Abstract
Using hypotonically permeabilized Toxoplasma gondii tachyzoites, we investigated the topology of the free glycosylphosphatidylinositols (GPIs) within the endoplasmic reticulum (ER) membrane. The morphology and permeability of parasites were checked by electron microscopy and release of a cytosolic protein. The membrane integrity of organelles (ER and rhoptries) was checked by protease protection assays. In initial experiments, GPI biosynthetic intermediates were labeled with UDP-[6-3H]GlcNAc in permeabilized parasites, and the transmembrane distribution of the radiolabeled lipids was probed with phosphatidylinositol-specific phospholipase C (PI-PLC). A new early intermediate with an acyl modification on the inositol was identified, indicating that inositol acylation also occurs in T. gondii. A significant portion of the early GPI intermediates (GlcN-PI and GlcNAc-PI) could be hydrolyzed following PI-PLC treatment, indicating that these glycolipids are predominantly present in the cytoplasmic leaflet of the ER. Permeabilized T. gondii parasites labeled with either GDP-[2-3H]mannose or UDP-[6-3H]glucose showed that the more mannosylated and side chain (Glc-GalNAc)-modified GPI intermediates are also preferentially localized in the cytoplasmic leaflet of the ER.
Toxoplasma gondii is one of the most widespread parasites causing toxoplasmosis, a disease that affects humans and a wide variety of mammals. As with most members of the Apicomplexa, this protozoan is obligately intracellular. Toxoplasma has the ability to invade nearly all nucleated cells in an active multistep process (2, 6, 42). Several surface proteins, including the major surface antigen SAG1, are proposed to establish the first contact between the parasite and the host cell (33, 37). As in the case of many other parasitic protozoa (9, 25, 26), glycosylphosphatidylinositol (GPI)-anchored proteins such as SAG1 dominate the plasma membranes of T. gondii tachyzoites, a stage that is associated with the acute phase of infection. Structural analysis of various glycolipid anchors initially described for Trypanosoma brucei showed the presence of a conserved core structure of phosphatidylinositol-lipid linked to a glycan consisting of nonacetylated glucosamine and three mannose residues (10, 17, 38). The terminal mannose carries an ethanolamine phosphate (EtNP), which can be attached to the C-terminal amino acids of proteins via an amide bond. Various side chain modifications by additional sugars or ethanolamine phosphates have been described (7, 14, 25). In the case of T. gondii, the side chain consists of glucose-α1-4N-acetylgalactosamine linked to the first mannose (48, 59). It has been shown that free GPIs (the so-called low-molecular-weight antigen) (41) present in large amounts on the parasite surface are highly immunogenic in humans (48). Additionally, Toxoplasma GPIs can induce tumor necrosis factor alpha production in macrophages (5).
GPI membrane anchors are transferred en bloc to proteins as preassembled glycolipid precursors in a transamidase reaction cleaving off the hydrophobic C-terminal GPI signal sequence (reviewed in references 19, 29, and 50). Synthesis of the precursor starts with the transfer of GlcNAc to PI from UDP-GlcNAc, followed by de-N-acetylation, resulting in the formation of GlcN-PI (reviewed in reference 8). Dolichyl-phosphomannose (Dol-P-Man) is the donor for the subsequent mannosylation (31), and synthesis is complete after the transfer of ethanolamine phosphate from phosphatidylethanolamine (30). In T. gondii, the addition of the side chain modification occurs before transfer to the protein, most likely before addition of the terminal ethanolamine phosphate. The direct donor for the glucose modification is UDP-Glc (47). The location of the glucose and GalNAc transferase and the donor of the GalNAc modification have not been characterized yet. The poor labeling efficiency of GalNAc using tritiated UDP-GalNAc points to the possibility that a lipophilic intermediate is involved (Natacha Hyams, unpublished observations).
