Abstract
Mycoplasma genitalium causes nonchlamydial nongonococcal urethritis. M. genitalium was detected by PCR in 17 urethral swabs obtained from 99 men with and without urethritis (J. S. Jensen, R. Orsum, B. Dohn, S. Uldum, A. M. Worm, and K. Lind, Genitourin. Med. 69:265-269, 1993), and later, four M. genitalium strains were isolated (J. S. Jensen, H. T. Hansen, and K. Lind, J. Clin. Microbiol. 34:286-291, 1996). The objective of this study was to characterize immunogenic proteins of M. genitalium by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting by using a hyperimmune rabbit serum against M. genitalium G37, determine their identity by mass spectrometry, and develop an M. genitalium-specific enzyme-linked immunosorbent assay (ELISA) free from cross-reactivity with M. pneumoniae antibodies. Using recombinant fragments of the C-terminal part of MgPa (rMgPa), we developed a specific ELISA for detection of M. genitalium antibodies. This antigen did not bind M. pneumoniae antibodies. Using serum samples from the 99 men with and without urethritis, we found that 26 had immunoglobulin G (IgG) antibodies to M. genitalium. There was a strong statistically significant correlation between PCR and IgG antibodies to M. genitalium (odds ratio [OR], 5.9; 95% confidence interval [CI], 2.3 to 21.5; P = 0.002). Furthermore, men with recurrent urethritis were more likely to have antibodies to M. genitalium than were those without recurrent urethritis (OR, 4.0; 95% CI, 1.1 to 14.5; P = 0.0383) and they had significantly higher antibody titers. By use of the rMgPa ELISA, this study further substantiates the importance of M. genitalium as a cause of male urethritis.
Mycoplasma genitalium is a pathogen of the human urogenital tract. It was isolated for the first time in 1981 from two men with urethritis. Transmission electron microscopy revealed the small size of M. genitalium and a flask-shaped body with an electron-dense tip structure (29, 41). M. genitalium belongs to the class Mollicutes, comprising small bacteria without a cell wall. Mycoplasmas, including M. genitalium, are characterized by the requirement for complex growth media and utilization of UGA as a tryptophan codon. The genome of M. genitalium is small (580 kbp) and was sequenced in 1995 (8).
M. genitalium appears to be an important cause of nonchlamydial nongonococcal urethritis, with an average prevalence of more than 20% in men with this condition (12, 15, 18). M. genitalium is sexually transmitted (7, 20) and may cause genital tract diseases in women, such as cervicitis (7, 28), pelvic inflammatory disease (35), and endometritis (5). M. genitalium G37 has been shown to induce urethritis in chimpanzees, followed by a sustained antibody response (39). Experimentally infected monkeys developed moderate to severe salpingitis (30). In humans, a fourfold rise in antibody titers measured by microimmunofluorescence was observed for 29% of men with nongonococcal urethritis compared to 12% of men without urethritis (37). This suggests that the infection of the human male urethra by M. genitalium causes the stimulation of an antibody response. Furthermore, an association between antibodies to M. genitalium in women with tubal factor infertility has been demonstrated (4).
M. genitalium is closely related to Mycoplasma pneumoniae, the cause of “walking pneumonia,” and they share many features, like shape and cell and genome structures. The attachment protein MgPa of M. genitalium has been identified as a homologue to the P1 adhesin of M. pneumoniae (13). The electron-dense core of M. pneumoniae comprises a network of proteins that localize and maintain P1 at the tip during adhesion to host cells (21, 22). Like P1 of M. pneumoniae, MgPa is the immunodominant protein of M. genitalium (14, 27, 31).
In order to analyze human serum samples for antibodies to M. genitalium, different serological methods with different combinations of M. genitalium antigens have been attempted. However, serological cross-reactions to M. pneumoniae are reported to be a major problem in many of the tests that are based on whole-cell antigens of M. genitalium. Examples hereof are the enzyme-linked immunosorbent assay (ELISA) described by Jacobs et al. with whole M. genitalium cells as the antigen (14), the complement fixation test with chloroform-methanol-extracted M. genitalium cells, indirect immunofluorescence on M. genitalium microcolonies, and the indirect hemagglutination test using sonicated M. genitalium cells described by Lind (25) and Lind and Kristensen (26). The metabolism inhibition test was described to be less cross-reactive (25, 38), but it is also a more complicated method to use than the more modern serological methods. More specific serological tests, like the ELISA on Triton X-114-extracted lipid-associated membrane proteins (LAMPs) (42) and purified full-length MgPa (14), have been published in the nineties, but since then, the methods have not been evaluated in other published studies except for one recent study using the LAMP ELISA (1). Therefore, the need for an easy, specific, and sensitive serological assay is obvious.
In the present study, we analyzed Triton X-114 phase partitioning and migration of immunogenic and high-molecular-mass proteins of M. genitalium and M. pneumoniae by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. The identities of the proteins were determined by mass spectrometry. Using hyperimmune rabbit sera, we determined the major immunogens to be P1 and MgPa of M. pneumoniae and M. genitalium, respectively. Due to cross-reaction between the hyperimmune sera, we developed an M. genitalium ELISA in which the antigen was composed of two recombinant fragments of MgPa that were free from cross-reactivity with M. pneumoniae antibodies. Using serum samples from 99 men with and without urethritis that were attending a Danish venereal disease clinic (18), we compared the results of immunoblotting and the developed ELISA and found that 20% were positive by both immunoblotting and ELISA, while 6% were positive by either immunoblotting or ELISA.
MATERIALS AND METHODS
Organisms and cultivation.
M. pneumoniae FH (ATCC), M. genitalium G37 (ATCC), and four Danish M. genitalium strains (M2288, M2300, M2321, and M2341) (collectively denoted “M strains”) isolated from the male urethra (17) were used for this study. Evidence that the four Danish strains were different was shown by partial sequencing of the mgpB gene (16, 33). All mycoplasma strains were grown in 100 ml SP-4 medium (40) in 150-cm2 tissue culture flasks (TPP, Trasadingen, Switzerland) and incubated at 37°C. The mycoplasmas were harvested in the exponential growth phase, which was indicated by a color change of the medium from red to orange. This occurred after 48 h for M. pneumoniae and M. genitalium G37 and after 3 to 4 days for the M strains. The SP-4 medium was poured off, and the mycoplasmas attached to the bottom were scraped off in phosphate-buffered saline (PBS) (0.01 M sodium phosphate, 0.85% NaCl, pH 7.4) and pelleted by centrifugation at 15,000 × g for 45 min. M. genitalium strain M2288 was harvested directly from the medium because it did not attach to plastic. The cells were resuspended in 4 ml PBS and centrifuged in Eppendorf tubes at 20,000 × g for 20 min. The supernatant was discarded and the pellets were stored at −70°C.
Triton X-114 phase partitioning of proteins.
Harvested logarithmic-phase cultures of M. pneumoniae FH and M. genitalium G37 were subjected to Triton X-114 phase partitioning based on the method described by Bordier (3). The mycoplasma pellets were suspended in 350 μl Tris buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl) and the protein concentration was adjusted to 1 mg/ml. The sample was sonicated for 10 seconds at 20% amplitude by use of Ultrasonic Processor Vibra Cell VCX 600 W (Sonics & Materials, Newton, CT). Triton X-114 (Boehringer, Germany) was added to a final concentration of 1% and the solution was incubated for one hour at 0°C. Then, the sample was centrifuged at 20,000 × g at 4°C for 15 min to remove undissolved protein. A 300-μl sucrose solution (6% [vol/wt] sucrose, 0.06% Triton X-114 in Tris buffer) was carefully located at the bottom of the tube with the supernatant. The sample was incubated for 3 min at 37°C, and the solution went turbid. After centrifugation at 3,000 rpm at 30°C for 5 min, three phases appeared: an upper aqueous phase with hydrophilic proteins, sucrose in the middle separating the other two phases, and, below, a detergent phase with hydrophobic proteins. The aqueous phase was separated from the detergent phase and the two phases were rinsed by the addition of 450 μl Tris buffer to the detergent phase and 50 μl Tris buffer containing 1% Triton X-114 to the aqueous phase. This wash procedure was then repeated twice.
