Abstract
We report a case of Fusarium solani mycetoma of the foot that could not be diagnosed by culture, but was correctly identified after amplification and sequence analysis of fungal internal transcribed spacers 1 and 2 and 5.8S ribosomal DNA regions.
CASE REPORT
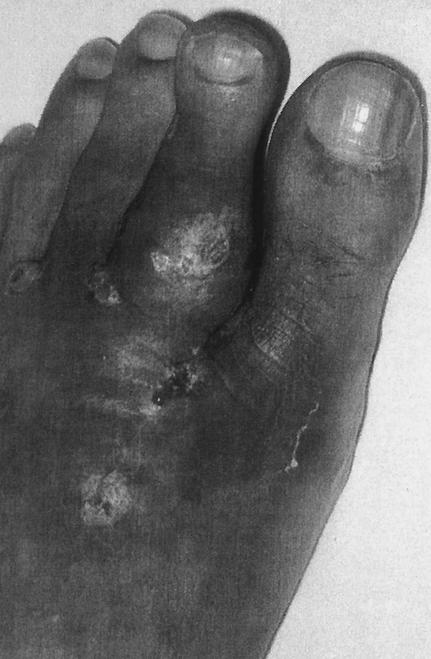
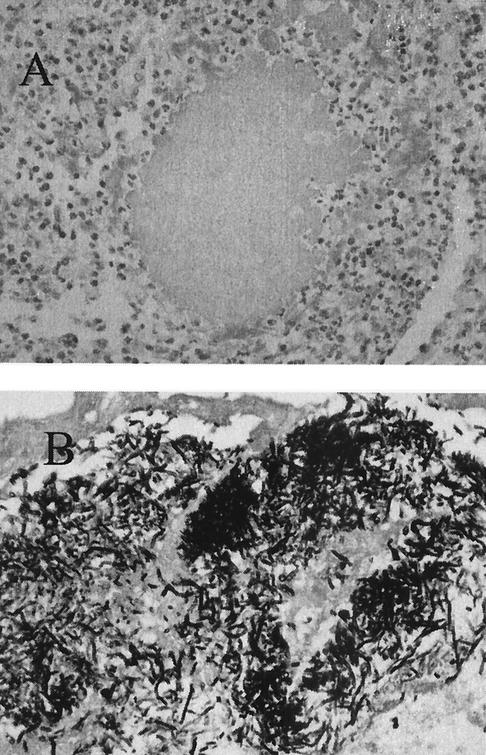
A 51-year-oldmale truck driver from Mauritius, who had been living in France since 1970, hurt his left foot with a handcart in April 2000. The wound was swollen and excoriated and was self-treated ineffectively with various local antiseptics for several months. In September 2000, he presented to the Orthopedic Department of Cochin University Hospital. Physical examination showed tumefaction of the dorsal face of the left forefoot, with a few nodules and fistulae (Fig. 1). A small fistula secreted whitish pus. The patient had trouble walking and could not wear shoes because of violent pain. X-ray films revealed a periosteal reaction involving the second and third metatarsals, a heterogeneous aspect of the first phalanx of the second toe, and involvement of the second metatarso-phalangeal joint. Magnetic resonance imaging confirmed the X-ray findings and also revealed massive dorso-plantar infiltration of the forefoot. The wound was biopsied by a dorsal approach. Direct examination of silver-stained imprints of the biopsy specimen showed septate hyphae. Culture slowly grew a beige filamentous fungus without spore formation. Identification was unsuccessful. Histologically, the specimen consisted of inflammatory tissue containing lymphocytes and altered polymorphonuclear neutrophils; no hyphae were seen. In October, a second biopsy done by a plantar approach was performed to confirm the fungal infection. Direct examination and culture were again positive, with a fungus of identical aspect, and careful microscopic examination of the histological section showed two small fungal grains (Fig. 2). Precise identification was impossible, even after the use of nutritionally deficient media (potato dextrose agar and malt agar). Treatment was started with oral itraconazole (400 mg/kg of body weight/day), and molecular identification of the fungus was attempted.
FIG. 1.
Dorsal view of the patient's left foot at initial presentation showing fistulae, nodules, and a surgical scar.
FIG. 2.
