Abstract
Recent efforts toward developing vaccines against group B streptococci (GBS) have focused on increasing the immunogenicity of GBS polysaccharides by conjugation to carrier proteins. However, partial depolymerization of GBS polysaccharides for the production of vaccines is a difficult task because of their acid-labile, antigenically critical sialic acids. Here we report a method for the partial depolymerization of type II and III polysaccharides by mild deaminative cleavage to antigenic fragments with reducing-terminal 2,5-anhydro-d-mannose residues. Through the free aldehydes of their newly formed end groups, the fragments were conjugated to tetanus toxoid by reductive amination. The resulting conjugates stimulated the production in animals of high-titer type II- and III-specific antibodies which induced opsonophagocytic killing of type II and III strains of group B streptococci. For the type II conjugates, immunogenicity increased as oligosaccharide size decreased, whereas for type III conjugates, the size of the oligosaccharides did not significantly influence immunogenicity. When oligosaccharides of defined size were conjugated through sialic acid residues, the resulting cross-linkages were shown to affect immunogenicity. When oligosaccharides were conjugated through terminal aldehyde groups generated by deamination, modification of the exocyclic chain of sialic acid did not influence immunogenicity.
Group B streptococci (GBS) are an important cause of neonatal sepsis and meningitis and of invasive infections in nonpregnant adults with underlying illnesses (7). Although antibodies directed to the capsular polysaccharide (CPS) antigens are protective, these antigens are variably immunogenic (6). The immunogenicity of GBS CPS antigens has been increased by covalent coupling to proteins to form CPS-protein conjugate vaccines (1-5, 18, 21, 23, 25, 29). Experimental GBS type III polysaccharide (GBSP-III)- and oligosaccharide-tetanus toxoid conjugate vaccines of different designs have been developed and their immunogenicity tested in animals (21, 29). (The term “oligosaccharide” is generally used to designate carbohydrates containing between 2 and 10 monosaccharide units per molecule [12]. For convenience and consistency with related published material [12, 15, 23, 25, 29], the term oligosaccharide is used in this paper to indicate a fragment obtained by chemical or enzymatic cleavage of a native polysaccharide.) Generating GBS oligosaccharides is a difficult task because of the acid-labile, antigenically critical sialic acid residues present at the termini of the side chains of all GBS CPS serotypes. Enzymatic digestion of the type III CPS with endo-β-galactosidase allowed the production of oligosaccharide-tetanus toxoid (TT) conjugates which proved to be immunogenic in animals (21). Unfortunately, the specificity of the endo-β-galactosidase is restricted to type III CPS. We sought a facile chemical degradation for the type III polysaccharide (PS) and possibly those of other CPS serotypes, keeping in mind the acid lability of the sialic acid residues. Recently, Laferriere and coworkers (15) applied a classical carbohydrate degradation technique, sequential N-deacetylation and nitrous acid deamination (16), to the fragmentation of the specific antigen of type 14 pneumococci, structurally related to the CPS of GBS type III. The oligosaccharides thus produced were used in the synthesis of conjugate vaccines. Here we report the practicability of preparative deaminative cleavage of GBS polysaccharides and the subsequent construction of conjugate vaccines for the characterization of immunologically important structural properties of GBS polysaccharides and conjugates. The resulting series of vaccines allowed the study of the influence of oligosaccharide chain length, modification of the side chain terminal sialic acid residues, and conjugate cross-linking on the immunogenicity and efficacy of type II and III GBS conjugate vaccines.
In previous studies, we concluded that the degree of the polysaccharide-protein cross-linking influenced the immunogenicity and protective efficacy of III-TT conjugate vaccine (30). The immunodominant conformational epitope of type III GBS (9, 13, 14, 20, 28) has recently been proposed to be exclusively located on extended helical domains of the polysaccharide (9). The terminal sialic acid residues control the orientation of the penultimate β-d-galactopyranose residues with respect to the backbone of the native antigen (13). These results have guided us to further investigate the optimal antibody response to III-TT conjugate vaccine through a series of vaccines whose preparation was based on the partial deamination method.
To test the effect of polysaccharide-protein cross-linking on immunogenicity, a series of vaccines was constructed by using a type III GBS oligosaccharide generated by partial deamination, having a defined molecular weight, and then reduced at the resulting 2,5-anhydromannose terminal. Sialic acid residues were then modified by periodate oxidation to varying degrees to give a series of oligosaccharides which were conjugated to tetanus toxoid.
To fully reveal the nature of the conformational epitope in terms of its overall structural requirements and its role in the antigenic and immunogenic properties of GBS-III polysaccharide, another series of vaccines was constructed using type III GBS polysaccharide in which various proportions of the sialic acid residues were modified by periodate oxidation. The oxidized components were then reduced with sodium borohydride. The resulting polysaccharide was then subjected to controlled partial nitrous acid deamination, which resulted in the degradation of GBS-III polysaccharide to oligosaccharides of defined molecular weight. These fragments were then coupled directly to tetanus toxoid to yield single-ended neoglycoconjugate vaccines.
MATERIALS AND METHODS
Bacterial strain.
GBS strains M781 (type III) and 18RS21 (type II) were used to prepare type-specific polysaccharide by methods described previously for the purification of type III polysaccharide (29).
Purification of tetanus toxoid monomer.
Tetanus toxoid (Statens Seruminstitut, Copenhagen, Denmark), obtained as a 1.6-mg/ml solution, was concentrated to 9 mg/ml through a Diaflo YM-30 membrane (Amicon, Inc., Beverly, MA). A 20-ml sample of this protein concentrate was passed through a 2.6- by 90-cm column of Superdex G-200 (Pharmacia) which was eluted with phosphate-buffered saline (PBS) containing 0.01% thimerosal. The purification proceeded as previously described (29) to yield tetanus toxoid monomer.