SDS-PAGE and silver staining.
To analyze the protein solutions by SDS-PAGE, the insoluble proteins were dissolved in 150 μl SDS sample buffer (62.5 mM Tris-HCl, pH 6.8, 10% [vol/vol] glycerol, 2.3% [wt/vol] SDS, 5% [vol/vol] β-mercaptoethanol, 0.05% [wt/vol] bromophenol blue). A 150-μl sample of total protein (1 mg/ml) was dissolved in 2× SDS buffer. The aqueous phase was made in a solution of 10% (vol/vol) glycerol, 2.3% (wt/vol) SDS, and 5% (vol/vol) β-mercaptoethanol, and the detergent phase was mixed with 150 μl Tris buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl) and 200 μl 2× SDS sample buffer.
All samples were boiled for 2 min before loading. The proteins were separated by SDS-PAGE using 7.5% SDS-polyacrylamide gels with a 5% stacking gel. Coomassie brilliant blue staining was performed as described by Birkelund and Andersen (2).
The silver staining procedure was based on that of Nesterenko et al. (32). The proteins in SDS-PAGE gels were fixed by adding a mixture of 50 ml 50% acetone, 3 ml 20% tetrachloroacetic acid, and 20 μl 37% formalin for 5 min. Then, the gel was washed with water for 5 min. Fifty milliliters of 50% acetone was added for 15 min and then replaced by 50 ml water and 80 μl 10% (wt/vol) sodium thiosulfite for 3 min. After a washing with water, a silver stain (50 ml water, 0.15 g silver nitrate, 125 μl 37% formalin) was added for 12 min. The gel was washed again. To visualize the proteins, a solution of 50 ml double-distilled water, 1 g sodium bicarbonate, and 20 μl 37% formalin was added. The reaction was stopped by adding 1% acetic acid. The gel was conserved first by a washing in 25% ethanol and 3% glycerol for 10 min and then by being stored in 50 ml 2% glycerol.
Preparation of proteins for mass spectrometry.
Immunogenic proteins separated by Triton X-114 and SDS-PAGE were excised from a Coomassie brilliant blue-stained gel. Protein bands of interest were stored at 4°C in an Eppendorf tube until further analysis. Prior to protein digestion, the gel pieces were washed twice for 15 min in 100 μl of acetonitrile-water (1:1) (both analyzed by high-performance liquid chromatography [Mallinckrodt Baker B.V., Deventer, The Netherlands]) and dried in a centrifugal vacuum concentrator. The dried gel pieces were rehydrated in 10 μl of 50 mM freshly prepared ammonium bicarbonate buffer containing 0.05 μg of sequencing-grade modified trypsin (Promega Corporation, Madison, WI). The buffer was added so as to completely submerge the gel pieces, and the digestion proceeded overnight at 37°C. Then, the supernatant containing the generated peptides was removed from the gel pieces and acidified by adding 5 μl of 10% trifluoroacetic acid in water. The obtained peptide mixtures were purified and concentrated on a small amount of added Poros 50 R2 beads (Boehringer Mannheim GmbH, Mannheim, Germany) and stored at −20°C or immediately used for matrix-associated laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS) analysis as described previously (9, 10).
MALDI-TOF MS peptide mass fingerprinting.
The mass spectrometric measurements were performed on a Bruker Reflex III MALDI-TOF MS instrument (Bruker Daltonik GmbH, Bremen, Germany) operating in reflectron mode (for details of operation, see reference 10). Purified peptides were deposited on a metal target together with a matrix substance (α-cyano-4-hydroxy cinnamic acid). The masses of each peptide were matched to theoretical trypsin digests according to a database using the program Mascot to identify proteins. As for peptide mass fingerprinting, peptide mass accuracies better than 100 ppm were considered in the database searches.
Patients.
The 99 patients included in this study were previously described by Jensen et al. (18). Briefly, serum and urethral swab specimens were obtained from men attending the Venereal Disease Clinic of Copenhagen. The swab specimens were tested for M. genitalium by PCR using two different mgpB loci as gene targets for amplification (18). The PCR results were used for comparison with the serological data of the present study. Furthermore, information about acute and previous urethritis, duration of urethritis, and the ages of the patients was collected and used for comparison with the laboratory analysis. For cross-reaction studies, serum (obtained in 1997) from a staff member who accidentally got infected in the laboratory with M. pneumoniae FH in 1984 was used. Primary atypical pneumonia was confirmed by X ray, and the patient seroconverted by immunoblotting to M. pneumoniae. The patient had never had signs of urethritis or any other sexually transmitted diseases.
Immunoblotting.
Proteins separated by SDS-PAGE were transferred to nitrocellulose (Schleicher & Schuell, Dassel, Germany) by electroblotting. The molecular weight standard Mark12 (Novex) was cut from the membrane, stained with amido black for 2 min, and destained with a solution of 30% ethanol and 7% acetic acid. The rest of the membrane was blocked with blocking buffer (20 mM Tris base, 500 mM NaCl, 3% gelatin) for 15 min at 37°C. After being washed in washing buffer (20 mM Tris base, 500 mM NaCl, 0.05% Tween 20), the membrane was incubated with primary antibodies for 1 h at 37°C. Human sera were diluted 1:200 while the rabbit sera were diluted 1:1,000 in antibody buffer (20 mM Tris base, 500 mM NaCl, 3% gelatin, 0.05% Tween 20). The secondary antibodies used were alkaline phosphatase-conjugated goat anti-human immunoglobulin G (IgG) (heavy plus light chains) or goat anti-rabbit IgG (heavy plus light chains) (Sigma, Saint Louis, Missouri), both diluted 1:30,000 and incubated with the membrane for another hour. The membrane was washed with washing buffer after each incubation. Finally, the membrane was developed for 10 min with p-nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate p-toluidium solution.
Patients' sera were tested for IgG antibodies by using whole cells of M. genitalium as the antigen in immunoblotting and were graded as absent, weak, medium, or strong for selected antigens.
Rabbit polyclonal antibodies were raised against whole cells of M. genitalium G37 [denoted Pab(G37)] as previously described (4). Pab against whole cells of M. pneumoniae FH [denoted Pab(FH)] was purchased from the WHO Mycoplasma Reference Center, Aarhus University, Denmark.
Monospecific polyclonal antibody against P1 of M. pneumoniae [denoted Pab(P1)] was made by immunizing a rabbit with P1 excised from a one-dimensional polyacrylamide gel. The rabbit was immunized subcutaneously with P1 (excised from 0.75 mg total protein) in Freund's incomplete adjuvant (day 0) and with P1 in sterile saline (day 14), boosted by intramuscular injections at days 30, 45, and 60 with P1 in saline, and terminally bled at day 67. IgG from the serum was purified using protein A and finally dialyzed against PBS (0.5 M NaCl at pH 7.2).
ELISA.
Maxisorp microtiter 96-well ELISA plates (Nunc, Roskilde, Denmark) were coated with 60 μl of antigen solution/well (4 μg/ml recombinant MgPa mixture [Medac, Germany], 0.1 M sodium carbonate, 0.02% sodium azide, pH 9.6) and incubated for 24 h at 4°C. The coating solution was discarded before the ELISA plates were blocked with 75 μl 15% fetal calf serum in PBS and incubated for 1 h at 37°C. The plates were washed with PBS containing 0.05% Tween 20 (washing buffer). Fifty microliters/well of serum sample (diluted 1:50 in sample diluent [Medac, Germany]) was added in duplicate and incubated for 1 h at 37°C. In the same way, 50 μl/well of secondary antibodies (horseradish peroxidase-conjugated goat anti-human IgG [Medac, Germany]) was added and incubated for another 1 h at 37°C. A cutoff value for the IgG ELISA was determined by comparing the optical density (OD) values with immunoblotting. For cross-reaction studies, primary rabbit sera were diluted 1:2,000, whereas the secondary horseradish peroxidase-conjugated goat anti-rabbit (Bio-Rad) antibodies were diluted 1:60,000.
