Abstract
Johne's disease (JD), or paratuberculosis, caused by Mycobacterium avium subsp. paratuberculosis, is one of the most widespread and economically important diseases of livestock and wild ruminants worldwide. Control of JD could be accomplished by diagnosis and good animal husbandry, but this is currently not feasible because commercially available diagnostic tests have low sensitivity levels and are incapable of diagnosing prepatent infections. In this study, a highly sensitive and subspecies-specific enzyme-linked immunosorbent assay was developed for the diagnosis of JD by using antigens extracted from the surface of M. avium subsp. paratuberculosis. Nine different chemicals and various intervals of agitation by vortex were evaluated for their ability to extract the surface antigens. Various quantities of surface antigens per well in a 96-well microtiter plate were also tested. The greatest differences in distinguishing between JD-positive and JD-negative serum samples by ethanol vortex enzyme-linked immunosorbent assay (EVELISA) were obtained with surface antigens dislodged from 50 μg/well of bacilli treated with 80% ethanol followed by a 30-second interval of agitation by vortex. The diagnostic specificity and sensitivity of the EVELISA were 97.4% and 100%, respectively. EVELISA plates that had been vacuum-sealed and then tested 7 weeks later (the longest interval tested) had diagnostic specificity and sensitivity rates of 96.9 and 100%, respectively. In a comparative study involving serum samples from 64 fecal culture-positive cattle, the EVELISA identified 96.6% of the low-level fecal shedders and 100% of the midlevel and high-level shedders, whereas the Biocor ELISA detected 13.7% of the low-level shedders, 25% of the mid-level shedders, and 96.2% of the high-level shedders. Thus, the EVELISA was substantially superior to the Biocor ELISA, especially in detecting low-level and midlevel shedders. The EVELISA may form the basis for a highly sensitive and subspecies-specific test for the diagnosis of JD.
Worldwide, Johne's disease (JD), caused by Mycobacterium avium subsp. paratuberculosis, is one of the most prevalent and economically important diseases of livestock and other ruminants. The USDA estimates that approximately 22% of all dairy herds and 8% of all beef herds in the United States have Johne's disease, causing an annual loss of more than $200 million to the dairy industry alone (16). Attempts to control Johne's disease by vaccination, chemoprophylaxis, and diagnosis have proven extremely difficult, primarily because of the nature of the disease. For example, even though animals become infected at an early age by ingesting organisms shed in the milk or feces by older animals, several years are usually required before they begin to pass organisms in their feces and to show signs of disease.
Johne's disease could be controlled by diagnosis coupled with good animal husbandry, but this is currently impractical because commercially available diagnostic tests have relatively low sensitivity levels and are incapable of detecting prepatent infections. Also, there is essentially no information on cross-reactivity with other bacteria, including mycobacteria, for the commercial ELISAs for M. avium subsp. paratuberculosis. The fecal culture test still is recognized as the gold standard for diagnosis, but it requires 5 to 16 weeks to complete, has a sensitivity rate of approximately 38% (17), and like the PCR test cannot distinguish between pass-through bacilli and those arising from colonization of the intestinal tract. In general, commercial ELISAs are capable of identifying approximately 50% of the animals found to be positive by the fecal culture test (reviewed in reference 17). The sensitivities of commercial ELISAs for cattle were reported to be 8.9 to 32.1% for low-level fecal shedders and 47.1 to 62.9% for midlevel shedders (called low and middle shedders) (10). However, a recent longitudinal study by Sweeney et al. (14) suggests that commercial ELISAs might have an even lower sensitivity rate of 13.5%.
Although feces from high shedders have the greatest potential individually to transmit M. avium subsp. paratuberculosis to other members of the herd, high shedders are fewer in number, easier to diagnose, and are likely to be culled. As pointed out by Sweeney (13), even a small dose of M. avium subsp. paratuberculosis organisms may cause infections in newborn calves. Since low and middle shedders may constitute approximately 80% of fecal culture-positive cattle (17), detection and proper herd management of low and middle shedders are extremely important for herd management in preventing transmission of M. avium subsp. paratuberculosis. Further, the Committee on Diagnosis and Control of Johne's Disease has emphasized the need to develop a rapid, economically feasible test (such as an enzyme-linked immunosorbent assay [ELISA]) for the purpose of prepurchase testing of replacement animals to detect low and middle shedders as well as high shedders (8). Considering the low sensitivities of current ELISAs, especially for low and middle shedders, it appears that little progress will be made in controlling Johne's disease until a substantially more sensitive ELISA is available commercially.
Recently, we discovered that a flow cytometric method (FCM) could be used to diagnose early as well as chronic Johne's disease by testing serum for antibodies against M. avium subsp. paratuberculosis (3). The FCM had diagnostic sensitivity and specificity levels greater than 95% and was capable of detecting Johne's disease 6 to 44 months earlier than the fecal test and 17 to 67 months earlier than a commercial ELISA. Detecting animals before they become patent would enable livestock producers to control Johne's disease by reducing environmental contamination.
After finding that surface antigens are the key to the high sensitivity and subspecies specificity of the FCM for detecting M. avium subsp. paratuberculosis infections, we concentrated our efforts on developing a user-friendly and less-expensive diagnostic test for Johne's disease. We then found that surface antigens could be extracted by treating bacilli with formalin and a brief period of sonication. The extracted antigens were then used for the diagnosis of Johne's disease in an ELISA that had specificity and sensitivity levels similar to those of the FCM (11). Recognizing the hazardous nature of formalin, we performed the studies reported here to evaluate other chemicals for their ability to extract surface antigens from M. avium subsp. paratuberculosis, which were then tested in an ELISA for the diagnosis of Johne's disease.
MATERIALS AND METHODS
Mycobacterial cultures.
