Abstract
Engagement of CD154 on activated T cells with CD40 on antigen-presenting cells (APCs) potentiates adaptive immune responses in mammals. Soluble multimeric forms of CD154 have been used as an adjuvant or in immunotargeting strategies to enhance vaccine responses. The objective of our study was to examine the ability of duck CD154 (DuCD154) to enhance DNA vaccine responses in the duck hepatitis B model. Constructs were generated to express the functional domain of DuCD154 (tCD154), truncated duck hepatitis B virus (DHBV) core antigen (tcore) and chimera of tcore fused to tCD154 (tcore-tCD154). Expression in LMH cells demonstrated that all proteins were secreted and that tCD154 and tcore-tCD154 formed multimers. Ducks immunized with the plasmid ptcore-tCD154 developed accelerated and enhanced core-specific antibody responses compared to ducks immunized with ptcore or ptcore plus ptCD154. Antibody responses were better sustained in both ptcore-tCD154- and ptcore plus ptCD154-immunized ducks. Core-specific proliferative responses of duck peripheral blood mononuclear cells were enhanced in ducks immunized with ptcore-tCD154 or ptcore alone. This study suggests that the role of CD154 in the regulation of adaptive immune responses had already evolved before the divergence of birds and mammals. Thus, targeting of antigens to APCs with CD154 is an effective strategy to enhance DNA vaccine responses not only in mammalian species but also in avian species.
The development of DNA vaccines for avian species, including ducks, has applications for both human and livestock disease. Ducks infected with duck hepatitis B virus (DHBV) serve as an important model for infection of humans with the hepatitis B virus (HBV) (43). Worldwide, more than 350 million individuals are chronically infected with HBV, and many of these carriers will eventually die from end-stage liver disease or hepatocellular carcinoma (1, 54). Despite considerable progress in the treatment of hepatitis B, posttherapy relapse and drug-resistant HBV mutants remain major challenges (34). Long-term disease remission requires effective immunological control of the HBV infection, emphasizing the need for therapies that stimulate a successful immune response (32, 39-41). Therapeutic vaccines have been shown to augment HBV-specific cellular immune responses in patients with chronic hepatitis B, but clinically relevant viral suppression could not be demonstrated (17, 28, 35, 36). Recently, the DHBV model has been used to explore protective and therapeutic immunization strategies (10, 24, 42, 46, 48). DNA vaccines that express the DHBV envelope or DHBV core protein either alone or in combination with nucleoside analogues have been associated with an accelerated viral clearance in established chronic DHBV infection in some of these studies (24, 42, 46).
Wild aquatic birds such as ducks, geese, and swans are regarded as the principal reservoir hosts of the avian influenza viruses (52). Domestic ducks that are in contact with wild waterfowl and avian livestock may function as a key intermediate in the transmission of avian influenza (25). Ducks can show few or no signs of disease even when carrying viruses that are highly pathogenic in chickens (20, 22). Reassortment of genes between avian and mammalian influenza in other hosts, such as pigs, may help to overcome host restrictions and result in pandemic outbreaks (51). The analysis of the reconstructed genome of the 1918 Spanish influenza virus suggests that this strain was likely an avian influenza-like virus that adapted to humans (49). Hence, the development of effective vaccines and large-scale vaccination programs for avian livestock, including domestic ducks, is an important goal to reduce the burden of human and livestock disease from influenza.
CD154, a member of the tumor necrosis factor superfamily, has a central role in the development and regulation of adaptive immune responses in mammals (2, 27). CD154 is a type II integral membrane glycoprotein that is rapidly up-regulated on activated CD4+ cells and, via CD40 engagement on dendritic cells (DC), promotes DC migration to lymph nodes, DC survival, and T-cell priming (6, 33, 38). Engagement of CD154 with CD40 on B cells stimulates increased expression of major histocompatibility complex and CD80/86 on B cells, which subsequently promotes reciprocal activation of antigen-stimulated B and T cells. Effects of this activation cascade include B-cell clonal expansion, germinal center formation, isotype switching, affinity maturation, and the generation of long-lived plasma cells (11). Furthermore, soluble CD154 generated through proteolytic cleavage of the extracellular domain is biologically active, especially in multimeric forms (7, 12, 37). Consequently, CD154 has been used in various vaccination strategies to enhance both humoral and cellular immune responses in mammals (14, 19, 29-31, 45, 53). We have recently identified the duck homologue of CD154 (DuCD154) and demonstrated biological activity of its extracellular domain (8). In this study, we examine the use of the extracellular domain of DuCD154 as an immunotargeting agent and immunostimulator to enhance DNA vaccine responses in ducks.
MATERIALS AND METHODS
Generation of plasmid constructs.
All reagents for molecular work were from Invitrogen Canada, Inc. (Burlington, ON, Canada). To generate plasmids expressing secreted, truncated duck CD154 (tCD154), clone 13-5 containing the complete open reading frame of DuCD154 (8) was digested with SalI and then treated with Klenow and deoxynucleoside triphosphates to blunt ends, followed by digestion with EcoRI (SalI/Klenow/EcoRI). This fragment contains the extracellular domain, including the endogenous carboxyl secretory leader of DuCD154. The vector pSecTag2C was treated with HindIII/Klenow/EcoRI, and then DuCD154 fragments were inserted. Plasmids expressing DHBV core protein were prepared in two steps. First, the DHBV core precursor construct, pSecTag2C EV-Xmn, was made by mobilizing EcoRV/XmnI fragments of DHBV-16 DNA from pCMVDHBV2 (9) into pSecTag2C digested with HindIII/BamHI/Klenow. Next, the construct pSecTag2C EV-Xmn was digested with NheI/HincII, and the fragment was inserted into NheI/EcoRV-treated pSecTag2C to generate ptcore. To prepare the fusion construct ptcore-tCD154, a NheI/HincII fragment from pSecTag2C EV-Xmn was mobilized into ptCD154 treated with HindIII/Klenow/NheI. Plasmids were propagated in Escherichia coli TOP10 F′ and then purified on QIAGEN columns (QIAGEN, Inc., Mississauga, ON, Canada) and diluted in phosphate-buffered saline (PBS).
Expression and purification of DuCD154 from E. coli.