A common modification of GPI anchor precursors is an acyl chain linked to position 2 of inositol, which leads to resistance to PI-specific phospholipase C (PI-PLC) (25, 36). In Plasmodium falciparum and mammalian cells, GPI biosynthesis and inositol acylation, respectively, are requirements for transfer of the first mannose (12, 13). In contrast, T. brucei inositol acylation occurs only after transfer of the first mannose (15, 44).
In trypanosomal microsomes as well as a mouse Thy-1 cell line, intermediates of GPI biosynthesis were primarily located in the cytoplasmic leaflet of the endoplasmic reticulum (ER) membrane (51, 52). These findings were supported by investigations of different GPI-deficient mutants in mammalian cells and Saccharomyces cerevisiae (19, 20). The first step of GPI anchor biosynthesis is mediated by GPI-N-acetylglucosaminyltransferase, a complex consisting of at least six proteins (55, 57). Two of the proteins (PIG-A, the catalytic component, and PIG-H) were shown to be orientated to the cytoplasm (56). The same was shown for the N-acetylglucosaminylphosphatidylinositol (GlcNAc-PI) de-N-acetylase, PIG-L, which is essential for the second step and also has enzyme activity on the cytoplasmic face of the ER membrane (35). On the other hand, the GPI α1-4-mannosyltransferase encoded by the PIG-M gene possesses a functionally essential DXD motif within an ER luminal domain, suggesting that transfer of the first mannose linked to the GlcN takes place on the luminal side of the ER (22). GPI biosynthesis starts on the cytoplasmic face of the ER and ends with the transfer of the mature GPI anchor precursor to nascent proteins on the luminal side, suggesting the transfer of intermediates across the ER membrane by specific proteins called flippases. Recently, an ATP-independent, protein-mediated flip-flop of GPI lipids was reported (3, 54).
Here we show that early GPI biosynthetic intermediates of T. gondii are located primarily in the cytoplasmic leaflet of the ER membrane. We show that 71% of the first biosynthetic intermediate (GlcNAc-PI) and 82% of the following intermediate (GlcN-PI) are found on the cytoplasmic face of the ER. Additionally, we show that large amounts of the two GPI anchor precursors, glycolipids II and III [EtNP-Man2-(GalNAc-Glc)Man-GlcN-PI and EtNP-Man2-(GalNAc)Man-GlcN-PI, respectively], are also located in the cytoplasmic leaflet. Although transfer to newly synthesized proteins takes place in the ER lumen, about 66% of these two glycolipids are present on the cytoplasmic side of the ER membrane.
(This communication is presented in partial fulfillment of the requirements of the doctoral thesis of J. Kimmel.)
MATERIALS AND METHODS
Materials.
d-[6-3H]glucosamine hydrochloride (25 Ci/mmol), UDP-[6-3H]GlcNAc (18.9 Ci/mmol), GDP-[2-3H]mannose (15.1 Ci/mmol), UDP-[1-3H]GalNAc, and UDP-[6-3H]glucose (15 Ci/mmol) were purchased from Amersham Bioscience. PI-PLC (EC 3.1.4.10) from Bacillus cereus was obtained from Boehringer, Bacillus thuringiensis PI-PLC, jack bean α-mannosidase, and jack bean β-N-acetylhexosaminidase were obtained from Glyco, and α-glucosidase was obtained from Roche. Antibodies raised against Trypanosoma brucei heavy chain binding protein (BiP) were a gift from J. D. Bangs (University of Wisconsin, Madison, WI).
Parasites and cell culture.
T. gondii strain RH tachyzoites and a clone of the RH strain stably expressing the cytosolic marker Escherichia coli β-galactosidase (40) were grown in Vero cells. Confluent cell cultures (175 cm2) were infected with 5 × 107 tachyzoites in Dulbecco's modified Eagle medium supplemented with 2% (vol/vol) fetal calf serum. Tachyzoites were harvested after 72 h. They were set free from their host cells by passing suspensions of infected cultures twice through a 20-gauge needle, twice through a 23-gauge needle, and three times through a 26-gauge needle. The suspension was run through a 20-ml glass wool column to remove cellular debris and filtered through 3-μm polycarbonate filters (Nuclepore). The purity of the tachyzoite suspension was monitored microscopically.
