Abstract
The Merck pneumococcal (Pn) enzyme-linked immunosorbent assays (ELISAs) for measuring antibodies to 12 serotypes (serotypes 1, 3, 4, 6B, 7F, 8, 9V, 12F, 14, 18C, 19F, and 23F) were validated in 1999. Merck Laboratories developed the Pn assays using 10 μg/ml C polysaccharide, 100 μg/ml Pn polysaccharide (PnPs) 25, and 100 μg/ml PnPs 72 for preadsorption of samples, standards, and controls in order to improve the specificity to the Pn serotypes in the vaccine. The Pn assays utilize postimmunization sera obtained from subjects immunized with PNEUMOVAX 23 as standards for measuring immunoglobulin G concentrations in sera obtained from vaccine clinical trials with adults and infants. This material was calibrated to the Pn reference standard serum, 89SF, subjected to the Merck Pn ELISA adsorbants. Comparisons were made between the Merck Pn assay and the international Pn assay, showing moderate agreement between the two assay formats. This work describes the test procedures and operating characteristics of the Merck Pn assays and the results of experiments performed to compare the Merck Pn ELISAs to the international Pn ELISAs.
PNEUMOVAX 23 (pneumococcal [Pn] polyvalent vaccine), which was licensed in 1983, is a sterile, liquid vaccine for intramuscular or subcutaneous injection. It consists of a mixture of highly purified capsular polysaccharides from 23 of the most prevalent or invasive serotypes of Streptococcus pneumoniae, including the six serotypes (serotypes 6B, 9V, 14, 19A, 19F, and 23F) that most frequently cause invasive drug-resistant Pn infections among children and adults in the United States (1). Bacterial capsular polysaccharides (Ps) induce antibodies primarily by T-cell-independent mechanisms (15); therefore, the antibody response to most Pn capsular polysaccharide types is generally poor or inconsistent in children <2 years of age, whose immune systems are immature and unable to respond effectively to the polysaccharide antigens.
The immunogenicity of PNEUMOVAX 23 in early clinical trials was determined using a radioimmunoassay measuring antibodies to the Pn polysaccharides (PnPs) (14). However, due to the practicality of supporting large-scale testing, the enzyme-linked immunosorbent assay (ELISA) became the preferred method for estimating antibody concentrations. In this respect, the first-generation ELISA showed a poor correlation of antibody concentration with the efficacy of the vaccines. This observation was attributed to an overestimation of the serotype-specific PnPs antibody concentrations due to the presence of contaminating C Ps in the antigen preparations used in the ELISA (4, 6). Subsequently, a second-generation ELISA was developed by taking steps to neutralize C Ps antibodies in the serum prior to ELISA measurements. These Pn assays, utilizing the steps with preadsorption of test sera with C Ps, were compared in a multicenter study (11). The study compared results among 12 laboratories, with each laboratory testing the same series of 48 Pn paired adult serum specimens from 24 individuals (quality control sera). The results from 12 laboratories were highly correlated (11). Subsequent to the interlaboratory studies, this second-generation ELISA was found to have insufficient specificity when sera from unimmunized adults were investigated (3).
Following the discovery that ELISA specificity could be further improved when test sera were preadsorbed with heterologous PnPs, a third-generation ELISA was developed. While Merck Laboratories focused on developing a third-generation ELISA that utilized Pn25 and Pn72 for serum preadsorption in order to improve the specificity to the Pn serotypes in the vaccine, other laboratories utilized Pn22F or Pn adsorbant from a capsule-negative variant of S. pneumoniae (13) for preadsorption of nonspecific reactivity (3, 9, 18). The Merck Pn assay was not developed utilizing Pn22F to preadsorb nonspecific reactivity, since this polysaccharide is a component of the PNEUMOVAX 23 vaccine. Furthermore, due to the imminent limited supply of the Pn reference standard serum, lot 89SF, sera from individuals vaccinated with PNEUMOVAX 23 were calibrated to 89SF and utilized as standards for the Merck Pn ELISAs. This work describes the development, validation, and performance of the Merck Pn ELISAs for measuring antibodies to 12 serotypes (serotypes 1, 3, 4, 6B, 7F, 8, 9V, 12F, 14, 18C, 19F, and 23F) and the correlation to the World Health Organization (WHO) international Pn assays (9, 18).
MATERIALS AND METHODS
C polysaccharides.
C Ps is a Pn cell wall polysaccharide obtained from the Statens Serum Institut, Copenhagen, Denmark. It is common to all Pn serotypes and used for preadsorbing human serum samples before quantitation of serotype-specific Pn capsular polysaccharide antibodies.
Pn polysaccharides.
All PnPs powders for serotypes 1, 3, 4, 6B, 7F, 8, 9V, 12F, 14, 18C, 19F, 23F, 25, and 72 were manufactured and received from the Merck Manufacturing Division, West Point, PA. Each PnPs was reconstituted in sterilized pyrogen-free water. The final concentration for each PnPs following reconstitution was 2 mg/ml.
89SF serum.