Preparation of single-ended neoglycoconjugate vaccines using type II and III fragments generated by deamination.
Native type II PS with an average molecular weight of ∼200,000 was partially N-deacylated as follows. The polysaccharide was dissolved in 3 ml of 0.5 N NaOH, and the solution was then divided into three parts. The samples (S1 to S3) were heated at 70°C for 60, 90, and 180 min, respectively, and then chilled in an ice-water bath. Glacial acetic acid (125 μl) was added to each sample to bring the pH to 4. The partially N-deacylated product was deaminated by the addition of 200 μl of 5% (wt/vol) NaNO2. The samples were stirred at 4°C for 2 h. Each of the three samples was then diluted to 5 ml with deionized water and diafiltered against deionized water through an Amicon Diaflo YM 10 membrane. The retentates were lyophilized. Three type II polysaccharide fragment preparations (II-1, II-2, and II-3) of different molecular weight distributions were obtained.
The fragments of type III polysaccharides were prepared by deamination as described above except that native type III PS (125 mg) was divided into five parts. The samples (S1 to S5) were heated at 70°C for 60, 90, 120, 180, and 240 min, respectively, to yield five fragment preparations (III-1 through III-5) of different molecular weight distributions.
Conjugates were typically prepared as follows. Dry tetanus toxoid monomer (4 mg) and fragmented polysaccharide (10 mg), which may have been further processed as described below, were dissolved in 200 μl of 0.2 M sodium phosphate buffer, pH 7.5, and recrystallized NaBH3CN (8 mg) was added to the solution. The reaction mixture was incubated at 37°C for 4 days. Conjugate reaction mixtures were purified by size exclusion chromatography on a column of Superdex G-200 (Pharmacia) eluted with PBS containing 0.01% (wt/vol) thimerosal. Fractions containing the conjugates were pooled, sterile filtered through a 0.22-μm Millipore membrane, and analyzed for their protein and sialic acid contents by the methods of Bradford (8) and Svennerholm (24), respectively. The average total amount of carbohydrate in each type II and III conjugate was calculated by multiplying the sialic acid content by correction factors based on the composition of their respective repeating units (4 for type II, 3.3 for type III).
Multisite cross-linked conjugate vaccines prepared from deamination-fragmented type III polysaccharide with varying degrees of oxidation.
Fragmentation of native type III polysaccharide preceded by deamination was performed as described above; deamination was carried out for 2 h at 70°C. Fragmented GBSP-III (72.69 mg) was dissolved in 2 ml of a solution of 0.1 M NaOH; NaBH4 (20 mg) was then added to the reaction solution. After 2 h at room temperature, the solution was neutralized with 50% acetic acid, dialyzed against deionized water at 4°C, and lyophilized to provide 69 mg of reduced GBSP-III polysaccharide fragments. Three 20-mg samples of the reduced polysaccharide were each dissolved in deionized water and treated with 400 μl, 800 μl, and 1.2 ml of 10 mM NaIO4. The targeted degrees of oxidation were 20%, 40%, and 60%, respectively. Reaction volumes were adjusted with deionized water to 2 ml. The reaction mixtures were stirred in the dark for 2 h at room temperature, and each reaction was quenched with 20 μl of ethylene glycol. The reaction mixtures were then dialyzed and lyophilized to provide 18.7 mg, 17.6 mg, and 17.5 mg of oxidized polysaccharide, respectively.
Conjugation of these oxidized polysaccharide fragments to tetanus toxoid monomer proceeded as previously described (30).
Multisite cross-linked conjugate vaccines prepared from fragments of the type III polysaccharide generated by ozonolysis.
GBS-III polysaccharide fragments were generated by ozonolysis (27) of native GBS-III polysaccharide and purified by ultrafiltration. Fragments (low molecular weight, with an average molecular weight of 10,000 and intermediate fragments with an average molecular weight of 40,000) were then oxidized to 25% with 2.5 mM NaIO4 solution. Oxidized fragments with multiple active sites were then conjugated to a recombinant streptococcal Cβ protein (rCβ) by reductive amination. Conjugates were then purified by gel filtration using a Superdex 200 column.
Single-ended neoglycoconjugate vaccines prepared from native type III polysaccharide oxidized to varying degrees and then fragmented by deamination.
A 50-mg portion of GBSP-III powder (30.2 mg of pure GBSP-III) was dissolved in 10 ml of deionized water. A 150-μl aliquot of 0.1 M NaIO4 was added, with the theoretical target degree of oxidation being 35%, and the reaction mixture was stirred for 2 h in the dark at room temperature. The reaction was quenched by the addition of 20 μl of ethylene glycol. The reaction mixture was then dialyzed against deionized water, and the retentate was lyophilized, yielding 37 mg of solid material. This material was then treated in 0.1 M NaBH4 in 0.1 M NaOH as described above. The product was lyophilized to yield 33 mg of oxidized and reduced GBSP-III ([O]/[H]-GBSP-III). Identical conditions were used, except with twice the amount of NaIO4, to produce [O]/[H]-GBS-III with a targeted degree of oxidation of 80%.
The fragments of [O]/[H]-GBSP-III polysaccharides were prepared by deamination as described above, except the fragment preparation was then fractionated by gel filtration chromatography on a Sephadex G75 column (Pharmacia). The material eluting at the left of the major peak was pooled, dialyzed against deionized water, and lyophilized to yield material of 10,000 average molecular weight.
Conjugation of the fragment of [O]/[H]-GBSP-III polysaccharide to tetanus toxoid monomer was accomplished by the well-characterized reductive amination coupling method as described above.
Sizing of polysaccharide fragments.
The average molecular weight of each fragment was estimated by size-exclusion chromatography on a Superose-12 column (Pharmacia) calibrated with a series of defined dextran standards (Pharmacia) of average molecular weights ranging from 10,000 to 2,000,000. The voided volume and total volume were determined with dextran of 2,000,000 average molecular weight and sodium azide, respectively.