Between each of the incubations, the wells were washed with washing buffer by using a single-plate washer (Columbus M16/2 Ch; Tecan, Mannedorf/Zurich, Switzerland). The reactions were visualized with 50 μl TMB substrate (Medac, Germany) per well and incubated for 30 min at 37°C. The development was stopped by adding 100 μl of 1 M HCl/well. The results were read immediately by photometric readings (Sunrise reader; Tecan, Mannedorf/Zurich, Switzerland). All OD values were measured at 450 nm with a reference wavelength of 620 nm.
M. pneumoniae IgG ELISA (Medac, Germany) and Chlamydia trachomatis IgG and IgA pELISA (Medac, Germany) were performed in accordance with the manufacturer's instructions.
Statistical methods.
Multiple logistic regression analysis with backward elimination was used to model M. genitalium IgG (ELISA) as a function of urethritis, previous urethritis, duration of symptoms, and age. Secondary objectives were to investigate the possible connection between M. genitalium IgG (ELISA) and M. genitalium PCR and M. genitalium IgG (immunoblotting). Firstly, Fischer's exact test was used to see if the two dichotomous variables were independent. Then, the levels of M. genitalium IgG (ELISA) were compared between the two subgroups, determined by M. genitalium PCR positivity. We also wanted to investigate the independence between M. genitalium IgG (ELISA) and M. pneumoniae IgG or C. trachomatis IgG (ELISA) both as continuous and dichotomous variables. Linear regression analysis was used on the continuous variables, and Fischer's exact test was used on the dichotomous variables. Finally, it was examined by t test whether those with present urethritis and who previously had urethritis had higher levels of M. genitalium IgG (ELISA) than patients who only had urethritis at the time or previously had urethritis.
RESULTS
Triton X-114 phase separation of mycoplasma proteins and identification by MALDI-TOF mass spectrometry.
Triton X-114 phase partitioning of M. genitalium was performed to examine the antigenic components of this organism and with the intention to make a crude antigen extract of the LAMPs for ELISA. M. pneumoniae was included as a control for the phase partitioning and to study cross-reacting antigens.
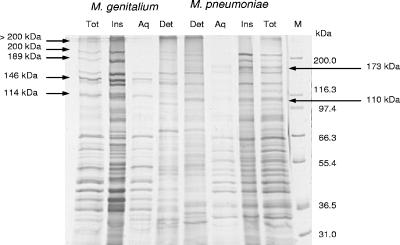
The Triton X-114 phase partitioning was performed on whole cells of M. genitalium and M. pneumoniae, and the protein contents of the resulting three phases (insoluble, aqueous, and detergent phases) were analyzed by SDS-PAGE and immunoblotting. Silver-stained proteins of both mycoplasma species appearing in the different phases were compared as shown in Fig. 1. In M. genitalium, these proteins were >200 kDa, 200 kDa, 189 kDa, 146 kDa, and 114 kDa in size. The >200-kDa, 200-kDa, and 189-kDa proteins were present in the insoluble phase, whereas the 146- and 114-kDa proteins were found in the aqueous phase. In M. pneumoniae, a 173-kDa protein and a 110-kDa protein were investigated. The 173-kDa protein was seen in the insoluble phase, while the 110-kDa protein was soluble in the detergent phase.
FIG. 1.
Silver staining/SDS-PAGE (7.5% acrylamide gel) of M. genitalium (first four lanes) and M. pneumoniae (second four lanes) proteins phase separated by Triton X-114. Lane M is the molecular size marker. Total (Tot) unfractionated protein extract from whole cells is shown. The proteins are separated into insoluble (Ins), aqueous (Aq), and detergent (Det) phases. The high-molecular-mass proteins that were further analyzed in this study are marked with arrows.
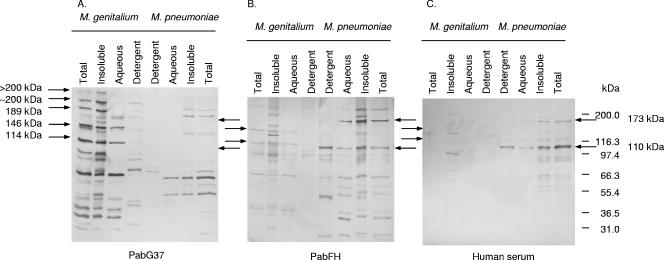
In order to identify the immunogenic proteins, rabbit polyclonal antibodies raised against whole cells of M. genitalium G37 [Pab(G37)] and M. pneumoniae FH [Pab(FH)] were used as the primary antibody in immunoblotting (Fig. 2). As could be identified by Pab(G37), the three most immunogenic proteins of M. genitalium, with molecular masses of 70, 114, and 146 kDa, partitioned in the aqueous and insoluble phases (Fig. 2). The detergent phase did not contain actual immunogenic proteins. In addition, the insoluble phase contained three intermediately immunogenic proteins with high molecular masses (189, 200, and >200 kDa) (Fig. 2A). Immunoblotting of M. pneumoniae using Pab(FH) also identified several immunogenic proteins (Fig. 2B). The two most immunogenic proteins of M. pneumoniae, a 110-kDa protein (P116) and a 173-kDa protein (P1), were, like those of M. genitalium, localized in the aqueous and insoluble phases. However, the majority of the 110-kDa protein (P116) appeared in the detergent phase (Fig. 2B).
FIG. 2.
Immunoblotting of M. genitalium and M. pneumoniae proteins phase separated by Triton X-114 (using a 7.5% acrylamide gel). (A) Rabbit polyclonal antibodies immunized with whole cells of M. genitalium [Pab(G37)] were reacted with M. genitalium and cross-reacted with M. pneumoniae. Note that Pab(G37) does not react with the major immunogenic proteins of M. pneumoniae, the 173- and 116-kDa proteins. (B) Rabbit polyclonal antibodies obtained after immunization with whole cells of M. pneumoniae [Pab(FH)] were reacted with M. pneumoniae and cross-reacted with M. genitalium. Note that Pab(FH) cross-reacts with the major immunogenic proteins of M. genitalium, the 146-, 114-, and 66-kDa proteins. The high-molecular-mass proteins that were further analyzed in this study are marked with arrows. (C) Serum from a healthy person known to have had an atypical pneumonia caused by M. pneumoniae, reacted with M. pneumoniae and cross-reacted with M. genitalium. Note that the human serum did not cross-react with M. genitalium major immunogenic proteins.
To identify the immunogenic proteins with high molecular masses, the proteins were excised from a Coomassie brilliant blue-stained gel and examined by mass spectrometry. The 114- and 146-kDa proteins of M. genitalium were cut from the lane containing the aqueous phase, whereas the less immunogenic proteins with molecular masses of 189, 200, and >200 kDa were cut from the lane with insoluble proteins. Proteins of 110 and 173 kDa of M. pneumoniae were cut from the aqueous and detergent phases, respectively. The excised proteins were digested by trypsin, and the resulting peptides were analyzed by MALDI-TOF mass spectrometry.
The identified proteins are summarized in Table 1. The 114-kDa protein of M. genitalium was identified as MG192 (mgpC gene product), which is the homologue protein of the cleaved ORF6 product of M. pneumoniae. The 146-kDa protein was identified as the adhesion protein MgPa (MG191 or mgpB). The high-molecular-mass proteins of M. genitalium with masses of 189, 200, and >200 kDa were identified as MG218, MG312, and MG386. These proteins are homologues of the cytoskeleton-like proteins P200, HMW1, and HMW2 of M. pneumoniae. The aqueous-phase protein of M. pneumoniae was identified as P1 and the detergent soluble protein as P116.
TABLE 1.