The Linda strain of M. avium subsp. paratuberculosis obtained from the USDA (Ames, IA) and the 706 strain of Mycobacterium avium subsp. avium obtained from P. Small at the University of Tennessee (Knoxville, TN) were cultured in Middlebrook 7H9 medium (Becton Dickinson, Cockeysville, MD) with 10% OADC (oleic acid-albumin-dextrose-NaCl) (Becton Dickinson Microbiology Systems, Franklin Lakes, NJ). In the case of M. avium subsp. paratuberculosis, the medium was supplemented with 2 μg/ml of Mycobactin J (Allied Monitor, Fayette, MO). The cultures were maintained at 37°C without shaking until they reached an optical density of approximately 0.7 at 600 nm. M. avium subsp. paratuberculosis and M. avium subsp. avium organisms were harvested from stationary-phase cultures that contained approximately 7 mg/ml of bacilli.
Serum samples.
Thirty-eight M. avium subsp. paratuberculosis-negative serum samples were collected from female dairy cattle (Holstein-Friesian; 5 months to 9 years old [mean ± standard deviation {SD} = 3.4 ± 2.7]) on herds that had tested negative for M. avium subsp. paratuberculosis infections by ELISA (Kyoritsu, Seiyako Co., Tokyo, Japan) for five consecutive years and by ELISA, fecal culture, PCR, and gamma interferon tests during the last year of sample collection. These samples also tested negative for M. avium subsp. paratuberculosis infections by the FCM, which is capable of detecting M. avium subsp. paratuberculosis infections 6 to 44 months earlier than the fecal culture test (3). Sixty-four M. avium subsp. paratuberculosis-positive serum samples were collected from female dairy cattle (Holstein-Friesian; 1 to 8 years old [mean ± SD = 4.1 ± 2.0]) that had tested positive for M. avium subsp. paratuberculosis infections by the fecal culture test.
Serum samples were also collected from male Holstein-Friesian calves that had been experimentally inoculated with M. avium subsp. paratuberculosis, M. avium subsp. avium, Mycobacterium bovis, or saline as described previously (15). In brief, 2-week-old calves were intratonsillarly inoculated four times with approximately 4 × 106 CFU of mycobacteria at 1-week intervals for 4 weeks, and serum samples were collected at 1- to 2-week intervals for up to 321 days after the first inoculation.
Serum samples were separated into aliquots of 20 to 1,000 μl and stored at −20°C for short-term storage (<6 months) or at −80°C for long-term storage (>6 months).
Preparation of antigens.
Various chemical extracting agents, including acetone, acetonitrile, chloroform, dichloromethane, ethanol, ether, hexane, methanol, and isopropanol, were evaluated for their ability to extract surface antigens of M. avium subsp. paratuberculosis, which were then used to prepare ELISA plates. M. avium subsp. paratuberculosis was harvested from liquid cultures at stationary phase and centrifuged at 2,600 × g for 10 min; the pellet was resuspended in extracting agent, agitated by vortex at room temperature for 2 min, and centrifuged at 10,621 × g for 10 min; and 50 μl of supernatant was inoculated into each well of a 96-well plate (PolySorp, Nunc-Immuno 96 microwell plate; Nalge Nunc International, Rochester, NY). Different volumes of extracting agent were used depending on the number of wells required for the various experiments; the details are explained in each figure legend. The plates were then incubated overnight with the covers removed in a fume hood at room temperature to allow the extracted antigens to adhere to the surfaces of the wells by evaporation. Because the plates prepared by ethanol extraction gave the best results, various ethanol concentrations (0 to 100%), durations of vortex (0 to 120 s), and amounts of M. avium subsp. paratuberculosis (2 to 4,000 μg/well) were used to prepare the ELISA plates. Because treatment with ethanol followed by a 30-second vortex agitation was used to prepare antigen for plate preparation, the assay was referred to as an ethanol vortex ELISA (EVELISA). The EVELISA plates were prepared by inoculating 50 μl of ethanol-extracted antigens into each well of a 96-well microtiter plate, which was then dried in a fume hood and usually used for experimentation within 3 days after preparation. To determine the shelf life of the EVELISA plates, several plates were prepared, vacuum-sealed, stored for up to 7 weeks, and then tested by the EVELISA for reactivity with 35 JD-positive and 34 JD-negative serum samples.
ELISAs and analysis.
Each well of a microtiter plate coated with antigens of M. avium subsp. paratuberculosis or M. avium subsp. avium was incubated with 200 μl of buffer A at room temperature for 1 hour, washed twice with 100 μl of PBST (10 mM phosphate-buffered saline, pH 7.0, containing 0.5% Tween 80), and inoculated with 50 μl of M. avium subsp. paratuberculosis-positive, M. avium subsp. paratuberculosis-negative, M. avium subsp. avium-positive, or M. bovis-positive serum that was prepared by diluting each serum sample 100 times with buffer A and incubating it at room temperature for 1 hour. After the wells were washed four times with 100 μl of PBST, each well was inoculated with 50 μl of horseradish peroxidase (HRP)-labeled goat anti-bovine immunoglobulin G (IgG) (heavy plus light chains) polyclonal antibody (1:500 dilution; Jackson ImmunoResearch Laboratories, Westgroup, PA) or with 50 μl of biotin-labeled goat anti-bovine IgG (heavy plus light chains) polyclonal antibody (1:500 dilution; Jackson ImmunoResearch Laboratories) and incubated at room temperature for 1 hour. Wells treated with biotin-labeled secondary antibody were further treated with horseradish peroxidase-labeled streptavidin (0.5 μg/ml; Pierce Biotechnology, Rockford, IL) at room temperature for 1 hour. After the wells were washed five times with 100 μl of PBST, ABTS tablets (2,2′-azinobis [3-ethylbenzthiazoline-6-sulfonic acid] and diammonium salt) were used to develop color reactions according to the manufacturer's instructions, and optical densities were determined by using a microplate reader (model 680; Bio-Rad, Hercules, CA) at 415 nm for 16 min at 2-min intervals. Aliquots of the same serum samples were also tested by a commercial ELISA (Biocor Animal Health, Omaha, NE) according to the manufacturer's instructions.