The U.S. FDA Pn reference standard, lot 89SF, is accepted as the international calibrator for quantitating antibody to serotype-specific Pn capsular polysaccharides. The 89SF serum is the Merck pneumococcal enzyme immunoassay control tested at three (twofold serial) dilutions on each plate within an assay run (low, medium, and high dilutions). This pool was prepared by Lederle-Praxis Biologicals from 17 adult individual high-titered sera, following vaccination with PNU-IMUNE (a 23-valent Pn vaccine; Lederle), MENOMUNE (a meningococcal polysaccharide vaccine; Connaught), and ProHIBIT (a Haemophilus influenzae conjugate vaccine; Connaught) (10, 13). The pooled serum is stored frozen at −70°C and is also available as lyophilized aliquots from CBER, U.S. FDA (U.S. Pn standard reference serum, lot 89SF).
Sera for standards.
Human immune sera from adult individuals following vaccination with PNEUMOVAX 23 are listed by polysaccharide type as follows: Pn1, Pn4, Pn8, Pn9V, and Pn18C (Giebink 30 [G30] standard); Pn3, Pn6B, Pn7F, Pn12F, and Pn23F (G11 standard); and Pn14 and Pn19F (G16 standard). These sera were obtained from S. Giebink, University of Minnesota, in November 1996. The antibody assignments for each polysaccharide are listed in Table 1, as calibrated to the Pn reference standard, lot 89SF.
TABLE 1.
Reassignment of serotype-specific antibody titers to the international reference standard, lot 89SF, for 12 Pn serotypes
| PnPs serotype | Titer (μg/ml) ± 95% CI for 89SF
|
Titer (μg/ml) (95% CI) for Giebink serac (CPS + Pn25 + Pn72) | |
|---|---|---|---|
| CPSa | CPS + Pn25 + Pn72b | ||
| 1 | 6.32 ± 0.85 | 3.43 ± 0.45 | 4.78 (3.21, 7.11) |
| 3 | 2.36 ± 0.30 | 0.53 ± 0.10 | 3.64 (3.18, 4.16) |
| 4 | 4.07 ± 0.47 | 3.95 ± 0.50 | 6.38 (5.92, 6.88) |
| 6B | 16.87 ± 2.77 | 18.28 ± 2.70 | 7.88 (7.25, 8.58) |
| 7F | 5.21 ± 1.25 | 3.45 ± 0.60 | 10.2 (9.26, 11.3) |
| 8 | 5.15 ± 0.88 | 6.42 ± 0.91 | 4.50 (3.41, 5.96) |
| 9V | 6.90 ± 1.85 | 6.93 ± 1.64 | 26.6 (21.9, 32.2) |
| 12F | 1.75 ± 0.39 | 1.69 ± 0.19 | 4.24 (3.66, 4.90) |
| 14 | 27.76 ± 3.65 | 21.46 ± 3.90 | 9.02 (7.12, 11.4) |
| 18C | 4.46 ± 0.94 | 6.50 ± 1.57 | 8.97 (8.27, 9.74) |
| 19F | 12.97 ± 2.67 | 11.39 ± 2.92 | 10.0 (8.10, 12.3) |
| 23F | 8.14 ± 1.07 | 7.28 ± 1.05 | 6.41 (5.91, 6.95) |
Goldblatt test sera.
The panel of “Goldblatt sera” utilized for concordance experimentation was provided by D. Goldblatt and consists of 12 calibration serum samples with known assigned antibody concentrations (9). These sera were obtained from adults vaccinated with a 23-valent Pn polysaccharide vaccine.
Assay method.
Medium-binding ELISA plates were coated with serotype-specific PnPs antigen or 1% bovine serum albumin (BSA) diluted in phosphate-buffered saline (PBS). Antigen-coated plates were sealed and incubated overnight at room temperature (RT). Assay standard, controls, and test sera were diluted at the appropriate dilutions in PBS containing 1% BSA, 10 μg/ml C Ps, 100 μg/ml Pn25, and 100 μg/ml Pn72 for each polysaccharide type and incubated overnight at 2 to 8°C. On the day of the assay, plates were washed three times and standard, controls, and test sera were added to the plates. Plates were covered and incubated for 2 h at RT on a rocker platform. Plates were washed three times. Alkaline phosphatase-conjugated goat anti-human immunoglobulin G (IgG) antibody diluted in PBS containing 0.05% Tween 20 was prepared and added to the plates. Plates were covered and incubated for 2 h at RT on a rocker platform. Plates were washed four times, and p-nitrophenyl phosphate substrate solution was added to the plates. Color development was stopped by adding 3.0 N NaOH to each well after the absorbance at 405 nm (A405) of the least-diluted standard concentration was at least 2.5. An assay run typically included multiple plates, with each plate containing the 89SF control sample at low, medium, and high dilutions as a quality control. Test sample titers were determined by referencing their optical density responses against a standard curve generated from a serially diluted reference serum. A four-parameter logistic regression function with a bounded fit (minimum parameter must be greater than zero) was fit to the standard curve. The four-parameter equation has the following form: 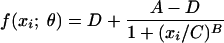 , where A, B, C, and D are the four parameters. The A and D parameters refer to the asymptotic maximum and minimum parameters, respectively, of the standard curve. The B and C parameters correspond to the slope and the concentration corresponding to the 50% response, respectively. The fit is carried out using the Solver tool in Excel by identifying the values of the parameters that minimize the weighted residual sum of squares. A weighted fit is employed where the squared residual at each standard curve concentration is weighted by the inverse of the squared predicted response at that concentration. The response that is modeled in the assay is the delta optical density (DOD), and it is the difference between the average of the duplicate antigen-coated wells and the average of the duplicate background wells at each dilution of the standard.