Permeabilization of T. gondii tachyzoites.
Tachyzoites (1 × 109) were permeabilized in 500 μl of ice-cold water containing 1 μg/ml leupeptin, 0.1 mM Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK), and 0.2 μg/ml tunicamycin for 10 min on ice, homogenized by 10 strokes in a Dounce homogenizer, mixed with an equal volume of double-isotonic incubation buffer (100 mM Na-HEPES at pH 7.4, 50 mM KCl, 10 mM MgCl2, 10 mM MnCl2, 1 μg/ml leupeptin, 0.1 mM TLCK, and 0.2 μg/ml tunicamycin), and homogenized by 5 strokes in the homogenizer.
Controls for permeability and integrity of hypotonically permeabilized parasites. (i) β-Galactosidase assay.
Supernatants corresponding to 1 × 105 parasites from permeabilized or intact RHβ-1 tachyzoites (40) were transferred to a 96-well plate (Nunc, Denmark). The corresponding pellets were resuspended in phosphate-buffered saline and transferred to the plate. Positive controls corresponding to the complete release of β-galactosidase were achieved by lysing the parasites with 0.1% Triton X-100. Each sample was supplemented with 50 μl chlorophenol red-β-d-galactopyranoside (substrate) (Boehringer Mannheim, Germany) in 100 mM HEPES, pH 8.0. Each plate was incubated for 10 to 30 min at 37°C. Adding a solution of 25 mM EDTA, 500 mM glycine, pH 11.2, terminated the reaction. Absorbance was measured in a microplate reader (Sanofi, Freiburg, Germany) at 570 nm.
(ii) Protease protection assay.
The integrity of the luminal contents (ER and rhoptries) of permeabilized parasites was determined using protease protection experiments with BiP, an ER luminal protein. Experiments were performed according to the method of Vidugiriene and Menon (52), using 5 × 107 parasites per sample.
Electron microscopy.
Purified tachyzoites (5 × 107 to 10 × 107) were centrifuged (1,000 × g, 2 min, 4°C), fixed at room temperature for 2 h with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4), and centrifuged again (13,000 × g, 15 min, room temperature). The cells were then washed with cacodylate buffer, resuspended in 0.25% tannic acid in cacodylate buffer, incubated for 1 h at room temperature, and centrifuged again. Parasites were postfixed for 2 h with 1% osmium tetroxide in cacodylate buffer, dehydrated in a graded series of ethanol followed by propylene oxide, and embedded in Epon 812 (Serva, Heidelberg, Germany). Ultrathin sections were stained with 1% uranyl acetate in 50% acetone, followed by lead citrate, and then examined using a Zeiss electron microscope (Zeiss, Göttingen, Germany).
In vitro labeling of GPI biosynthesis intermediates. (i) Labeling of early, nonmannosylated glycolipids.
In vitro labeling was performed according to a method for cell-free systems (23). Briefly, hypotonically permeabilized parasites were centrifuged (1,000 × g, 10 min, 4°C) and washed twice with incubation buffer A (50 mM Na-HEPES at pH 7.4, 25 mM KCl, 5 mM MgCl2, 8 mM EDTA, 0.1 mM TLCK, 1 μg/ml leupeptin, 0.2 μg/ml tunicamycin). Labeling was performed in the absence of Mn2+ (which is needed for dolichyl-phosphomannose synthase activity) to prevent the formation of Dol-P-Man (39). Aliquots of 1 × 108 tachyzoites were labeled in buffer A supplemented with 1 mM ATP, 1 mM coenzyme A (CoA), and 2 μCi UDP-[6-3H]GlcNAc.
(ii) Labeling of mannosylated glycolipids.