An AccI/Klenow/HincII fragment from DuCD154 clone 13-5, comprising the majority of the DuCD154 ectodomain, was inserted into BamHI/Klenow-treated pET21b (EMD Biosciences, Inc., Mississauga, ON, Canada). This insertion places a polyhistidine tract in frame and downstream of the DuCD154 sequence. Overexpression of DuCD154 (23-kDa predicted size) was achieved by transformation into E. coli BL21(DE3). Transformed cultures were grown overnight with aeration at 37°C in LB broth containing 100 μg/ml ampicillin and then induced with 1 mM isopropyl-β-d-thiogalactopyranoside (all from Invitrogen Canada, Inc.) for the final 2 h of culture. Bacteria were pelleted at 2,000 × g for 10 min, and pellets were suspended in 6 M guanidine Tris-Cl lysis buffer. Mechanical lysis was done with shaking for 1 h, and then cellular debris was pelleted at 10,000 × g for 30 min. Polyhistidine-tagged DuCD154 was purified from the supernatant by immobilized metal affinity chromatography using denaturing conditions with 8 M urea as the chaotrope in accordance with the manufacturer's protocol (QIAGEN, Inc.). DuCD154 was eluted from the matrix using decreasing pH as the elution variable. Positive fractions as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were pooled, dialyzed extensively against 0.85% saline, and used as antigens for antibody (Ab) production. Protein concentrations were determined using the DC protein assay (Bio-Rad Laboratories Ltd., Mississauga, Ontario, Canada) with bovine serum albumin (BSA, fraction V; Sigma-Aldrich Co., Oakville, ON, Canada) as a standard.
Generation and purification of E. coli-expressed DHBV core protein.
The sequence encoding amino acids 1 to 199 of the DHBV (Alberta 16 strain) core protein was amplified from a plasmid containing a 1.3-genome-length copy of DHBV. Primers were used that introduced a BamHI restriction site at the beginning (5′-CTTGGGATCCGATGGATATCAATGCTTCTAGAGC-3′) and a KpnI site at the end (5′-TTGTGGTACCGCCTCCCTGAGCCACCTGG-3′) of the amplicon (restriction enzyme recognition sites are underlined). PCR conditions were 2 min at 94°C, followed by 10 cycles of 30 s at 94°C, and then 90 s at 68°C using the Expand high-fidelity PCR kit (Roche Diagnostics Corp., Laval, PQ, Canada). The PCR product was digested with BamHI and KpnI and then ligated into similarly digested pRSET-B (Invitrogen Canada, Inc.). Plasmids were cloned into DH5α and used to transform E. coli BL21(DE3). Transformed cultures were grown and induced, and the polyhistidine-tagged DHBV core was purified using metal affinity chromatography as described for recombinant DuCD154. The purity of the core was evaluated by separating each fraction on 10% SDS-PAGE gels and staining with Coomassie blue. The affinity-purified core was used for immunization of mice and enzyme-linked immunosorbent assays (ELISAs). For peripheral blood mononuclear cell (PBMC) assays, the core was filter sterilized and then urea was removed by four rounds of ultrafiltration using Amicon Ultra-15 filters (5,000-molecular-weight cutoff; Millipore Corp.).
Antibody production.
Monoclonal antibodies (MAbs) to E. coli-produced DHBV core and polyclonal Abs to the DuCD154 ectodomain were generated by standard procedures (16). Briefly, 100 μg/mouse of recombinant protein was emulsified in an equal volume of complete Freund's adjuvant (Sigma-Aldrich Co.) and injected subcutaneously (s.c.) into BALB/c mice, followed by two s.c. booster injections at 2-week intervals with 100 μg/mouse of recombinant proteins emulsified in incomplete Freund's adjuvant (Sigma-Aldrich Co.). For production of MAbs, 100 μg/mouse of recombinant DHBV core protein in PBS was injected intravenously. Spleens were removed 3 days later, and single-cell suspensions were prepared. Spleen cells were mixed 10:1 with SP0/2 myeloma cells and fused using polyethylene glycol (Sigma-Aldrich Co.). Supernatants from hybridomas were screened by ELISA using DHBV core adsorbed to the solid phase. Core-reactive MAb specificity was verified by immunoblot analysis using untagged core protein expressed in transfected LMH cells. Immune sera from DuCD154-immunized mice were collected 10 days after a final s.c. injection of 100 μg/mouse of recombinant CD154. Titers were determined by ELISA using recombinant CD154 adsorbed to the solid phase, and Ab specificity was verified by immunoblotting using untagged CD154 expressed in transfected LMH cells.
Expression and characterization of recombinant proteins in LMH cells.
To generate recombinant DHBV core and DuCD154 lacking the polyhistidine tract, LMH cells (ATCC CRL-2117) were transfected as previously described (9) with ptcore, ptCD154, ptcore-tCD154, or pSecTag2C, and supernatants were collected after 72 to 96 h of culture. If supernatants required concentration, Microcon YM-10 (10,000 nominal molecular weight limit; Millipore Corp., Bedford, MA) filters were used. Supernatants were mixed 1:1 with SDS-PAGE loading buffer in the presence (reducing) or absence (nonreducing) of 5% 2-mercaptoethanol, boiled for 10 min, and separated by PAGE using 10% gels. Proteins were blotted onto nitrocellulose membranes using semidry electrophoretic transfer. Membrane blocking and Ab incubations were done in PBS containing 5% skim milk protein and 0.1% Tween 20. Blots were washed in PBS containing 1% skim milk protein and 0.1% Tween 20. DHBV core proteins were detected using mouse MAb 9G8, and DuCD154 was detected using polyclonal mouse antiserum.
ELISA to quantify DHBV core-specific Ab in immunized ducks.
Falcon 96-well, flat-bottom, flexible polyvinyl chloride plates (Becton Dickinson, Franklin Lakes, NJ) were coated with 2 μg/ml recombinant, metal affinity-purified DHBV core antigen diluted in PBS, pH 7.4, and aliquoted at 50 μl/well. Antigen was adsorbed overnight at 4°C or for 30 min at 37°C. After incubation, fluid containing unadsorbed antigen was removed, and 100 μl/well of sterile filtered PBS containing 3% BSA was added. Plates were incubated at 37°C for 30 to 60 min and then washed twice by immersion in PBS containing 0.1% Tween 20 (PBS-T). Duck sera were diluted in PBS containing 1% BSA and 0.1% Tween 20 (assay diluent) and added to plates in triplicate at 50 μl/well. Incubation was for 1 h at 37°C. Thereafter, plates were washed by immersion four times in PBS-T and then affinity-purified goat anti-duck immunoglobulin Y (IgY; heavy plus light chain specific) conjugated to horseradish peroxidase (KPL, Gaithersburg, MD) was diluted in assay diluent 1:3,000 and added at 50 μl/well. Plates were incubated for 30 min at 37°C and then washed four times in PBS-T as described. Following the addition of 3,3′,5,5′-tetramethylbenzidine (KPL) at 100 μl/well, plates were read within 30 min at the optical density at 650 nm. Averages were determined for each sample, and then endpoint titers were calculated using an arbitrarily defined cutoff value equivalent to the conjugate background + 3 standard deviations on each ELISA plate. A dilution series of a positive reference serum from a DHBV-infected duck was routinely included on each plate to verify that the cutoff value was equivalent to the endpoint optical density of the reference serum. Comparisons were made between vaccination groups using endpoint titers. Statistical significance was determined by the unpaired Student t test.