1H NMR analysis of the polysaccharide fragments.
The structural integrity of each polysaccharide fragment preparation with respect to its parent native polysaccharide was established by high resolution one-dimensional 1H nuclear magnetic resonance (NMR) spectroscopy at 500 MHz. Spectra were acquired at a molecular weight of 300,000 for samples dissolved in D2O on a Bruker AMX 500 spectrometer. Critical resonances examined were those signals at δ 2.82 ppm and 1.83 ppm corresponding to H3e and H3a of terminal sialic acid residues. The intensity of these signals was compared with one at 2.09 ppm corresponding to the overlapping N-acetyl (N-COCH3) resonance of sialic acid and N-acetyl-d-glucosamine residues of type II and III repeating units. For these signals, a ratio of intensity of ca. 1:6 was observed in each polysaccharide fragment.
Immunogenicity of conjugates in mice.
Groups of 10 female Swiss Webster outbred mice (4 to 6 weeks old) (Harlan Sprague-Dawley, Inc., Indianapolis, IN) were injected subcutaneously with 2 μg of native type II or III polysaccharide or their corresponding oligosaccharide tetanus toxoid conjugates. The vaccines were adsorbed on aluminum hydroxide gel (Alhydrogel; Superfos Biosector a/s, Vedbaek, Denmark) at 1 mg of elemental Al/ml in 10 mM PBS containing 0.01% thimerosal. Mice received the vaccine at days 0, 21, and 42 and finally were exsanguinated at day 52. Sera were collected and stored at −70°C.
Antiserum pools.
Aliquots of serum samples from individual mice were pooled for each vaccine condition for further analyses. Pooled sera were chosen rather than geometric or arithmetic means of individual serum samples as representative of experimental outcomes from the immunizations. Typically, in our hands, titer values obtained from pooled sera were relatively representative of the mean values of individual serum assays, perhaps slightly higher on average than the geometric mean titers and very similar to arithmetic mean titers.
ELISA.
Direct enzyme-linked immunosorbent assay (ELISA) titers of polysaccharide-specific antibody were determined using native type II or III GBSP-human serum albumin conjugates as coating antigens (11, 26). Microtiter plates (NUNC PolySorp; Nalge Nunc International Corp., Rochester, NY) were coated by adding 100 μl per well of native type II or III polysaccharide-human serum albumin conjugate (1 μg/ml) in PBS with 0.02% azide. The plates were incubated at 37°C for 1 h. The plates were washed with PBS containing 0.05% Tween 20 (PBS-T) and blocked with 0.5% bovine serum albumin in PBS for 1 h at room temperature. The wells were then filled with 100 μl of twofold serial dilutions of primary mouse antisera in PBS-T, and the plates were incubated for 1 h at room temperature. The plates were washed five times with PBS-T, and the wells were filled with 100 μl of peroxidase-labeled goat anti-mouse immunoglobulin G (IgG) heavy plus light chains diluted 1:2,500 in PBS-T (Kirkegaard & Perry Laboratories [KPL], Gaithersburg, MD) and incubated for 1 h at room temperature. The plates were again washed five times with PBS-T. Finally, 50 μl of TMB peroxidase substrate (KPL) was added to each well, and following incubation of the plates for 10 min at room temperature, the reaction was stopped by the addition of 50 μl of 1 M H3PO4. The plates were read immediately at 450 nm, with the reference wavelength set at 650 nm.
Opsonic activity of conjugate antisera.
The functional activity of the sera was determined in an opsonophagocytic assay using the HL-60 cell line as described previously (10, 19, 22). Briefly, 200 CFU of GBS type II strain 18RS21 cells or type III strain M781 cells were mixed in an equal volume with serum antibodies, and samples were shaken for 15 min at 35°C in an incubator in 5% CO2. Baby rabbit complement and HL-60 cells (5 × 105) cultured for 5 days in the presence of 90 mM dimethyl formamide were added to the mixture and incubated at 37°C for 1 h under shaking. Aliquots were removed for quantitative culture. Titers were determined by extrapolating the antibody dilution corresponding to 50% live bacteria.
Efficacy of GBS vaccines in mice.
The protective efficacy of GBS conjugate vaccines was measured with the maternal immunization-neonatal mouse challenge model of GBS infection, as described previously (17, 30).
RESULTS
Depolymerization and activation of native GBS type II and III PS.
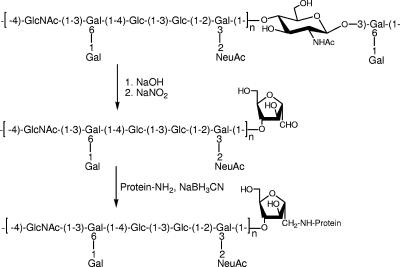
The backbone 2-deoxy-2N-acetamido-β-d-glucopyranosyl residues in the type II and III GBS polysaccharides (Fig. 1 and 2, respectively) were partially N-deacetylated in 0.5 M NaOH. The resulting glucosamine residue was then susceptible to nitrosation with nitrous acid and acetic acid to form an unstable N-nitrosoamine. The subsequent deaminative cleavage resulted in the production of fragments having terminal aldehyde groups.
FIG. 1.
Deaminative cleavage of GBS type II polysaccharide and conjugation to tetanus toxoid.
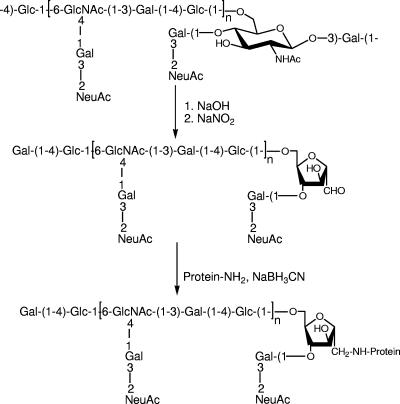
FIG. 2.
