Abstract
Interleukin-12 (IL-12) functions as a representative lipopolysaccharide (LPS) mediator in both innate and adaptive immunity. We investigated the regulation of LPS-induced IL-12 production by mouse macrophages. In response to LPS, peritoneal macrophages produced bioactive IL-12 p70, a heterodimer (p40/p35) of subunits, but macrophage lines such as J774.1 and RAW264.7 did not. Induction of the p35 subunit was impaired in both cell lines, and additional impairment of p40 induction was observed in RAW264.7 cells. These results suggest that some negative regulatory mechanisms against LPS-induced IL-12 p40 production are constitutively functioning in RAW264.7 cells but not in the other types of cells. Activation of GA-12 (a repressor element of IL-12 p40), rather than suppression of promoter elements, such as binding sites for NF-κB, AP-1, and IRF-1, was detected in LPS-stimulated RAW264.7 cells, accompanying hyperactivation of extracellular signal-related kinase (ERK). When ERK activation was suppressed by an inhibitor (U0126), production of p40 rose from an undetectable to a substantial level and GA-12 activation decreased. In peritoneal macrophages, stimulation with a high dose of LPS reduced p40 production with enhanced activation of ERK. Pretreatment of the cells with phorbol myristate acetate to enhance ERK activation reduced p40 production in response to the optimal LPS stimulation. Taken together, these results demonstrate that hyperactivation of the ERK pathway plays a role in upstream signaling for the activation of GA-12, leading to the repression of IL-12 p40 production in mouse macrophages.
In response to microbial infections, host cells recognize key molecular signatures of invading pathogens, so-called pathogen-associated molecular patterns, by pattern recognition receptors, such as Toll-like receptors, and activate the innate immune system for host defense against the pathogenic actions of the infective agents (12). Bacterial lipopolysaccharide (LPS), a cell wall component in gram-negative bacteria, is the best-characterized pathogen-associated molecular pattern that is recognized by host immune cells, such as macrophages, for the induction of various mediators, including proinflammatory cytokines (1). Under normal physiological conditions, the induction of mediators by LPS is well regulated and fine tuned by several negative and positive control mechanisms, and comparably low and balanced mediator levels are induced, leading to the activation of general antimicrobial, antiviral, and antitumor defense mechanisms. In contrast to such beneficial effects on host immunity, dysregulation of the control mechanisms induces unbalanced mediator levels, leading to pathological situations, as observed in severe forms of sepsis and endotoxin shock. Interleukin-12 (IL-12) is a typical example of an LPS mediator that is a proinflammatory cytokine and functions in both innate and adaptive immune systems (38). This cytokine takes part in the production of gamma interferon (IFN-γ) by NK cells and T cells (14), induction of Th1 responses (21), and enhancement of resistance to intracellular infections (23, 33). Although IL-12 contributes to the establishment of an effective immune response, its overproduction has been implicated in endotoxemia (40) as well as granuloma formation from delayed-type hypersensitivity responses (13) and autoimmune disorder (9, 37). Elucidation of the underlying regulatory mechanisms of LPS-induced IL-12 production may enable the development of new strategies for the prevention of detrimental effects of LPS on the host and for beneficial application of LPS and its agonists.
Bioactive IL-12 is a heterodimeric 70-kDa glycoprotein (p70) comprising disulfide-linked p40 and p35 subunits encoded by separate genes (36). The expression of each subunit is regulated differently from that of the other. Regulation of IL-12 p40 production occurs predominantly at the level of transcription, while that of IL-12 p35 occurs at both the transcriptional and the posttranscriptional levels by atypical processing mechanisms (2, 26). In the promoter of the IL-12 p40 gene, multiple control elements, including binding sites of NF-κB (5, 27), of Ets-2 (19), and of family members in IFN regulatory factor (IRF) (32) and C/EBP (30), have been implicated. Participation of mitogen-activated protein kinases (MAPKs), including p38, extracellular signal-related kinases (ERKs), and Jun N-terminal protein kinase (JNK), in the upstream signaling pathways has been indicated. It was demonstrated that activation of p38 promoted LPS-induced IL-12 p40 production, but enhanced activation of ERKs negatively regulated production (11, 28, 35). Other than those transcription factors that participate in the activation of inducible IL-12 p40 gene expression, a transcription factor (GAP-12) that binds to repressor element GA-12 has recently been reported (3). Much remains to be elucidated regarding the regulatory mechanisms of LPS-induced IL-12 production in relation to the roles of these factors and upstream signals that activate or suppress the factors.
In a previous paper (22), we demonstrated that mouse RAW264.7 cells did not produce IL-12 in response to LPS while they responded normally in inducing several cytokines other than IL-12. The impaired responses of RAW264.7 cells to LPS-induced IL-12 production were investigated in this study to elucidate the regulatory mechanisms. Neither p35 nor p40 subunits of IL-12 were induced by LPS in the cells. Activation of repressor element GA-12 by LPS, rather than suppression of the promoter elements of the IL-12 p40 gene, was found, and hyperactivation of the ERK pathway was indicated to play a role in upstream signaling leading to the activation of GA-12. It was also suggested that this pathway contributes to the negative regulation of LPS-induced IL-12 p40 production in mouse peritoneal macrophages.
MATERIALS AND METHODS
Animals and cells.
C3H/HeN female mice obtained from Japan Charles River (Tokyo, Japan) were used at ages of 7 to 10 weeks according to the guidelines of the Laboratory Animal Center, Jichi Medical University. Peritoneal exudate cells obtained from mice treated intraperitoneally with 2 ml of thioglycolate broth (Difco Laboratories, Detroit, MI) 4 days previously were cultured for 2 h to obtain adherent cells used as peritoneal macrophages (N-PM). The murine macrophage lines J774.1 and RAW264.7 from the American Type Culture Collection (Manassas, VA) were also used. Cells were cultured in RPMI-1640 medium (ICN Biomedicals, Inc., Aurora, OH) supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.2% NaHCO3, and 5% heat-inactivated fetal bovine serum (Flow Laboratories Inc., Rockville, MD) at a cell density of 1 × 106/ml by use of 24-well culture plates (1 ml/well) or 60-mmφ culture dishes (3 ml/dish) (Corning Inc., Corning, NY) in a humidified chamber at 37°C with 5% CO2 and 95% air.
Reagents.