M. genitalium and M. pneumoniae proteins identified by MALDI-TOF MS
| Identified protein (homologue) | Calculated mol mass (kDa)a | Protein accession no.b | No. of identified peptides | % of identified protein |
|---|---|---|---|---|
| M. genitalium | ||||
| MgPa (ORF2) | 159.7 | AAC71410 | 24 | 21.47 |
| P114 (ORF3) | 114.3 | P22747 | 17 | 20.91 |
| MG218 (P200) | 185.7 | Q49429 | 19 | 19.59 |
| MG312 (HMW1) | 130.5 | Q49413 | 15 | 18.70 |
| MG386 (HMW2) | 219.3 | P47460 | 19 | 19.06 |
| M. pneumoniae | ||||
| P1 | 179.6 | A41480 | 34 | 33.33 |
| P116 | 116.1 | S73944 | 11 | 15.24 |
The molecular masses of the identified proteins were calculated by using the Compute pI/Mw tool (http://www.expasy.ch/tools/pi_tool.html).
Accession numbers were obtained from GenBank.
Pab(G37) was used in immunoblotting to analyze reactions with the proteins of whole cells of M. pneumoniae (Fig. 2A). Interestingly, PabG37 did not recognize the two most immunogenic proteins of M. pneumoniae, the 173-kDa protein (P1) and the 110-kDa protein (P116). Pab(G37) cross-reacted most intensively with proteins of 53 kDa and 68 kDa. In the same way, Pab(FH) was used to analyze for cross-reaction with proteins of whole cells of M. genitalium (Fig. 2B). In contrast to Pab(G37), Pab(FH) cross-reacted with the two major immunogenic proteins of M. genitalium, MgPa and P114, and also with 66-kDa and 36-kDa proteins.
In addition, a human serum sample obtained from a man who was accidentally infected during laboratory work with M. pneumoniae FH was reacted with the proteins of M. genitalium and M. pneumoniae (Fig. 2C). The human serum reacted strongly with two bands of M. pneumoniae, the 173-kDa and 110-kDa proteins, which supported the identification of P1 and P116 in the correct phases. Surprisingly, the human serum did not cross-react with the most immunogenic proteins of M. genitalium. Instead, two weak protein bands, 136 and 98 kDa, were seen. The two proteins of M. genitalium appeared only in the insoluble phase.
Comparison of M. genitalium strains.
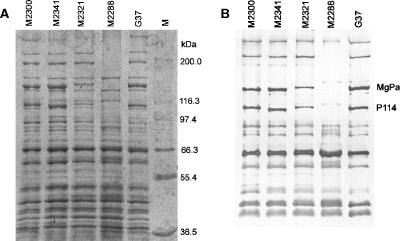
Protein profiles of the M strains were compared to that of type strain G37 by SDS-PAGE and immunoblotting (Fig. 3A and B). Pab(G37) was used as the primary antibody against all the strains in immunoblotting. Compared to G37, only minor variations in the protein profiles were observed for strains M2300, M2321, and M2341. The size of MgPa in type strain G37, M2300, and M2341 was calculated to be 144 kDa, but MgPa was slightly greater in M2321 (Fig. 3B). A major difference, however, was seen in strain M2288, in which MgPa, P114, and the protein homologues P200, HMW1, and HMW2 could not be detected. This was in agreement with the lack of hemadsorption of this strain.
FIG. 3.
Protein profiles of the four Danish M. genitalium M strains and of the type strain G37. (A) Coomassie brilliant blue-stained SDS-PAGE (7.5% acrylamide gel) of total protein extracts of M2300, M2341, M2321, M2288, and type strain G37. M represents the molecular size marker. (B) Immunoblotting of total protein extract of the M strains and G37. Polyclonal rabbit antibodies [Pab(G37)] were used as the primary antibodies.
Evaluation of patients' sera.
Because we failed to identify immunogenic proteins of M. genitalium in the Triton X-114 detergent phase, this fraction was not used for ELISA. The hydrophilic phase containing immunogenic proteins was not suitable for ELISA because of the presence of cross-reacting cytoplasmic proteins (Fig. 2). Instead, proteins of M. genitalium were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were cut in strips of 3 mm and reacted with serum samples from 99 males. Preimmune rabbit serum and Pab(G37) were used as negative and positive controls, respectively. Pab(G37) was tested in each assay and for each membrane. In addition, the 99 serum samples were tested by ELISA using a mixture of two recombinant MgPa fragments. We used the following three controls in the ELISA which were tested on each plate: sample diluents without primary antibodies (blank), with positive human serum, and with negative human serum. The OD value of the blank control was subtracted from all other OD values. All readings of immunoblots and ELISA were performed without knowledge of the patient status regarding PCR and clinical findings.
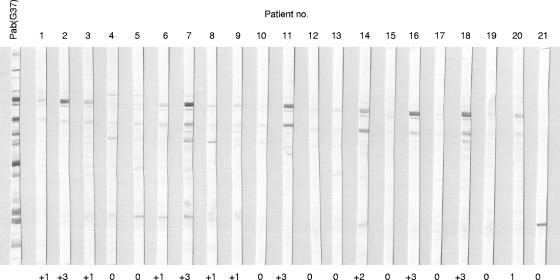
Examples of immunoblotting and gradings are shown in Fig. 4. The human serum IgG reacted mainly with MgPa and P114 like the rabbit serum Pab(G37). The preimmune rabbit serum did not react with any proteins of M. genitalium (data not shown). Negative reactions with MgPa were graded as 0 (absent reaction) or 1+ (weak), and positive reactions were graded as 2+ (medium) or 3+ (strong). In general, the reactions to MgPa were stronger than those to P114. Therefore, the seropositivity of the patient sera to M. genitalium was determined alone on reactions with this protein as done previously (4). Of the 99 men, 26 had a medium or strong IgG response against MgPa of M. genitalium in immunoblotting.
FIG. 4.
Immunoblotting of whole-cell protein extract of M. genitalium using a 7.5% acrylamide gel. The first lane on the left shows immunogenic proteins using rabbit polyclonal antibodies immunized with whole cells of M. genitalium [Pab(G37)]. Strips labeled 1 to 21 are examples of patient's serum reacted with M. genitalium proteins. The most immunogenic proteins are MgPa, P114, and less frequently, a 30-kDa protein.
The four Danish M. genitalium strains were previously isolated from patients no. 61, 71, 76, and 86 (17). Sera from these patients were analyzed in immunoblotting for reactions with their own M. genitalium strains. Patient no. 61, from whom strain M2288 was isolated, reacted with MgPa of type strain G37, but since M2288 lacked MgPa, no reaction could be observed. Patient no. 71, from whom strain M2300 was isolated, did not react with MgPa of G37 or of M2300. Patient no. 76, from whom M2321 was isolated, reacted with both MgPa of G37 and M2321. Lastly, patient no. 86, from whom M2341 was obtained, showed only weak reactions with MgPa of G37 and M2341. Thus, in regards to MgPa, there was an absolute agreement between immunoblotting with type strain G37 and the patient's own isolate, indicating that MgPa of M. genitalium G37 can be used as a target for serological testing.
rMgPa ELISA.
Two recombinant proteins of the C-terminal part of MgPa (rMgPa) were produced and purified as recombinant proteins, mixed, and used as antigens in the ELISA. A concentration of rMgPa of 4 μg/μl gave a satisfying dynamic range. The OD values of blank and negative controls were always less than 0.100. Between the ELISA plates, OD values of the positive control ranged from 1.3 to 1.6. The OD measurements of the rMgPa ELISA were compared to the immunoblotting results (Fig. 5). Of the negative immunoblot reactions evaluated as 0 or 1+, 92% had an OD value below 0.4. Therefore, this cutoff value was chosen to discriminate positive and negative samples. In total, 26 samples were positive by ELISA, of which 20 (77%) were positive for MgPa in immunoblotting. Of the 99 patients, 20% were positive for MgPa in both immunoblot and ELISA, 6% were positive in immunoblotting but negative in the ELISA and 6% were positive by ELISA but negative by immunoblotting. By comparing the two serological tests, a sensitivity of 77% and a specificity of 92% were achieved.