To label mannosylated GPI biosynthetic intermediates, hypotonically permeabilized parasites were processed as described above. Aliquots of 1 × 108 tachyzoites were labeled in buffer B supplemented with 1 mM ATP, 1 mM CoA, 1 mM UDP-GlcNAc, and (depending on the radioactive component used) 1 mM UDP-GalNAc, 1 mM UDP-Glc, and/or 1 mM GDP-Man. Four microcuries of GDP-[2-3H]Man, 4 μCi UDP-[1-3H]GalNAc, or 4 μCi UDP-[6-3H]Glc was added per incubation tube.
Metabolic labeling of tachyzoites.
After infection with T. gondii tachyzoites (at 72 h postinfection), cell cultures were washed twice with glucose-free Dulbecco's modified Eagle medium containing 20 mM sodium pyruvate. Labeling was performed using the same medium supplemented with 0.5 mCi d-[6-3H]glucosamine for 6 hours at 37°C. After being labeled, parasites were liberated from host cells with 20 strokes in a Dounce homogenizer. Tachyzoites were purified as described above.
Extraction of glycolipids.
All in vitro labeled preparations were extracted in a one-step procedure (modified from the method of McDowell and Schwarz [27]). Briefly, 1 × 108 tachyzoites were extracted three times with 800 μl chloroform-methanol-water (C/M/W). C/M/W extracts were dried in a Speedvac concentrator (Savant) and partitioned between water and water-saturated butan-1-ol. Washed glycolipid extracts from UDP-[6-3H]GlcNAc labeling were analyzed on silica gel 60 thin-layer chromatography (TLC) plates (Merck) with solvent system A (10:10:3 chloroform-methanol-1 M ammonium hydroxide by volume) (11). All other extracts with mannosylated glycolipids were developed in solvent system B (3:10:10:2:1 n-hexane-C/M/W-acetic acid by volume) (48). Plates run in solvent system B were neutralized in a chamber equilibrated with 32% ammonia for 15 min. Dried plates were then scanned for radioactivity using a Berthold LB2842 linear analyzer, and single glycolipids were quantified with Chroma Berthold software supplied by the manufacturer.
PI-PLC treatment of permeabilized parasites.
The in vitro labeled parasites were treated with 3 U/ml PI-PLC from B. thuringiensis for 30 min on ice. As a positive control, parasite membranes were disrupted with a final concentration of 0.1% Triton X-100. Negative controls were supplemented with PI-PLC buffer (25 mM Tris-acetate, pH 7.4, 50% glycerol, 0.01% azide) instead of enzyme solution. Incubation was terminated by the addition of 700 μl chloroform-methanol (1:1 by volume) to give a final mixture of chloroform-methanol-water (10:10:3 by volume).
Statistical analysis.
PI-PLC-insensitive material {GlcN-(acyl)-PI for UDP-[6-3H]GlcNAc labeling, Dol-P-Man for GDP-[2-3H]Man labeling, and Dol-P-Glc for UDP-[6-3H]Glc labeling} was used to normalize samples from one labeling experiment. To normalize the samples, the counts of samples from one experiment were adjusted to that of the sample with the highest PI-PLC-resistant peak. All results were then expressed as percentages of cleavage, with 100% being the amount of glycolipids from the negative control (without PI-PLC and without detergent). Statistical analysis of the data was performed using SigmaPlot 4.0 (Jandel Scientific) software. The Student t test was used for comparison of two groups. Results were considered significant at P values of <0.05.
Enzyme digestion and chemical treatment of glycolipids.
Deamination and enzyme digestion with jack bean mannosidase and GPI-PLD were performed as described by Smith et al. (44, 45).
Glycan headgroup analysis.