PBMC proliferation assay.
PBMC proliferation assays were done as previously described with adaptations for the duck (18, 50). Prior to performance of the experiments reported here, proliferative responses were optimized with respect to cell density, incubation time and temperature, antigen concentration, and growth medium. Briefly, venous blood was added to heparinized tubes, diluted in an equal volume of PBS, and layered over Ficoll (lympholyte M; Cedarlane Laboratories, Hornby, ON, Canada), and then PBMCs were separated by centrifugation at 800 × g for 20 min. Cells at the interface were collected, washed, and pelleted twice by centrifugation at 700 × g for 8 min using Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (Invitrogen Canada, Inc.), 5% DHBV-negative duck serum, 2 mM l-alanyl-l-glutamine, 50 μg/ml gentamicin, and ITS liquid medium supplement (all from Sigma-Aldrich Co.) and 55 μM 2-mercaptoethanol (complete medium) to wash the cells. Cells were finally suspended in complete medium, counted by hemacytometer, and then seeded in triplicate to Falcon 96-well, flat-bottom tissue culture plates (Becton Dickinson) at 2 × 106 cells/well, and doubling dilutions were done. Cells were left unstimulated or were stimulated with 100 μg/ml recombinant DHBV core. Tissue culture plates were incubated at 37°C in a 5% CO2, humidified atmosphere for 6 days. Tritiated thymidine (specific activity, 85 Ci/mM; Amersham Biosciences, Piscataway, NJ) was added at 0.5 μCi/well for the final 6 h of incubation. Proliferation was assessed as a direct correlation of tritium uptake, and stimulation indices (SI) were calculated by dividing the average cpm of sample wells by the average cpm for unstimulated cells. Statistical significance was determined by the unpaired Student t test.
Animals and immunization protocols.
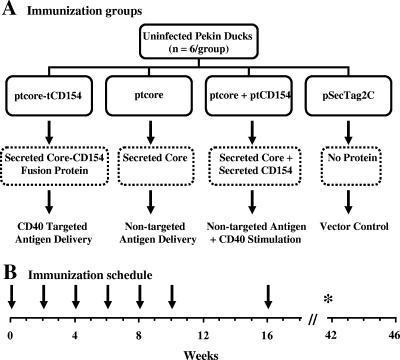
Newborn domestic Pekin ducks (Anas platyrhynchos) were obtained from an uninfected breeding colony at the University of Alberta that was maintained according to the regulations of the Canadian Council on Animal Care. Sera from all animals were screened for the presence of DHBV infection by dot blot analysis prior to use for these studies. Blots were probed for DHBV DNA as previously described (23). Five- to six-week-old domestic Pekin ducks were allocated to four immunization groups (6/group) as shown in Fig. 1A. Ducks were immunized with plasmids every 2 weeks up to week 10 as illustrated in Fig. 1B. A final boost was administered 16 weeks after the primary immunization. Purified plasmid DNA was injected intramuscularly alternating between the right and left thighs. The total plasmid dose was kept constant at 400 μg/injection in 400 μl of PBS. Ducks received, per injection, either 200 μg of ptcore-tCD154 (49 nmol) plus 200 μg the control vector pSecTag2C (59.2 nmol) or 200 μg of ptcore (54.4 nmol) plus 200 μg of pSecTag2C or 200 μg ptcore plus 200 μg of ptCD154 (53.6 nmol) or 400 μg of pSecTag2C. The total number of moles of plasmid per injection per duck was 113 ± 5 nmol. Serum was collected pre- and postimmunization to determine core-specific Ab titers. PBMCs were isolated for analysis of core-specific proliferative responses of the remaining ducks at weeks 32 and 42. Seven ducks died of causes unrelated to the experimental protocol during the course of the experiment. Two ducks were euthanized after developing foot problems. Autopsies performed on the other 5 ducks indicated death due to heart failure or pneumonia but no obvious pathology related to vaccination.
FIG. 1.
Immunization strategy and schedule. (A) Experimental outline and rationale of each vaccination group; (B) timeline depicting the immunization schedule. Plasmids were injected at each time point indicated (↓). At week 32 and 42 (*), PBMCs were isolated and stimulated in vitro to assess DHBV core-specific proliferation.
Nucleotide sequence accession numbers.
The nucleotide sequence data corresponding to the constructs have been submitted to the GenBank database. The accession numbers are DQ267671 for DuCD154, AF047045 for the Alberta strain of DHBV, and K01834 for DHBV-16.
RESULTS
In vitro expression of tcore, tCD154, and tcore-tCD154 in LMH cells.
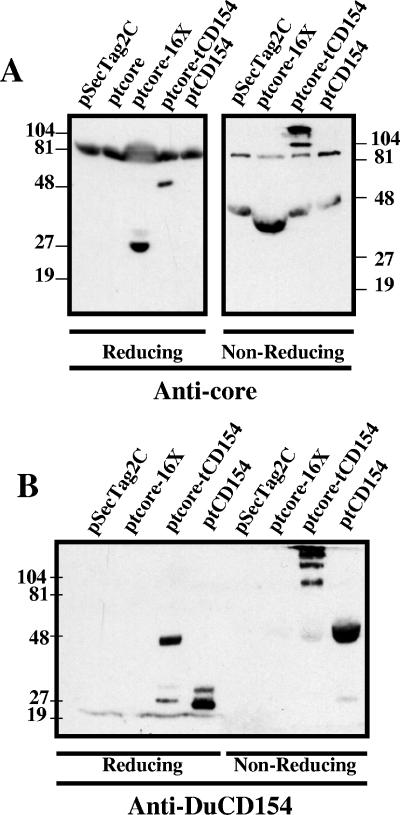
Plasmid constructs were designed to encode tCD154; a truncated, secreted DHBV core protein (tcore); and a secreted chimeric protein of tcore fused to tCD154 (tcore-tCD154) (Fig. 2). Protein expression and secretion were verified by transfection of LMH cells with plasmids lacking the polyhistidine tract and analysis of supernatants by immunoblotting. Supernatants of ptcore- and ptcore-tCD154-transfected LMH cells separated under reducing conditions and probed with anti-core MAb 9G8 contained proteins consistent with the predicted size of 27 kDa (Fig. 3A, left panel, ptcore 16X) and 46 kDa (ptcore-tCD154). Cross-reactive proteins of 81 kDa were detected in supernatants from all transfectants. Under nonreducing conditions, tcore had an apparent molecular mass of approximately 38 kDa (Fig. 3A, right panel, ptcore 16X), presumably due to conformational changes that altered protein mobility, whereas the apparent molecular masses of tcore-tCD154 bands were consistent with dimers (92 kDa) and trimers (138 kDa).