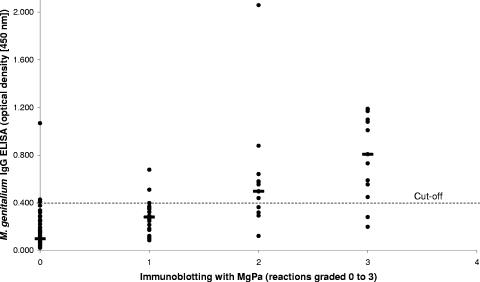
FIG. 5.
Chart showing a comparison of immunoblotting (reactions graded 0 to 3) and ELISA (OD values) results for the patient sera. Of the serum samples with no reaction (0) or a weak reaction (+1) to MgPa in immunoblotting, 92% showed an OD value less than 0.4. Therefore, a cutoff value of 0.4 was used to discriminate positive and negative serum samples in the ELISA (dotted line at 0.4). The median OD for each immunoblotting grade is marked by a horizontal line.
Evaluation of cross-reactivity to M. pneumoniae.
Cross-reactions between MgPa and P1 of M. pneumoniae were analyzed by testing the M. pneumoniae Pab(FH) and Pab(P1) sera against whole cells of M. genitalium in immunoblotting and against rMgPa in the ELISA. Immunoblotting with Pab(FH) and Pab(P1) against M. pneumoniae is shown in Fig. 6, where Pab(P1) was also reacted with M. genitalium whole cells. Pab(P1) reacted strongly with P1 of M. pneumoniae and did not react with MgPa of M. genitalium. As shown in Fig. 2A, Pab(FH) cross-reacted slightly with full-length MgPa in immunoblotting. However, testing rabbit Pab(FH) and Pab(P1) in rMgPa ELISA resulted in signals far below the cutoff, with OD values of 0.130 and 0.028, whereas the positive rabbit control [Pab(G37)] (diluted 1:2,000) showed an OD value of 1.372. This was consistent with previous findings (4), in which it was shown that Pab(FH) did not react with the C-terminal part of MgPa. In addition, the serum sample from an M. pneumoniae-infected person was analyzed in the M. genitalium ELISA in a 1:50 dilution and showed an OD value of 0.038. Thus, there appeared to be no cross-reaction with the M. genitalium rMgPa antigen using rabbit or one human serum against M. pneumoniae.
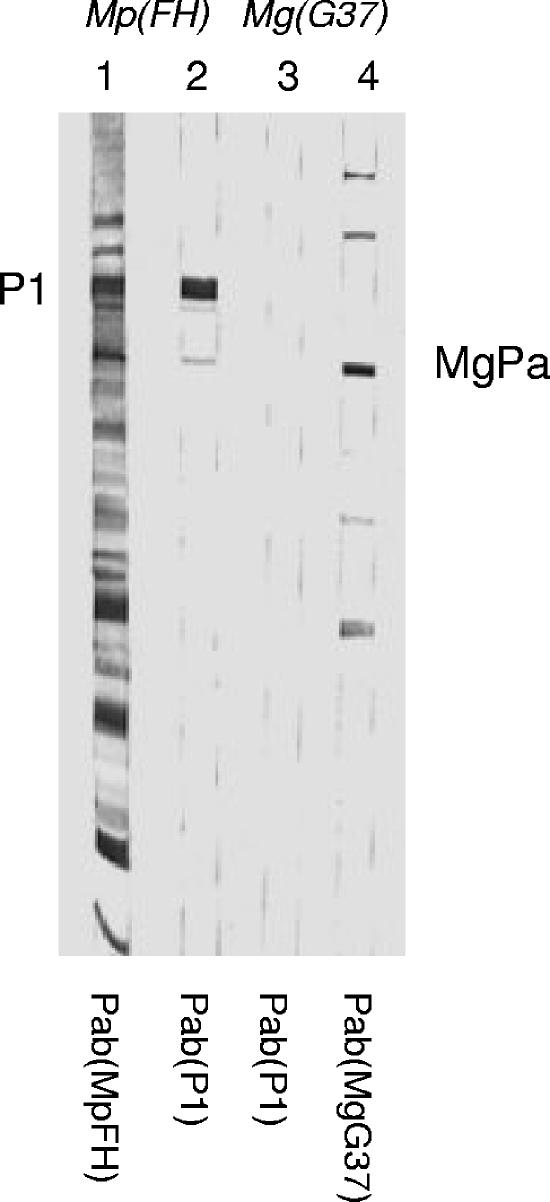
FIG. 6.
Immunoblot of M. pneumoniae FH [Mp(FH); strips 1 and 2] and M. genitalium G37 [Mg(G37); strips 3 and 4] using Pab(FH) (strip 1), Pab(P1) (strips 2 and 3), and Pab(G37) (strip 4). There was no cross-reaction of Pab(P1) to any of the M. genitalium proteins.
M. pneumoniae ELISA and comparison with rMgPa ELISA.
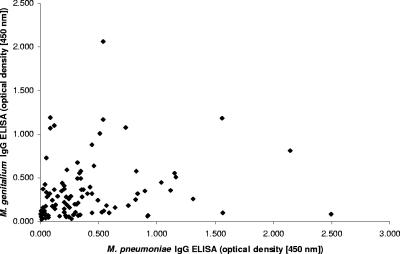
To further study the potential cross-reaction with M. pneumoniae all human sera were analyzed in an M. pneumoniae commercial ELISA (Medac, Germany) containing a recombinant antigen mixture. Of the 99 patient samples, 42 (42%) were IgG seropositive for M. pneumoniae. Of the M. genitalium-seropositive men, 65% (17/26) were also seropositive for M. pneumoniae, and of the M. pneumoniae-seropositive men, 60% (25/42) were seropositive for M. genitalium. The linear regression analysis of the rMgPa ELISA and the M. pneumoniae ELISA showed a significant correlation with a P value of 0.049 but a poor correlation coefficient (R2), only 0.039. The dichotomous M. genitalium and M. pneumoniae ELISA variables were also significantly correlated with a P value of 0.010 (Fisher's exact test). A scatter plot of the M. genitalium rMgPa ELISA results against the M. pneumoniae ELISA results is shown in Fig. 7.
FIG. 7.
Scatter plot of rMgPa ELISA results versus M. pneumoniae IgG ELISA results. There was a statistically significant but poor correlation between the M. genitalium IgG antibodies and M. pneumoniae IgG antibodies.
Comparison of serological data with PCR data.
Table 2 lists the 17 men who tested positive for M. genitalium by PCR and shows the serological results obtained by immunoblotting and ELISA. Among the PCR-positive men, 13 were seropositive for MgPa in immunoblotting, which resulted in a sensitivity of 77% and a specificity of 84%. Fewer PCR-positive men (n = 10) were seropositive by ELISA, and this resulted in slightly lower sensitivity and specificity, 59% and 81%.
TABLE 2.
Antibody response against M. genitalium and C. trachomatis in M. genitalium PCR-positive patientsa
| Patient no. | Age (yr) | Presence ofb:
|
Duration of AU (days) | M. genitalium isolate | IMc (MgPa) | M. genitalium IgG OD |
C. trachomatis OD
|
||
|---|---|---|---|---|---|---|---|---|---|
| PU | AU | IgG | IgA | ||||||
| 24811 | 31 | + | + | 2 | 3 | 0.808 | 0.210 | 0.091 | |
| 24853 | 27 | + | + | 2 | 2 | 0.439 | 0.936 | 0.198 | |
| 24888 | 30 | + | + | 3 | 2341 | 1 | 0.084 | 1.334 | 0.429 |
| 24898 | 37 | + | + | 3 | 3 | 1.188 | 0.188 | 0.743 | |
| 24857 | 28 | − | + | 4 | 2282 | 2 | 0.494 | 0.140 | 0.324 |
| 24873 | 21 | + | + | 4 | 2300 | 0 | 0.158 | 0.144 | 0.036 |
| 24891 | 39 | − | + | 4 | 2 | 0.362 | 0.480 | 0.102 | |
| 24824 | 26 | + | + | 5 | 3 | 0.280 | 0.068 | 0.085 | |
| 24892 | 31 | + | + | 7 | 3 | 0.447 | 0.196 | 0.220 | |
| 24871d | 23 | + | + | 10 | 3 | 1.183 | 0.582 | 0.035 | |
| 24876 | 29 | + | + | 14 | 0 | 0.282 | 0.741 | 0.072 | |
| 24878 | 47 | + | + | 30 | 2321 | 3 | 1.098 | 1.478 | 0.255 |
| 24863 | 25 | − | + | 60 | 2288 | 2 | 0.496 | 0.054 | 0.038 |
| 24819 | 23 | + | − | 1 | 0.360 | 0.304 | 0.263 | ||
| 24833 | 24 | + | − | 3 | 1.078 | 0.073 | 0.061 | ||
| 24840 | 35 | − | − | 3 | 0.198 | 0.432 | 0.022 | ||
| 24884 | 30 | − | − | 2 | 0.640 | 0.126 | 0.084 | ||
The data are sorted by acute urethritis and duration of symptoms.