Serum samples from female Holstein-Friesian cattle on the University of Tennessee Cherokee Dairy farm that had tested M. avium subsp. paratuberculosis negative or M. avium subsp. paratuberculosis positive by the FCM (3) were used as M. avium subsp. paratuberculosis-negative controls (NC) and M. avium subsp. paratuberculosis-positive controls (PC), respectively. These serum samples were used to optimize conditions for the EVELISA and to determine SOD/POD values, which were calculated by using the following formula: [(SOD − NOD)/(POD − NOD)] × 10, where SOD is the optical density of a sample, NOD is the optical density obtained using the NC serum, and POD is the optical density obtained using the PC serum. The diagnostic specificity and sensitivity of the EVELISA were determined using the following formulae: diagnostic specificity was determined by dividing the number of cattle tested negative by the EVELISA by the number of cattle tested negative by the fecal culture test and multiplying the result by 100 (%); and diagnostic sensitivity was determined by dividing the number of cattle tested positive by the EVELISA by the number of cattle tested positive by the fecal culture test and multiplying the result by 100 (%).
Statistical analysis.
All experiments were conducted in duplicate or triplicate and repeated at least twice. Data from triplicate experiments are presented as the mean ± SD. Wilcoxon's rank sum test was used to compare SOD/POD values for fecal culture-negative and -positive samples. P values below 0.05 were considered statistically significant. JMP software (release 5.1.2; SAS Institute Inc., Cary, NC) was used for the statistical analysis.
RESULTS
Chemical extracting agents.
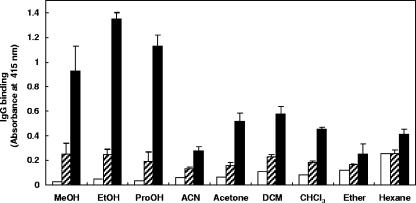
Surface antigens extracted from M. avium subsp. paratuberculosis by use of various organic solutions were immobilized on 96-well plates and tested for IgG binding by using NC and PC serum samples. The greatest level of IgG binding with PC serum was observed when ethanol-extracted antigens were used (Fig. 1). The greatest difference (5.5-fold) between M. avium subsp. paratuberculosis antigen-IgG binding by NC and PC serum samples occurred with antigens extracted in ethanol. Methanol and propanol showed similar results, whereas other less-polar organic solutions resulted in lower levels of M. avium subsp. paratuberculosis antigen-IgG binding and less differentiation between NC and PC serum samples (Fig. 1). Relatively low levels of IgG binding to alcohol-extracted M. avium subsp. paratuberculosis antigens occurred when serum treatment was omitted (Fig. 1). Based on these results, ethanol was used thereafter to extract M. avium subsp. paratuberculosis antigens.
FIG. 1.
IgG binding to M. avium subsp. paratuberculosis antigens extracted with various organic solutions. Eight milligrams of M. avium subsp. paratuberculosis was suspended in 400 μl of each organic solution, agitated by vortex at room temperature for 2 min, and centrifuged, and 50-μl volumes of the supernatants were inoculated into each well of a 96-well microtiter plate. The supernatants were allowed to evaporate at room temperature overnight, after which the plate was used in an ELISA. After incubation of the wells with a buffer (open bars), NC serum (hatched bars), or PC serum (solid bars), IgG binding was detected by using biotin-labeled anti-bovine IgG antibody and HRP-labeled streptavidin. Abbreviations: MeOH, methanol; EtOH, ethanol; ProOH, isopropanol; ACN, acetonitrile; DCM, dichloromethane; CHCl3, chloroform. Each hatched and solid bar represents the mean ± standard deviation (n = 3) of absorbance values at 415 nm. Each open bar represents the mean (n = 2) absorbance value at 415 nm. This experiment was repeated twice with similar results.
Effects of ethanol concentrations.
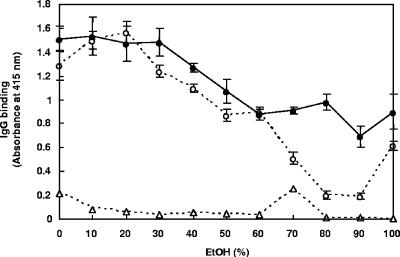
Different concentrations of ethanol were tested for their efficacy in extracting M. avium subsp. paratuberculosis and M. avium subsp. avium antigens and in IgG binding by PC serum. Similar levels of IgG binding were obtained with M. avium subsp. paratuberculosis and M. avium subsp. avium antigens extracted in 0 to 30% ethanol, which progressively declined with 40 to 60% ethanol (Fig. 2). Compared to the results with M. avium subsp. avium, high IgG binding levels were obtained when M. avium subsp. paratuberculosis antigens were extracted with 70 to 90% ethanol, showing the greatest difference (4.9-fold) with 80% ethanol (Fig. 2). Low levels of IgG binding occurred in wells treated with different concentrations of ethanol only (Fig. 2). Based on this finding, 80% ethanol was used thereafter to prepare mycobacterial antigens for the EVELISA.
FIG. 2.
Effects of various ethanol concentrations on IgG binding to antigens of M. avium subsp. paratuberculosis and M. avium subsp. avium determined by EVELISA. Twelve milligrams of M. avium subsp. paratuberculosis (solid circles) or M. avium subsp. avium (open circles) was suspended in 300 μl of different concentrations of ethanol, agitated by vortex at room temperature for 1 min, and centrifuged, and 50 μl of the supernatant was inoculated into each well of a 96-well microtiter plate. After the solution evaporated at room temperature overnight, the plate was used in an ELISA. As a negative control, each concentration of ethanol (open triangle) was also evaporated in the wells of 96-well plates. After incubation of the wells with PC serum, IgG binding was detected by using biotin-labeled anti-bovine IgG antibody and HRP-labeled streptavidin. Each point represents the mean ± standard deviation (n = 3) of absorbance at 415 nm. This experiment was repeated twice with similar results. EtOH, ethanol.