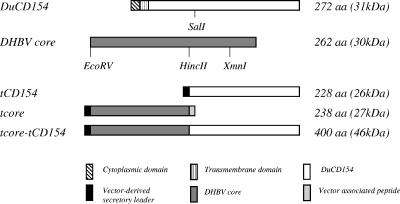
FIG. 2.
Design of plasmid constructs and expressed proteins. A 595-bp SalI/EcoRI fragment of DuCD154 was inserted into pSecTag2C (ptCD154) or was fused with a 512-bp EcoRV/HincII fragment of the DHBV core in pSecTag2C (ptcore-tCD154). The EcoRV-HincII fragment alone was also ligated into pSecTag2C (ptcore). aa, amino acids.
FIG. 3.
Expression of secreted tcore, tcore-tCD154, and tCD154 in LMH cells. LMH cells were transiently transfected with pSecTag2C, ptcore, ptcore-tCD154, or ptCD154. Proteins in supernatants were concentrated (ptcore 16×) or left unconcentrated, separated by SDS-PAGE under reducing or nonreducing conditions, and then immunoblotted with anti-core MAb 9G8 (A) or anti-CD154 Abs (B). Plasmids used for transfection are indicated above the corresponding lane. Molecular weight markers (in thousands) are indicated on the right and left (A) or on the left (B).
Supernatants of ptCD154- and ptcore-tCD154-transfected LMH cells separated under reducing conditions and then probed with polyclonal mouse anti-DuCD154 serum contained proteins consistent with the predicted molecular mass of 26 kDa for tCD154 (Fig. 3B, reducing, ptCD154) and 46 kDa for tcore-tCD154 (ptcore-tCD154). A minor protein band of approximately 26 kDa was present in the supernatants of ptcore-tCD154 transfectants and might represent a proteolytic cleavage product of the fusion protein. In addition, a minor protein of 32 kDa was detected in supernatants of ptCD154 transfectants. The latter species might be a glycosylated form of DuCD154. Under nonreducing conditions, a very faint 46-kDa band of monomeric tcore-tCD154 was apparent, but the sizes of the predominant bands were consistent with multimeric forms of tcore-CD154 (Fig. 3B, nonreducing, ptcore-tCD154). Supernatants from ptCD154 transfectants contained a predominant anti-DuCD154-reactive band of 52 kDa consistent with dimeric tCD154. Monomeric tCD154 was detected as a very faint 27-kDa band, but trimeric tCD154 was not detected. These experiments indicated that all expressed proteins were secreted and that CD154 proteins formed multimers. Furthermore, the observed high-molecular-mass structures were absent under reducing conditions, consistent with monomers associating through intermolecular disulfide bonds.
Early DHBV core-specific antibody responses in individual ducks.
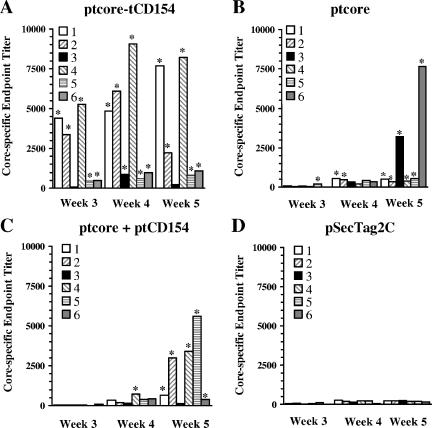
Core-specific Ab titers from individual ducks 3, 4, and 5 weeks following primary immunization are shown in Fig. 4. Ducks immunized with control plasmids had a low level of Ab reactivity to the affinity-purified core throughout the course of the vaccination study (Fig. 4D and 5). Therefore, a seropositivity cutoff value was calculated using the mean + 3 standard deviations of the vector control group at each time point. Ducks with endpoint titers greater than this value for the corresponding time point were considered seropositive. In the group immunized with ptcore-tCD154, 5/6 ducks were seropositive 3 weeks after primary immunization (Fig. 4A). In contrast, only one of the ducks vaccinated with ptcore was seropositive (Fig. 4B), and none of the ducks immunized with ptcore plus tCD154 were seropositive (Fig. 4C). Four weeks following primary immunization, all 6 ducks vaccinated with ptcore-tCD154 were seropositive; however, only 2/6 ducks in the ptcore group and only one duck in the ptcore plus ptCD154 group had seroconverted. Five weeks following the primary immunization, 5/6 ducks immunized with ptcore-tCD154 were seropositive. One previously slightly positive duck at week 4 had an Ab titer that had declined below the seropositivity cutoff at week 5. At this time point, all 6 ducks immunized with ptcore were seropositive and 5/6 ducks vaccinated with ptcore plus ptCD154 were seropositive. At week 6, all ducks in the ptcore-tCD154 and ptcore groups were seropositive and remained seropositive for the duration of the experiment (data not shown). In the group immunized with ptcore plus ptCD154, all ducks had seroconverted by week 15 and remained seropositive for the duration of the experiment.
FIG. 4.
Early DHBV core-specific antibody responses of individual ducks. Ducks were immunized with DNA plasmid ptcore-tCD154 (A), ptcore (B), ptcore plus ptCD154 (C), or pSecTag2C (D). Core-specific endpoint titers were determined by ELISA. Each bar represents an individual duck. Seropositive animals are indicated by an asterisk.
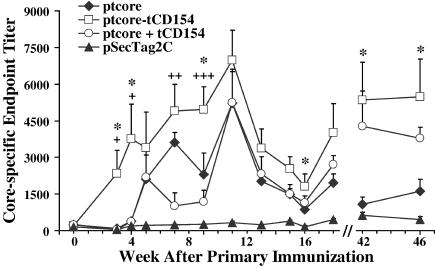
FIG. 5.
Comparison of mean antibody titers of immunized groups. Mean endpoint titers ± standard errors are indicated for ducks immunized with ptcore-tCD154 (□), ptcore (⧫), ptcore plus ptCD154 (○), or pSecTag2C (▴). Statistical comparisons at the indicated time points were between ptcore-tCD154- and ptcore-immunized groups (*) or between ptcore-tCD154- and ptcore plus ptCD154-immunized groups (+). * and +, P ≤ 0.05; ++, P ≤ 0.01; +++, P ≤ 0.005.