, where A, B, C, and D are the four parameters. The A and D parameters refer to the asymptotic maximum and minimum parameters, respectively, of the standard curve. The B and C parameters correspond to the slope and the concentration corresponding to the 50% response, respectively. The fit is carried out using the Solver tool in Excel by identifying the values of the parameters that minimize the weighted residual sum of squares. A weighted fit is employed where the squared residual at each standard curve concentration is weighted by the inverse of the squared predicted response at that concentration. The response that is modeled in the assay is the delta optical density (DOD), and it is the difference between the average of the duplicate antigen-coated wells and the average of the duplicate background wells at each dilution of the standard.
Assay validity criteria.
An assay run typically includes multiple plates, with each plate containing a control sample (89SF) that is tested at three twofold serial dilutions. Within each plate, the geometric mean titer (GMT) and the ratio of the maximum titer to the minimum titer are calculated across the dilutions of the control (QC geomean and QC ratio, respectively). A plate is considered invalid if the QC geomean or QC ratio is outside predetermined specification ranges or if more than one of the positive control dilutions fails on a plate. If either of these occurs on the standard curve plate, the entire run is invalid. Furthermore, for an assay run to be valid, the root mean square error of the standard curve must be within the predetermined acceptance limits. Preset criteria regarding the number of points that can be dropped from the standard curve are also established.
Method for assessing inhibition of nonspecific reactivity to C Ps when using Pn25 plus Pn72 in the serum diluent.
Twenty sera were preadsorbed with either 10 μg/ml C Ps, 10 μg/ml C Ps plus 10 μg/ml Pn22F, 10 μg/ml C Ps plus 100 μg/ml Pn25 plus 100 μg/ml Pn72, or no preadsorbant. Samples were tested at two dilutions, 1:50 and 1:250. Plates were coated with 10 μg/ml C Ps in C Ps-coated wells and 1% BSA in background wells. Assay methods were followed as stated above except that standards and controls were not utilized. Inhibition of nonspecific reactivity to C Ps was determined by calculating the DOD response and comparing the DOD response for preadsorbed sera to the DOD response for nonpreadsorbed sera. Statistical analysis was done using Student's t test.
Method for reassignment of serotype-specific antibody titers to the international reference standard 89SF using Pn25 plus Pn72 in the serum diluent.
The quantitation of antibodies in the 89SF serum following the addition of the preadsorbants C Ps, Pn25, and Pn72 was performed by evaluating the equivalence of adsorbance between total IgG ELISA (henceforth called reference ELISA) and anti-PnPs ELISA. The two assays were performed under identical conditions of assay buffers (PBS, 1% BSA, 0.05% Tween 20, 10 μg/ml C Ps, 100 μg/ml Pn25, and 100 μg/ml Pn 72), incubations, and alkaline phosphatase-conjugated secondary antibodies and completed concurrently. The ELISA for the anti-PnPs assay was performed as described above. The reference ELISA for total IgG was performed by coating microtiter plates with goat anti-human light-chain-specific reagents (κ and λ) (0.8 μg/ml each in PBS, catalog numbers 4306 and 4308, respectively; Biosource), followed by the addition of U.S. National Reference Preparation IS1644 reference serum from the CDC (IgG concentration, 11.28 mg/ml). The absorbance of the wells containing the highest concentration of IS1644 was monitored at 405 nm using a microtiter plate reader, and the experiments were completed when the absorbances of these wells were >1.3 and <2.0. The known concentrations (in micrograms per milliliter) of the reference preparation at the endpoint dilution are used to assign antibody concentrations to the endpoint dilution of 89SF standard serum in the anti-PnPs ELISA. The concentration of IgG in the anti-PnPs ELISA was calculated by parallel-line fit analysis. Briefly, resultant absorbances were plotted against the serially diluted standard curve of the IS1644 reference standard to check for parallelism. Only those data points that were parallel to the reference standard curve were used for quantitation. Resulting titers, including 95% confidence intervals, were averaged and reported.
Concordance of Merck Pn assay with the international Pn assay. (i) Concordance panel.
CBER and the WHO developed a set of sera from adults vaccinated with a 23-valent Pn polysaccharide vaccine (11; D. Goldblatt, personal communication). These sera, also known as Goldblatt sera, have been used by several laboratories to compare different assays with respect to their abilities to produce the assigned antibody concentrations to within an acceptable level of tolerance. In addition to the Goldblatt sera, 120 randomly selected adult serum samples (60 pre- and 60 postvaccination samples) from an internal clinical protocol were analyzed for IgG antibodies to the 12 serotypes using the Merck Pn assay and a slightly modified version of the international Pn assay. The 120 sera were tested across five assay runs. Each of the five runs contained 24 samples: 12 randomly assigned prevaccination samples and 12 randomly assigned postvaccination samples. The Merck Pn assay was performed at Merck Laboratories, and the international Pn assay was performed in a contract laboratory, as described previously (9, 18), with the exception that Pn22F was replaced with Pn25 to preadsorb the test sera. The main reagent differences between the Merck assay and the international WHO assay are indicated in Table 2.