The LPS prepared from Salmonella enterica serovar Aabortus-equi (8), a kind gift from C. Galanos (MIP für Immunobiologie, Freiburg, Germany), was used. The antibodies (Abs) used were anti-NF-κB p50 or p65 (Santa Cruz Biotechnology Inc., Santa Cruz, CA), anti-phospho-ERK1/2, anti-phospho-MEK1/2, anti-phospho-p38 MAPK, anti-phospho-stress-activated protein kinase (SAPK)/JNK (Cell Signaling Technology, Inc., Beverly, MA), anti-MAPK (Seikagaku Co., Tokyo, Japan), anti-tubulin-α (NeoMarkers, Fremont, CA), and anti-murine IL-10 (BD Biosciences, San Jose, CA). Recombinant murine IL-10 was obtained from PeproTech EC, Inc. (London, United Kingdom), phorbol myristate acetate (PMA) from Sigma-Aldrich Co. (St. Louis, MO), and U0126, a synthetic inhibitor of MEK1/2, from Cell Signaling Technology, Inc.
Assay of IL-12 p70 and IL-12 p40.
Production of IL-12 p70 (active form) and IL-12 p40 in the culture supernatant at 24 h after LPS stimulation was measured by specific sandwich enzyme-linked immunosorbent assays (ELISAs), according to the manufacturer's instructions (Endogen, Woburn, MA), by use of matched Ab pairs and 96-well enzyme immunoassay/radioimmunoassay plates (Corning Inc.). Quantification of each form (in pg/ml for IL-12 p70 and in ng/ml for IL-12 p40) was performed based on the standard curve obtained in each assay.
RNA isolation and semiquantitative reverse transcription PCR (RT-PCR).
Total RNA was isolated from unstimulated or stimulated cells by use of ISOGEN (Nippon Gene Co., Ltd. Tokyo, Japan), according to the manufacturer's instructions. A portion (1 μg) of total RNA was reverse transcribed using SuperScript II RT (Invitrogen Co., Carlsbad, CA). Equal aliquots of cDNA equivalent to 50 ng of RNA were subsequently amplified for the indicated genes by use of the following primers: mouse IL-12 p35, 5′-GGC TAC TAG AGA GAC TTC TTC C-3′ and 5′-GTG AAG CAG GAT GCA GAG CTT C-3′; mouse IL-12 p40, 5′-TGC TCA TGG CTG GTG CAA AG-3′ and 5′-TGG ACT TCG GTA GAT GTC TTC-3′; mouse IL-1β, 5′-GCA ACT GTT CCT GAA CTC AA-3′ and 5′-CTC GGA GCC TGT AGT GCA G-3′; mouse IL-6, 5′-TTC CTC TCT GCA AGA GAC T-3′ and 5′-TGT ATC TCT CTG AAG GAC T-3′; mouse IL-10, 5′-TAC CTG GTA GAA GTG ATG CC-3′ and 5′-CAT CAT GTA TGC TTC TAT GC-3′; mouse tumor necrosis factor alpha (TNF-α), 5′-ATG AGC ACA GAA AGC ATG ATC-3′ and 5′-TAC AGG CTT GTC ACT CGA ATT-3′; mouse IRF-8/IFN consensus sequence binding protein (ICSBP), 5′-GAT CAA GGA ACC TTC TGT GG-3′ and 5′-GAA GCT GAT GAC CAT CTG GG-3′; and mouse GAPDH (glyceraldehyde-3-phosphate dehydrogenase), 5′-CCT CAA CTA CAT GGT CTA C-3′ and 5′-CCT TCC ACA ATG CCA AAG T-3′. PCR conditions for each primer were set as shown in Table 1 to obtain the products within the logarithmic phases of the PCR amplifications. The resulting PCR products were separated by electrophoresis on 1.2% agarose gels and stained with ethidium bromide. The densities of the stained bands were quantified using the ImageJ 1.36b program (National Institutes of Health). The expression level of each gene was assessed by normalization of the density to that of the housekeeping GAPDH gene in the same sample and represented as the relative mRNA level.
TABLE 1.
Conditions of PCR
| Primer | No. of cycles | Annealing temp (°C) |
|---|---|---|
| Mouse IL-12 p35 | 35 | 60 |
| Mouse IL-12 p40 | 30 | 60 |
| Mouse IL-1β | 25 | 60 |
| Mouse IL-6 | 30 | 53 |
| Mouse IL-10 | 30 | 56 |
| Mouse TNF-α | 23 | 55 |
| Mouse IRF-8/ICSBP | 30 | 56 |
| Mouse GAPDH | 23 | 63 |
EMSA.
Nuclear proteins (5 μg) from the cells, prepared as described previously (31), were mixed with the appropriate radiolabeled synthetic oligonucleotide probes for 30 min at room temperature, and the complexes were subjected to nondenaturing 5% polyacrylamide gel electrophoresis. The gel was dried and analyzed by a BAS 3000 bio-imaging analyzer (Fuji Photo Film Co. Ltd., Tokyo, Japan). The sequences of oligonucleotide probes (top strands) (Santa Cruz Biotechnology) used in the electrophoretic mobility shift assay (EMSA) were as follows: NF-κB, 5′-AGT TGA GGG GAC TTT CCC AGG C-3′, and a mutant NF-κB with a substitution for G (underlined) by C in the NF-κB/Rel DNA binding motif; AP-1, 5′-CGC TTG ATG ACT CAG CCG GAA-3′, and a mutant AP-1 with a substitution for CA (underlined) by TG in the AP-1 binding motif; IRF-1, 5′-GGA AGC GAA AAT GAA ATT GAC T-3′, and a mutant IRF-1 with a substitution for two pairs of AA (underlined) by two pairs of GG in the IRF-1 binding motif; and GA-12, 5′-CCT CGT TAT TGA TAC ACA CAC A-3′, and a mutant GA-12 with a substitution for GA (underlined) by TC in the DNA binding motif (3). For competition experiments to check the binding specificities of the probes, nuclear extracts were incubated with a 100-fold excess of unlabeled normal probes or mutant probes before the EMSA. Supershift analyses were performed to identify transcription factor NF-κB by use of specific rabbit Abs against NF-κB p50 or p65.
Immunoblot analysis of MAPK family members.