Mean antibody titers of immunized groups.
Consistent with the rapid seroconversion of ducks immunized with ptcore-tCD154, the mean endpoint titer was significantly higher than for the vector control group by week 3 (P = 0.017; P values comparing test groups with the vector control are cited in the text only). At all subsequent time points up to week 46, ducks in the ptcore-tCD154 group had mean endpoint titers significantly higher than those of the vector control group (P values ranged between 0.0003 and 0.032). In contrast, mean serum titers of ptcore- and ptcore plus ptCD154-immunized groups were not significantly different than the vector control group until week 4 (P = 0.004 and P = 0.037, respectively). By week 6, mean titers for the ptcore group remained significantly higher than those for the vector control group for the duration of the experiment (P values ranged between 3.9 × 10−6 and 0.05). In the group immunized with ptcore plus ptCD154, mean Ab titers fluctuated until week 9 and then remained significantly higher than the vector control group for the duration of the experiment (P values ranged between 0.0004 and 0.044).
Ducks immunized with ptcore-tCD154 had a more rapid and vigorous Ab response throughout the course of the experiment than the groups immunized with ptcore or ptcore plus ptCD154 (Fig. 5). Mean Ab titers of the ptcore-tCD154 group were significantly greater than titers of the ptcore group as early as 3 weeks following primary immunization. Mean Ab titers of the ptcore-tCD154 group remained significantly higher than in the ptcore-immunized ducks to weeks 42 and 46 (termination of experiment), several months after the final boost at week 16. Hence, ptcore-tCD154 generated an anti-core Ab response that was faster, more vigorous, and better maintained than that induced by ptcore. Comparison of the mean Ab titers of the ptcore-tCD154 group with the titers of the ptcore plus ptCD154-immunized ducks indicated that the fusion protein mounted a significantly more robust core-specific Ab response by week 3. The Ab response stimulated by ptcore-tCD154 remained higher than the response stimulated by ptcore plus ptCD154, and these differences were significant for several time points between weeks 3 and 10. Similar to the ptcore-tCD154 group, Ab titers for the ptcore plus ptCD154-immunized group were significantly higher than in ptcore-immunized animals at weeks 42 and 46 (P = 0.026 and 0.012, respectively), indicating that DuCD154 alone or as a fusion protein promoted the longevity of the Ab response.
DHBV core-specific proliferative responses of PBMC in immunized ducks.
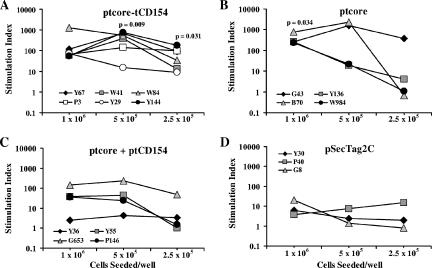
Core-specific cellular immune responses were evaluated by quantifying PBMC proliferative responses to DHBV core antigen in vitro. The SI of each duck at three different cell seeding concentrations is shown in Fig. 6. PBMCs from ducks immunized with ptcore-tCD154 had significantly greater proliferation at lower seeding densities than the vector control group (Fig. 6A and D), whereas ptcore-immunized animals had significant proliferative responses only at the highest seeding concentration (Fig. 6B). Although stimulation indices of three of four ptcore plus ptCD154-immunized ducks appeared numerically greater than those of the vector control group, the differences were not statistically significant (Fig. 6C).
FIG. 6.
DHBV core-specific proliferative responses of PBMCs. Ducks were immunized with DNA plasmid ptcore-tCD154 (A), ptcore (B), ptcore plus ptCD154 (C), or pSecTag2C (D). PBMCs from vaccinated ducks were isolated and stimulated in vitro with DHBV core antigen. Each point represents the SI of an individual duck at the indicated seeding concentration. Statistical comparisons are between the test group and the pSecTag2C control group.
DISCUSSION
The immunostimulatory functions of CD154 (CD40L) have been exploited to enhance B- and T-cell responses in various vaccination strategies in mammalian species. We have recently cloned DuCD154 and demonstrated that the extracellular domain enhances proliferation of duck splenocytes (8). The objective of the current study was to determine if the functional domain DuCD154 can enhance DNA vaccine responses in ducks either via coexpression with viral antigens or as a fusion protein to target viral antigen to antigen-presenting cells (APCs). The plasmids generated for this immunization study expressed tCD154, tcore, and a secreted chimeric protein, tcore-tCD154. Supernatants from transfected LMH cells were analyzed by immunoblotting with Ab to DHBV core or DuCD154. Each of the three plasmid constructs produced secreted proteins of the expected size under reducing conditions. Under nonreducing conditions, dimeric tCD154 and dimeric plus trimeric tcore-tCD154 proteins were detected. This suggests that these proteins multimerize through the formation of intermolecular disulfide bonds. Dimeric and trimeric forms of soluble CD154 bind more efficiently to cell surface CD40 on APCs than does monomeric CD154 (12, 37). Thus, our data indicated that ptCD154 and ptcore-tCD154 encoded proteins that were likely able to bind to and activate duck APCs.
We hypothesized that tcore-tCD154 fusion proteins would direct DHBV core antigen to APCs via CD40 and activate APCs, thereby promoting induction of core-specific T- and B-cell responses more effectively. Immunization with a combination of ptcore plus ptCD154 would enhance immune responses compared to the expression of core alone because of the adjuvant effect of DuCD154. Chimeric proteins of tcore-tCD154 did in fact accelerate the Ab response by 2 weeks and generated titers that, by week 3, were 30- to 65-fold above those observed with tcore and tcore plus tCD154 proteins, respectively. Ab responses to tcore were similar in kinetics and titer to responses generated by a DNA vaccine encoding full-length DHBV core protein (46). The response to tcore-tCD154 remained elevated relative to the other groups throughout the experiment. In contrast, coexpressed CD154 only promoted a better sustained B-cell response than immunization with ptcore. Examination of core-specific proliferative responses of PBMCs several months after the final immunization indicated that both ptcore-tCD154 and ptcore stimulated strong T-cell responses in vitro. This suggested that core-specific T memory cells were better induced by immunization with ptcore-tCD154 and ptcore than with ptcore plus ptCD154.