TABLE 2.
Differences between Merck assay and international Pn ELISA
| Serum or preadsorbant | Merck assay | International assay |
|---|---|---|
| Standard sera | Giebink sera | Lot 89SF |
| G30, types 1, 4, 8, 9V, and 18C | ||
| G11, types 3, 6B, 7F, 12F, and 23F | ||
| G16, types 14 and19F | ||
| Preadsorbant for standard | 10 μg/ml C Ps, 100 μg/ml Pn25, and 100 μg/ml Pn72 | 5 μg/ml C Ps |
| Preadsorbant for test samples | 10 μg/ml C Ps, 100 μg/ml Pn25, and 100 μg/ml Pn72 | 5 μg/ml C Ps and 10 μg/ml Pn22F |
(ii) Concordance of Merck Pn assay titers to published results for a set of 12 Goldblatt sera.
For each serotype, the 12 Goldblatt sera were tested across six assay runs. Within each run, the 12 sera were tested across three plates. On each plate, samples were tested at two dilutions, 1:250 and 1:1,250. The comparison was done between the concentrations obtained using the Merck Pn assay and the assigned antibody concentrations reported by D. Goldblatt (9). Runs within each assay that failed established assay validity criteria were repeated. Concordance slopes and changes (n-fold) in titers (pre- and postvaccination) were determined.
RESULTS
The ability of the pneumococcal ELISA to measure antibodies to serotype-specific Pn polysaccharides is dependent on the ability to block nonspecific binding (9). Reactivity to C Ps has been shown to decrease specificity in the pneumococcal ELISA (19). To this end, many laboratories have used Pn22F to reduce nonspecific binding reactivity to C Ps and other noncapsular antigens that contaminate capsular Ps preparations. In order to evaluate the ability of Pn25 and Pn72 to reduce nonspecific binding reactivity, an experiment to compare preadsorption with Pn22F to preadsorption with Pn25 and Pn72 was conducted as described in Materials and Methods. Results from this experimentation demonstrated that preadsorption of sera with C Ps plus Pn25 and Pn72 provides additional inhibition of nonspecific reactivity to noncapsular components compared to when sera are preadsorbed with either C Ps alone or with C Ps plus Pn22F (data not shown) (P < 0.01).
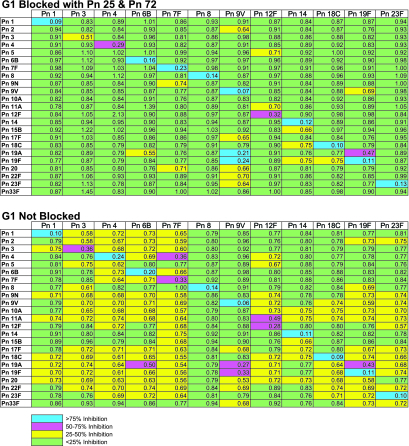
Experiments were performed in order to characterize and quantify the assay specificities among PnPs serotypes when preadsorption was done with Pn25 plus Pn72. For this purpose, aliquots of five individual Giebink sera were treated separately with 100 μg/ml of PnPs from each of the 23 Pn serotypes, and cross-reactivity was measured in the 12 Pn ELISAs. The ability of each serotype to reduce the amount of antibody detected in the ELISA using the serotype-specific Pn-coated plates was evaluated. Specificity was defined as the extent to which liquid-phase polysaccharides from homologous serotypes were able to block the detection of antibodies to plate-bound PnPs of that serotype. Conversely, nonspecific reactivity was defined as the extent to which polysaccharides from heterologous serotypes could block the detection of antibodies to that serotype. The estimated mean specificity improvement when preadsorbing with Pn25 and Pn72 ranged from 5.0% to 20.1% across serotypes. As an example, Fig. 1 illustrates the cross-reactivity assessment performed for one serum sample, G1, in the presence or absence of Pn25 and Pn72 adsorbants. Homologous polysaccharides inhibited binding by >75%. These studies show that (i) considerable nonspecificity was observed in several of the Pn antibody assays, (ii) this interference was reduced by the pretreatment of sera with Pn C Ps as well as a heterologous adsorbant comprised of Pn25 and Pn72, and (iii) the degree of improvement was both serotype and serum dependent.
FIG. 1.
Cross-reactivity of heterologous PnPs with and without adsorbant. Nonspecificity assessment for one representative serum sample, G1, in the presence or absence of Pn25 and Pn72 adsorbants is shown (top and bottom panels, respectively). Within a grid, the percent inhibition in the DOD response for a particular combination of serotypes is coded with one of four colors.