High-performance TLC (HPTLC)-purified radiolabeled glycolipids were delipidated, deaminated, reduced, dephosphorylated with aqueous HF, and desalted by passage through AG50X12 (H+) and AG3X4 (OH−) ion-exchange resins. The resulting neutral glycan headgroups were analyzed before and after various glycosidic digests either by Bio-Gel P4 gel filtration (see reference 44 and references therein) or by high-pH anion-exchange chromatography on a Dionex Basic chromatography system (Dionex Corp., Sunnyvale, CA), using a gradient elution program as described by Mayor and Menon (24). For both types of analysis, the radiolabeled glycans were detected by scintillation counting and correlated with the elution positions of the coinjected individual glucose oligomer standards.
Exoglycosidase digestion of glycans.
Glycans obtained from HPTLC-purified radiolabeled glycolipids were digested with either jack bean α-mannosidase, jack bean β-N-acetylhexosaminidase, or α-glucosidase (maltase). All enzymatic digests were terminated by heating at 100°C for 5 min. The samples were desalted by passage through 0.25 ml of AG50X12 (H+) resin, dried, and flash coevaporated with toluene to remove residual acetic acid.
RESULTS
Hypotonic permeabilization of T. gondii tachyzoites.
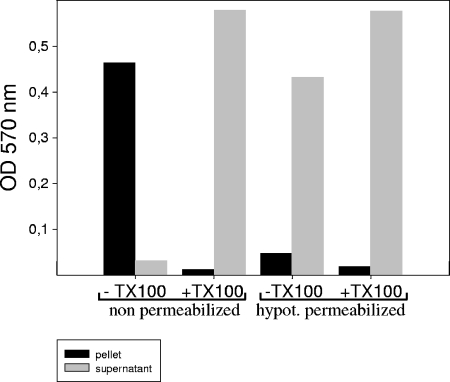
To examine whether the membranes of parasites are actually permeabilized by treatment with hypotonic buffer, we used a strain of T. gondii (RHβ-1) stably transfected with the lacZ gene expressing soluble β-galactosidase. After permeabilization and centrifugation of the parasites, the β-galactosidase, detected with the help of color reaction, was found mainly in the supernatant, with the exception of a small portion (Fig. 1). For cells that were not hypotonically permeabilized, the β-galactosidase activity was found in the pellet (Fig. 1) and recovered in the supernatant only after treatment with Triton X-100.
FIG. 1.
Release of β-galactosidase into medium after hypotonic treatment of T. gondii. T. gondii (RHβ-1) stably transfected with the lacZ gene expressing soluble β-galactosidase was permeabilized as described in Materials and Methods. After centrifugation, β-galactosidase was measured in the supernatant and in the pellet before and after the addition of Triton X-100.
Hypotonic treatment did not destroy cell compartments.
For the planned experiments on the topology of GPI biosynthesis at the ER level, the intactness of the ER was monitored by determining the extent to which a luminal ER protein, BiP (ER chaperon GRP78), was protected from the action of exogenously added proteinase K. We used T. brucei anti-BiP serum, which was shown to cross-react with T. gondii BiP and other protozoan homologues (1). This fact underscores the occurrence of cross-reactivity with different species. As a negative control, cells were treated with proteinase K buffer, and in parallel, as a positive control, cells were treated with Triton X-100 (final concentration, 0.1%). Each lane was loaded with the same cell equivalent of 5 × 107 parasites. As shown in Fig. 2A, the BiP luminal protein was not cleaved in hypotonically permeabilized cells (Fig. 2A, lane 1) and became accessible to proteinase K only after the addition of detergent (Fig. 2A, lane 3). Figure 2A, lane 6, shows the total BiP released after treatment with a detergent. Additionally, parasites were tested for ER integrity under conditions used for GPI labeling (37°C incubation) (Fig. 2B, lane 4) and following PI-PLC treatment (Fig. 2B, lane 5). The same protection was obtained after these additional treatments. The data indicate that hypotonic permeabilization as well as PI-PLC treatment has no effect on the integrity of the ER membranes. As an additional control, rhoptry proteins (ROP2, -3, and -4) were also found to be resistant to proteinase K treatment but were sensitive after the addition of detergent, also indicating the intactness of the rhoptries (data not shown). Together, these data show that the integrity of most organelles and the ER remains intact following hypotonic permeabilization.