Cells were lysed in lysis buffer (1% Triton X-100, 20 mM Tris-HCl at pH 7.5, 120 mM NaCl, 10% glycerol, 1 mM Na3VO4, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, 5 μg/ml aprotinin, and 5 μg/ml pepstatin) and maintained on ice for 20 min. Triton X-100 soluble protein (10 μg) was separated on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred to a polyvinyldifluoride membrane. MAPKs (ERK1, ERK2, p38 MAPK, and SAPK/JNK) were analyzed for phosphorylated proteins by use of Abs against their phosphorylated forms. Immunoreactive bands were visualized by ECL Western blotting detection reagent (Amersham, Buckinghamshire, United Kingdom).
RESULTS
Impairment of mouse macrophage lines RAW264.7 and J774.1 in LPS-induced production of bioactive IL-12.
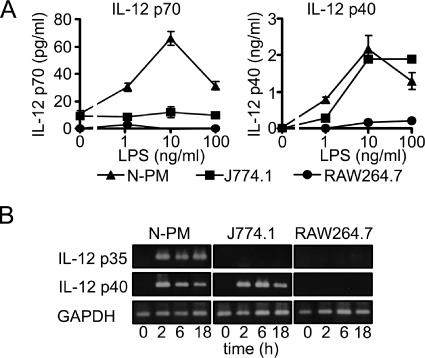
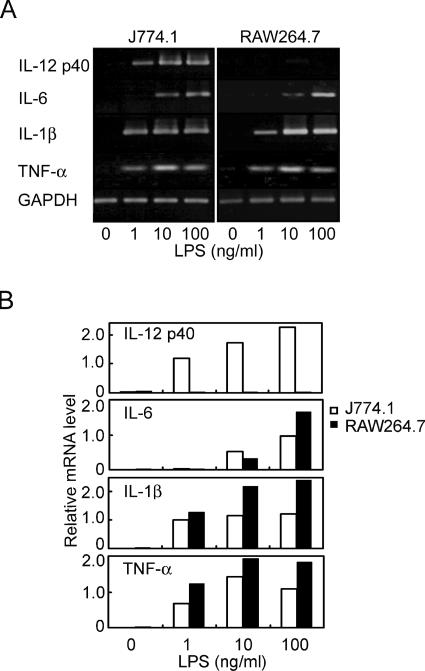
Mouse macrophages, i.e., peritoneal macrophages of C3H/HeN mice (N-PM), RAW264.7 cells, and J774.1 cells, were stimulated with LPS, and production of IL-12 in the culture supernatant was determined. As shown in Fig. 1A, bioactive IL-12 (IL-12 p70) was significantly produced by N-PM but not by RAW264.7 or J774.1 cells. The production by N-PM was detectable (>20 pg/ml) at 1 ng/ml of LPS, reached a maximum (>60 pg/ml) at 10 ng/ml, and decreased at 100 ng/ml. Production of IL-12 p40, a subunit of IL-12 p70, was detected also in the culture supernatant of J774.1 cells but not in that of RAW264.7 cells. Protein production correlated well with expression of mRNA. In N-PM, mRNAs of both p35 and p40 subunits were clearly expressed at 2 h after LPS stimulation, and expression was sustained for at least 18 h (Fig. 1B). In J774.1 cells, only mRNAs of the p40 subunit were detected (high levels at 2 and 6 h and a lower level at 18 h); mRNAs of the p35 subunit were not detected. In RAW264.7 cells, mRNAs of neither p35 nor p40 subunits were detectable in response to LPS. It is interesting that LPS-induced IL-12 production is regulated differently in these three types of macrophages. In LPS-induced cytokines other than IL-12 p40, e.g., IL-6, IL-1β, and TNF-α, the expression patterns of their mRNAs in RAW264.7 cells were similar to those in J774.1 cells (Fig. 2). These results suggest that RAW264.7 cells have the characteristic feature of selective down-modulation in LPS-induced IL-12 p40 production.
FIG. 1.
LPS-induced IL-12 production by peritoneal macrophages of C3H/HeN mice (N-PM) and by macrophage lines J774.1 and RAW264.7. (A) Mouse macrophages N-PM (▴), J774.1 (▪), and RAW264.7 (•) were cultured with LPS at the indicated doses. Production of IL-12 p70 and p40 in the culture supernatant at 24 h was determined by ELISA. Data represent the means ± standard errors of the means for triplicate samples. (B) Mouse macrophages were stimulated with LPS (10 ng/ml) for the indicated periods, and total RNAs isolated from the cells were subjected to RT-PCR to determine the mRNA expression levels of IL-12 p35 and p40 subunits. A representative result from three independent experiments is shown.
FIG. 2.
mRNA expression levels of the LPS-induced mediators in J774.1 and RAW264.7 cells. Cells were stimulated with LPS for 2 h at the indicated doses, and total RNAs isolated were subjected to RT-PCR to determine the mRNA expression levels of IL-12 p40, IL-6, IL-1β, and TNF-α. (A) Resulting PCR products were separated by electrophoresis on 1.2% agarose gels and stained with ethidium bromide. (B) Densities of the stained bands were quantified using the ImageJ 1.36b program (National Institutes of Health) and normalized to that of the housekeeping GAPDH gene in the same sample to represent the relative mRNA level. A representative result from three independent experiments is shown.
Activation of repressor element GA-12 in the IL-12 p40 promoter by stimulation of RAW264.7 cells with LPS.
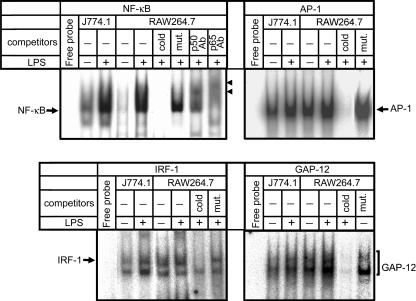
Nuclear extracts of cells stimulated or unstimulated with LPS were subjected to EMSA to evaluate the DNA-binding activities of NF-κB, AP-1, IRF-1, or GAP-12 that have been shown to be involved in the regulation of IL-12 p40 transcription (3, 18, 27, 30, 41). Figure 3 shows that the DNA-binding activities of NF-κB were clearly enhanced by LPS in both J774.1 and RAW264.7 cells. The activities of AP-1 and IRF-1 were kept at similar levels or somewhat enhanced by LPS but never reduced. These results suggest that the activation of these factors may contribute to LPS-induced promotion but not to repression of IL-12 p40 production in RAW264.7 cells, since these transcription factors are positive promoters. The binding activity of GAP-12 in RAW264.7 cells without LPS was already somewhat stronger than that in J774.1 cells with LPS, and it was further activated by LPS stimulation (Fig. 3). Since GAP-12 is a negative regulator that binds to repressor element GA-12 in the IL-12 p40 promoter, this result suggests that the activation of GAP-12 contributes largely to the induction of impaired IL-12 p40 production in RAW264.7 cells.