Skin biopsy specimen showing a grain consisting of fungal elements. (A) Hematoxylin and eosin staining. Original magnification, ×400. (B) Gomori methenamine silver staining. Original magnification, ×400.
The lesions failed to improve on itraconazole at 400 mg/day, and serum drug levels were found to be inadequate. The patient was hospitalized in late December 2000 with excruciating pain of the left foot. The itraconazole dose was increased. Pain diminished after a few days, and the patient was able to walk after a few weeks. An itraconazole regimen of 600 mg/day was pursued for 3 months, yielding the following levels in serum: itraconazolemia, 275 μg/liter; hydroxy-itraconazolemia, 620 μg/liter. These values were close to usual therapeutic levels (itraconazole, >250 μg/liter; hydroxy-itraconazole, >1,000 μg/liter), but we preferred to increase the daily dose to 800 mg for 4 months. Serum drug levels on this regimen were not measured. Itraconazole was clinically and biologically well tolerated, with normal liver enzyme activities and blood cell counts. The itraconazole susceptibility of the isolate could not be tested because of slow growth in vitro. However, the itraconazole treatment was effective, so surgery was not required. An X -ray film obtained in September 2001 was normal; 12 months after completion of treatment, the patient was pain free and able to walk.
Mycetoma is a chronic pseudotumorous infection of the skin and subcutaneous tissue, occasionally including bone, caused by fungi (eumycetoma) or bacteria (actinomycetoma), generally inoculated traumatically. Mycetoma is usually localized to the foot and principally occurs in tropical and subtropical regions. We describe here a case of eumycetoma of the foot that was diagnosed by PCR and DNA sequencing.
Fungal DNA was extracted from culture material by the technique described by Hennequin et al. (11) with Chelex 100 resin (Bio-Rad Laboratories, Marne-la-Coquette, France) (21). The fungus-specific universal primers internal transcribed spacer 1 (ITS1) and ITS4 were used to amplify the internal transcribed spacer and 5.8S regions of fungal ribosomal genes (22). PCR amplification was performed in a reaction mixture (final volume, 50 μl) containing 50 mM KCl, 15 mM Tris-HCl (pH 8.0), 2.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 0.4 μM each primer, 2.5 U of AmpliTaq Gold DNA polymerase (Applied Biosystems, Paris, France), and 10 μl of fungal DNA. Ten microliters of sterile water was tested as a negative control. DNA amplification was performed with the following temperature cycles: 95°C for 5 min; then 30 cycles of 20 s at 95°C, 60 s at 55°C, and 60 s at 72°C; and a final cycle at 72°C for 5 min. PCR products and a molecular weight maker were submitted to electrophoresis on 2.5% agarose gel containing ethidium bromide. A specific amplified fragment of 509 bp was obtained and purified and then was cycle sequenced in both directions with an automated sequencing system. A sequence of 509 bp was determined and compared to GenBank nucleotide sequences by using BLAST-N software (National Center for Biotechnology Information). Total identity was obtained with Fusarium solani (ITS1-5.8S rRNA-ITS2) sequences given under accession no. AF165874, AF150467, and AF440567. The molecular identification was repeated with each isolate obtained from the two biopsies and was carried out in two different laboratories.
About 23 cases of eumycetoma due to Fusarium species have been reported (Table 1). The patients' origins were the tropics (2-7, 10, 14, 16-20), Israel (15), and Italy (1) or were not specified (8, 9). Morphological identification of the Fusarium genus was often difficult, and species-level identification was only achieved in eight cases. Classically, identification is based on the white-yellowish color of the grains, the light-brown colonies (with a reddish diffusing pigment in some cases), and genus-characteristic sickle-shape spores (these can be absent). Five cases of mycetoma due to F. solani have previously been reported (5, 9, 17, 19, 20).
TABLE 1.