The assigned values for the human Pn reference international standard, lot 89SF, have been determined by using C Ps to preadsorb nonspecific reactivity. In this respect, Quataert et al. (12) and Concepcion and Frasch (2) previously established weight-based antibody units for the international Pn reference standard, lot 89SF, for all 23 Pn serotypes. Since the Merck Pn assay utilizes 89SF as the assay control to show day-to-day reproducibility and serotypes Pn25 and Pn72 for preadsorption, it was necessary to reassign the IgG assignments for the international reference standard, 89SF, across serotypes 1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F following preadsorption with C Ps, Pn25, and Pn72 as described in Materials and Methods. The resulting concentration reassignments are shown in Table 1. Eight out of the 12 serotypes exhibited a slight to moderate decrease in serotype-specific IgG concentrations following preadsorption with Pn25 plus Pn72.
Calibration of the Pn antibody concentrations in the Giebink sera for specific serotypes (Giebink serum G30, Pn1, Pn 4, Pn8, Pn9V, and Pn18C; Giebink serum G11, Pn3, Pn 6B, Pn7F, Pn 12F, and Pn23F; and Giebink serum G16, Pn 14 and Pn 19F) relative to the international reference standard, 89SF, was performed. Six calibration runs, which included a 12-point, twofold dilution series of 89SF and the respective Giebink serum, were performed for each serotype. The Giebink sera were preadsorbed with 10 μg/ml C Ps, 100 μg/ml Pn25, and 100 μg/ml Pn72, while 89SF was preadsorbed with 5 μg/ml C Ps. Relative potency (i.e., the calibration factor) between pairs of standards was assessed by parallel-line analysis using the four-parameter logistic regression function. The concentration assignments for the Giebink sera, based on the most recent assessment and Quataert IgG assignments for 89SF, are indicated in Table 1.
Validation of the Merck Pn antibody assays for serotypes 1, 3, 4, 6B, 7F, 8, 9V, 12F, 14, 18C, 19F, and 23F was performed as described in Materials and Methods. We followed the procedures and methodology outlined previously by Findley et al. (5) in order to establish the quantifiable range, estimate assay precision, assess the ruggedness of the assay, assess the effect of dilution on titer response, and establish quality control specifications for determining the validity of an assay run and of individual test samples within that run. The key characteristics of the validated assays are as follows.
(i) LOD and LOQ.
The limit of detection (LOD) of an assay was defined as the lowest concentration of serum that had the highest probability of producing a response that could be distinguished from the background response. The lower and upper limits of quantitation were defined as the highest and lowest concentrations between which test samples could be measured with acceptable precision (≤20% relative standard deviation [RSD]) (5). The standard curve was fit according to the procedures noted in Materials and Methods. The results from fitting the standard curves in the four runs performed for each serotype-specific assay were used to determine the LOD and limit of quantitation (LOQ). For each serotype, the LOD and the lower LOQ were below 0.002 μg/ml. This means that for each of the 12 serotypes, the lower LOQ does not exceed 0.1 μg/ml for a sample tested at the 1:50 dilution.
(ii) Precision.
Precision of test samples was addressed by testing nine samples across four assay runs. Within each run, samples were tested across a series of four to six twofold dilutions. The precision of the Pn antibody assays (expressed as percent RSD) was estimated using variance component analysis of the logarithm of the dilution-corrected titers. Furthermore, assay variability was also assessed based on the assay controls, three serial twofold dilutions of the international reference, lot 89SF, tested across a large number (>30) of clinical runs. Overall, an RSD of <30% was observed for all serotypes.
(iii) Dilutability.
Dilutability is an attribute of a biological assay that demonstrates that a test sample can be diluted through a series, yielding equivalent dilution-corrected titers across that series. Sample matrices contribute significantly to the dilutability, with the dilution effect (i.e., matrix interference) typically being more pronounced at the lower dilutions of the sera. For each serotype, nine samples were tested across a series of four to six twofold dilutions. The dilution effect was estimated by the bias in dilution-corrected titers per twofold dilution of a test sample. In general, acceptable dilutability (i.e., dilution effect of less than 30% per twofold dilution) was observed in samples with titer levels exceeding 0.1 μg/ml for each serotype.
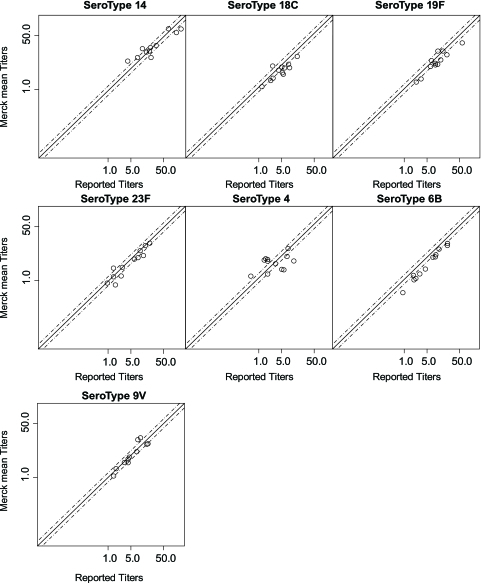
The main reagent differences between the Merck assay and the international assay are indicated in Table 2. The objective of a concordance analysis was to benchmark the performance of the Merck Pn ELISA (sera preadsorbed with 10 μg/ml C Ps plus 100 μg/ml Pn25 and 100 μg/ml Pn72) against the results obtained when the internationally accepted Pn assay was used (sera preadsorbed with 5 μg/ml C Ps plus 10 μg/ml Pn22F). For this purpose, two sets of experiments were performed. The first set compared Goldblatt test sample titers generated with the Merck Pn assay to the corresponding titers obtained using the international Pn assay reported by Goldblatt (9). The working group at the WHO agreed that when Pn IgG ELISAs are evaluated, 9 of the 12 selected Goldblatt sera needed to be within 1.4-fold of the assigned titers for the assay to be considered acceptably accurate (11). Figure 2 displays the plots of the Merck titers against the reported Goldblatt titers (9). Although the Merck assay did not meet the defined acceptance criteria for four of the seven serotypes evaluated, there was a moderate level of agreement between the two sets of results for each of the seven serotypes as evidenced by the concordance plots displayed in Fig. 2.