FIG. 2.
Determination of integrity of the ER. (A) Cells were hypotonically permeabilized and subjected to proteinase K digestion before (lane 1) and after (lanes 2 to 5) the addition of Triton X-100. Lane 6 shows the total BiP released after treatment with detergent. After treatment, proteins were precipitated and analyzed by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with specific monoclonal antibodies raised against the ER BiP protein. (B) Lanes 1 to 3, assays with permeabilized tachyzoites; lane 4, mock assay with permeabilized tachyzoites under the conditions used for the labeling procedure (37°C incubation); lane 5, permeabilized tachyzoites that were mock labeled and treated with 3 U/ml B. thuringiensis PI-PLC. Each lane was loaded with a cell equivalent of 5 × 107 tachyzoites.
Ultrastructure analysis after hypotonic permeabilization.
Further analyses by light microscopy showed no differences between hypotonically permeabilized cells and intact cells (data not shown). On an ultrastructural level, intact free T. gondii (Fig. 3A) shows a typical apicomplexan ultrastructure, with a conoid (C), rhoptries (R), micronemes (m), nucleus (N), mitochondrion (M), and Golgi body (G). The cell is enclosed and filled out with the subpellicular microtubule (P) and electron-dense cytosolic structures. After hypotonic permeabilization (Fig. 3B), cells have lost their typical curved form, but clear outside cell delimitations are still present. All cytoplasmic and ribosome contents seem to be withdrawn from the cells and washed out. All organelles, such as the rhoptries, micronemes, mitochondrion, and apicoplast, are held back in the cells and are still recognizable. Due to this observation, these cells are permeabilized but are not “ghosts” in a proper sense because the structure seems intact and is still biosynthetically active. We also investigated the effects of different preparation procedures, including labeling with radioactive sugar precursors and treating cells with B. thuringiensis PI-PLC. In all cases, the same ultrastructures were observed (data not shown). Altogether, the data demonstrate that the ER membranes are intact following hypotonic permeabilization and are suitable for experiments concerning the topology of different GPI intermediates.
FIG. 3.
Electron microscopy of free intact T. gondii tachyzoites (A) and hypotonically permeabilized tachyzoites (B). A, apicoplast; V, vesicles; C, conoid; D, dense granules; R, rhoptries; m, micronemes; N, nucleus; M, mitochondrion; P, pellicula; and G, Golgi body.
Comparison of T. gondii GPI intermediates labeled in vitro and in vivo.
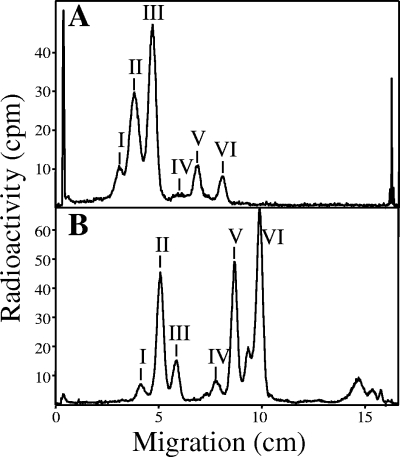
In vitro labeling was performed on hypotonically permeabilized cells by using GDP-[2-3H]mannose. In parallel, T. gondii tachyzoites (at 48 to 72 h postinfection) were labeled in vivo with [3H]glucosamine for 9 h at 37°C. After a period of labeling, GPI glycolipids were extracted from both systems and analyzed by thin-layer chromatography. The profiles of [3H]glycolipids (I to VI) observed with in vivo labeling (Fig. 4A) have almost identical migration differences between each other as those observed for [3H]glycolipids synthesized in vitro (Fig. 4B). However, the Rf values for the in vitro [3H]glycolipids are higher than those for the corresponding [3H]glycolipids synthesized in vivo, indicating a common difference in either their glycans or, more likely, their lipid moieties. These differences were not recognized in previous studies (47, 49). Only by using new two-dimensional methods with much better resolution (Bio-Imager; Raytest, Straubenhardt, Germany) can one reveal the heterogeneity of glycolipids synthesized in vivo and in vitro. Therefore, we investigated the hydrophilic glycan parts of the [3H]glycolipids synthesized in vitro and compared them to the well-characterized corresponding glycans from the glycolipids synthesized in vivo.