FIG. 3.
DNA-binding activities of various nuclear factors in LPS-stimulated J774.1 and RAW264.7 cells. Nuclear extracts of the cells stimulated with LPS (1 ng/ml) for 1 h were subjected (5 μg of protein/lane) to EMSA to evaluate the DNA-binding activities of NF-κB, AP-1, IRF-1, or GAP-12. To check the binding specificities of the radiolabeled probes used, nuclear extracts were incubated with a 100-fold excess of unlabeled normal probes (cold) or mutant probes (mut.) before the EMSA. Supershift analyses were also performed by incubation of nuclear extracts with 1 μg of Abs against NF-κB subunits, such as p50 (p50 Ab) and p65 (p65 Ab), before the EMSA. The arrowheads in the NF-κB panel indicate supershifted bands. Lanes labeled “Free probe” are negative controls without nuclear extract. Similar results were obtained in three independent experiments.
Hyperactivation of ERK in RAW264.7 cells in response to LPS stimulation.
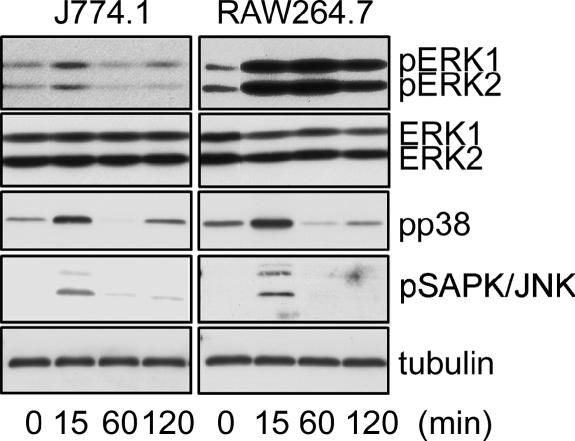
Activation of MAPK family members is known to play an important role in the transduction of LPS signals. J774.1 and RAW264.7 cells were stimulated with LPS, and activation of MAPK family members, such as ERK1/2, SAPK/JNK, and p38, was analyzed by immunoblot analysis. Activation of ERK1/2 in J774.1 cells was clearly observed at 15 min after LPS stimulation and decreased to normal levels thereafter. This seems to be the usual pattern of LPS-induced activation of ERK1/2 (Fig. 4). Activation of ERK1/2 in RAW264.7 cells was detected very strongly at 15 min, sustained over 60 min, and somewhat reduced at 120 min. Such activation of ERK1/2 in LPS-stimulated RAW264.7 cells is unusually high. This hyperactivation of ERK1/2 was characteristic in RAW264.7 cells but not in J774.1 cells, while the activation patterns of the other MAPKs (SAPK/JNK and p38) were comparable in both types of cells (Fig. 4).
FIG. 4.
Time course of LPS-induced phosphorylation of MAPKs in J774.1 and RAW264.7 cells. Whole-cell lysates prepared from the cells stimulated with 10 ng/ml of LPS for the indicated period were immunoblot analyzed with Abs against phospho-ERK1/2 (top row, pERK1/2), ERK1/2 (second row), phospho-p38 MAPK (third row, pp38), phospho-SAPK/JNK (fourth row, pSAPK/JNK), or tubulin-α (bottom row). A representative result from three independent experiments is shown.
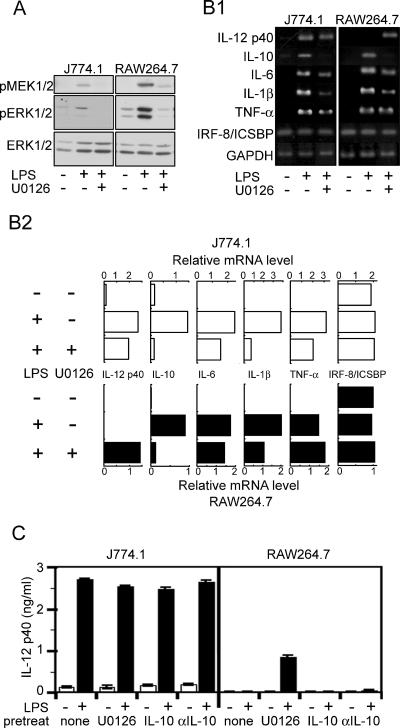
Suppression of LPS-induced hyperactivation of ERK by MEK inhibitor U0126 in RAW264.7 cells, leading to the production of IL-12 p40.
To estimate the role of LPS-induced ERK1/2 hyperactivation in the negative regulation of IL-12 p40 production, the cells were stimulated with LPS in the presence of U0126, a synthetic inhibitor of the ERK pathway, by attack on MEK1/2, which leads to activation of ERK1/2. U0126 suppressed the LPS-induced hyperactivation of ERK1/2 in RAW264.7 cells (Fig. 5A), accompanying the appearance of LPS-induced gene expression of IL-12 p40 and the production of protein as well (Fig. 5B and C). No suppressive effect of U0126 on the LPS-induced activation of the other MAPK members (SPAK/JNK and p38) was observed in either type of cell (data not shown). Signals, such as the activation of p38 MAPK seen in both J774.1 and RAW264.7 cells (Fig. 4), may be responsible for the promotion of IL-12 p40 production. In J774.1 cells, the gene expression and protein production clearly detected in the absence of U0126 were not affected by the inhibitor. In the presence of U0126, expression of the LPS-induced IL-10 gene decreased remarkably and that of the IL-1β gene decreased substantially (Fig. 5B). Since IL-10 is known to act as a suppressive cytokine for the production of proinflammatory cytokines, including IL-12, the relationship between the decreased IL-10 gene expression and the appearance of IL-12 p40 production in RAW264.7 cells may be an interesting subject for further investigation. Neither IL-10 nor anti-IL-10 supplied exogenously to the cell cultures showed any effect on the protein production of IL-12 p40 (Fig. 5C). These results suggest that hyperactivation of the ERK pathway in RAW264.7 cells participates in the negative regulation of IL-12 p40 and that activation of the ERK pathway in both RAW264.7 and J774.1 cells participates positively in the induction of IL-10 gene expression while the regulatory effect of IL-10 on IL-12 p40 production is negligible.
