Abstract
Persistent infection with hepatitis B virus (HBV) likely depends on viral inhibition of host defenses. We report that chronic hepatitis B e antigen-positive HBV infection is associated with a significant reduction in peripheral blood monocyte expression of Toll-like receptor 2, a key component of innate immunity, thereby providing a mechanism by which wild-type HBV may establish persistent infection.
Mechanisms by which hepatitis B virus (HBV) establishes persistent infection remain unclear, although viral inhibition of host defenses is likely to be important. Most studies of the immunological aspects of persistent HBV infection have focused on adaptive immunity, with little research on the innate immune response to HBV (5). One of the key components of the innate immune response is the family of Toll-like receptors (TLRs), evolutionarily conserved pattern recognition receptors (1). Activation of TLRs by various motifs common to microorganisms, known as pathogen-associated molecular patterns, triggers the release of inflammatory mediators such as tumor necrosis factor alpha (TNF-α) (6). Recent studies have shown that some viruses suppress TLR-mediated immune mechanisms, thereby disabling an important aspect of the host's antiviral defenses (2-4). The expression of TLRs in patients with HBV and any functional consequences of disturbed TLR expression in this group have not previously been investigated.
We studied 17 consecutive noncirrhotic patients with chronic HBV infection (hepatitis B surface antigen positive, as measured by reverse passive hemagglutination [Serodia-HBs; Fujirebio Inc., Japan]) and ongoing viral replication (median HBV DNA level, 1.5 × 103 copies/ml; range, 210 to 1.62 × 109 copies/ml, as measured by reverse transcription-PCR and, when serum levels were <2,000 copies/ml, using the Cobas Amplicor HBV Monitor test [Roche]). Nine (53%) patients were hepatitis B e antigen (HBeAg) positive, and eight (47%) patients were HBeAg negative, as determined using the Abott Axsym HbE 2.0 assay (Abbott Laboratories, Illinois). No patient had clinical or laboratory evidence of other infection or immunodeficiency that may have confounded the interpretation of TLR levels. The HBeAg-positive and HBeAg-negative groups were comparable in terms of gender (ratios of males to females, 7/2 and 6/2, respectively), age (median of 35 years and range of 21 to 55 years and median of 40 years and range of 18 to 56 years, respectively), Ishak histological activity index (median of 4 and range of 2 to 6 and median of 4 and range of 2 to 6, respectively), and histological stage (median of 2 and range 1 of 3 and median of 2 and range of 1 to 3, respectively). Thirty-two asymptomatic, age- and sex-matched subjects with negative viral serology, normal liver function tests, and no history of liver disease or immunodeficiency served as controls.
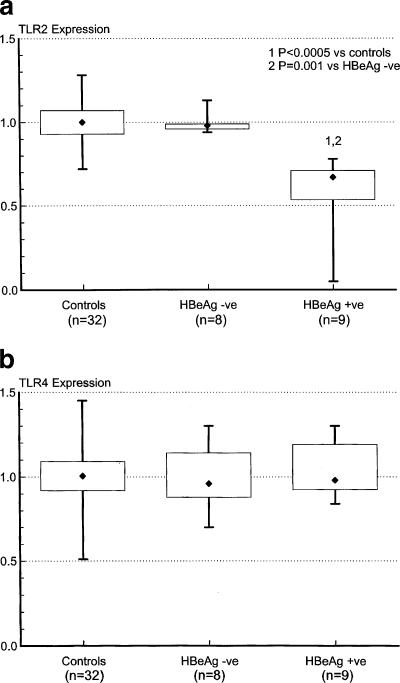
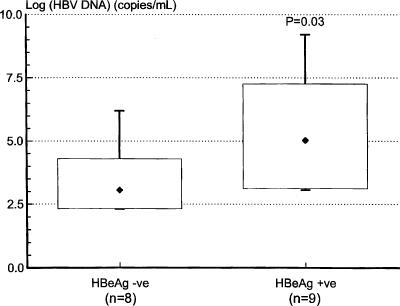
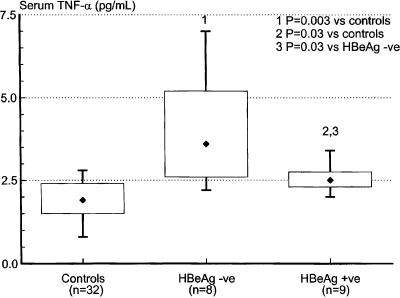
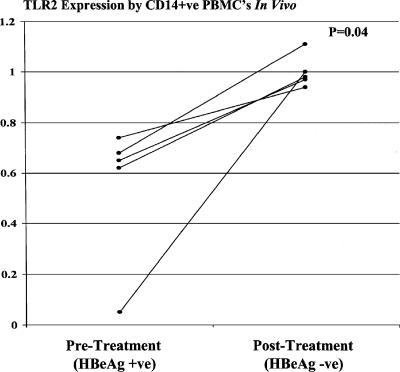