PU, previous urethritis; AU, acute urethritis; +, present; −, absent.
IM, immunoblotting score.
C. trachomatis culture positive.
Moreover, the M. genitalium PCR positivity was evaluated against the continuous ELISA data by using logistic regression. The PCR correlated significantly with ELISA with an odds ratio (OR) of 5.9 (95% confidence interval [CI] of 2.3 to 21.5; P = 0.0017). The mean OD of PCR-positive men was 0.564, as opposed to 0.282 for PCR-negative men. The difference of the mean OD values was statistically significant with a P value of 0.002 and a t value of 3.3 (t test). Thus, there was a very good correlation between PCR and the rMgPa ELISA.
Comparison of serological, PCR, and clinical data.
The M. genitalium-seropositive men as determined by immunoblotting or ELISA were studied in relation to PCR and clinical data. Table 3 compares the two serological assays and PCR for patients with acute urethritis. Of the 52 men with urethritis, 15 (29%) were seropositive for MgPa in immunoblotting, compared to 11 (23%) men without urethritis (P = 0.649) (Table 3). Correspondingly, 13 (25%) men with urethritis were seropositive by rMgPa ELISA, compared to 13 (28%) men without urethritis (P = 0.821). Thus, both serological methods failed to identify men with acute urethritis. Contrarily, the PCR for M. genitalium identified 13 (25%) men with acute urethritis and only 4 (9%) men without urethritis (18).
TABLE 3.
Prevalence of positive test results in serological M. genitalium assays and in PCR among men with or without acute or recurrent urethritis
| Test | Acute urethritis
|
Recurrent urethritis
|
||||
|---|---|---|---|---|---|---|
| % of men positive | % of men negative | P value | % of men positive | % of men negative | P value | |
| Immunoblotting (MgPa) | 29 | 23 | 0.649 | 37 | 22 | 0.199 |
| ELISA (IgG) | 25 | 28 | 0.821 | 37 | 22 | 0.199 |
| PCR | 25 | 9 | 0.035 | 33 | 11 | 0.015 |
Previous episodes of urethritis were recorded for 50 men. The two serological tests and PCR were compared for patients with recurrent urethritis (Table 3). The prevalence rate markedly increased when men with recurrent urethritis were considered. The prevalence rate for men with recurrent urethritis was equally high (37%) by immunoblotting and ELISA, but the difference between the seropositive men with recurrent urethritis and men without recurrent urethritis did not reach statistical significance.
The seropositivity for M. genitalium measured by ELISA was analyzed by multiple logistic regression. There was no correlation to acute urethritis, duration of symptoms, or age, and the variables were stepwise removed from the model. In contrast, men with previous urethritis had a 2.3-times-higher risk of being seropositive than men without a history of urethritis, although this was not significant (CI = 0.9 to 5.8; P = 0.081).
Reactivity to MgPa in both immunoblotting and ELISA indicated that men with recurrent urethritis had the most antibodies. Therefore, we tested for a correlation between acute urethritis and previous urethritis by rMgPa IgG ELISA using logistic regression on continuous ELISA data. Men with recurrent urethritis had a four-times-higher risk of having antibodies to M. genitalium (OR = 4.0; CI = 1.1 to 14.5; P = 0.038). In contrast to the ELISA data, the PCR data correlated significantly with acute urethritis (OR = 3.5; CI = 1.1 to 11.9; P = 0.037). However, in agreement with the ELISA results, there was an even stronger association between PCR positivity for M. genitalium and recurrent urethritis (OR = 4.0; CI = 1.3 to 11.9; P = 0.012). The mean OD of men with recurrent urethritis was 0.454 compared with 0.285 of those without recurrent urethritis. The mean OD difference was statistically significant with a P value of 0.026 and a t value of 2.264 (t test).
Chlamydia trachomatis IgG and IgA ELISA and comparison with culture.
Chlamydia trachomatis is a frequent cause of urethritis, and therefore all serum samples were tested for antibodies to C. trachomatis with the pELISA (Medac) specific for C. trachomatis major outer membrane protein. Thirty-seven (31.9%) were C. trachomatis IgG positive. A total number of 14 men were IgA positive for C. trachomatis, of whom 7 were also IgG positive for C. trachomatis. As described in the previous study by Jensen et al. (18), 18 specimens were C. trachomatis culture positive. By comparing IgG ELISA with culture, a sensitivity of 39% (7/18) and a specificity of 63% (51/81) were found. Likewise, a comparison of IgA seropositivity and culture resulted in a sensitivity of 28% (5/18) and a specificity of 90% (72/81).
Twenty-two (42%) IgG-positive men had acute urethritis, and 15 (32%) were without urethritis. By using Fisher's exact test the difference did not reach statistical significance (P = 0.31). Seven (14%) IgA-positive men had acute urethritis, and 7 (15%) were without urethritis.
Eleven (41%) of the M. genitalium IgG-positive men were also C. trachomatis IgG positive. Of those men with urethritis, 5 (10%) were seropositive for both microorganisms and 8 (15%) were seropositive for M. genitalium only. There was no significant correlation between M. genitalium IgG and C. trachomatis IgG when tested by linear regression or Fisher's exact test.
DISCUSSION
Triton X-114 was used to separate the proteins according to hydrophobicity while maintaining the native and folded structure of the proteins. Triton X-114 has been used in several studies to characterize membrane proteins of M. pneumoniae. Most of the studies were concentrating on P1 and P116, which was one reason to include these proteins in the present study. Proteins of 180 kDa and 120 kDa of M. pneumoniae have been characterized as LAMP antigens, which are defined as lipid-associated membrane proteins soluble in the Triton X-114 detergent phase (42). These proteins were identified with human sera in immunoblotting. When the immunogenicity and the sizes of these proteins are considered, the 180-kDa protein is most likely P1 and the 120-kDa protein is P116. A similar study supported these findings (11). There seems to be some disagreement, however, about the solubility of P1. In agreement with our study, Proft and Herrmann (34) found P1 mainly in the aqueous phase, but P1 was also present in the insoluble phase. P1 was not in the detergent phase, as described in the other studies. The connection of P1 to the cytoskeleton could explain why P1 is detected in both phases. When Triton X-100 was used as a detergent, P1 remained in a Triton-insoluble shell of tip structure proteins (19).
Proteins from whole cells of M. genitalium were also separated by Triton X-114 in the study of Wang et al. (42). Immunogenic proteins of 150/160 kDa and 112 kDa, corresponding (by size) to MgPa and P114, respectively, were identified as LAMPs. This was opposite to our findings that MgPa and P114 were located in the hydrophilic Triton X-114 phase. Critical factors in the Triton X-114 partitioning are the concentration of protein and salts, the separation temperature, and the purity of the Triton X-114. Apparently, the studies mentioned above all performed the Triton X-114 partitioning according to the method of Bordier (3) as we did, but small deviations from the critical factors could be decisive for the result. P1 has been shown to be cross-linked to other adhesion and cytoskeleton-like proteins (24), and therefore, it is likely that cross-linking to other adhesion or Triton-shell proteins may influence the outcome. We identified P116, the positive control of our Triton X-114 phase separation, mainly in the detergent phase as described by Duffy et al. (6). Accordingly, we were not able to identify immunogenic proteins of M. genitalium in the Triton X-114 detergent phase.