FIG. 5.
Effect of U0126, a MEK1/2 inhibitor, on LPS-induced responses in J774.1 and RAW264.7 cells. (A) Cells pretreated with U0126 (10 μM) for 30 min were stimulated with LPS (10 ng/ml) for 20 min and whole-cell lysates prepared for immunoblot analyses with Abs against phospho-MEK1/2 (top panels), phospho-ERK 1/2 (middle panels), or ERK1/2 (bottom panels). (B1) Total RNAs isolated from the cells pretreated with U0126 (10 μM) for 30 min and stimulated with LPS (10 ng/ml) for 2 h were subjected to RT-PCR to determine the mRNA expression levels of IL-12 p40, IL-10, IL-6, IL-1β, TNF-α, and IRF-8/ICSBP. Resulting PCR products were separated by electrophoresis on 1.2% agarose gels and stained with ethidium bromide. A representative result from three independent experiments is shown. (B2) Densities of the stained bands were quantified and normalized to that of the GAPDH gene in the same sample to represent the relative mRNA level. (C) Cells were pretreated with the indicated substances (U0126, 10 μM; IL-10, 10 ng/ml; anti-IL-10 Ab, 5 μg/ml) for 30 min and then stimulated with LPS (10 ng/ml) for 24 h. Production of IL-12 p40 in the culture supernatant was determined by ELISA. Data represent the means ± standard errors of the means for triplicate samples.
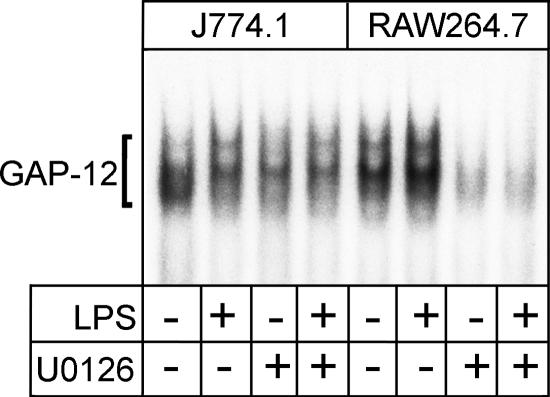
Suppression of DNA-binding activity of GAP-12 by U0126 in RAW264.7 cells.
Participation of GAP-12 in the negative regulation of IL-12 p40 production in RAW264.7 cells was suggested by the results shown in Fig. 3. The effect of U0126 on the DNA-binding activity of GAP-12 in the macrophages stimulated or unstimulated with LPS was investigated. As shown in Fig. 6, DNA-binding activities of GAP-12 in J774.1 cells with and without LPS stimulation were detected and the activities were reduced by the presence of U0126. In RAW264.7 cells, much stronger activity than that found in J774.1 cells was observed in the cells without LPS stimulation and further activation was observed with LPS stimulation. These strong activations were remarkably reduced by the presence of U0126; the reduction in the activities of the LPS-stimulated cells was especially striking. These results suggest that the LPS-induced negative regulation of IL-12 p40 production in RAW264.7 cells is exhibited through hyperactivation of the ERK pathway, leading to the activation of GAP-12 binding to repressor element GA-12 in the IL-12 p40 promoter.
FIG. 6.
Effect of U0126 on the DNA-binding activity of GAP-12. Nuclear extracts of the cells pretreated with U0126 and stimulated with LPS (as described in the legend for Fig. 5) for 1 h were prepared and subjected to EMSA to evaluate the DNA-binding activities of GAP-12. Similar results were obtained in three independent experiments.
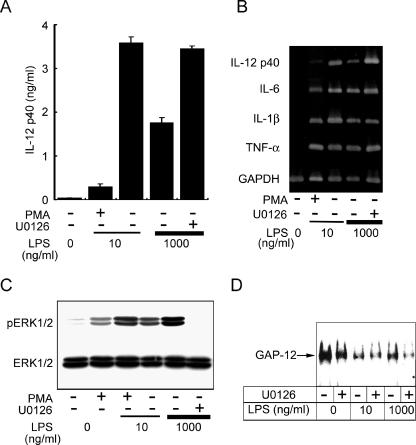
Participation of ERK hyperactivation in the regulation of LPS-induced IL-12 p40 production in mouse peritoneal macrophages (N-PM).
As shown in Fig. 1A, production of IL-12 by N-PM was highest at an LPS dose of 10 ng/ml and decreased at higher LPS doses. This suggests that production of IL-12 is negatively regulated at higher LPS doses in N-PM culture. We then investigated whether or not ERK hyperactivation also participates in this regulatory mechanism. The decreased production of IL-12 p40 protein by N-PM at a high LPS dose (1,000 ng/ml) was recovered in the presence of U0126 to a comparable level of production by the optimal LPS dose of 10 ng/ml (Fig. 7A). In the gene expression of IL-12 p40, the effect of either a high LPS dose or the presence of U0126 on expression was clear and a result similar to that for protein production was obtained (Fig. 7B). Such a clear influence by these treatments was not observed in the expression of the other cytokine genes. Activation of ERK1/2 at the optimal LPS dose was enhanced at the high dose and suppressed remarkably by U0126 (Fig. 7C), suggesting the participation of ERK hyperactivation in the negative regulation of IL-12 p40 in N-PM. High DNA-binding activity of GAP-12 in N-PM without stimulation was strongly reduced by stimulation with the optimal LPS dose. Compared to this weak activation level, the activity by stimulation with the high LPS dose was obviously strong and decreased greatly with U0126 (Fig. 7D). These results suggest that the negative regulatory mechanism of IL-12 p40 production similar to that observed in RAW264.7 cells also functions in N-PM.
FIG. 7.