Effects of intervals of agitation by vortex.
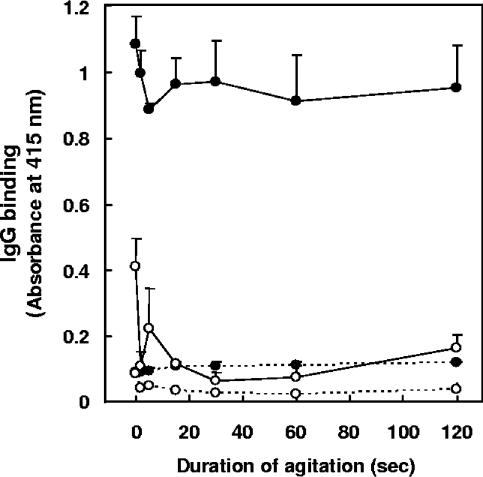
Antigens of M. avium subsp. paratuberculosis and M. avium subsp. avium, prepared by agitating bacilli suspended in 80% ethanol by vortex for various time intervals, were tested for IgG binding in NC and PC serum samples. Binding of IgG in PC serum to M. avium subsp. paratuberculosis antigens (Fig. 3) occurred even without agitation, and the levels of IgG-M. avium subsp. paratuberculosis binding were relatively consistent for all durations of agitation. IgG binding levels in NC serum (Fig. 3) were 8.2- to 12.2-fold lower than those in PC serum. In PC serum, the levels of IgG binding to M. avium subsp. avium antigens (Fig. 3) varied depending on the duration of agitation, with the lowest levels of nonspecific binding occurring at 30 seconds. Therefore, in subsequent experiments, M. avium subsp. paratuberculosis antigens were extracted by agitating bacilli in 80% ethanol for 30 seconds.
FIG. 3.
Effects of duration of agitation on IgG binding to antigens of M. avium subsp. paratuberculosis and M. avium subsp. avium by EVELISA. Eighteen milligrams of M. avium subsp. paratuberculosis (solid circles) or M. avium subsp. avium (open circles) bacilli were suspended in 600 μl of 80% ethanol, agitated by vortex at room temperature for various intervals, and centrifuged, and 50 μl of the supernatant was inoculated into each well of a 96-well microtiter plate. After evaporation at room temperature overnight, the plate was used in an ELISA. After incubation of the wells with NC (dotted line) or PC (solid line) serum, IgG binding was detected by using biotin-labeled anti-bovine IgG antibody and HRP-labeled streptavidin. Each point represents the mean ± standard deviation (n = 3) of absorbance at 415 nm. This experiment was repeated twice with similar results.
Quantification of mycobacteria.
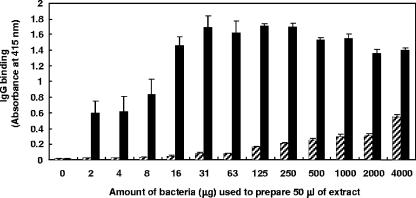
Different quantities of M. avium subsp. paratuberculosis were used to prepare M. avium subsp. paratuberculosis antigens, which were then tested for IgG binding in NC and PC serum samples. IgG in PC serum bound to M. avium subsp. paratuberculosis antigens at the lowest quantity (2 μg/well) of antigen tested, and the level of IgG binding increased coincidentally with an increase in quantity of M. avium subsp. paratuberculosis antigen (Fig. 4). The binding of IgG in PC serum increased with increasing quantities of M. avium subsp. paratuberculosis antigens, reaching saturation at 16 μg/well. The maximum level (31-fold) of differentiation between NC and PC serum samples was observed at 16 μg/well (Fig. 4). Fifty micrograms/well of antigen was used in all subsequent experiments. In addition, since similar levels of differentiation were obtained between NC and PC serum samples when either an HRP-labeled secondary antibody only or a combination of biotin-labeled secondary antibody and HRP-labeled streptavidin was used (data not shown), the single-step HRP-labeled antibody was used in the subsequent experiments.
FIG. 4.
Effects of quantity of bacteria on IgG binding to antigens of M. avium subsp. paratuberculosis analyzed by EVELISA. After M. avium subsp. paratuberculosis was suspended in 80% ethanol to adjust the cell density to 4,000 μg of M. avium subsp. paratuberculosis/50 μl, agitated by vortex at room temperature for 30 seconds, and centrifuged, the supernatant was collected. After the supernatant was serially diluted in 80% ethanol, 50 μl of the diluted supernatant was inoculated into each well of a 96-well microtiter plate and allowed to evaporate at room temperature overnight before being used in an ELISA. After incubation of the wells with NC (hatched bars) or PC (solid bars) serum samples, IgG binding was detected by using biotin-labeled anti-bovine IgG antibody and HRP-labeled streptavidin. Each bar represents the mean ± standard deviation (n = 3) of absorbance at 415 nm. This experiment was repeated twice with similar results.
Diagnostic specificity and sensitivity.