Deaminative cleavage of GBS type III polysaccharide and conjugation to tetanus toxoid.
Three type II fragments of 51,000, 33,000, and 15,000 average molecular weights and five type III fragments of 41,000 to 11,000 average molecular weights were obtained (Table 1). Comparison of the 500 MHz 1H NMR spectra of the type II and III fragments with those of their respective native polysaccharides indicated that no structural change had occurred during the chemical processes and, most importantly, that terminal sialic acid residues had been preserved during the nitrosation treatment process.
TABLE 1.
Average molecular weights of GBS type II and III fragments
| PS fragment | Kava (range) | Avg mol wt | Range |
|---|---|---|---|
| II-1 | 0.23 (0.13-0.34) | 51,000 | 26,000-99,000 |
| II-2 | 0.30 (0.19-0.40) | 33,000 | 17,000-68,000 |
| II-3 | 0.43 (0.30-0.50) | 15,000 | 9,000-33,000 |
| III-1 | 0.21 (0.11-0.27) | 41,000 | 28,000-81,000 |
| III-2 | 0.26 (0.17-0.38) | 30,000 | 13,000-53,000 |
| III-3 | 0.29 (0.18-0.40) | 24,000 | 12,000-50,000 |
| III-4 | 0.37 (0.23-0.44) | 14,000 | 9,000-36,000 |
| III-5 | 0.41 (0.31-0.47) | 11,000 | 8,000-21,000 |
Kav = (ve − vo/(vt − vo), where vt is the total volume of gel in the column, vo is the void volume, and ve is the volume at peak maximum.
Synthesis of single-ended GBS type II and III conjugates with variations in size of their oligosaccharide chains.
The aldehyde group in the resulting 2,5-anhydro-d-mannose residue formed following deamination at the reducing end of the polysaccharide fragment was used directly, without further chemical manipulation for linking through reductive amination to the tetanus toxoid monomer. The resulting neoglycoconjugates contain saccharide chains attached to the carrier protein by a single covalent bond. The biochemical characteristics of a series of such type II and III single-ended oligosaccharide neoglycoconjugates which vary in the size of their oligosaccharide are listed in Table 2. Conjugates containing the larger fragments had approximately one oligosaccharide chain per molecule of TT, but conjugates prepared from smaller fragments contained larger numbers of chains per molecule of TT. As expected, in a competitive binding ELISA using antibodies induced by whole cells of type II and III GBS organisms, all of these conjugates, regardless of the size of their saccharide chains, had similar binding to their homologous antibodies.
TABLE 2.
Biochemical characteristics of GBS type II and III single-ended conjugate vaccines
| Conjugate | Avg mol wt of PS chains | Protein (μg/ml) | CHO (μg/ml) | % CHO on conjugate | No. of PS chains |
|---|---|---|---|---|---|
| II-1-TT | 51,000 | 120 | 15 | 11 | 0.4 |
| II-2-TT | 33,000 | 140 | 42 | 23 | 1.4 |
| II-3-TT | 15,000 | 110 | 26 | 19 | 2.4 |
| III-1-TT | 41,000 | 190 | 70 | 27 | 1.2 |
| III-2-TT | 30,000 | 140 | 61 | 29 | 1.8 |
| III-3-TT | 24,000 | 100 | 39 | 28 | 2.0 |
| III-4-TT | 14,000 | 70 | 17 | 20 | 2.0 |
| III-5-TT | 11,000 | 80 | 21 | 21 | 3.0 |
Preparation of cross-linked type III oligosaccharide conjugates.
To test the effect of conjugate cross-linking (multisite coupling) on the immunogenicity of the above-described conjugates, we prepared a type III fragment of ca. 9,000 Da (nine repeat units) by deamination of the native PS. The generated oligosaccharide was first treated with sodium borohydride to reduce the 2,5-anhydromannose terminal aldehyde. Aldehyde groups were then introduced to varying degrees into the terminal sialic acids of the oligosaccharide (30) to achieve theoretical oxidation levels of 20, 40, and 60%. The resulting oxidized oligosaccharides were then conjugated to tetanus toxoid to produce a series of lattice conjugates with different degrees of carbohydrate-to-protein cross-linking. A conjugate with no oxidation in its sialic acid was also prepared as a control. The biochemical characterization of these conjugates is reported in Table 3. The relative compositions of carbohydrate and protein were comparable among all conjugate preparations.
TABLE 3.
Biochemical characteristics of GBS cross-linked tetanus toxoid and rCβ conjugate vaccines
| Conjugate | Avg mol wt of PS chains | Protein (μg/ml) | CHO (μg/ml) | % CHO in conjugatea |
|---|---|---|---|---|
| III0%-TT | 9,000 | 78 | 35 | 31 |
| III20%-TT | 9,000 | 230 | 177 | 44 |
| III40%-TT | 9,000 | 297 | 218 | 42 |
| III60%-TT | 9,000 | 259 | 206 | 44 |
| III-Cβ | 10,000 | 259 | 92 | 26 |
| III-Cβ | 40,000 | 172 | 105 | 37 |
| III-Cβ | Native | 340 | 520 | 60 |
For Cβ conjugates, percentages of CHO in conjugate are wt/wt.
Preparation of single-ended conjugates containing variations in the degree of modification of their sialic acid residues.