The enhanced B-cell response compared to immunization with DHBV core alone might be due to engagement of secreted DuCD154 with CD40 on antigen-activated B cells and direct induction of B memory cells. Furthermore, soluble DuCD154-mediated activation of APCs might enhance T-cell priming, T-cell help to B cells, and induction of B memory cells. In mammalian systems, interaction of antigen-stimulated B cells via CD40 with CD154 on activated CD4+ T cells promotes B-cell activation, including upregulation of CD80/86 and major histocompatibility complex class II, B-cell differentiation, and sustained Ab secretion (11, 21). Reciprocal interaction of CD80/86 on activated B cells with CD28 on T cells promotes clonal expansion of primed T cells and cytokine secretion (13). We observed strong core-specific Ab responses in the ptcore plus ptCD154 group but relatively weak core-specific T-cell proliferative responses. Since Ab responses were well maintained by this group, soluble CD154 might act as a surrogate for conventional T-cell help and directly stimulate B-cell maturation, decreasing the requirement for induction of antigen-specific T cells and the subsequent generation of T memory cells (21). In addition, hepadnaviral core antigen has been shown to induce core-specific IgM secretion of B cells (5). Furthermore, the priming of T cells might have been inhibited in the presence of DuCD154-activated B cells. Inadequate induction of antigen-specific T-cell proliferative responses for ptcore plus ptCD154-immunized ducks might explain the considerable initial lag in the Ab response. The weak responses of PBMCs from ptcore plus ptCD154-vaccinated animals to in vitro antigen stimulation supports this possibility.
DNA vaccines offer many advantages over conventional vaccines but can be poorly immunogenic and inefficient at inducing long-lived memory (15). To improve DNA vaccine efficacy, coinjection of plasmids encoding antigens and CD154 has been reported previously (3, 4, 14, 31, 44, 47). Although some groups have shown that this strategy can enhance immune responses, we did not observe a significant improvement of Ab responses between ptcore and ptcore plus ptCD154-immunized ducks until several months after the final boost. This could be related to lower levels of expression of tcore than of tCD154. Activation of APC prior to antigen uptake might predominate when there is a paucity of antigen, leading to inefficient immune induction. Our observations are consistent with a recent report in which coinjection of plasmids that expressed multivalent trimerized CD154 with viral antigens in mice failed to enhance antigen-specific Ab responses and proliferation of splenocytes (45). Nevertheless, cytotoxic T-cell responses were markedly enhanced. Other groups reported that coinjection of antigen and CD154-expressing plasmids enhanced cellular immune responses (4, 44, 47). However, in these studies, cellular immune responses were exclusively assessed by CD8+ cytolytic responses or gamma interferon production. Our in vitro assay measured a proliferating population of PBMCs most likely comprised of CD4+ cells, since antigen was added exogenously. At this time, we cannot perform in vitro cytotoxicity assays with the duck model.
Our results indicate that CD154 fusion proteins improved DNA vaccine responses most efficiently. Other reports indicate that CD40 targeting using DNA plasmids that express proteins such as green fluorescent protein (GFP), viral antigens, and tumor antigens fused to CD154 improve B- and T-cell responses (26, 29, 30, 53). Similar to our results, GFP fused to CD154 dramatically enhanced early B-cell responses to GFP compared to coinjection with GFP plus CD154 plasmids, but long-term Ab responses and T-cell responses between the two groups were not compared (26). Ab responses of sheep immunized with bovine herpesvirus (BHV) glycoprotein fused to bovine CD154 were not significantly enhanced compared with animals immunized with BHV glycoprotein alone until 12 weeks after the primary immunization (29). However, serum IgA and nasal IgA titers in BHV-challenged cattle were earlier and significantly higher in cattle previously immunized with bovine CD154 fusion constructs (30). Together, these studies suggest that CD40-targeted antigen delivery is an effective strategy to improve the kinetics of immune induction and memory. Our work emphasizes that, similar to what occurs in mammalian systems, DuCD154 likely has an important immunoregulatory role that promotes the induction of adaptive immune responses in avian species.
The potential of this vaccination strategy to resolve an established chronic infection with DHBV can now be assessed in the duck model, which might lead to more effective approaches to treat chronic hepatitis B in humans. The observed positive effect on induction and maintenance of Ab responses with our vaccine might also be utilized to develop more effective DNA vaccines to protect against avian pathogens such as avian influenza, a disease of global importance from both a health and economic perspective.
Acknowledgments
We thank Gerald Lachance for expert assistance with the care and handling of the ducks and Babita Agrawal for critically reviewing the manuscript.
This work was supported by grants to K.S.G. from the National Sciences and Engineering Research Council (NSERC) of Canada, the Canadian Liver Foundation, and the University Hospital Foundation and by a grant to D.L.T. from the Canadian Institutes of Health Research (CIHR). S.L.G. was supported by a postdoctoral fellowship awarded by the Canadian Association for the Study of the Liver (CASL)/Biotec Ortho, and S.E.C. was supported by summer studentships awarded by the Canadian Liver Foundation.
REFERENCES
- 1.Beasley, R. P., L. Y. Hwang, C. C. Lin, and C. S. Chien. 1981. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22,707 men in Taiwan. Lancet ii:1129-1133. [DOI] [PubMed] [Google Scholar]
- 2.Bodmer, J. L., P. Schneider, and J. Tschopp. 2002. The molecular architecture of the TNF superfamily. Trends Biochem. Sci. 27:19-26. [DOI] [PubMed] [Google Scholar]