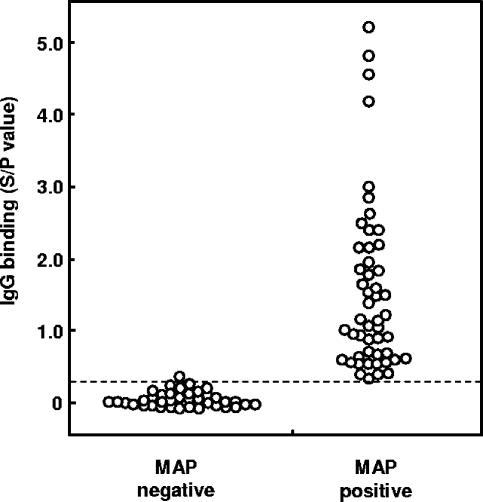
After determining the optimal conditions for preparing mycobacterial antigens (i.e., 80% ethanol, 30 seconds of agitation by vortex, and 50 μg bacilli/well), serum samples from 38 JD-free cattle and serum samples from 51 fecal culture-positive cattle were tested for IgG binding to determine the diagnostic specificity and sensitivity of the EVELISA. Ethanol-extracted antigens, obtained from 50 μg of M. avium subsp. paratuberculosis, contained approximately 112 ± 22 ng of carbohydrate (mean ± standard deviation of results of triplicate experiments; glucose equivalent, phenol sulfuric assay) (2) and 53 ± 2 ng of protein (mean ± standard deviation for triplicate experiments; bovine serum albumin equivalent, Coomassie protein assay kit; Pierce Biotechnology, Rockford, IL). Ethanol-extracted antigens, obtained from 50 μg of M. avium subsp. avium, contained 250 ± 55 ng of carbohydrate and 26 ± 1 ng of protein. To avoid possible false positives that might arise from pass-through bacilli, cattle whose feces produced fewer than 5 colonies by fecal culture were excluded from the analyses of diagnostic specificity and sensitivity. With a cutoff value of 0.24 (Fig. 5), all except one animal tested negative by the EVELISA, whereas all fecal culture-positive cattle tested positive (Fig. 5). The diagnostic specificity and sensitivity of the EVELISA were 97.4 and 100%, respectively. The difference between the SOD/POD values of Mycobacterium avium subsp. paratuberculosis-negative and Mycobacterium avium subsp. paratuberculosis-positive cattle was statistically significant (Wilcoxon's rank sum test, P < 0.0001).
FIG. 5.
Diagnostic specificity and sensitivity of EVELISA. M. avium subsp. paratuberculosis-negative serum samples were obtained from cattle on dairy farms that had tested JD negative by ELISA for 5 years and by fecal culture, PCR, gamma interferon tests, and ELISAs performed during the fifth year. M. avium subsp. paratuberculosis (MAP)-positive serum samples were obtained from cattle whose feces resulted in formation of more than 4 colonies by the fecal culture test. A cutoff value of 0.24 was used to distinguish M. avium subsp. paratuberculosis-negative and -positive results. Similar results were obtained in three separate experiments.
The sensitivity of the EVELISA was then compared with that of a commercial ELISA (Biocor) by testing serum samples from 64 fecal culture-positive cattle categorized as low (<10 colonies; n = 29), middle (10 to 50 colonies; n = 8), and high (>50 colonies; n = 27) shedders as described previously (16). In this analysis, all of the fecal culture-positive cattle were included, regardless of the number of colonies formed in the fecal culture test. The EVELISA identified 96.6% of the low shedders and 100% of the middle and high shedders, whereas the Biocor ELISA detected only 13.7% of the low shedders, 25% of the middle shedders, and 96.2% of the high shedders. Overall, the EVELISA had a sensitivity of 98.4%, detecting all but one fecal culture-positive animal, whereas the Biocor ELISA detected 50% of the fecal culture positives.
The diagnostic specificity and sensitivity of EVELISA plates that had been vacuum-sealed and stored for 7 weeks were 96.9% and 100%, respectively. These data suggest that the EVELISA plates have an adequate shelf life.
Specificity of anti-M. avium subsp. paratuberculosis IgG detection.
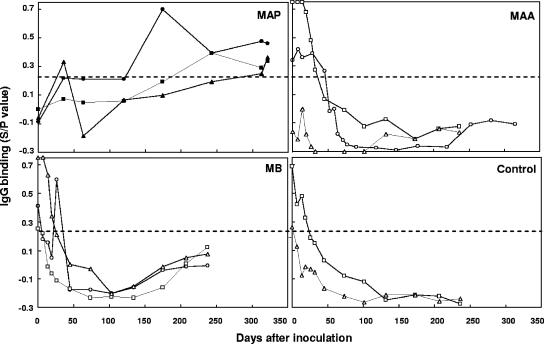
To determine if detection of anti-M. avium subsp. paratuberculosis IgG by EVELISA was specific for infections of M. avium subsp. paratuberculosis, we tested serum samples obtained from calves experimentally inoculated with M. avium subsp. paratuberculosis and other mycobacteria. With a cutoff SOD/POD value of 0.24 (Fig. 5), two calves (5902 and 5904) inoculated with M. avium subsp. paratuberculosis showed positive IgG binding at 174 and 313 days, respectively, after M. avium subsp. paratuberculosis inoculation (Fig. 6). IgG binding by serum samples from the third M. avium subsp. paratuberculosis-inoculated calf (5903) was positive at 35 days postinoculation, then dropped below the cutoff value, and became positive again at 174 days postinoculation (Fig. 6). Although the levels of IgG binding in calves inoculated with M. avium subsp. avium, M. bovis, or saline (control) were positive at the beginning of the experiment, probably due to the presence of maternal antibodies (3), they dropped markedly during the first 50 days and stayed lower than the cutoff value at 50 to 316 days (Fig. 6).
FIG. 6.
Specific detection of M. avium subsp. paratuberculosis infections by EVELISA in calves experimentally inoculated with M. avium subsp. paratuberculosis (MAP), M. avium subsp. avium (MAA), and M. bovis (MB). Serum samples were obtained from calves intratonsillarly inoculated with M. avium subsp. paratuberculosis (n = 3; squares, calf 5903; circles, calf 5902; triangles, calf 5904), Mycobacterium avium subsp. avium (n = 3; squares, calf 193; circles, calf 6137; triangles, calf 2016), M. bovis (n = 3; squares, calf 202; circles, calf 2354; triangles, calf 203) and saline (control; n = 2; squares, calf 191; triangles, calf 192) at 1- or 2-week intervals for up to 321 days. A cutoff value of 0.24 (dotted line) was used to distinguish M. avium subsp. paratuberculosis-negative and -positive results. Similar results were obtained in two separate experiments.
