S. Ringler, D. M. Rings, and G. Allan. 2001. Activation of the immune system is the pivotal event in the production of wasting disease in pigs infected with porcine circovirus-2 (PCV-2). Vet. Pathol. 38:31-42. [DOI] [PubMed] [Google Scholar]
- 22.Li, H., and H. Yang. 2003. Infection of porcine reproductive and respiratory syndrome virus suppresses the antibody response to classical swine fever virus vaccination. Vet. Microbiol. 95:295-301. [DOI] [PubMed] [Google Scholar]
- 23.Madec, F., E. Eveno, P. Morvan, L. Hamon, P. Blanchard, R. Cariolet, N. Amenna, H. Morvan, C. Truong, D. Mahé, E. Albina, and A. Jesting. 2000. Post-weaning multisystemic wasting syndrome (PMWS) in pigs in France: clinical observations from follow-up studies on affected farms. Livestock Prod. Sci. 63:223-233. [Google Scholar]
- 24.Mavromatis, I., S. K. Kritas, C. Alesopoulos, A. Tsinas, and S. C. Kyriakis. 1999. Field evaluation of a live vaccine against porcine reproductive and respiratory syndrome in fattening pigs. J. Vet. Med. B 46:603-612. [DOI] [PubMed] [Google Scholar]
- 25.Mengeling, W. L., K. M. Lager, and A. C. Vorwald. 1995. Diagnosis of porcine reproductive and respiratory syndrome. J. Vet. Diagn. Investig. 7:3-16. [DOI] [PubMed] [Google Scholar]
- 26.Mengeling, W. L., K. M. Lager, and A. C. Vorwald. 1998. Clinical consequences of exposing pregnant gilts to strains of porcine reproductive and respiratory syndrome (PRRS) virus isolated from field cases of “atypical” PRRS. Am. J. Vet. Res. 59:1540-1544. [PubMed] [Google Scholar]
- 27.Nawagitgul, P., P. A. Harms, I. Morozov, B. J. Thacker, S. D. Sorden, C. Lekcharoensuk, and P. S. Paul. 2002. Modified indirect porcine circovirus (PCV) type 2-based and recombinant capsid protein (ORF2)-based enzyme-linked immunosorbent assays for detection of antibodies to PCV. Clin. Diagn. Lab. Immunol. 9:33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann, E. J., J. B. Kliebenstein, C. K. Johnson, J. W. Mabry, E. J. Bush, A. H. Seitzinger, A. L. Green, and J. J. Zimmermann. 2005. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med. Assoc. 227:385-392. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen, J., I. E. Vincent, A. Bøtner, A. S. Ladekær-Mikkelsen, G. Allan, A. Summerfield, and K. C. McCullough. 2003. Association of lymphopenia with porcine circovirus type 2 induced postweaning multisystemic wasting syndrome (PMWS). Vet. Immunol. Immunopathol. 92:97-111. [DOI] [PubMed] [Google Scholar]
- 30.Núñez, A., F. McNeilly, A. Perea, P. J. Sánchez-Cordón, B. Huerta, G. Allan, and L. Carrasco. 2003. Coinfection by Cryptosporidium parvum and porcine circovirus type 2 in weaned pigs. J. Vet. Med. B 50:255-258. [DOI] [PubMed] [Google Scholar]
- 31.Olvera, A., M. Sibila, M. Calsamiglia, J. Segalés, and M. Domingo. 2004. Comparison of porcine circovirus type 2 load in serum quantified by a real time PCR in postweaning multisystemic wasting syndrome and porcine dermatitis and nephropathy syndrome naturally affected pigs. J. Virol. Methods 117:75-80. [DOI] [PubMed] [Google Scholar]
- 32.Opriessnig, T., E. L. Thacker, S. Yu, M. Fenaux, X. J. Meng, and P. G. Halbur. 2004. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual-infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet. Pathol. 41:624-640. [DOI] [PubMed] [Google Scholar]
- 33.Opriessnig, T., F. J. Pallarés, D. Nilubol, A. L. Vincent, E. L. Thacker, E. M. Vaughn, M. Roof, and P. G. Halbur. 2005. Genomic homology of ORF5 gene sequence between modified live vaccine virus and porcine reproductive and respiratory syndrome virus challenge isolates is not predictive of vaccine efficacy. J. Swine Health Prod. 13:246-253. [Google Scholar]
- 34.Opriessnig. T., M. Fenaux, S. Yu, R. B. Evans, D. Cavanaugh, G. M. Gallup, F. J. Pallares, E. L. Thacker, K. M. Lager, X. J. Meng, and P. G. Halbur. 2004. Effect of porcine parvovirus vaccination on the development of PMWS in segregated early weaned pigs coinfected with type 2 porcine circovirus and porcine parvovirus. Vet. Microbiol. 98:209-220. [DOI] [PubMed] [Google Scholar]