- 3.Boyer, J. D., T. M. Robinson, M. A. Kutzler, R. Parkinson, S. A. Calarota, M. K. Sidhu, K. Muthumani, M. Lewis, G. Pavlakis, B. Felber, and D. Weiner. 2005. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques J. Med. Primatol. 34:262-270. [DOI] [PubMed] [Google Scholar]
- 4.Burger, J. A., R. B. Mendoza, and T. J. Kipps. 2001. Plasmids encoding granulocyte-macrophage colony-stimulating factor and CD154 enhance the immune response to genetic vaccines. Vaccine 19:2181-2189. [DOI] [PubMed] [Google Scholar]
- 5.Cao, T., U. Lazdina, I. Desombere, P. Vanlandschoot, D. Milich, M. Sällberg, and G. Lerroux-Roels. 2001. Hepatitis B virus core antigen binds and activates naïve human B cells in vivo: studies with a human PBL-NOD/SCID mouse model. J. Virol. 75:6359-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castle, B. E., K. Kishimoto, C. Stearns, M. L. Brown, and M. R. Kehry. 1993. Regulation of expression of the ligand for CD40 on T helper lymphocytes. J. Immunol. 151:1777-1788. [PubMed] [Google Scholar]
- 7.Fanslow, W. C., S. Srinivasan, R. Paxton, M. G. Gibson, M. K. Spriggs, and R. J. Armitage. 1994. Structural characteristics of CD40 ligand that determine biological function. Semin. Immunol. 6:267-278. [DOI] [PubMed] [Google Scholar]
- 8.Fischer, K. P., S. L. Gares, D. Wang, D. L. Tyrrell, and K. S. Gutfreund. 6 June 2006, posting date. Identification and characterization of functional CD154 (CD40 ligand) in the Pekin duck. Dev. Comp. Immunol. [Online.] doi: 10.1016/j.dci.2006.05.001. [DOI] [PubMed]
- 9.Fischer, K. P., and D. L. Tyrrell. 1996. Generation of duck hepatitis B virus polymerase mutants through site-directed mutagenesis which demonstrate resistance to lamivudine [(−)-beta-l-2′, 3′-dideoxy-3′-thiacytidine] in vitro. Antimicrob. Agents Chemother. 40:1957-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster, W. K., D. S. Miller, P. L. Marion, R. J. Colonno, I. Kotlarski, and A. R. Jilbert. 2003. Entecavir therapy combined with DNA vaccination for persistent duck hepatitis B virus infection. Antimicrob. Agents Chemother. 47:2624-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garside, P., E. Ingulli, R. R. Merica, J. G. Johnson, R. J. Noelle, and M. K. Jenkins. 1998. Visualization of specific B and T lymphocyte interactions in the lymph node. Science 281:96-99. [DOI] [PubMed] [Google Scholar]
- 12.Graf, D., S. Muller, U. Korthauer, C. Van Kooten, C. Weise, and R. A. Kroczek. 1995. A soluble form of TRAP (CD40 ligand) is rapidly released after T cell activation. Eur. J. Immunol. 25:1749-1754. [DOI] [PubMed] [Google Scholar]
- 13.Grewal, I. S., and R. A. Flavell. 1996. A central role of CD40 ligand in the regulation of CD4+ T-cell responses. Immunol. Today 17:410-414. [DOI] [PubMed] [Google Scholar]
- 14.Gurunathan, S., K. R. Irvine, C. Y. Wu, J. I. Cohen, E. Thomas, C. Prussin, N. P. Restifo, and R. A. Seder. 1998. CD40 ligand/trimer DNA enhances both humoral and cellular immune responses and induces protective immunity to infectious and tumor challenge. J. Immunol. 161:4563-4571. [PMC free article] [PubMed] [Google Scholar]
- 15.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18:927-974. [DOI] [PubMed] [Google Scholar]
- 16.Harlow, E., and D. Lane. 1988. Monoclonal antibodies, p. 139-281. In E. Harlow and D. Lane (ed.), Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 17.Heathcote, J., J. McHutchison, S. Lee, M. Tong, K. Benner, G. Minuk, T. Wright, J. Fikes, B. Livingston, A. Sette, R. Chestnut, et al. 1999. A pilot study of the CY-1899 T-cell vaccine in subjects chronically infected with hepatitis B virus. Hepatology 30:531-536. [DOI] [PubMed] [Google Scholar]
- 18.Higgins, D. A., and C. S. Teoh. 1988. Duck lymphocytes. II. Culture conditions for optimum transformation response to phytohaemagglutinin. J. Immunol. Methods 106:135-145. [DOI] [PubMed] [Google Scholar]
- 19.Huang, H. I., P. Y. Wu, C. Y. Teo, M. N. Chen, Y. C. Chen, D. Silin, and M. H. Tao. 2004. Improved immunogenicity of a self tumor antigen by covalent linkage to CD40 ligand. Int. J. Cancer 108:696-703. [DOI] [PubMed] [Google Scholar]
- 20.Hulse-Post, D. J., K. M. Sturm-Ramirez, J. Humberd, P. Seiler, E. A. Govorkova, S. Krauss, C. Scholtissek, P. Puthavathana, C. Buranathai, T. D. Nguyen, H. T. Long, T. S. Naipospos, H. Chen, T. M. Ellis, Y. Guan, J. S. Peiris, and R. G. Webster. 2005. Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proc. Natl. Acad. Sci. USA 102:10682-10687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins, M. K. 1994. The ups and downs of T cell costimulation. Immunity 1:443-446. [DOI] [PubMed] [Google Scholar]
- 22.Kishida, N., Y. Sakoda, N. Isoda, K. Matsuda, M. Eto, Y. Sunaga, T. Umemura, and H. Kida. 2005. Pathogenicity of H5 influenza viruses for ducks. Arch. Virol. 150:1383-1392. [DOI] [PubMed] [Google Scholar]
- 23.Lee, B., W. X. Luo, S. Suzuki, M. J. Robins, and D. L. Tyrrell. 1989. In vitro and in vivo comparison of the abilities of purine and pyrimidine 2′,3′-dideoxynucleosides to inhibit duck hepadnavirus. Antimicrob. Agents Chemother. 33:336-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Guerhier, F., A. Thermet, S. Guerret, M. Chevallier, C. Jamard, C. S. Gibbs, C. Trepo, L. Cova, and F. Zoulim. 2003. Antiviral effect of adefovir in combination with a DNA vaccine in the duck hepatitis B virus infection model. J. Hepatol. 38:328-334. [DOI] [PubMed] [Google Scholar]
- 25.Li, K. S., Y. Guan, J. Wang, G. J. Smith, K. M. Xu, L. Duan, A. P. Rahardjo, P. Puthavathana, C. Buranathai, T. D. Nguyen, A. T. Estoepangestie, A. Chaisingh, P. Auewarakul, H. T. Long, N. T. Hanh, R. J. Webby, L. L. Poon, H. Chen, K. F. Shortridge, K. Y. Yuen, R. G. Webster, and J. S. Peiris. 2004. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 430:209-213. [DOI] [PubMed] [Google Scholar]
- 26.Li, W. 2005. Synergistic antibody induction by antigen-CD40 ligand fusion protein as improved immunogen. Immunology 115:215-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 28.Mancini-Bourgine, M., H. Fontaine, D. Scott-Algara, S. Pol, C. Brechot, and M. L. Michel. 2004. Induction or expansion of T-cell responses by a hepatitis B DNA vaccine administered to chronic HBV carriers. Hepatology 40:874-882. [DOI] [PubMed] [Google Scholar]