FIG. 2.
Plots of the Merck titers (averaged across the runs) versus the reported titers for Goldblatt sera (9). Results for the Goldblatt serum calibration are reported for only 7 of the 12 serotypes (available at the following website: http://www.vaccine.uab.edu/qc3.pdf). The dotted lines represent the agreement criterion ±1.4-fold. The comparison was performed as described in Materials and Methods.
Given the limited number of Goldblatt serum samples available for the assay comparison, a second set of experiments was conducted to compare the Merck Pn assay to the international Pn assay, as executed in a contractual laboratory. For this comparison, a randomized set of 120 samples, comprised of 60 prevaccinated sera and 60 sera from individuals vaccinated with PNEUMOVAX 23, was tested in the two assays. The test sample titer results obtained within each of the assays are summarized in Table 3. As evidenced in Table 3, the individual titer results and the rises (n-fold) in titer responses from prevaccination to postvaccination were fairly comparable between the two assays. The concordance slopes in terms of titer responses ranged from 0.70 to 1.02 across the 12 serotypes evaluated.
TABLE 3.
Comparison of the Merck Pn assay with the international Pn assaya
| PnPs Serotype | Prevaccination
|
Postvaccination
|
Fold rise
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| International assay
|
MRL
|
International assay
|
MRL
|
International assay
|
MRL
|
|||||||
| GMT | SD | GMT | SD | GMT | SD | GMT | SD | GMT | SD | GMT | SD | |
| 1 | 0.2 | 1.06 | 0.2 | 1.15 | 2.8 | 1.38 | 2.4 | 1.21 | 13.0 | 1.11 | 12.3 | 1.29 |
| 3 | 0.4 | 1.01 | 0.5 | 0.92 | 1.5 | 1.09 | 1.3 | 0.86 | 4.3 | 0.93 | 2.5 | 0.86 |
| 4 | 0.2 | 1.25 | 0.3 | 1.27 | 2.4 | 1.33 | 3.9 | 1.30 | 10.6 | 1.00 | 11.8 | 1.06 |
| 6B | 0.7 | 1.10 | 1.0 | 1.15 | 3.2 | 1.48 | 5.7 | 1.32 | 4.5 | 1.09 | 5.9 | 1.01 |
| 7F | 0.6 | 1.24 | 0.6 | 1.27 | 7.0 | 1.33 | 7.2 | 1.29 | 11.8 | 1.23 | 12.2 | 1.23 |
| 8 | 0.3 | 1.07 | 1.1 | 0.83 | 4.6 | 1.10 | 6.4 | 0.83 | 13.3 | 1.16 | 5.7 | 0.92 |
| 9V | 0.7 | 1.37 | 0.8 | 1.47 | 5.7 | 1.32 | 6.0 | 1.19 | 8.4 | 1.03 | 7.5 | 1.05 |
| 12F | 0.1 | 0.97 | 0.1 | 1.17 | 1.0 | 1.62 | 2.0 | 1.64 | 8.7 | 1.32 | 13.7 | 1.52 |
| 14 | 1.8 | 1.55 | 4.5 | 1.18 | 15.1 | 1.79 | 26.1 | 1.48 | 8.4 | 1.40 | 5.8 | 1.24 |
| 18C | 0.9 | 1.19 | 1.7 | 1.25 | 7.9 | 1.31 | 13.2 | 1.26 | 8.6 | 1.15 | 7.7 | 1.33 |
| 19F | 0.9 | 1.00 | 2.0 | 0.96 | 4.3 | 1.48 | 11.2 | 1.35 | 4.7 | 1.07 | 5.7 | 1.07 |
| 23F | 0.5 | 1.24 | 1.0 | 1.08 | 3.2 | 1.64 | 5.5 | 1.44 | 6.8 | 1.33 | 5.4 | 1.19 |
Pre- and postimmunization adult serum samples (n = 120) were quantitated for total IgG as described in Materials and Methods. MRL, Merck Research Laboratory Pn assay.
DISCUSSION
The polysaccharide capsules that surround Streptococcus pneumoniae and other bacterial pathogens are potent virulence factors, protecting these bacteria from phagocytosis. Capsular Ps-specific antibodies, when combined with complement binding to the capsule, mediate opsonization of these microorganisms for subsequent phagocytosis. In this respect, the opsonophagocytic killing (OPK) assay has been utilized to measure functional antibody responses (7). However, it is not clear how much antibody is sufficient for protection against Streptococcus pneumoniae infections in vivo. Serum concentrations ranging from 0.1 to 3.5 μg/ml have been suggested by various efficacy studies. These levels of antibodies vary depending on the serotype, age of the subject (i.e., adult versus pediatric sera), and type of infection (lung and/or systemic bacteremia).