FIG. 4.
Comparison between T. gondii GPIs synthesized in vivo and in vitro (permeabilized cells). Tachyzoites were labeled in vivo with [3H]glucosamine (A) or in vitro via GDP-[2-3H]Man (B). Glycolipids were extracted and analyzed by TLC. Radioactivity was detected by a Berthold TLC scanner (LB 1842). See Table 1 for characterization of the [3H]glycolipids.
Characterization of T. gondii GPI intermediates synthesized in vitro.
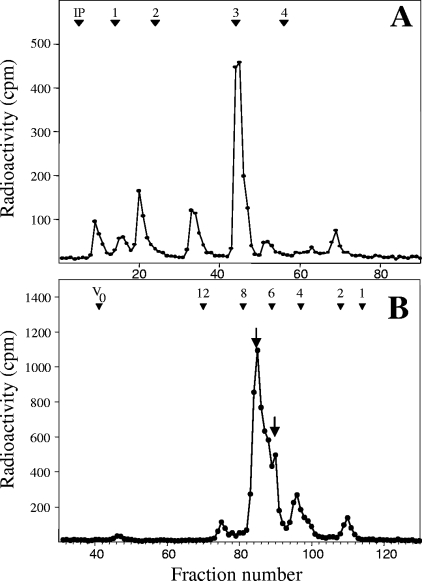
The glycan moieties of the GPIs synthesized in vitro by permeabilized T. gondii cells were obtained from the labeled lipid extracts by dephosphorylation, deamination, and reduction. The released GPI core glycans were then analyzed by anion-exchange chromatography (Dionex) with an internal dextran hydrolysate standard (elution positions of individual glucose oligomers are given in Dionex units) (Fig. 5A). The major product coeluted at 3 Dionex units, but when analyzed further by Bio-Gel P4 size exclusion chromatography (Fig. 5B), the single Dionex peak was resolved into two glycans, eluting at ∼6 and ∼7 glucose units (GU), corresponding to the previously characterized glycans generated by the same treatment of glycolipid extracts labeled in vivo (48), namely, Manα1-2Manα1-6(GalNAcβ1-4)Manα1-4anhydromannitol and Manα1-2Manα1-6(Glcα1-4GalNAcβ1-4)Manα1-4anhydromannitol, respectively.
FIG. 5.
High-pH anion-exchange chromatography and Bio-Gel P4 size exclusion chromatography analyses of GPI core glycans generated from glycolipids synthesized in vitro. Neutral core glycans were generated by dephosphorylation, deamination, and reduction from crude GPI extracts labeled with GDP-[2-3H]Man. Neutral glycans were analyzed by high-pH anion-exchange chromatography (Dionex) (A) and Bio-Gel P4 size exclusion column chromatography (B). The elution positions of the coinjected glucose oligomer standards are indicated at the top of each profile. The arrows in panel B indicate that two glycolipid headgroups were resolved from the major peak shown in the Dionex analysis (A). Abbreviations: IP, injection peak; V0, void volume.