Participation of enhanced ERK activation, leading to the activation of GAP-12 in the negative regulation of IL-12 p40 production in N-PM. (A) N-PM were stimulated with optimal (10 ng/ml) and high (1,000 ng/ml) doses of LPS for 24 h. Production of IL-12 p40 in the culture supernatant was determined. In some experiments, cells were pretreated with U0126 (10 μM) or PMA (10 μM) for 30 min prior to LPS stimulation. Data represent the means ± standard errors of the means for triplicate samples. (B) N-PM were stimulated with LPS for 2 h and total RNAs isolated to determine mRNA expression levels. Pretreatment with U0126 or PMA was conducted as described for panel A. (C) Whole-cell lysates of N-PM pretreated with U0126 or PMA for 15 min and stimulated with LPS for 20 min were immunoblot analyzed with Abs against phospho-ERK1/2 or ERK1/2. (D) Nuclear extracts of N-PM pretreated with U0126 for 30 min and stimulated with LPS for 1 h were subjected to EMSA for evaluation of GAP-12 activities. All data are representative of two or three independent experiments.
PMA is known as a potent activator of the ERK pathway that transduces activation signals independent from those of LPS (39). Pretreatment of N-PM with PMA and stimulation with the optimal LPS dose induced hyperactivation of ERK (Fig. 7C) and remarkable reductions in both the protein production and the gene expression of IL-12 p40 (Fig. 7A and B). A similar effect of PMA on the suppression of IL-12 p40 production in LPS-stimulated J774.1 cells was also observed (data not shown). These results support the important role of ERK hyperactivation in the repression mechanism of p40 production common in various types of macrophages.
DISCUSSION
The p40 subunit is often produced in large excess over the p70 heterodimer as seen in this study (Fig. 1A), i.e., levels of LPS-induced IL-12 p70 in N-PM were no higher than 80 pg/ml at the peak, while the peak level of IL-12 p40 was around 2 ng/ml. The p40 subunit was shown to associate not only with the p35 subunit to form bioactive IL-12 (IL-12 p70) but also with a p19 subunit to form a novel cytokine, IL-23 (29), which has some overlapping and some distinct functions in comparison to IL-12 (4, 20, 24, 34). In addition, the p40 subunits can form a homodimer to function as an antagonist of bioactive IL-12 (10, 17). The production of a large amount of the p40 subunit may be required to supply the essential component for such multiple forms and suggests the important role of this subunit under physiological conditions. A good understanding of the regulatory mechanisms for the induction of this subunit is therefore very important.
The present study shows that LPS-induced production of p40 is negatively regulated in RAW264.7 cells through the activation of transcription factor GAP-12, which binds to repressor element GA-12 (3) in the promoter region of the IL-12 p40 gene. No suppression of transcription factors that promote p40 gene expression was detected in this investigation. This study points out for the first time that in addition to the activation of GAP-12, the hyperactivation of the ERK pathway, one of the MAPK pathways, plays a role in the upstream signaling pathway leading to the activation of GAP-12. This conclusion was supported by use of an inhibitor (U0126) of MEK, an upstream signaling factor of ERK, which showed the decreased binding of GAP-12 to GA-12 in RAW264.7 cells (Fig. 6) and the appearance of LPS-induced p40 in levels of both mRNA expression (Fig. 5B) and protein production (Fig. 5C). Activation of N-PM with a high dose of LPS, e.g., 1,000 ng/ml, induced the suppression of p40 production accompanying the enhanced activation of ERK (Fig. 7). Production of p40 by N-PM upon stimulation with the optimal LPS dose (10 ng/ml) was suppressed by PMA pretreatment, which enhanced the activation level of ERK (Fig. 7). Similar effects of high-dose LPS and of PMA pretreatment on the suppression of p40 production and the enhancement of ERK activation were also observed in J774.1 cells (data not shown). These results demonstrate that the underlying mechanisms for repression of IL-12 p40 through hyperactivation of the ERK pathway function not only in RAW264.7 cells but also in various types of cells such as peritoneal macrophages and J774.1 cells.
In RAW264.7 cells, ERK seemed to be constitutively activated (Fig. 5). Stimulation of the cells with LPS enhanced the activation level further and maintained the hyperactive state for a long period. In J774.1 cells, activation of ERK weakly detected without stimulation was strengthened after LPS stimulation, although the activated level was comparable to that detected in RAW264.7 cells without LPS stimulation. Suppression of this LPS-induced ERK activation in J774.1 cells by U0126 (Fig. 5A) showed no effect on augmentation of IL-12 p40 production (Fig. 6). In N-PM, a moderate level of ERK activation by stimulation with an optimal LPS dose was enhanced by stimulation with a high LPS dose or by PMA pretreatment. In accordance with the enhanced activation, production of p40 was reduced (Fig. 7). Taken together, these results suggest that hyperactivation rather than moderate activation of ERK is required to induce repression of LPS-induced IL-12 p40 production.
It was reported that LPS-induced IL-12 p40 production by macrophages is negatively regulated by ERK at the level of transcription/message stability (7) and that the enhanced activation of ERK targets p40 transcription by suppressing the synthesis of transcription factor IRF-1 (11). Our results are consistent with the above-mentioned reports in that enhanced activation of ERK by LPS-stimulated macrophages leads to negative regulation of p40 production, but they do not agree on the point that this negative regulation is caused by the suppressed synthesis of IRF-1. In our study, reduction of IRF-1 activity was not observed in LPS-stimulated RWA264.7 cells, where ERK was hyperactivated and production of p40 was undetectable, in comparison to LPS-stimulated J774.1 cells, where ERK was moderately activated and p40 was sufficiently produced (Fig. 3). The earlier reports examined neither recovery of IRF-1 synthesis in relation to recovery of p40 production nor activation of GAP-12 in relation to LPS stimulation. Our study clearly revealed that GAP-12 is activated by LPS stimulation in RAW264.7 cells (Fig. 6) and that reduction of the activation by U0126 leads to the appearance of p40 production (Fig. 5C). In peritoneal macrophages (N-PM), the effect of GAP-12 activation on reduction of p40 production was also observed when the stimulation dose of LPS was heightened from 10 ng/ml (optimal dose) to 1,000 ng/ml (high dose) and this reduction of p40 was recovered by treatment with U0126, leading to the suppression of GAP-12 activity (Fig. 7). The present authors believe it important to clarify whether or not GAP-12 is also activated in the experimental system of the earlier studies.