To test whether modification of the side chain between carbons 8 and 9 of the terminal sialic acids would have any influence on the immunogenicity of the resulting oligosaccharide conjugate, a series of type III fragments containing various degrees of oxidation, i.e., various amounts of 8-carbon sialic acid residues, were prepared. The type III PS was first oxidized with sodium periodate to generate modified PSs with degrees of oxidation of their sialic acid of 35 and 80%. Thereafter, the terminal C-8 aldehydes were reduced with sodium borohydride into their corresponding C-8 primary hydroxyl groups. The resulting modified polysaccharides were then deaminated to generate a PS fragment of ca. 10,000 average molecular weight. These fragments were then conjugated to tetanus toxoid. The biochemical characterization of the resulting single-ended neoglycoconjugates is reported in Table 4. The carbohydrate content of these conjugates was 18% for the fragment preparation originating from native PS and 31 and 33%, respectively, for the corresponding modified 35 and 80% oxidized-reduced fragments.
TABLE 4.
Biochemical characteristics of GBS type III oligosaccharide conjugate vaccines with a modification in their sialic acid residues
| Conjugate | Avg mol wt of PS chains | Protein (μg/ml) | CHO (μg/ml) | % CHO in conjugate |
|---|---|---|---|---|
| III0%-TT | 10,000 | 267 | 59 | 18 |
| III35%-TT | 10,000 | 348 | 153 | 31 |
| III80%-TT | 10,000 | 436 | 218 | 33 |
Influence of the size of the oligosaccharide chains on the immunogenicity of single-ended and cross-linked conjugates.
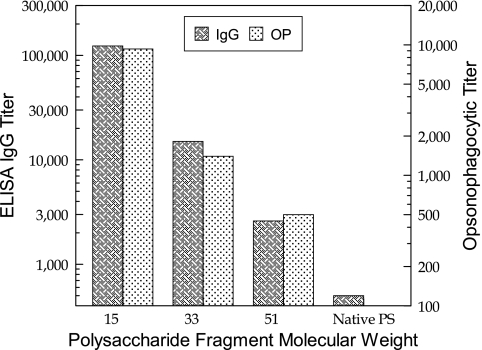
Type II PS-specific IgG titers of mice vaccinated with native type II PS or type II fragment PS-tetanus toxoid conjugates are shown in Fig. 3. There was a significant increase in PS-specific IgG levels as the size of the oligosaccharide chain of the conjugates decreased from 51,000 average molecular weight (titer of 2,600), through 33,000 average molecular weight (titer of 15,000), and to 15,000 average molecular weight (titer of 123,000). Similarly, the opsonophagocytic (OP) titers of these antisera, as measured with an HL-60 human cell line as a source of polymorphonuclear leukocytes, followed the same trend, where the antisera raised against the conjugate with the smallest oligosaccharide gave the highest OP titer (9,300) and the sera raised against the largest oligosaccharide gave the smallest titer (<500) (Fig. 3). For these antisera, there was a good positive correlation between PS antibody levels and corresponding OP activity. Thus, it is clear that the size of the oligosaccharide chains in those conjugates significantly influenced their immunogenicity. The native uncoupled type II PS was not immunogenic.
FIG. 3.
Effect of oligosaccharide size on immunogenicity of GBS type IIOS-TT conjugates in mice. Molecular weight is shown in thousands.
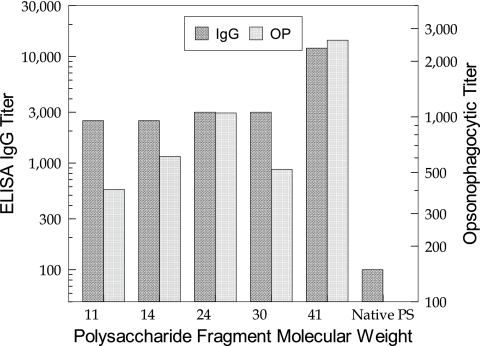
Type III PS-specific IgG titers of mice vaccinated with either native type III PS or type III fragment PS-tetanus toxoid conjugates are shown in Fig. 4. Unlike for the type II fragment-TT conjugates, and perhaps lending more clarity to a previous report (20), the size of the oligosaccharide chains in the type III conjugates did not significantly influence the functional activity of the immune response. There was no real significant difference in type III PS-specific IgG titers between the sera raised against the smallest oligosaccharide (11,000 average molecular weight) conjugate (titer of 2,800) and the sera raised against the 30,000-average-molecular-weight oligosaccharide conjugate (titer of 3,000), with perhaps a modest increase in type III PS-IgG toward the largest oligosaccharide (41,000 average molecular weight). Again, as for the type II, OP titers correlated well with antibody levels (Fig. 4) with no real significant difference in the antibody titers among the different groups (range, 400 to 2,600). The native type III PS was not immunogenic.
FIG. 4.
Effect of oligosaccharide size on immunogenicity of GBS type IIIOS-TT conjugates in mice. Molecular weight is shown in thousands.
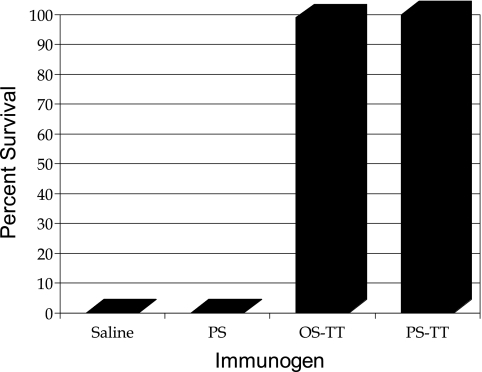
The efficacy in a neonatal mouse model of single-ended conjugate with a short type III oligosaccharide chain (11,000 average molecular weight) was compared with its homologous lattice (multisite coupling) form constructed with the native PS in which aldehyde groups were introduced into the terminal sialic acids (29). The native uncoupled type III PS was also used as a control. Both conjugates were highly potent in the model, and no significant difference in protection was observed between the two constructs (Fig. 5). The uncoupled PS was not protective.
FIG. 5.
Efficacy of GBS type III conjugate vaccines in the neonatal mouse model.