Cell surface staining was performed on whole blood using anti-TLR2 and anti-TLR4 (eBioscience) and anti-CD14 (Becton Dickinson) monoclonal antibodies. After red cell lysis, monocytes were gated on the basis of their scatter profile, and 10,000 CD14-positive events were acquired from each sample using a FACSCalibur flow cytometer (Becton Dickinson). At least two control patients were used for standardization purposes for each acquisition. The geometric mean fluorescence of TLR2 and TLR4 in individual study patients was expressed as the ratio of their individual results to the mean of control values obtained on the day of acquisition. We found that CD14-positive peripheral blood monocyte expression of TLR2 was significantly reduced in HBeAg-positive patients compared to both controls and HBeAg-negative patients, with values ranging from 80% to as low as 5% of normal. TLR2 expression in HBeAg-negative patients was not significantly different from that found in controls (Fig. 1a). Peripheral blood monocyte expression of TLR4 was not significantly different in the three groups (Fig. 1b). Although serum HBV DNA levels were significantly higher in the HBeAg-positive group than in the HBeAg-negative group (Fig. 2), multivariate linear regression analysis showed that peripheral blood monocyte expression of TLR2 correlated significantly with patients' HBeAg status rather than HBV DNA levels (for HBeAg status [positive or negative], the r value was 0.75 and the P value was 0.001; for log HBV DNA copies per milliliter, the r value was −0.08 and the P value was 0.71). Notably, TLR2 expression was reduced to values as low as 67% of normal in HBeAg patients with HBV DNA levels as low as 1.1 × 103 copies/ml, while TLR2 expression was normal in HBeAg-negative patients with HBV DNA levels of up to 1.56 × 106 copies/ml. Serum levels of TNF-α were measured by immunoassay (R&D Systems, Minneapolis, Minn.). Although elevated compared to values in control patients, serum TNF-α levels were significantly lower in HBeAg-positive patients than in HBeAg-negative patients (Fig. 3). Peripheral blood monocyte expression of TLR2 was remeasured in five HBeAg-positive patients who became HBeAg negative/anti-HBeAg positive following treatment with the antiviral agent lamivudine (GlaxoSmithKline, Melbourne, Australia). Peripheral blood monocyte expression of TLR2 normalized in each case (Fig. 4).
FIG. 1.
Expression of TLR2 (a) and TLR4 (b) on CD14-positive peripheral blood monocytes of patients with chronic replicative HBV infection, stratified according to HBeAg status, and controls. The boxes represent the interquartile ranges, the whiskers indicate the ranges, and the diamonds indicate the medians. Significantly reduced TLR2 values were found in HBeAg-positive (HBeAg +ve) patients (Mann-Whitney rank-sum test [Systat for Windows, version 5.02]). HBeAg -ve, HBeAg negative.
FIG. 2.
Serum HBV DNA levels in HBeAg-negative (HBeAg -ve) and HBeAg-positive (HBeAg +ve) patients with chronic replicative HBV infection. Statistical analysis was performed using the Mann-Whitney rank-sum test (Systat for Windows, version 5.02).
FIG. 3.
Serum TNF-α levels in control subjects and HBeAg-negative (HBeAg -ve) and HBeAg-positive (HBeAg +ve) patients with chronic replicative HBV infection. Statistical analysis was performed using the Mann-Whitney rank-sum test (Systat for Windows, version 5.02).
FIG. 4.
Serial assessment of TLR2 expression on peripheral blood monocytes from five HBeAg-positive (HBeAg +ve) patients who became HBeAg negative (HBeAg −ve)/anti-HBeAg positive following treatment with the antiviral agent lamivudine. TLR2 expression improved substantially in all patients. Statistical analysis was performed using the Wilcoxon test (Systat for Windows, version 5.02). CD14+ve, CD14 positive; PBMC's, peripheral blood mononuclear cells.