IL-4 and prostaglandin E2 (PGE2) have been shown to be mediators of a Th2-like immune response that suppresses IL-12 production (6, 15, 16, 25) via enhancement of GAP-12 binding to GA-12 (3). Participation of IL-4 as a mediator in the present regulatory mechanism is difficult to assume since this cytokine has not been found to be an LPS mediator produced by macrophages, while that of PGE2 is worth considering since this chemical mediator is known to be one of the LPS mediators produced by macrophages. In the presence of AH6809, an antagonist of PGE2 receptor EP2, RAW264.7 cells were stimulated with LPS and production of p40 was determined. Production of p40 was not observed (data not shown), unlike in the presence of U0126, suggesting a low possibility of PGE2 participation in the present regulatory mechanism. This regulatory mechanism may be induced directly by LPS without the participation of any mediators.
In the present study, we showed that LPS could induce repression signals of IL-12 p40 production in addition to the promotion signals. The repression signals were suggested to be transduced via activation of transcription factor GAP-12, which binds to repressor element GA-12 in the promoter region of the IL-12 p40 gene. This study notes for the first time that hyperactivation of the ERK pathway plays a role as an upstream signal for the activation of GAP-12. This repression mechanism may function as a feedback repression pathway to prevent LPS-induced overproduction of bioactive IL-12.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research 17590397 (to M.M.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Alexander, C., and E. T. Rietschel. 2001. Bacterial lipopolysaccharide and innate immunity. J. Endotoxin Res. 7:167-202. [PubMed] [Google Scholar]
- 2.Babik, J. M., E. Adams, Y. Tone, P. J. Fairchild, M. Tone, and H. Waldmann. 1999. Expression of murine IL-12 is regulated by translational control of the p35 subunit. J. Immunol. 162:4069-4078. [PubMed] [Google Scholar]
- 3.Becker, C., S. Wirtz, X. Ma, M. Blessing, P. R. Galle, and M. F. Neurath. 2001. Regulation of IL-12 p40 promoter activity in primary human monocytes: roles of NF-κB, CCAAT/enhancer-binding protein β, and PU.1 and identification of a novel repressor element (GA-12) that responds to IL-4 and prostaglandin E2. J. Immunol. 167:2608-2618. [DOI] [PubMed] [Google Scholar]
- 4.Belladonna, M. L., J. C. Renauld, R. Bianchi, C. Vacca, F. Fallarino, C. Orabona, M. C. Fioretti, U. Grohmann, and P. Puccetti. 2002. IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells. J. Immunol. 168:5448-5454. [DOI] [PubMed] [Google Scholar]
- 5.Cameron, P., A. McGachy, M. Anderson, A. Paul, G. H. Coombs, J. C. Mottram, J. Alexander, and R. Plevin. 2004. Inhibition of lipopolysaccharide-induced macrophage IL-12 production by Leishmania mexicana amastigotes: the role of cysteine peptidases and the NF-κB signaling pathway. J. Immunol. 173:3297-3304. [DOI] [PubMed] [Google Scholar]
- 6.D'Andrea, A., M. Rengaraju, N. M. Valiante, J. Chehimi, M. Kubin, M. Aste, S. H. Chan, M. Kobayashi, D. Young, E. Nickbarg, R. Chizzonite, S. F. Wolf, and G. Trinchieri. 1992. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J. Exp. Med. 176:1387-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng, G. J., H. S. Goodridge, M. M. Harnett, X. Q. Wei, A. V. Nikilaev, A. P. Higson, and F. Y. Liew. 1999. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J. Immunol. 163:6403-6412. [PubMed] [Google Scholar]
- 8.Galanos, C., O. Lüderitz, and O. Westphal. 1979. Preparation and properities of standardized lipopolysaccharide from Salmonella aborutus equi. Zentbl. Bakteriol. Mikrobiol. Hyg. Abt. 1 Orig. A 243:226-244. [PubMed] [Google Scholar]
- 9.Germann, T., J. Szeliga, H. Hess, S. Storkel, F. Podlaski, M. Gately, E. Schmitt, and E. Rude. 1995. Administration of interleukin-12 in combination with type II collagen induces severe arthritis in DBA/1 mice. Proc. Natl. Acad. Sci. USA 92:4823-4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillessen, S., D. Carvajal, P. Ling, F. J. Podlaski, D. L. Stremlo, P. C. Familletti, U. Gubler, D. H. Presky, A. S. Stern, and M. K. Gately. 1995. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur. J. Immunol. 25:200-206. [DOI] [PubMed] [Google Scholar]
- 11.Goodridge, H. S., W. Harnett, F. Y. Liew, and M. M. Harnett. 2003. Differential regulation of interleukin-12 p40 and p35 induction via Erk mitogen-activated protein kinase-dependent and -independent mechanisms and the implications for bioactive IL-12 and IL-23 responses. Immunology 109:415-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janeway, C. A. J., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi, K., K. Kaneda, and T. Kasama. 2001. Immunopathogenesis of delayed-type hypersensitivity. Microsc. Res. Tech. 53:241-245. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi, M., L. Fitz, R. Ryan, R. Hewick, S. Clark, S. Chan, R. Loudon, F. Sherman, B. Perussia, and G. Trinchieri. 1989. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 170:827-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch, F., U. Stanzl, P. Jennewein, K. Janke, C. Heufler, E. Kampgen, N. Romani, and G. Schuler. 1996. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 184:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levings, M. K., and J. W. Schrader. 1999. IL-4 inhibits the production of TNF-alpha and IL-12 by STAT6-dependent and independent mechanisms. J. Immunol. 162:5224-5229. [PubMed] [Google Scholar]
- 17.Ling, P., M. K. Gately, U. Gubler, A. S. Stern, P. Lin, K. Hollfelder, C. Su, Y. C. Pan, and J. Hakimi. 1995. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J. Immunol. 154:116-127. [PubMed] [Google Scholar]