DISCUSSION
Various serological diagnostic tests have been developed for JD, including ELISAs, agar gel immunodiffusion tests, and complement fixation tests. The most frequently used tests for JD are conventional ELISAs, because of their relatively short turnaround time and low cost. However, some recent reports have shown that conventional ELISAs are capable of detecting only 37 to 45% of fecal culture-positive cattle (12, 17). Further, McKenna et al. (7) found recently that sensitivities of commercial ELISAs for detecting M. avium subsp. paratuberculosis infections ranged from 13.9 to 27.8% compared to the fecal culture test. In addition, a very recent report involving a longitudinal study performed on nearly 3,000 serum samples from known JD-positive and JD-negative cattle as determined by fecal culture found that the Biocor ELISA had a sensitivity level of only 13.5% (14). Considering that the fecal culture test has been shown to have a diagnostic sensitivity level of 38% (17), actual sensitivities of commercial ELISAs to detect M. avium subsp. paratuberculosis infections may be less than 10%, especially since a recent study found that commercial ELISAs detect only 6.9 to 8.8% of tissue culture-positive cattle (7). Furthermore, the extent to which commercial ELISAs for M. avium subsp. paratuberculosis are contaminated with nonspecific, cross-reacting antigens is not known. Cross-reacting antigens are likely shared with other mycobacteria and perhaps other bacteria. Since commercial ELISAs are unable to detect early infections and have low detection rates for low and middle shedders, the effectiveness of conventional ELISAs in JD control is questionable.
The antigens used in conventional ELISAs generally consist of purified protein derivative (PPD) (18) or lipoarabinomannan (LAM) (4) that is obtained by extensive physical and chemical disruption of M. avium subsp. paratuberculosis cells. The use of harsh conditions to extract mycobacterial antigens is likely partially responsible for the low levels of diagnostic sensitivity and specificity of conventional ELISAs. None of the tests are based on antigens extracted from the mycobacterial surface. Recently, we discovered that an FCM using whole bacilli with a complete repertoire of surface antigens provided a highly sensitive and subspecies-specific means of serological testing for JD (3). The FCM provided diagnostic sensitivity and specificity levels of 95.2% and 96.7%, respectively. In a retrospective study, the FCM detected M. avium subsp. paratuberculosis infections 6 to 44 months earlier than the fecal culture test and 17 to 67 months earlier than the Biocor ELISA. Also, with the FCM, no cross-reactivity occurred when M. avium subsp. paratuberculosis-positive serum was reacted with Escherichia coli and other closely related mycobacteria, including M. avium subsp. avium, M. bovis, Mycobacterium gordonae, Mycobacterium phlei, Mycobacterium scrofulaceum, Mycobacterium smegmatis, and Mycobacterium szulgai (unpublished data).
In a follow-up study, we converted the FCM to an ELISA format using antigens that were first fixed with formalin and then gently dislodged from the mycobacterial surface by a brief interval of sonication (11). An ELISA consisting of wells coated with surface antigens had diagnostic sensitivity and specificity levels greater than 95%. Surprisingly, the greatest difference between M. avium subsp. paratuberculosis-positive and -negative serum samples was obtained with full-strength formalin (37% formaldehyde and 10 to 15% methanol). We used formalin to chemically fix M. avium subsp. paratuberculosis because it is known to maintain antigenic integrity and reactivity against specific antibodies (1). However, formalin is extremely hazardous, even at low concentrations.
In this study, various chemical and physical treatments were evaluated for their ability to extract and maintain the diagnostic specificity and sensitivity of surface antigens from M. avium subsp. paratuberculosis when the antigens were tested in an ELISA-based format for reactivity against JD-positive and JD-negative cattle sera. Bacilli were chemically treated and then subjected to a mild physical agitation to dislodge the surface antigens, which were then recovered in the supernatant and used in the EVELISA. Interestingly, after chemical treatment, a mild agitation by vortex for only 30 seconds dislodged the surface antigens. Although we used a short duration of agitation to dislodge M. avium subsp. paratuberculosis antigens with ethanol solution, our data also showed that the antigens could be extracted by ethanol treatment without agitation. Perhaps centrifugation to pellet the bacilli was sufficient to cause dislodgement of the antigens from the surfaces of the bacilli. It is unknown why the level of IgG binding in M. avium subsp. paratuberculosis-negative serum samples was higher for antigens extracted without agitation than for antigens extracted with short durations of agitation. Binding of IgG in M. avium subsp. paratuberculosis-negative serum samples was also slightly higher with longer periods of agitation (120 s), indicating that antigens were being released from M. avium subsp. paratuberculosis which reacted nonspecifically with bovine IgG or that longer periods of agitation caused structural changes in the antigens, resulting in cross-reactivity.