Comparison of the protein profiles of the four isolated M. genitalium strains showed only minor variations. Nonetheless, it was interesting that one of the isolated strains, M2288, lacked MgPa, P114, and the HMW2 homologue and produced a reduced amount of the P200 and HMW1 homologues. These proteins are homologous to the M. pneumoniae adhesion and cytoskeleton-like proteins, and the deficiency of these proteins was consistent with the failure of M2288 to attach to the plastic bottom of the culture flasks and to hemadsorb. It is noteworthy that M2288 multiplied as fast in cell culture as the adhering strains. Strain M2288 is similar to the M. pneumoniae class IV mutant lacking P1 and the P40/P90 proteins (23). The class IV mutant of M. pneumoniae expressed all HMW proteins; however, the amount was reduced compared to the wild type. M. genitalium strain M2321 expressed a reduced amount of MgPa and P114 compared to the type strain, but it was not similar to any of the described mutants of M. pneumoniae. The observable fact that the sizes of MgPa and P114 of strain M2321 were increased has not been described for any of the M. pneumoniae mutants. The increase in molecular masses compared to the type strain indicates that the proteins were elongated.
We used whole cells of M. genitalium and recombinant MgPa as antigens in immunoblotting and ELISA. There was a good correlation between results obtained by immunoblotting and ELISA of M. genitalium, but the outcomes of the two tests were not identical. The samples positive by immunoblot but negative by ELISA could be explained by reactions to epitopes located outside the region of MgPa used for ELISA. M. pneumoniae cross-reactivity of these samples was unlikely because the M. pneumoniae results were either negative or weakly positive (OD < 0.5). The protein refolding conditions in the ELISA and immunoblotting are very dissimilar, and differences in antigen refolding may explain why some serum samples were ELISA positive and immunoblot negative.
Because of the high amino acid similarity, cross-reactions between MgPa and P1 are likely; however, we showed that there were no cross-reactions between MgPa and P1 by using rabbit antisera and one human antiserum. The risk of cross-reaction is probably greater in immunoblotting using whole cells of M. genitalium than in the ELISA using only a minor part of MgPa, yet the immunoblotting results with the patient samples did not show any pattern of cross-reaction. The lack of cross-reactivity between purified P1 and MgPa has also been reported by Jacobs et al. (14). Consistent with this, M. pneumoniae-infected mice failed to give protection against a genital infection with M. genitalium (36).
This study showed a close-to-significant correlation between previous urethritis and antibodies to M. genitalium. Additionally, men with recurrent urethritis had a significantly higher IgG response than men without urethritis or acute urethritis. The serological testing of a single serum sample as performed in this study, however, does not discriminate between a previous, recurrent, or acute M. genitalium infection. Thus, serological testing of follow-up serum samples is necessary to determine if M. genitalium is causing the acute urethritis. Demonstration of a titer increase in men with recurrent urethritis, however, might be impaired by a high antibody titer from a previous infection with M. genitalium.
In conclusion, this study showed that the rMgPa ELISA did not cross-react with antibodies against the P1 protein of M. pneumoniae, and therefore cross-reactions from more distantly related Mollicutes are highly unlikely. As a consequence, we considered the positive ELISA results for specific M. genitalium reactions. The serological studies showed that men with recurrent urethritis were more likely to have IgG antibodies to MgPa, which emphasizes the important role of M. genitalium in male urethritis.
Acknowledgments
Søren A. Uldum and Jan Søndergaard Andersen are thanked for kindly providing the rabbit monospecific polyclonal antibody against P1 for the cross-reaction studies. Likewise, we are grateful to Helle Hartvig for helping with the statistical analysis. We are also thankful to Karin Skovgaard Sørensen for the assistance with laboratory procedures and Lisbet Wellejus Pedersen for linguistic assistance with this article.
The ELISA equipment was funded by “Novo Nordisk Plus Prizes” (journal no. SUN-2002-653). K. Gevaert is a Postdoctoral Fellow of the Fund for Scientific Research-Flanders (Belgium) (F.W.O.-Vlaanderen). H. F. Svenstrup initiated the study, designed, optimized, and evaluated the rMgPa ELISA, performed the laboratory experiments and data management, and wrote the first draft of the manuscript. J. S. Jensen provided the clinical specimens with known M. genitalium PCR status and provided major contributions to the design of the study and analysis of the data. K. Gevaert performed the MALDI-TOF mass spectrometry analysis, including protein preparation and protein identification. G. Christiansen and S. Birkelund supervised the study, provided research facilities, and commented on the manuscript.
REFERENCES
- 1.Baseman, J. B., M. Cagle, J. E. Korte, C. Herrera, W. G. Rasmussen, J. G. Baseman, R. Shain, and J. M. Piper. 2004. Diagnostic assessment of Mycoplasma genitalium in culture-positive women. J. Clin. Microbiol. 42:203-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birkelund, S., and H. Andersen. 1988. Comparative studies of mycoplasma antigens and corresponding antibodies, p. 25-33. In O. J. Bjerrum and N. H. H. Heegaard (ed.), Handbook of immunoblotting of proteins, vol. II. CRC Press, Inc., Boca Raton, Fla. [Google Scholar]
- 3.Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256:1604-1607. [PubMed] [Google Scholar]
- 4.Clausen, H. F., J. Fedder, M. Drasbek, P. K. Nielsen, B. Toft, H. J. Ingerslev, S. Birkelund, and G. Christiansen. 2001. Serological investigation of Mycoplasma genitalium in infertile women. Hum. Reprod. 16:1866-1874. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, C. R., L. E. Manhart, E. A. Bukusi, S. Astete, R. C. Brunham, K. K. Holmes, S. K. Sinei, J. J. Bwayo, and P. A. Totten. 2002. Association between Mycoplasma genitalium and acute endometritis. Lancet 359:765-766. [DOI] [PubMed] [Google Scholar]
- 6.Duffy, M. F., I. D. Walker, and G. F. Browning. 1997. The immunoreactive 116 kDa surface protein of Mycoplasma pneumoniae is encoded in an operon. Microbiology 143:3391-3402. [DOI] [PubMed] [Google Scholar]
- 7.Falk, L., H. Fredlund, and J. S. Jensen. 2005. Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sex. Transm. Infect. 81:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser, C. M., J. D. Gocayne, O. White, M. D. Adams, R. A. Clayton, R. D. Fleischmann, C. J. Bult, A. R. Kerlavage, G. Sutton, J. M. Kelley, et al. 1995. The minimal gene complement of Mycoplasma genitalium. Science 270:397-403. [DOI] [PubMed] [Google Scholar]
- 9.Gevaert, K., H. Demol, M. Puype, D. Broekaert, S. De Boeck, T. Houthaeve, and J. Vandekerckhove. 1997. Peptides adsorbed on reverse-phase chromatographic beads as targets for femtomole sequencing by post-source decay matrix assisted laser desorption ionization-reflectron time of flight mass spectrometry (MALDI-RETOF-MS). Electrophoresis 18:2950-2960. [DOI] [PubMed] [Google Scholar]