A review of cases of eumycetoma due to Fusarium species
| Patient origin | No. of cases | Location | Species | Treatment | Reference |
|---|---|---|---|---|---|
| Cameroon | 1a | Fusarium sp. | 14 | ||
| Cameroon | 1a | Foot | Fusarium sp. | Surgery | 7 |
| Senegal | 1 | Foot | Fusarium solani var. minus | 17 | |
| Somalia | 1 | Fusarium sp. | 6 | ||
| 1 | Fusarium solani | 9 | |||
| 1 | Fusarium oxyporum | ||||
| 2b | Fusarium spp. | ||||
| 1b | Fusarium sp. | 8 | |||
| Jamaica | 1 | Fusarium sp. | 10 | ||
| India | 4 | Fusarium spp. | |||
| Thailand | 1 | Ankle | Fusarium solani var. coeruleum | Surgery | 19 |
| Italy | 1 | Foot | Fusarium moniliforme | Antibacterial therapy | 1 |
| Mexico | 1 | Fusarium sp. + Acremonium sp. | 4 | ||
| 1 | Fusarium sp. | ||||
| Nigeria | 1 | Foot | Fusarium sp. | Ketoconazole | 2 |
| Surinam | 1c | Foot | Fusarium sp. | Itraconazole + surgery | 3 |
| Surinam | 1c,d | Foot | Fusarium solani | Itraconazole | 5 |
| Brazil | 1 | Lung | Fusarium sp. | AMBe + itraconazole + surgery | 16 |
| Colombia | 1 | Fusarium oxyporum | Itraconazole | 18 | |
| Israel | 1 | Renal pelvis | Fusarium sp. | AMB + 5FCf + surgery | 15 |
| Brazil | 1 | Hand | Fusarium solani | Ketoconazole | 20 |
Probably the same case.
Probably one shared case.
Same case (13).
Granules were not observed.
Amphotericin B.
5-Fluorocytosine.
Advances in molecular technology using sequence areas within the ribosomal DNA gene complex have shown promise for the rapid and accurate identification of fungal pathogens (12, 22). Particularly, ribosomal DNA sequencing (11) can readily identify Fusarium species, as confirmed in the case of foot mycetoma described here. These techniques are particularly welcomed when conventional methods used in routine practice have failed and when fungal pathogens cannot be cultivated. Development of molecular technology could potentially impact care and improve clinical treatment decisions.
Fusarium species are cosmopolitan fungi, which are thought to be inoculated into the skin by penetrative trauma. Our patient with a recent history of foot injury probably acquired the infection from the local French environment, because he had only spent 1 month (in 1996) in Mauritius since 1970. Interestingly, however, he had hurt the same foot 10 years previously while living in France, with a fracture of the first phalanx of the second toe. It is unlikely that the fungus was inoculated during his first injury, because he had no local clinical signs before the second injury. However, the first injury could have sensitized the tissues, allowing an easier development of the mycetoma.
Medical treatment of fungal mycetoma is usually disappointing. A case due to Fusarium has been treated with itraconazole (18), but extensive surgical excision could be necessary (3, 16). In our case, as noted above, high-dose oral itraconazole was effective without surgery, because the follow-up X-ray film obtained in September 2001 was normal (a magnetic resonance imaging control could not be performed because the patient was lost at follow up), and the patient was pain free and able to walk 12 months after completing treatment.
REFERENCES
- 1.Ajello, L., A. A. Padhye, F. W. Chandler, M. R. McGinnis, L. Morganti, and F. Alberici. 1985. Fusarium moniliforme, a new mycetoma agent. Restudy of a European case. Eur. J. Epidemiol. 1:5-10. [DOI] [PubMed] [Google Scholar]
- 2.Baudraz-Rosselet, F., M. Monod, L. Borradori, J. M. Ginalsky, B. Vion, C. Boccard, and E. Frenk. 1992. Mycetoma of the foot due to Fusarium sp. treated with oral ketoconazole. Dermatology 184:303-305. [DOI] [PubMed] [Google Scholar]