- 29.Manoj, S., P. J. Griebel, L. A. Babiuk, and S. van Drunen Littel-van Den Hurk. 2004. Modulation of immune responses to bovine herpesvirus-1 in cattle by immunization with a DNA vaccine encoding glycoprotein D as a fusion protein with bovine CD154. Immunology 112:328-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manoj, S., P. J. Griebel, L. A. Babiuk, and S. van Drunen Littel-van Den Hurk. 2003. Targeting with bovine CD154 enhances humoral immune responses induced by a DNA vaccine in sheep. J. Immunol. 170:989-996. [DOI] [PubMed] [Google Scholar]
- 31.Mendoza, R. B., M. J. Cantwell, and T. J. Kipps. 1997. Immunostimulatory effects of a plasmid expressing CD40 ligand (CD154) on gene immunization. J. Immunol. 159:5777-5781. [PubMed] [Google Scholar]
- 32.Mizukoshi, E., J. Sidney, B. Livingston, M. Ghany, J. H. Hoofnagle, A. Sette, and B. Rehermann. 2004. Cellular immune responses to the hepatitis B virus polymerase. J. Immunol. 173:5863-5871. [DOI] [PubMed] [Google Scholar]
- 33.Moodycliffe, A. M., V. Shreedhar, S. E. Ullrich, J. Walterscheid, C. Bucana, M. L. Kripke, and L. Flores-Romo. 2000. CD40-CD40 ligand interactions in vivo regulate migration of antigen-bearing dendritic cells from the skin to draining lymph nodes. J. Exp. Med. 191:2011-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrillo, R. P. 2005. Current treatment of chronic hepatitis B: benefits and limitations. Semin. Liver Dis. 25(Suppl. 1):20-28. [DOI] [PubMed] [Google Scholar]
- 35.Pol, S., B. Nalpas, F. Driss, M. L. Michel, P. Tiollais, J. Denis, and C. Brecho. 2001. Efficacy and limitations of a specific immunotherapy in chronic hepatitis B. J. Hepatol. 34:917-921. [DOI] [PubMed] [Google Scholar]
- 36.Pol, S., F. Driss, M. L. Michel, B. Nalpas, P. Berthelot, and C. Brechot. 1994. Specific vaccine therapy in chronic hepatitis B infection. Lancet 344:342. [DOI] [PubMed] [Google Scholar]
- 37.Pullen, S. S., M. E. Labadia, R. H. Ingraham, S. M. McWhirter, D. S. Everdeen, T. Alber, J. J. Crute, and M. R. Kehry. 1999. High-affinity interactions of tumor necrosis factor receptor-associated factors (TRAFs) and CD40 require TRAF trimerization and CD40 multimerization. Biochemistry 38:10168-10177. [DOI] [PubMed] [Google Scholar]
- 38.Quezada, S. A., L. Z. Jarvinen, E. F. Lind, and R. J. Noelle. 2004. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22:307-328. [DOI] [PubMed] [Google Scholar]
- 39.Rehermann, B., C. Ferrari, C. Pasquinelli, and F. V. Chisari. 1996. The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat. Med. 2:1104-1108. [DOI] [PubMed] [Google Scholar]
- 40.Rehermann, B., D. Lau, J. H. Hoofnagle, and F. V. Chisari. 1996. Cytotoxic T lymphocyte responsiveness after resolution of chronic hepatitis B virus infection. J. Clin. Investig. 97:1655-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rehermann, B., P. Fowler, J. Sidney, J. Person, A. Redeker, M. Brown, B. Moss, A. Sette, and F. V. Chisari. 1995. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J. Exp. Med. 181:1047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rollier, C., C. Sunyach, L. Barraud, N. Madani, C. Jamard, C. Trepo, and L. Cova. 1999. Protective and therapeutic effect of DNA-based immunization against hepadnavirus large envelope protein. Gastroenterology 116:658-665. [DOI] [PubMed] [Google Scholar]
- 43.Schultz, U., E. Grgacic, and M. Nassal. 2004. Duck hepatitis B virus: an invaluable model system for HBV infection. Adv. Virus Res. 63:1-70. [DOI] [PubMed] [Google Scholar]
- 44.Sin, J. I., J. J. Kim, D. Zhang, and D. B. Weiner. 2001. Modulation of cellular responses by plasmid CD40L: CD40L plasmid vectors enhance antigen-specific helper T cell type 1 CD4+ T cell-mediated protective immunity against herpes simplex virus type 2 in vivo. Hum. Gene Ther. 12:1091-1102. [DOI] [PubMed] [Google Scholar]
- 45.Stone, G. W., S. Barzee, V. Snarsky, K. Kee, C. A. Spina, X. F. Yu, and R. S. Kornbluth. 2006. Multimeric soluble CD40 ligand and GITR ligand as adjuvants for human immunodeficiency virus DNA vaccines. J. Virol. 80:1762-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thermet, A., C. Rollier, F. Zoulim, C. Trepo, and L. Cova. 2003. Progress in DNA vaccine for prophylaxis and therapy of hepatitis B. Vaccine 21:659-662. [DOI] [PubMed] [Google Scholar]
- 47.Tripp, R. A., L. Jones, L. J. Anderson, and M. P. Brown. 2000. CD40 ligand (CD154) enhances the Th1 and antibody responses to respiratory syncytial virus in the BALB/c mouse. J. Immunol. 164:5913-5921. [DOI] [PubMed] [Google Scholar]
- 48.Triyatni, M., A. R. Jilbert, M. Qiao, D. S. Miller, and C. J. Burrell. 1998. Protective efficacy of DNA vaccines against duck hepatitis B virus infection. J. Virol. 72:84-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tumpey, T. M., C. F. Basler, P. V. Aguilar, H. Zeng, A. Solorzano, D. E. Swayne, N. J. Cox, J. M. Katz, J. K. Taubenberger, P. Palese, and A. Garcia-Sastre. 2005. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 310:77-80. [DOI] [PubMed] [Google Scholar]
- 50.Vickery, K., Y. Cossart, X. Gu, and R. Dixon. 1997. Antigen-specific blastogenesis assays for duck hepatitis B virus using duck peripheral blood and splenic mononuclear cells. Vet. Immunol. Immunopathol. 59:349-358. [DOI] [PubMed] [Google Scholar]
- 51.Webster, R. G. 1997. Influenza virus: transmission between species and relevance to emergence of the next human pandemic. Arch. Virol. Suppl. 13:105-113. [DOI] [PubMed] [Google Scholar]
- 52.Webster, R. G., W. J. Bean, O. T. Gorman, T. M. Chambers, and Y. Kawaoka. 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56:152-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiang, R., F. J. Primus, J. M. Ruehlmann, A. G. Niethammer, S. Silletti, H. N. Lode, C. S. Dolman, S. D. Gillies, and R. A. Reisfeld. 2001. A dual-function DNA vaccine encoding carcinoembryonic antigen and CD40 ligand trimer induces T cell-mediated protective immunity against colon cancer in carcinoembryonic antigen-transgenic mice. J. Immunol. 167:4560-4565. [DOI] [PubMed] [Google Scholar]
- 54.Yim, H. J., and A. S. Lok. 2006. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology 43:S173-S181. [DOI] [PubMed] [Google Scholar]