- 10.Gevaert, K., H. Demol, T. Sklyarova, J. Vandekerckhove, and T. Houthaeve. 1998. A peptide concentration and purification method for protein characterization in the subpicomole range using matrix assisted laser desorption/ionization-postsource decay (MALDI-PSD) sequencing. Electrophoresis 19:909-917. [DOI] [PubMed] [Google Scholar]
- 11.Hirschberg, L., T. Holme, and N. Hyden. 1989. Demonstration of membrane association and surface location of Mycoplasma pneumoniae antigens using monoclonal antibodies. J. Gen. Microbiol. 135:613-621. [DOI] [PubMed] [Google Scholar]
- 12.Horner, P. J., C. B. Gilroy, B. J. Thomas, R. O. Naidoo, and D. Taylor-Robinson. 1993. Association of Mycoplasma genitalium with acute non-gonococcal urethritis. Lancet 342:582-585. [DOI] [PubMed] [Google Scholar]
- 13.Inamine, J. M., S. Loechel, A. M. Collier, M. F. Barile, and P. C. Hu. 1989. Nucleotide sequence of the MgPa (mgp) operon of Mycoplasma genitalium and comparison to the P1 (mpp) operon of Mycoplasma pneumoniae. Gene 82:259-267. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs, E., T. Watter, H. E. Schaefer, and W. Bredt. 1991. Comparison of host responses after intranasal infection of guinea-pigs with Mycoplasma genitalium or with Mycoplasma pneumoniae. Microb. Pathog. 10:221-229. [DOI] [PubMed] [Google Scholar]
- 15.Jensen, J. S. 2004. Mycoplasma genitalium—the etiological agent of urethritis and other sexually transmitted diseases. J. Eur. Acad. Dermatol. Venereol. 18:1-11. [DOI] [PubMed] [Google Scholar]
- 16.Jensen, J. S., E. Bjornelius, B. Dohn, and P. Lidbrink. 2004. Use of TaqMan 5′ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J. Clin. Microbiol. 42:683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen, J. S., H. T. Hansen, and K. Lind. 1996. Isolation of Mycoplasma genitalium strains from the male urethra. J. Clin. Microbiol. 34:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen, J. S., R. Orsum, B. Dohn, S. Uldum, A. M. Worm, and K. Lind. 1993. Mycoplasma genitalium: a cause of male urethritis? Genitourin. Med. 69:265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahane, I., S. Tucker, D. K. Leith, J. Morrison-Plummer, and J. B. Baseman. 1985. Detection of the major adhesin P1 in triton shells of virulent Mycoplasma pneumoniae. Infect. Immun. 50:944-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keane, F. E., B. J. Thomas, C. B. Gilroy, A. Renton, and D. Taylor-Robinson. 2000. The association of Chlamydia trachomatis and Mycoplasma genitalium with non-gonococcal urethritis: observations on heterosexual men and their female partners. Int. J. STD AIDS 11:435-439. [DOI] [PubMed] [Google Scholar]
- 21.Krause, D. C. 1998. Mycoplasma pneumoniae cytadherence: organization and assembly of the attachment organelle. Trends Microbiol. 6:15-18. [DOI] [PubMed] [Google Scholar]
- 22.Krause, D. C., and M. F. Balish. 2001. Structure, function, and assembly of the terminal organelle of Mycoplasma pneumoniae. FEMS Microbiol. Lett. 198:1-7. [DOI] [PubMed] [Google Scholar]
- 23.Krause, D. C., D. K. Leith, R. M. Wilson, and J. B. Baseman. 1982. Identification of Mycoplasma pneumoniae proteins associated with hemadsorption and virulence. Infect. Immun. 35:809-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Layh-Schmitt, G., A. Podtelejnikov, and M. Mann. 2000. Proteins complexed to the P1 adhesin of Mycoplasma pneumoniae. Microbiology 146:741-747. [DOI] [PubMed] [Google Scholar]
- 25.Lind, K. 1982. Serological cross-reactions between “Mycoplasma genitalium” and M. pneumoniae. Lancet ii:1158-1159. [DOI] [PubMed] [Google Scholar]
- 26.Lind, K., and G. B. Kristensen. 1987. Significance of antibodies to Mycoplasma genitalium in salpingitis. Eur. J. Clin. Microbiol. 6:205-207. [DOI] [PubMed] [Google Scholar]
- 27.Mader, B., P. C. Hu, C. H. Huang, E. Schilz, and E. Jacobs. 1991. The mature MgPa-adhesin of Mycoplasma genitalium. Zentbl. Bakteriol. 274:507-513. [DOI] [PubMed] [Google Scholar]
- 28.Manhart, L. E., C. W. Critchlow, K. K. Holmes, S. M. Dutro, D. A. Eschenbach, C. E. Stevens, and P. A. Totten. 2003. Mucopurulent cervicitis and Mycoplasma genitalium. J. Infect. Dis. 187:650-657. [DOI] [PubMed] [Google Scholar]
- 29.Mernaugh, G. R., S. F. Dallo, S. C. Holt, and J. B. Baseman. 1993. Properties of adhering and nonadhering populations of Mycoplasma genitalium. Clin. Infect. Dis. 17(Suppl. 1):S69-S78. [DOI] [PubMed] [Google Scholar]
- 30.Moller, B. R., D. Taylor-Robinson, P. M. Furr, and E. A. Freundt. 1985. Acute upper genital-tract disease in female monkeys provoked experimentally by Mycoplasma genitalium. Br. J. Exp. Pathol. 66:417-426. [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison-Plummer, J., A. Lazzell, and J. B. Baseman. 1987. Shared epitopes between Mycoplasma pneumoniae major adhesin protein P1 and a 140-kilodalton protein of Mycoplasma genitalium. Infect. Immun. 55:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nesterenko, M. V., M. Tilley, and S. J. Upton. 1994. A simple modification of Blum’s silver stain method allows for 30 minute detection of proteins in polyacrylamide gels. J. Biochem. Biophys. Methods 28:239-242. [DOI] [PubMed] [Google Scholar]
- 33.Peterson, S. N., C. C. Bailey, J. S. Jensen, M. B. Borre, E. S. King, K. F. Bott, and C. A. Hutchison. 1995. Characterization of repetitive DNA in the Mycoplasma genitalium genome: possible role in the generation of antigenic variation. Proc. Natl. Acad. Sci. USA 92:11829-11833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proft, T., and R. Herrmann. 1994. Identification and characterization of hitherto unknown Mycoplasma pneumoniae proteins. Mol. Microbiol. 13:337-348. [DOI] [PubMed] [Google Scholar]
- 35.Simms, I., K. Eastick, H. Mallinson, K. Thomas, R. Gokhale, P. Hay, A. Herring, and P. A. Rogers. 2003. Associations between Mycoplasma genitalium, Chlamydia trachomatis, and pelvic inflammatory disease. Sex. Transm. Infect. 79:154-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor-Robinson, D., and P. M. Furr. 2001. Failure of Mycoplasma pneumoniae infection to confer protection against Mycoplasma genitalium: observations from a mouse model. J. Med. Microbiol. 50:383-384. [DOI] [PubMed] [Google Scholar]
- 37.Taylor-Robinson, D., P. M. Furr, and N. F. Hanna. 1985. Microbiological and serological study of non-gonococcal urethritis with special reference to Mycoplasma genitalium. Genitourin. Med. 61:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor-Robinson, D., P. M. Furr, and J. G. Tully. 1983. Serological cross-reactions between Mycoplasma genitalium and M. pneumoniae. Lancet i:527. [DOI] [PubMed] [Google Scholar]
- 39.Taylor-Robinson, D., J. G. Tully, and M. F. Barile. 1985. Urethral infection in male chimpanzees produced experimentally by Mycoplasma genitalium. Br. J. Exp. Pathol. 66:95-101. [PMC free article] [PubMed] [Google Scholar]
- 40.Tully, J. G., D. L. Rose, R. F. Whitcomb, and R. P. Wenzel. 1979. Enhanced isolation of Mycoplasma pneumoniae from throat washings with a newly-modified culture medium 60. J. Infect. Dis. 139:478-482. [DOI] [PubMed] [Google Scholar]
- 41.Tully, J. G., D. Taylor-Robinson, D. L. Rose, R. M. Cole, and J. M. Bove. 1983. Mycoplasma genitalium, a new species from the human urogenital tract. Int. J. Syst. Bacteriol. 33:387-396. [Google Scholar]
- 42.Wang, R. Y., T. Grandinetti, J. W. Shih, S. H. Weiss, C. L. Haley, M. M. Hayes, and S. C. Lo. 1997. Mycoplasma genitalium infection and host antibody immune response in patients infected by HIV, patients attending STD clinics and in healthy blood donors. FEMS Immunol. Med. Microbiol. 19:237-245. [DOI] [PubMed] [Google Scholar]