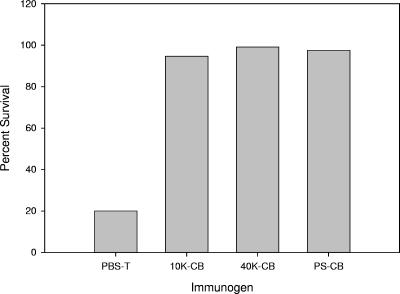
Efficacy in the neonatal mouse model was also examined, with conjugates prepared using type III fragments that were produced by ozonolysis (17, 27) and in which aldehydes were introduced into the terminal sialic acid (Table 3). No significant differences in protection were observed between the different constructs regardless of their size (Fig. 6).
FIG. 6.
Efficacy of GBS type III conjugate vaccines produced by ozonolysis in the neonatal mouse model. CB, rCβ; 10K, 10,000 molecular weight; 40K, 40,000 molecular weight.
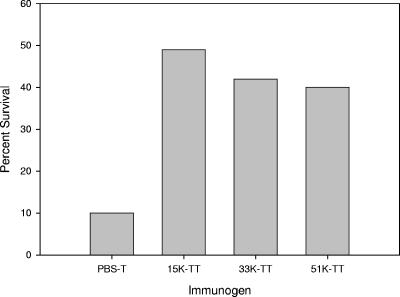
Efficacy determinations for the type II single-ended neoglycoconjugates with different saccharide sizes were also carried out in the mouse model. The conjugate with the small oligosaccharide size (15,000 average molecular weight) appeared to offer the best protection (Fig. 7); however, the difference in efficacy between this conjugate and the other two was not as dramatic as the difference observed in the antibody and OP titers in the immunogenicity study reported above. The GBS type II PS conjugate was comparable in its efficacy to the small oligosaccharide size conjugate. In this study, however, there was a clear indication that the type II PS-specific antibody, as measured by ELISA, had been transported across the placenta from the mothers to their offspring.
FIG. 7.
Efficacy of GBS type II conjugate vaccines in the neonatal mouse model. 15K, 15,000 molecular weight; 33K, 33,000 molecular weight; 51K, 51,000 molecular weight.
Effect of oligosaccharide-protein cross-linking on immunogenicity.
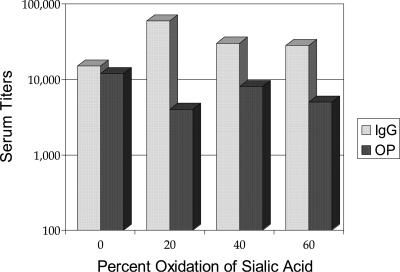
To test the effect of oligosaccharide-protein cross-linking on immunogenicity, a series of vaccines was constructed by using a type III GBS oligosaccharide (ca. 9,000 average molecular weight or nine repeat units) generated by deamination. The levels of oxidation in the sialic acids were 20, 40, and 60%; a control conjugate with no oxidation in its sialic acid was also prepared. All of these conjugates had similar oligosaccharide-to-protein ratios, indicating similar loading scenarios regardless of the degree of OS oxidation. Immunogenicity testing of these conjugate vaccines was carried out in mice. As shown in Fig. 8, the cross-linked (or lattice-structured) conjugates were more immunogenic, producing more type III PS-specific IgG. However, the opsonic activity produced by the oxidized conjugates was not significantly different than or even slightly reduced compared to that produced by the non-cross-linked PS conjugate (Fig. 8).
FIG. 8.
Effect of oligosaccharide-to-protein cross-linking on immunogenicity.
Effect of sialic acid modification on immunogenicity.
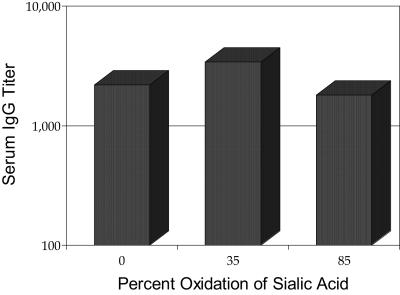
A study was designed to examine whether or not cleaving the bond from C-8 to C-9 in the exocyclic chain of the terminal sialic acids of a short GBS type III oligosaccharide (10,000 average molecular weight) would affect immunogenicity. We constructed two single-ended conjugates (i.e., linked through their terminal reducing 2,5-anhydromannose) that had 35 and 80% of their sialic acid oxidized and reduced, thus leaving primary hydroxyl groups at C-8. The immunogenicity of these conjugates was compared to that of the control single-ended conjugate described above. As shown in Fig. 9, the three conjugates had similar immunogenicities, indicating that shortening the sialic acids from 9 to 8 carbons had no effect on the immunogenicity of the resulting vaccines.
FIG. 9.
Effect of sialic acid modification (percentage of C-8 NeuAc) on immunogenicity.
DISCUSSION
Similar to what was reported for the production of pneumococcal type 14 conjugates (15), the deaminative cleavage of the native GBS type II and III polysaccharides allowed for the simple and well-characterized preparation of single-ended neoglycoconjugates, which gave sufficient uniformity to their structures to enable a legitimate comparison to be made. All of the GBS PS fragments were terminally linked exclusively at the reducing end. The methodology is a nice extension to what is already available to successfully depolymerize the GBS polysaccharides (21, 27) with the absolute requirement of preserving the important sialic acids that control the shape of the protective epitopes (9, 13, 14). In addition, the methodology offers the added advantage of arming the PS fragments with a reactive aldehyde group readily available for subsequent coupling to proteins by reductive amination. This methodology is of course applicable only to GBS II and III serotypes due to the presence of N-acetyl-d-glucosamine residues in the side chains of the other GBS serotypes.