Extensive studies have evaluated the concentrations of Pn antibodies, measured by ELISA, and their ability to correlate functional activity, measured by OPK activity in vitro. The correlation between OPK and ELISA, however, has not been consistent. It has been established that antibodies to the cross-reactive domains of the Ps antigens and contaminating C Ps contribute to the decreased correlation between ELISA and OPK assays (8, 16, 17). Furthermore, immunogenic non-C-Ps contaminants have also been implicated in the nonspecific reactivity (19). Preadsorption of sera with heterologous Ps has been shown to reduce nonspecific reactivity and increase the correlation with functional antibody responses (3). In this paper, we describe the use of heterologous Ps, which are not components of the 23-valent PNEUMOVAX 23 vaccine, to effectively inhibit nonspecific reactivity and increase the specificity of measuring type-specific antibodies.
Various studies have demonstrated that sera from children and adults can have high levels of anti-C Ps antibodies, which account for the nonspecific binding reactivity in the assay. The specificity of the ELISA for measuring serotype-specific antibodies to the PnPs is dependent on the ability to block nonspecific binding. In order to minimize the contribution of nonspecific reactivity, we have preadsorbed sera with Pn25 and Pn72 polysaccharides, in addition to preadsorption with C Ps, to increase specificity to the individual capsular serotypes. However, the degree to which preadsorption influences specificity is serotype as well as serum specific. The average improvement in specificity for the 12 serotypes in this study ranged from 5 to 20%. Concepcion and Frasch (3) previously reported a similar range of percent inhibition with heterologous PnPs. Those authors speculated that a common epitope involving the linkage region between C Ps and the type-specific PnPs could be involved in the cross-reactivity.
The international Pn assay evaluated heterologous serotypes 12F, 15B, 22F, and 33F to inhibit nonspecific reactivity. The Pn22F heterologous Ps was chosen, since it was not one of the Ps in the licensed pneumococcal conjugate vaccine (Prevnar) for pediatric patients. Since the 23-valent PNEUMOVAX 23 vaccine contains serotype 22F, we have utilized polysaccharides from Pn25 and Pn72 as preadsorbants for blocking nonspecific reactivity. In order to accurately measure antibody concentrations in sera from vaccinated individuals, IgG concentrations for the international reference standard, lot 89SF, following preadsorption with C Ps, Pn25, and Pn72 were quantified. The methodology used for antibody quantitation is consistent with the method previously adapted for the assignment of weight-based antibody units to 89SF reference serum (12). The overall reduction in titers reflects the removal of nonspecific components from the response. Due to the imminently limited supply of 89SF sera, sera from individuals vaccinated with the 23-valent vaccine were prepared and utilized as standards for the Pn ELISA following calibration to the international standard.
The Goldblatt sera have been used as a “gold standard” for comparisons of various platforms for Pn assays across laboratories. In order to benchmark the performance of the Merck Pn assay with the international Pn assay, the reported serotype-specific IgG concentrations of the Goldblatt sera were compared to those obtained using the Merck Pn assay. There was good concordance between the two assay formats, as observed by the concordance plots; however, the criteria of the WHO were not met for three (serotypes 23F, 6B, and 9V) of the seven serotypes. For most of the serotypes, the Merck assay measured lower titers, as would be expected due to a higher degree of preadsorption of nonspecific antibodies, with the average difference (n-fold) ranging from 0.54 to 1.90 for the 12 serotypes. In order to expand this assessment, a blinded set of 120 pre- and postvaccination sera was utilized for further evaluation. The results showed that the Merck assay and the international assay yielded comparable results with regard to subject-to-subject variability in titers and in the rise in responses (n-fold) following vaccination.
In conclusion, we have shown that preadsorption of sera with Pn25 and Pn72 polysaccharides, in addition to CPs, provides an adequate reduction of nonspecific reactivity. Furthermore, the degree to which preadsorption influences specificity is serotype as well as serum specific. The Merck Pn ELISAs demonstrate agreement with the internationally accepted ELISA. The assay is utilized at Merck and Co., Inc., for evaluating immune responses to the PNEUMOVAX 23 vaccine.
Acknowledgments
We acknowledge the contributions of Y. Wang, K. Nadig, C. Tan, P. Johansen, T. Schofield, S. Reed, M. Caulfield, L. Rubinstein, C. Abegunawardena, H. Pujar, and T. Morley. In addition, we thank the Merck Manufacturing Division for the supply of the purified polysaccharides and D. Goldblatt for the supply of the “Goldblatt sera.”