- 35.Opriessnig, T., P. G. Halbur, S. Yu, E. L. Thacker, M. Fenaux, and X. J. Meng. 2006. Effects of the timing of the administration of Mycoplasma hyopneumoniae bacterin on the development of lesions associated with porcine circovirus type 2. Vet. Rec. 158:149-154. [DOI] [PubMed] [Google Scholar]
- 36.Opriessnig, T., S. Yu, J. M. Gallup, R. B. Evans, M. Fenaux, F. Pallares, E. L. Thacker, C. W. Brockus, M. R. Ackermann, P. Thomas, X. J. Meng, and P. G. Halbur. 2003. Effect of vaccination with selective bacterins on conventional pigs infected with type 2 porcine circovirus. Vet. Pathol. 40:521-529. [DOI] [PubMed] [Google Scholar]
- 37.Pallarés, F. J., P. G. Halbur, T. Opriessnig, S. D. Sorden, D. Villar, B. H. Janke, M. J. Yaeger, D. J. Larson, K. J. Schwartz, K. J. Yoon, and L. J. Hoffman. 2002. Porcine circovirus type 2 (PCV-2) coinfections in US field cases of postweaning multisystemic wasting syndrome (PMWS). J. Vet. Diagn. Investig. 14:515-519. [DOI] [PubMed] [Google Scholar]
- 38.Quintana, J., J. Segalés, C. Rosell, M. Calsamiglia, G. M. Rodríguez-Arrioja, F. Chianini, J. M. Folch, J. Maldonado, M. Canal, J. Plana-Durán, and M. Domingo. 2001. Clinical and pathological observations on pigs with postweaning multisystemic wasting syndrome. Vet. Rec. 149:357-361. [DOI] [PubMed] [Google Scholar]
- 39.Rovira, A., M. Balasch, J. Segalés, L. García, J. Plana-Durán, C. Rosell, H. Ellerbrok, A. Mankertz, and M. Domingo. 2002. Experimental inoculation of conventional pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus 2. J. Virol. 76:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarli, G., L. Mandrioli, M. Laurenti, L. Sidoli, C. Cerati, G. Rolla, and P. S. Marcato. 2001. Immunohistochemical characterisation of the lymph node reaction in pig post-weaning multisystemic wasting syndrome (PMWS). Vet. Immunol. Immunopathol. 83:53-67. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt, C. S., P. G. Halbur, J. A. Roth, J. M. Kinyon, C. Kasorndorkbua, and B. Thacker. 2001. Influence of ampicillin, ceftiofur, attenuated live PRRSV vaccine, and reduced dose Streptococcus suis exposure on disease associated with PRRSV and S. suis coinfection. Vet. Microbiol. 78:29-37. [DOI] [PubMed] [Google Scholar]
- 42.Segalés, J., F. Alonso, C. Rosell, J. Pastor, F. Chianini, E. Campos, L. López-Fuertes, L. Quintana, G. Rodríguez-Arrioja, M. Calsamiglia, J. Pujols, J. Domínguez, and M. Domingo. 2001. Changes in peripheral blood leukocyte populations in pigs with natural postweaning multisystemic wasting syndrome (PMWS). Vet. Immunol. Immunopathol. 81:37-44. [DOI] [PubMed] [Google Scholar]
- 43.Segalés, J., J. Pastor, R. Cuenca, and M. Domingo. 2000. Haematological parameters in postweaning multisystemic wasting syndrome-affected pigs. Vet. Rec. 46:675-676. [DOI] [PubMed] [Google Scholar]
- 44.Segalés, J., M. Domingo, M. Collell, H. E. Jensen, and J. L. Blanco. 2003. Pulmonary aspergillosis in a post-weaning multisystemic wasting syndrome (PMWS) affected pig. Pig J. 52:41-47. [Google Scholar]
- 45.Sorden, S. D. 2000. Update on porcine circovirus and postweaning multisystemic wasting syndrome (PMWS). Swine Health Prod. 8:133-136. [Google Scholar]
- 46.Sorden, S. D., P. A. Harms, P. Nawagitgul, D. Cavanaugh, and P. S. Paul. 1999. Development of a polyclonal-antibody-based immunohistochemical method for the detection of type 2 porcine circovirus in formalin-fixed, paraffin-embedded tissue. J. Vet. Diagn. Investig. 11:528-530. [DOI] [PubMed] [Google Scholar]
- 47.Sornsen, S. A., J. J. Zimmerman, D. D. Polson, and M. B. Roof. 1998. Effect of PRRS vaccination on average daily gain: a comparison of intranasal and intranasal-intramuscular administration. Swine Health Prod. 6:13-19. [Google Scholar]
- 48.Tischer, I., H. Glederblom, W. Vettermann, and M. A. Koch. 1982. A very small porcine virus with circular single stranded DNA. Nature 295:64-66. [DOI] [PubMed] [Google Scholar]
- 49.Wellenberg, G. J., N. Stockhofe-Zurwieden, W. J. Boersma, M. F. De Jong, and A. R. Elbers. 2004. The presence of coinfections in pigs with clinical signs of PMWS in The Netherlands: a case-control study. Res. Vet. Sci. 77:177-184. [DOI] [PMC free article] [PubMed] [Google Scholar]