In this study, a comparison of the performance of various configurations of type II and III GBS tetanus toxoid conjugates as vaccine immunogens was undertaken. These conjugates varied in the size of their oligosaccharide chains or in the way they were linked, whether through a single site as neoglycoproteins or as cross-linked or lattice-structured through multiple-site attachment to the carrier protein. There was a clear indication, for the type II neoglycoconjugates, that decreasing the size of the PS fragment was accompanied by an increase in immunogenicity that also correlated with OP activity. This could have been suggestive of two things: either that, as the length of the oligosaccharide increased, the resulting conjugate had more T-cell-independent antigen properties (20) or that, unlike for the type III polysaccharide, there is less of a conformational protective epitope in the GBS type II polysaccharide, regardless of the presence of sialic acid in its structure.
For the GBS type III neoglycoconjugates, however, the results were markedly different, with no real impact on opsonophagocytic activity observed with increased size of the PS fragment, and perhaps some indication for an increase in immunogenicity and OP activity toward the higher size of the type III PS fragment (beyond 30 repeats). These data are in good agreement with immunogenicity data obtained with neoglycoconjugates of the pneumococcal type 14 PS fragments (24); however, it contrasts with an earlier report that suggested an optimum immunogenicity for a size of 13 repeats in the case of the type III fragment (20), although the immunogenicity studies were conducted in rabbits and the conjugates were prepared by a slightly different chemistry (21). In the previous study (20), the type III oligosaccharide fragments were obtained by digestion of the native PS with an endo-β-galactosidase, followed by the introduction of a galactosyl spacer arm into the terminal reducing end of the generated fragment. Single-ended neoglycoconjugates were then produced after treatment of the galactosylated fragment with galactose oxidase to generate aldehyde groups at C-6 of the spacer terminal galactose, followed by subsequent coupling to TT by reductive amination.
In this study, we also examined the effect of oligosaccharide to protein cross-linking on immunogenicity. In a previous study (30), the effect of polysaccharide to protein cross-linking was examined, and it was demonstrated that higher PS-specific antibody responses were obtained as the extent of cross-linking increased. OP activity, however, was greatest in mouse antisera raised to a moderately (under 66%) cross-linked conjugate, suggesting that some antibodies evoked by highly cross-linked conjugates were directed to a nonprotective epitope (30). In support of the previous study and further examining a range of moderate cross-linking of oligosaccharides, our data indicated that increasing the oligosaccharide to protein cross-linking through terminal sialic acids (from 20 to 60%) had no significant effect on both the PS-specific antibody response or on the opsonic activity. It is also possible that perhaps because of the smaller size of the oligosaccharide chain (10,000 Da), no nonprotective epitopes had been induced.
To verify whether or not cleaving the exocyclic chain of the sialic acid residues from 9 to 8 carbons would have any effect on immunogenicity, several neoglycoconjugate constructs of GBS type III oligosaccharide with various amounts of modified sialic acids were prepared. The results indicated that such modification of the sialic acids, in an oligosaccharide conjugate context (i.e., with short oligosaccharide chains), did not influence immunogenicity. It is worth noting that such sialic acid modification in a polysaccharide protein conjugate context with a much larger saccharide might have been the reason for the induction of nonprotective epitopes.
All of these results could have important implications to improve the design of a multivalent GBS conjugate vaccine. A GBS oligosaccharide conjugate formulation would certainly be advantageous from a manufacturing standpoint, with a product easier to process and characterize, and thus better defined.
REFERENCES
- 1.Anderson, P. 1983. Antibody responses to Haemophilus influenzae type b and diphtheria toxin induced by conjugates of oligosaccharides of the type b capsule with the nontoxic protein CRM197. Infect. Immun. 39:233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, P., M. E. Pichichero, and R. A. Insel. 1985. Immunogens consisting of oligosaccharides from the capsule of Haemophilus influenzae type b coupled to diphtheria toxoid or CRM197. J. Clin. Investig. 76:52-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, P. W., M. E. Pichichero, E. C. Stein, S. Porcelli, R. F. Betts, D. M. Connuck, D. Korones, R. A. Insel, J. M. Zahradnik, and R. Eby. 1989. Effect of oligosaccharide chain length, exposed terminal group, and hapten loading on the antibody response of human adults and infants to vaccines consisting of Haemophilus influenzae type b capsular antigen uniterminally coupled to the diphtheria protein CRM197. J. Immunol. 142:2464-2468. [PubMed] [Google Scholar]
- 4.Anderson, P. W., M. E. Pichichero, R. A. Insel, R. Betts, R. Eby, and D. H. Smith. 1986. Vaccines consisting of periodate-cleaved oligosaccharides from the capsule of Haemophilus influenza type b coupled to a protein carrier: structural and temporal requirements for priming in the human infant. J. Immunol. 137:1181-1186. [PubMed] [Google Scholar]
- 5.Avery, O. T., and W. F. Goebel. 1931. Chemo-immunological studies on conjugated carbohydrate-proteins. V. The immunological specificity of an antigen prepared by combining the capsular polysaccharide of type III pneumococcus with foreign protein. J. Exp. Med. 54:437-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker, C. J., and D. L. Kasper. 1985. Group B streptococcal vaccines. Rev. Infect. Dis. 7:458-467. [DOI] [PubMed] [Google Scholar]
- 7.Baker, C. J., and M. S. Edwards. 1990. Group B streptococcal infections, p. 742-811. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and the newborn, 3rd ed. The W. B. Saunders Co., Philadelphia, Pa.