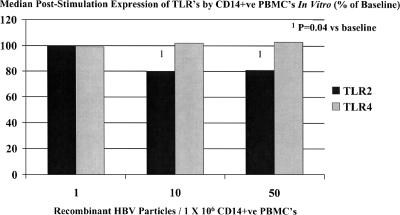
CD14-positive peripheral blood monocytes were isolated from lithium-heparinized blood of five healthy controls and cocultured in vitro with various numbers of purified recombinant HBV (genotype A) particles per million CD14-positive peripheral blood monocytes. After 20 h, cells were stained for CD14, TLR2, and TLR4 for flow cytometry and analyzed as described above. Purified recombinant HBV was produced in HepG2 cells transduced with recombinant baculovirus expressing wild-type HBV genotype A (HBeAg positive). CD14-positive peripheral blood monocyte expression of TLR2 was found to be significantly reduced following exposure to recombinant HBeAg-positive HBV. Peripheral blood monocyte expression of TLR4 was not significantly different following stimulation with recombinant HBV (Fig. 5).
FIG. 5.
CD14-positive (CD14+ve) peripheral blood mononuclear cell (PBMC) expression of TLRs in vitro measured in five control subjects at baseline and following stimulation for 20 h by various numbers of purified HBeAg-positive recombinant HBV particles per million PBMCs. A significant reduction in TLR2 expression was documented, even at low HBV concentrations. No change in TLR4 expression was noted. Statistical analysis was performed using the Wilcoxon test (Systat for Windows, version 5.02).
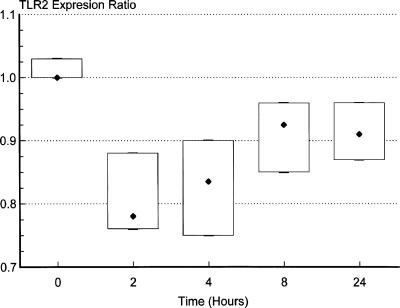
In order to further assess the relationship between HBeAg and peripheral blood monocyte expression of TLR2, we performed a longitudinal analysis with normal peripheral blood monocytes to determine the time course of down-regulation of TLR2 expression following exposure to HBeAg. Aliquots of lithium-heparinized blood (0.5 ml) were obtained from four healthy controls and mixed with either 0.5 ml normal medium (10% Dulbecco's modified Eagle medium) or 0.5 ml “conditioned” medium containing HBeAg (obtained from the culture supernatant from HepG2 cells transduced with recombinant baculovirus expressing wild-type HBV [HBeAg positive] and cultured in 10% Dulbecco's modified Eagle medium for 8 days; HBeAg concentration, 2,678 Paul Ehrlich international units/ml, measured by enzyme immunoassay [Murex; Abbott Diagnostics] in relation to a reference HBeAg preparation obtained from the Paul Ehrlich Institute, Langen, Germany). Blood and media were incubated at 37°C with gentle rotation for 2, 4, 8, and 24 h. CD14-positive peripheral blood monocyte expression of TLR2 at each of these time points was determined as described above and expressed as the ratio of values following incubation with “conditioned” medium containing HBeAg to those following incubation with medium alone. Peripheral blood monocyte expression of TLR2 was found to be substantially reduced within 2 h of exposure to HBeAg (Fig. 6).
FIG. 6.
Longitudinal analysis of CD14-positive peripheral blood mononuclear cell expression of TLR2 following exposure to HBeAg for various time periods ranging from 2 h to 24 h. Results are expressed as the “TLR2 expression ratio,” which represents the ratio of values following incubation of whole blood with “conditioned” medium containing HBeAg to those following incubation with medium alone.
Taken together, our cross-sectional and longitudinal findings, both in vivo and in vitro, suggest that in the presence of HBeAg, HBV down-regulates the expression of TLR2 on peripheral blood monocytes, even at a low viral load. This reduced expression of TLR2 in patients with HBeAg-positive HBV infection is associated with a blunted TNF-α response, as reflected by circulating cytokine concentrations. The proposed interaction between wild-type HBV and TLR2 may provide an important clue as to the reasons for the establishment of persistent HBV infection.
REFERENCES
- 1.Akira, S. 2003. Toll-like receptor signaling. J. Biol. Chem. 278:38105-38108. [DOI] [PubMed] [Google Scholar]
- 2.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. O'Neill. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and Toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97:10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foy, E., K. Li, C. Wang, et al. 2003. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300:1145-1148. [DOI] [PubMed] [Google Scholar]
- 4.Harte, M. T., I. R. Haga, G. Maloney, et al. 2003. The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defence. J. Exp. Med. 197:343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kakimi, K., L. G. Guidotti, Y. Koezuka, and F. V. Chisari. 2000. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 192:921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]