REFERENCES
- 1.Butler, J. C., J. Hofmann, M. S. Cetron, J. A. Elliott, R. R. Facklam, and R. F. Breiman. 1996. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention's Pneumococcal Sentinel Surveillance System. J. Infect. Dis. 174:986-993. [DOI] [PubMed] [Google Scholar]
- 2.Concepcion, N., and C. E. Frasch. 1998. Evaluation of previously assigned antibody concentrations in pneumococcal polysaccharide reference serum 89SF by the method of cross-standardization. Clin. Diagn. Lab. Immunol. 5:199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Concepcion, N., and C. E. Frasch. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coughlin, R. T., A. C. White, C. A. Anderson, G. M. Carlone, D. L. Klein, and J. Treanor. 1998. Characterization of pneumococcal specific antibodies in healthy unvaccinated adults. Vaccine 16:1761-1767. [DOI] [PubMed] [Google Scholar]
- 5.Findley, J. W. A., W. C. Smith, J. W. Lee, G. D. Nordblom, I. Das, B. S. DeSilva, M. N. Khan, and R. R. Bowsher. 2000. Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J. Pharm. Biomed. Anal. 21:1249-1273. [DOI] [PubMed] [Google Scholar]
- 6.Koskela, M. 1987. Serum antibodies to pneumococcal C polysaccharide in children: response to acute pneumococcal otitis media or to vaccination. Pediatr. Infect. Dis. J. 6:519-526. [DOI] [PubMed] [Google Scholar]
- 7.Musher, D., A. J. Chapman, A. Goree, S. Jonsson, D. E. Briles, and R. E. Baughn. 1986. Natural and vaccine-related immunity to Streptococcus pneumoniae. J. Infect. Dis. 154:254-256. [DOI] [PubMed] [Google Scholar]
- 8.Musher, D. M., D. A. Watson, and R. E. Baughn. 1990. Does naturally acquired IgG antibody to cell wall polysaccharides protect human subjects against pneumococcal infection? J. Infect. Dis. 161:736-740. [DOI] [PubMed] [Google Scholar]
- 9.Nahm, M., and D. Goldblatt. 26. November 2002, posting date. Training manual for enzyme linked immunosorbent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG (PnPg ELISA). [Online.] http://www.vaccine.uab.edu.
- 10.Phipps, D. C., S. Strohmeyer, S. A. Quataert, G. Siber, and D. V. Madore. 1990. Standardization of ELISA for the quantitation of antibodies to S. pneumoniae capsular polysaccharides (PnPs). Pediatr. Res. 27:179A. [Google Scholar]
- 11.Plikaytis, B. D., D. Goldblatt, C. Frasch, C. Blondeau, M. J. Bybel, G. S. Giebink, I. Jonsdottir, H. Kayhty, H. B. Konradsen, D. V. Madore, M. H. Nahm, C. A. Schulman, P. F. Holder, T. Lezhava, C. M. Elie, and G. M. Carlone. 2000. An analytical model applied to a multicenter pneumococcal enzyme-linked immunosorbent assay study. J. Clin. Microbiol. 38:2043-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quataert, S. A., C. S. Kirch, L. J. Q. Wiedl, D. C. Phipps, S. Strohmeyer, C. O. Cimino, J. Skuse, and D. V. Madore. 1995. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin. Diagn. Lab. Immunol. 2:590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quataert, S. A., K. Rittenhouse-Olson, C. S. Kirsch, B. Hu, S. Secor, N. Strong, and D. V. Madore. 2004. Assignment of weight-based antibody units for 13 serotypes to a human antipneumococcal standard reference serum, lot 89-S(F). Clin. Diagn. Lab. Immunol. 11:1064-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffman, G., R. M. Douglas, M. J. Bonner, M. Robbins, and R. Austrian. 1980. A radioimmunoassay for immunologic phenomena in pneumococcal disease and for the antibody to pneumococcal vaccines. I. Method for the radioimmunoassay of anticapsular antibodies and comparison with other techniques. J. Immunol. Methods 33:133-144. [DOI] [PubMed] [Google Scholar]
- 15.Snapper, C. M., Y. Shen, A. Q. Khan, J. Colino, P. Zelazowski, J. J. Mond, W. C. Gause, and Z. Q. Wu. 2001. Distinct types of T-cell help for the induction of a humoral immune response to Streptococcus pneumoniae. Trends Immunol. 22:308-311. [DOI] [PubMed] [Google Scholar]
- 16.Vitharsson, G., I. Jonsdottir, S. Jonsson, and H. Valdimarsson. 1994. Opsonization and antibodies to capsular cell wall polysaccharides of Streptococcus pneumoniae. J. Infect. Dis. 170:592-599. [DOI] [PubMed] [Google Scholar]
- 17.Watson, D. A., and D. M. Musher. 1993. Assessment of responses to pneumococcal infection and vaccination using enzyme linked immunoassay. Serodiagn. Immunother. Dis. 5:131-133. [Google Scholar]
- 18.Wernette, C. M., C. E. Frasch, D. V. Madore, G. Carlone, D. Goldblatt, B. Plikaytis, W. Benjamin, S. A. Quataert, S. Hildreth, D. J. Sikkema, H. Kayhty, I. Jonsdottir, and M. H. Nahm. 2003. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin. Diagn. Lab. Immunol. 10:514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu, X., Y. Sun, C. Frasch, N. Concepcion, and M. H. Nahm. 1999. Pneumococcal capsular polysaccharide preparations may contain non-C-polysaccharide contaminants that are immunogenic. Clin. Diagn. Lab. Immunol. 6:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]