- 18.Ma, X., J. M. Chow, G. Gri, G. Carra, F. Gerosa, S. F. Wolf, R. Dzialo, and G. Trinchieri. 1996. The interleukin 12 p40 gene promoter is primed by interferon γ in monocytic cells. J. Exp. Med. 183:147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma, X., M. Neurath, G. Gri, and G. Trinchieri. 1997. Identification and characterization of a novel Ets-2-related nuclear complex implicated in the activation of the human interleukin-12 p40 gene promoter. J. Biol. Chem. 272:10389-10395. [DOI] [PubMed] [Google Scholar]
- 20.Ma, X. T., X. J. Zhang, B. Zhang, Y. Q. Geng, Y. M. Lin, G. Li, and K. F. Wu. 2004. Expression and regulation of interleukin-23 subunits in human peritoneal blood mononuclear cells and hematopoietic cell lines in response to various inducers. Cell Biol. Int. 28:689-697. [DOI] [PubMed] [Google Scholar]
- 21.Magram, J., S. Connaughton, R. Warrier, D. Carvajal, C.-Y. Wu, J. Ferrante, C. Stewart, U. Sarmiento, D. Faherty, and M. Gately. 1996. IL-12 deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity 4:471-481. [DOI] [PubMed] [Google Scholar]
- 22.Matsuura, M., S. Saito, Y. Hirai, and H. Okamura. 2003. A pathway through interferon-γ is the main pathway for induction of nitric oxide upon stimulation with bacterial lipopolysaccharide in mouse peritoneal cells. Eur. J. Biochem. 270:4016-4025. [DOI] [PubMed] [Google Scholar]
- 23.Mattner, F., J. Magram, J. Ferrante, P. Launois, K. Dipadova, R. Behin, M. Gately, J. Louis, and G. Alber. 1996. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur. J. Immunol. 26:1553-1559. [DOI] [PubMed] [Google Scholar]
- 24.Middleton, M. K., T. Rubinstein, and E. Pure. 2006. Cellular and molecular mechanisms of the selective regulation of IL-12 production by 12/15-lipoxygenase. J. Immunol. 176:2513-2520. [DOI] [PubMed] [Google Scholar]
- 25.Monteleone, G., T. Parrello, I. Monteleone, S. Tammaro, F. Luzza, and F. Pallone. 1999. Interferon-gamma (IFN-gamma) and prostaglandin E2 (PGE2) regulate differently IL-12 production in human intestinal lamina propria mononuclear cells (LPMC). Clin. Exp. Immunol. 117:469-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy, F. J., M. P. Hayes, and P. R. Burd. 2000. Disparate intracellular processing of human IL-12 preprotein subunits: atypical processing of the p35 signal peptide. J. Immunol. 164:839-847. [DOI] [PubMed] [Google Scholar]
- 27.Murphy, T. L., M. G. Cleveland, P. Kulesza, J. Magram, and K. M. Murphy. 1995. Regulation of interleukin 12 p40 expression through an NF-κB half-site. Mol. Cell. Biol. 15:5258-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ningfeng, T., L. Liu, K. Kang, P. K. Mukherejee, M. Takahara, G. Chen, T. S. McCormic, and K. D. Cooper. 2004. Inhibition of monocytic interleukin-12 production by Candida albicans via selective activation of ERK mitogen-activated protein kinase. Infect. Immun. 72:2513-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oppmann, B., R. Lesley, B. Blom, J. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715-725. [DOI] [PubMed] [Google Scholar]
- 30.Plevy, S. E., J. H. M. Gemberling, S. Hsu, A. J. Dorner, and S. T. Smale. 1997. Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters: evidence of functional synergy between C/EBP and Rel proteins. Mol. Cell. Biol. 17:4572-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito, S., M. Matsuura, K. Tominaga, T. Kirikae, and M. Nakano. 2000. Important role of membrane-associated CD14 in the induction of IFN-β and subsequent nitric oxide production by murine macrophages in response to bacterial lipopolysaccharide. Eur. J. Biochem. 267:37-45. [DOI] [PubMed] [Google Scholar]
- 32.Salkowski, C. A., K. Kopydlowski, J. Blanco, M. J. Cody, R. McNally, and S. N. Vogel. 1999. IL-12 is dysregulated in macrophages from IRF-1 and IRF-2 knockout mice. J. Immunol. 163:1529-1536. [PubMed] [Google Scholar]
- 33.Scharton-Kersten, T., T. Wynn, E. Denkeres, S. Bala, E. Grunvald, S. Hieny, R. Gazzinelli, and A. Sher. 1996. In the absence of endogenous IFN-γ, mice develop unimpaired IL-12 response to Toxoplasma gondii while failing to control acute infection. J. Immunol. 157:4045-4054. [PubMed] [Google Scholar]
- 34.Schuetze, N., S. Schoeneberger, U. Mueller, M. A. Freudenberg, G. Alber, and R. K. Straubinger. 2005. IL-12 family members: differential kinetics of their TLR4-mediated induction by Salmonella enteritidis and the impact of IL-10 in bone marrow-derived macrophages. Int. Immunol. 17:649-659. [DOI] [PubMed] [Google Scholar]
- 35.Tomczak, M. F., M. Gadjeva, Y. Y. Wang, K. Brown, I. Maroulakou, P. N. Tsichlis, S. E. Erdman, J. G. Fox, and B. H. Horowitz. 2006. Defective activation of ERK in macrophages lacking p50/p105 subunit of NF-κB is responsible for elevated expression of IL-12 p40 observed after challenge with Helicobacter hepaticus. J. Immunol. 176:1244-1251. [DOI] [PubMed] [Google Scholar]
- 36.Tone, Y., S. A. Thompson, J. M. Babik, K. F. Nolan, M. Tone, C. Raven, and H. Waldmann. 1996. Structure and chromosomal location of the mouse interleukin-12 p35 and p40 subunit genes. Eur. J. Immunol. 26:1222-1227. [DOI] [PubMed] [Google Scholar]
- 37.Trembleau, S., G. Penna, E. Bosi, A. Mortara, M. Gately, and L. Adorini. 1995. Interleukin-12 administration induces T helper type 1 cells and accelerates autoimmune diabetes in NOD mice. J. Exp. Med. 181:817-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trinchieri, G. 1998. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv. Immunol. 70:83-243. [DOI] [PubMed] [Google Scholar]
- 39.Weinstein, S. L., J. S. Sanghera, K. Lemke, A. L. DeFranco, and S. L. Pelech. 1992. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J. Biol. Chem. 267:14955-14962. [PubMed] [Google Scholar]
- 40.Wysocka, M., M. Kubin, L. Vieira, L. Ozmen, G. Garotta, P. Scott, and G. Trinchieri. 1995. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur. J. Immunol. 25:672-676. [DOI] [PubMed] [Google Scholar]
- 41.Zhu, C., K. Gagnidze, J. H. M. Gemberling, and S. E. Plevy. 2001. Characterization of an activation protein-1-binding site in the murine interleukin-12 p40 promoter. J. Biol. Chem. 276:18519-18528. [DOI] [PubMed] [Google Scholar]