Six of the nine chemicals tested resulted in ELISAs with low sensitivity, whereas relatively high sensitivity levels were obtained when surface antigens were extracted with ethanol, methanol, or isopropyl alcohol. Although methanol and isopropyl alcohol gave acceptable results, antigens extracted with ethanol provided the greatest difference between serum samples from JD-positive and JD-negative cattle. Based on these results, extraction of surface antigens was tested by using distilled water and various concentrations of ethanol ranging from 10 to 100%. Our data showed that antigens of M. avium subsp. paratuberculosis could be extracted by distilled water only; however, there was no difference between serum IgG levels of binding to extracts of M. avium subsp. paratuberculosis and M. avium subsp. avium, indicating that the water extracts of the mycobacteria contained mostly cross-reactive antigens. The most significant differences in sensitivity between JD-positive and JD-negative serum samples were obtained by treating bacilli with 70 to 90% ethanol, especially 80% ethanol. Interestingly, when 80 or 90% ethanol was used for antigen extraction, higher levels of IgG binding occurred with antigen extracts from M. avium subsp. paratuberculosis than with those from M. avium subsp. avium. This indicates that antigens extracted with 80 to 90% ethanol contained M. avium subsp. paratuberculosis-specific antigens; however, it is also possible that bacterial extracts of M. avium subsp. paratuberculosis contained more antigens or that there were more sites available for IgG binding than were present in M. avium subsp. avium. Since the concentrations of carbohydrate and protein in bacterial extracts of M. avium subsp. paratuberculosis and M. avium subsp. avium were similar, it is unlikely that the high-level IgG binding to M. avium subsp. paratuberculosis extract was due to differences in amounts of antigens. It is more likely that ethanol-extracted antigens of M. avium subsp. paratuberculosis are structurally different from those of M. avium subsp. avium. While none of the extracted antigens were defined in this study, biochemical and proteomics approaches are being pursued, aided by the recent completion of the genome of M. avium subsp. paratuberculosis (6).
The effects of various quantities of mycobacteria were also tested by the EVELISA to determine the optimum starting quantity of bacilli/well. Starting quantities ranging from 31 to 250 μg of bacilli/well gave the best results when tested by the EVELISA. Since there were only minor differences in IgG binding levels when 31 to 250 μg/well of mycobacteria was used, 50 μg of bacilli/well was used in all of the EVELISAs. Further analysis revealed that when 50 μg bacilli/well was used, each well contained 112 ng of carbohydrate and 53 ng of protein, which are comparable to the amounts reported earlier for a PPD-ELISA (5) and a LAM-ELISA (15) for M. avium subsp. paratuberculosis. However, it is known that PPD and LAM antigens are not specific to M. avium subsp. paratuberculosis, and it is likely that few surface antigens are present in PPD and LAM antigen preparations. In our previous report concerning a sonication ELISA (SELISA) (11), we used formalin and sonication to extract surface antigens of M. avium subsp. paratuberculosis. When 1 mg bacteria/well was used, chemical analysis showed that each well of a 96-well plate contained 3,200 ng protein and 2,300 ng carbohydrate (11). In the present study involving the EVELISA, each well contained 53 ng protein and 112 ng carbohydrate, which are 60 times less protein and 23 times less carbohydrate than the SELISA had. Assays for lipid content were not performed in either study. It is not known whether the subspecies-specific antigens are protein, carbohydrate, or lipid. Further biochemical characterization of the ethanol-extracted M. avium subsp. paratuberculosis antigens is currently being conducted in our laboratory.
When serum samples from JD-free cattle and from fecal culture-positive cattle were used, the diagnostic specificity and sensitivity of the EVELISA were 97.4 and 100%, respectively. With the same serum samples, a commercial ELISA (Biocor) detected only 50% of the fecal culture-positive cattle. This sensitivity of the commercial ELISA is consistent with the sensitivity rate of 57% reported earlier by Sockett et al. (10) and that of 45% reported by Sweeney et al. (12). Further, it was reported that the diagnostic sensitivities of a commercial ELISA for low and middle shedders are 8.9 to 32.1% and 47.1 to 62.9%, respectively (9). In this study, the Biocor ELISA detected 13.7% of the low shedders and 25% of the middle shedders. In contrast, the EVELISA detected 96.6% of the low shedders and 100% of the middle shedders, demonstrating that the diagnostic sensitivity of the EVELISA, especially for low shedders, is much greater than that of the commercial ELISA.
Further, the present study showed that the EVELISA is capable of detecting anti-M. avium subsp. paratuberculosis IgG in calves inoculated with M. avium subsp. paratuberculosis as early as 174 days after inoculation, whereas from 50 to 320 days after inoculation, no positive levels of IgG binding were detected in serum samples from calves inoculated with M. avium subsp. avium or M. bovis. These results indicate that detection of anti-M. avium subsp. paratuberculosis IgG by the EVELISA is subspecies specific for Johne's disease.
A recommendation made by the Committee on Diagnosis and Control of Johne's Disease (8) stated that “early identification of infected animals would support control programs, especially for prepurchase testing of replacement animals. Exposure to other mycobacteria, such as M. avium subsp. avium, is likely to be common in cattle, so it is essential that any test to identify animals in the early stages of infection be highly specific for Map [sic].” Our data show that the EVELISA may satisfy these requirements as a diagnostic test for JD control by detecting early M. avium subsp. paratuberculosis infections and by being subspecies specific. However, further evaluation and validation of the EVELISA by using numerous samples are required.
Although the diagnostic specificity and sensitivity of the EVELISA were equivalent to those of the SELISA, the EVELISA has several advantages compared to the SELISA. For example, the EVELISA (i) uses ethanol as an extraction agent, which is less hazardous than formalin; (ii) requires 20-times-fewer bacteria for plate preparation; and (iii) can be completed in a shorter time period because it utilizes an HRP-labeled secondary antibody instead of a combination of biotin-labeled secondary antibody and HRP-labeled streptavidin. In addition, EVELISA plates that had been vacuum-sealed and stored for seven weeks (the longest interval tested) had diagnostic specificity and sensitivity rates of 96.9 and 100%, respectively, showing that storage of the plates had no adverse effect on the specificity and sensitivity of the EVELISA.
In conclusion, this study shows that the EVELISA is subspecies specific and highly sensitive in detecting early as well as late stages of M. avium subsp. paratuberculosis infections. Plates for the EVELISA can be prepared quickly and safely with a relatively small amount of bacteria and have a shelf-life of at least 7 weeks. This novel ELISA will likely form the basis for the development of a highly sensitive and specific diagnostic test for JD.