- 8.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram amounts of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 9.Brisson, J.-R., S. Uhrinova, R. J. Woods, M. van der Zwan, H. C. Jarrell, L. C. Paoletti, D. L. Kasper, and H. J. Jennings. 1997. NMR and molecular dynamics studies of the conformational epitope of the type III group B Streptococcus capsular polysaccharide and derivatives. Biochemistry 36:3278-3292. [DOI] [PubMed] [Google Scholar]
- 10.Fusco, P. C., J. W. Perry, S.-M. Liang, M. S. Blake, F. Michon, and J. Y. Tai. 1997. Bactericidal activity elicited by the beta C protein of group B streptococci contrasted with capsular polysaccharides. Adv. Exp. Med. Biol. 418:841-845. [DOI] [PubMed] [Google Scholar]
- 11.Guttormsen, H.-K., C. J. Baker, M. S. Edwards, L. C. Paoletti, and D. L. Kasper. 1996. Quantitative determination of antibodies to type III group B streptococcal polysaccharide. J. Infect. Dis. 173:142-150. [DOI] [PubMed] [Google Scholar]
- 12.IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). 1982. Abbreviated terminology of oligosaccharide chains. Recommendations 1980. Eur. J. Biochem. 126:433-437. [PubMed] [Google Scholar]
- 13.Jennings, H. J., C. Lugowski, and D. L. Kasper. 1981. Conformational aspects critical to the immunospecificity of the type III group B streptococcal polysaccharide. Biochemistry 20:4511-4518. [DOI] [PubMed] [Google Scholar]
- 14.Jennings, H. J., E. Katzenellenbogen, C. Lugowski, F. Michon, R. Roy, and D. L. Kasper. 1984. Structure, conformation and immunology of sialic acid. Pure Appl. Chem. 56:893-905. [Google Scholar]
- 15.Laferriere, C. A., R. K. Sood, J. M. De Muys, F. Michon, and H. J. Jennings. 1998. Streptococcus pneumoniae type 14 polysaccharide-conjugate vaccines: length stabilization of opsonophagocytic conformational polysaccharide epitopes. Infect. Immun. 66:2441-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindberg, B., J. Lonngren, and S. Svensson. 1975. Specific degradation of polysaccharides. Adv. Carbohydr. Chem. Biochem. 31:185-240. [Google Scholar]
- 17.Madoff, L. C., J. L. Michel, E. W. Gong, A. K. Rodewald, and D. L. Kasper. 1992. Protection of neonatal mice from group B streptococcal infection by maternal immunization with beta C protein. Infect. Immun. 60:4989-4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mäkelä, O., F. Peterfy, I. G. Outschoorn, A. W. Richter, and I. Seppälä. 1984. Immunogenic properties of alpha(1→6) dextran, its protein conjugates, and conjugates of its breakdown products in mice. Scand. J. Immunol. 19:541-550. [DOI] [PubMed] [Google Scholar]
- 19.Michon, F., P. C. Fusco, C. A. Minetti, M. Laude-Sharp, C. Uitz, C. H. Huang, A. J. D'Ambra, S. Moore, D. P. Remeta, I. Heron, and M. S. Blake. 1998. Multivalent pneumococcal capsular polysaccharide conjugate vaccines employing genetically detoxified pneumolysin as a carrier protein. Vaccine 16:1732-1741. [DOI] [PubMed] [Google Scholar]
- 20.Paoletti, L. C., D. L. Kasper, F. Michon, J. DiFabio, H. J. Jennings, T. D. Tosteson, and M. R. Wessels. 1992. Effects of chain length on the immunogenicity in rabbits of group B Streptococcus type III oligosaccharide-tetanus toxoid conjugates. J. Clin. Investig. 89:203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paoletti, L. C., D. L. Kasper, F. Michon, J. DiFabio, K. Holme, H. J. Jennings, and M. R. Wessels. 1990. An oligosaccharide-tetanus toxoid conjugate vaccine against type III group B Streptococcus. J. Biol. Chem. 265:18278-18283. [PubMed] [Google Scholar]
- 22.Perry, J. W., P. C. Fusco, F. Michon, and J. Y. Tai. 1996. An opsonophagocytic assay using HL-60 cells to measure potency of group B streptococcal (GBS) and pneumococcal conjugate vaccines. Abstr. 96th Gen. Meet. Am. Soc. Microbiol., abstr. E-64.
- 23.Seppälä, I., and O. Mäkelä. 1989. Antigenicity of dextran-protein conjugates in mice. Effect of molecular weight of the carbohydrate and comparison of two modes of coupling. J. Immunol. 143:1259-1264. [PubMed] [Google Scholar]
- 24.Svennerholm, L. 1957. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim. Biophys. Acta 24:604-611. [DOI] [PubMed] [Google Scholar]
- 25.Svenson, S. B., and A. A. Lindberg. 1983. Artificial Salmonella vaccines. Prog. Allergy 33:120-143. [DOI] [PubMed] [Google Scholar]
- 26.Wang, J. Y., A. H. C. Chang, H.-K. Guttormsen, A. L. Rosas, and D. L. Kasper. 2003. Construction of designer glycoconjugate vaccines with size-specific oligosaccharide antigens and site-controlled coupling. Vaccine 21:1112-1117. [DOI] [PubMed] [Google Scholar]
- 27.Wang, J. Y., R. I. Hollingsworth, and D. L. Kasper. 1999. Ozonolytic depolymerization of polysaccharides in aqueous solution. Carbohydr. Res. 319:141-147. [DOI] [PubMed] [Google Scholar]
- 28.Wessels, M. R., A. Munoz, and D. L. Kasper. 1987. A model of high-affinity antibody binding to type III group B Streptococcus capsular polysaccharide. Proc. Natl. Acad. Sci. USA 84:9170-9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wessels, M. R., L. C. Paoletti, D. L. Kasper, J. L. DiFabio, F. Michon, K. Holme, and H. J. Jennings. 1990. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J. Clin. Investig. 86:1428-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wessels, M. R., L. C. Paoletti, H. K. Guttormsen, F. Michon, A. J. D'Ambra, and D. L. Kasper. 1998. Structural properties of group B streptococcal type III polysaccharide conjugate vaccines that influence immunogenicity and efficacy. Infect. Immun. 66:2186-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]