- 3.Buiting, A. G., L. G. Visser, R. M. Barge, and J. W. van 't Wout. 1993. Mycetoma of the foot; a disease from the tropics. Ned. Tijdschr. Geneeskd. 137:1513-1515. [PubMed] [Google Scholar]
- 4.Buot, G., P. Lavalle, F. Mariat, and P. Suchil. 1987. Etude épidémiologique des mycétomes au Mexique. A propos de 502 cas. Bull. Soc. Pathol. Exot. Fil. 80:329-339. [PubMed] [Google Scholar]
- 5.de Hoog, G. S., A. Buiting, C. S. Tan, A. B. Stroebel, C. Ketterings, E. J. de Boer, B. Naafs, R. Brimicombe, M. K. Nohlmans-Paulssen, G. T. Fabius et al. 1993. Diagnostic problems with imported cases of mycetoma in The Netherlands. Mycoses 36:81-87. [DOI] [PubMed] [Google Scholar]
- 6.Destombes, P., F. Mariat, L. Rosati, and G. Segretain. 1977. Les mycétomes en Somalie—conclusions d'une enquête menée de 1959 de 1964. Acta Trop. 34:355-373. [PubMed] [Google Scholar]
- 7.Gamet, A., H. Brottes, and R. Essomba. 1964. Nouveaux cas de mycétomes dépistés au Cameroun. Bull. Soc. Pathol. Exot. Fil. 57:1191-1195. [PubMed] [Google Scholar]
- 8.Hay, R. J., and M. J. Collins. 1983. An ultrastructural study of pale eumycetoma grains. Sabouraudia 21:261-269. [DOI] [PubMed] [Google Scholar]
- 9.Hay, R. J., and D. W. Mackenzie. 1982. The histopathological features of pale grain eumycetoma. Trans. R. Soc. Trop. Med. Hyg. 76:839-844. [DOI] [PubMed] [Google Scholar]
- 10.Hay, R. J., and D. W. Mackenzie. 1983. Mycetoma (madura foot) in the United Kingdom—a survey of forty-four cases. Clin. Exp. Dermatol. 8:553-562. [DOI] [PubMed] [Google Scholar]
- 11.Hennequin, C., E. Abachin, F. Symoens, V. Lavarde, G. Reboux, N. Nolard, and P. Berche. 1999. Identification of Fusarium species involved in human infections by 28S rRNA gene sequencing. J. Clin. Microbiol. 37:3586-3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwen, P. C., S. H. Hinrichs, and M. E. Rupp. 2002. Utilization of the internal transcribed spacer regions as molecular targets to detect and identify human fungal pathogens. Med. Mycol. 40:87-109. [DOI] [PubMed] [Google Scholar]
- 13.Klokke, A. H. 1993. Mycetoma of the foot; a disease from the tropics. Ned. Tijdschr. Geneeskd. 137:2056-2057. [PubMed] [Google Scholar]
- 14.Mariat, F. 1963. Sur la distribution géographique et la répartition des agents de mycétomes. Bull. Soc. Pathol. Exot. 56:35-45. [PubMed] [Google Scholar]
- 15.Nakar, C., G. Livny, I. Levy, Z. Samra, N. Linder, S. Ashkenazi, P. Livne, and L. Sirota. 2001. Mycetoma of the renal pelvis caused by Fusarium species. Pediatr. Infect. Dis. J. 20:1182-1183. [DOI] [PubMed] [Google Scholar]
- 16.Nucci, M., W. Pulcheri, N. Spector, and H. P. Oliveira. 1993. Pulmonary mycetoma treatment in neutropenic patients. Rev. Assoc. Med. Bras. 39:188-189. [PubMed] [Google Scholar]
- 17.Peloux, Y., and G. Segretain. 1966. Mycétomes à Fusarium. Bull. Soc. Fr. Mycol. Med. 1:31-32. [Google Scholar]
- 18.Restrepo, A. 1994. Treatment of tropical mycoses. J. Am. Acad. Dermatol. 31:S91-S102. [DOI] [PubMed] [Google Scholar]
- 19.Thianprasit, M., and A. Sivayathorn. 1984. Black dot mycetoma. Mykosen 27:219-226. [DOI] [PubMed] [Google Scholar]
- 20.Tomimori-Yamashita, J., M. M. Ogawa, S. H. Hirata, O. Fischman, N. S. Michalany, H. K. Yamashita, and M. Alchorne. 2002. Mycetoma caused by Fusarium solani with osteolytic lesions on the hand: case report. Mycopathologia 153:11-14. [DOI] [PubMed] [Google Scholar]
- 21.Walsh, P. S., D. A. Metzger, and R. Higuchi. 1991. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 10:506-513. [PubMed] [Google Scholar]
- 22.White, T. J., et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gefland, J. J. Sninsky, and T. J. White (ed.), PCR protocols. A guide to methods and applications. Academic Press, Inc., San Diego, Calif.