Acknowledgments
This work was supported by the following grants to C.A.S.: a veterinary services grant from the Animal and Plant Health Inspection Service, U.S. Department of Agriculture, and grants from the Food Safety Center of Excellence and Veterinary Medicine at the University of Tennessee, the B. Ray Thompson Fund, and the Tennessee Agricultural Experiment Station. We also acknowledge funding by USDA CSREES-NRI grants to J.P.B. and by the USDA Agricultural Research Service.
REFERENCES
- 1.Brown, W. J., and M. G. Farquhar. 1989. Immunoperoxidase methods for the localization of antigens in cultured cells and tissue sections by electron microscopy. Methods Cell Biol. 31:553-569. [DOI] [PubMed] [Google Scholar]
- 2.Dubois, M., K. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1951. A colorimetric method for the determination of sugars. Nature 168:167. [DOI] [PubMed] [Google Scholar]
- 3.Eda, S., B. Elliott, M. C. Scott, W. R. Waters, J. P. Bannantine, R. H. Whitlock, and C. A. Speer. 2005. New method of serological testing for Mycobacterium avium subsp. paratuberculosis (Johne's disease) by flow cytometry. Foodborne Pathog. Dis. 2:250-262. [DOI] [PubMed] [Google Scholar]
- 4.Jark, U., I. Ringena, B. Franz, G. F. Gerlach, and M. Beyerbach. 1997. Development of an ELISA technique for serodiagnosis of bovine paratuberculosis. Vet. Microbiol. 57:189-198. [DOI] [PubMed] [Google Scholar]
- 5.Koets, A. P., V. P. M. G. Rutten, M. de Boer, D. Bakker, P. Valentin-Weigand, and W. van Eden. 2001. Differential changes in heat shock protein-, lipoarabinomannan-, and purified protein derivative-specific immunoglobulin G1 and G2 isotype responses during bovine Mycobacterium avium subsp. paratuberculosis infection. Infect. Immun. 69:1492-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li, L., J. P. Bannantine, Q. Zhang, A. Amonsin, B. J. May, D. Alt, N. Banerji, S. Kanjilal, and V. Kapur. 2005. The complete genome sequence of Mycobacterium avium subspecies paratuberculosis. Proc. Natl. Acad. Sci. USA 102:12344-12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKenna, S. L., G. P. Keefe, H. W. Barkema, and D. C. Sockett. 2005. Evaluation of three ELISAs for Mycobacterium avium subsp. paratuberculosis using tissue and fecal culture as comparison standards. Vet. Microbiol. 110:105-111. [DOI] [PubMed] [Google Scholar]
- 8.National Research Council. 2003. Diagnosis and control of Johne's disease, p. 129. National Academies Press, Washington, DC. [PubMed]
- 9.Reichel, M. P., R. Kittelberger, M. E. Penrose, R. M. Meynell, D. Cousins, T. Ellis, L. M. Mutharia, E. A. Sugden, A. H. Johns, and G. W. de Lisle. 1999. Comparison of serological tests and faecal culture for the detection of Mycobacterium avium subsp. paratuberculosis infection in cattle and analysis of the antigens involved. Vet. Microbiol. 66:135-150. [DOI] [PubMed] [Google Scholar]
- 10.Sockett, D. C., T. A. Conrad, C. B. Thomas, and M. T. Collins. 1992. Evaluation of four serological tests for bovine paratuberculosis. J. Clin. Microbiol. 30:1134-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speer, C. A., M. C. Scott, J. P. Bannantine, W. R. Waters, Y. Mori, R. H. Whitlock, and S. Eda. 2006. A novel enzyme-linked immunosorbent assay for diagnosis of Mycobacterium avium subsp. paratuberculosis infections (Johne's disease) in cattle. Clin. Vaccine Immunol. 13:535-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweeney, R. W., R. H. Whitlock, C. L. Buckley, and P. A. Spencer. 1995. Evaluation of a commercial enzyme-linked immunosorbent assay for the diagnosis of paratuberculosis in dairy cattle. J. Vet. Diagn. Investig. 7:488-493. [DOI] [PubMed] [Google Scholar]
- 13.Sweeney, R. W. 1996. Transmission of paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 12:305-312. [DOI] [PubMed] [Google Scholar]
- 14.Sweeney, R. W., R. H. Whitlock, S. McAdams, and T. Fyock. 2006. Longitudinal study of ELISA seroreactivity to Mycobacterium avium subsp. paratuberculosis in infected cattle and culture-negative herd mates. J. Vet. Diagn. Investig. 18:2-6. [DOI] [PubMed] [Google Scholar]
- 15.Waters, W. R., J. M. Miller, M. V. Palmer, J. R. Stabel, D. E. Jones, K. A. Koistinen, E. M. Steadham, M. J. Hamilton, W. C. Davis, and J. P. Bannantine. 2003. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 71:5130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells, S. J., S. L. Ott, and A. H. Seitzinger. 1998. Key health issues for dairy cattle—new and old. J. Dairy Sci. 81:3029-3035. [DOI] [PubMed] [Google Scholar]
- 17.Whitlock, R. H., S. J. Wells, R. W. Sweeney, and J. Van Tiem. 2000. ELISA and fecal culture for paratuberculosis (Johne's disease): sensitivity and specificity of each method. Vet. Microbiol. 77:387-398. [DOI] [PubMed] [Google Scholar]
- 18.Yokomizo, Y., R. S. Merkal, and P. A. Lyle. 1983. Enzyme-linked immunosorbent assay for detection of bovine immunoglobulin G1 antibody to a protoplasmic antigen of Mycobacterium paratuberculosis. Am. J. Vet. Res. 44:2205-2207. [PubMed] [Google Scholar]