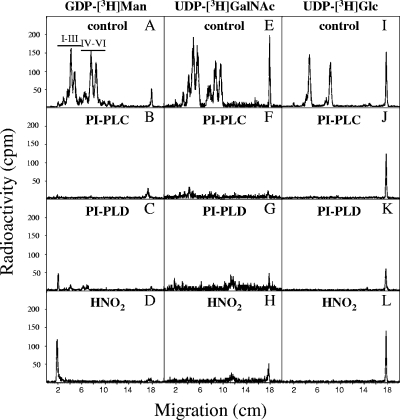
Using hypotonically permeabilized cells, glycolipids were labeled with either GDP-[2-3H]Man, UDP-[1-3H]GalNAc, or UDP-[6-3H]Glc. All six glycolipids were labeled with [3H]Man and [3H]GalNAc, while only glycolipids I, II, IV, and V were labeled with [3H]Glc (Fig. 6). Additional information concerning the structures of the [3H]mannosylated glycolipids formed from in vitro labeling (Fig. 4B) showed that they were all sensitive to deamination, GPI-PLD, PI-PLC (Fig. 6 and Table 1), and base treatment (data not shown), indicating that they are all GPI intermediates containing GlcN and a diacylglycerol-PI lipid anchor. However, only glycolipids IV, V, and VI were partially sensitive to α-mannosidase, indicating the presence of at least one free terminal α-mannose group, while glycolipids I, II, and III were resistant to jack bean mannosidase treatment (Table 1), implying the presence of an ethanolamine phosphate group on the terminal mannose, thus preventing digestion.
FIG. 6.
Characterization of GPIs synthesized in vitro. Hypotonically permeabilized tachyzoites were labeled in vitro via GDP-[2-3H]Man (A to D), UDP-[1-3H]GalNAc (E to H), or UDP-[6-3H]Glc (I to L). Glycolipids were extracted and analyzed by TLC before (A, E, and I) and after enzymatic digestion and B. cereus PI-PLC (B, F, and J), PI-PLD (C, G, and K), or chemical (HNO2) (D, H, and L) treatment. Radioactivity was detected by a Berthold TLC scanner (LB 1842).
TABLE 1.
Characterization of [3H]glycolipids and corresponding neutral [3H]glycans formed in Toxoplasma gondii
| Glycolipida | αManc | Digestion result (size [GU] of neutral glycan products)b
|
Glycan size (GU)d | Assignment | ||
|---|---|---|---|---|---|---|
| αGlc | βHexNAc | αMan | ||||
| II (in vivo)e | − | + (6.0) | − | + (4.9) | 7.0 | EtNP-Man2(Glc-GalNAc)ManGlcN-PI |
| III (in vivo)e | − | − | + (4.3) | + (4.3) | 6.0 | EtNP-Man2(GalNAc)ManGlcN-PI |
| I (in vitro) | − | + (ND) | − | + | 6.9 | lyso-EtNP-Man2(Glc-GalNAc)ManGlcN-PI |
| II (in vitro) | − | + (5.9) | − | + (4.9) | 6.9 | EtNP-Man2(Glc-GalNAc)ManGlcN-PI |
| III (in vitro) | − | − | + (ND) | + (ND) | 6.0 | EtNP-Man2(GalNAc)ManGlcN-PI |
| IV (in vitro) | + | + (ND) | − | + (ND) | 6.8 | lyso-Man2(Glc-GalNAc)ManGlcN-PI |
| V (in vitro) | + | + (5.9) | − | + (4.9) | 6.8 | Man2(Glc-GalNAc)ManGlcN-PI |
| VI (in vitro) | + | − | + (4.2) | + (4.4) | 5.9 | Man2(GalNAc)ManGlcN-PI |
See Fig. 4 for corresponding bands. All of the glycolipids listed here were sensitive to GPI-PLD, PI-PLC, base, and deamination.
+, positive digestion, i.e., loss (or shift) of the radiolabeled band; −, negative digestion, i.e., no loss or shift of the band; ND, not determined.
Radiolabeled glycolipids were treated with α-mannosidase as described in Materials and Methods.
The sizes of neutral glycans terminating in 2,5-anhydromannitol (AHM) were determined as described in Materials and Methods.
Characterization of glycolipids II and III from in vivo labeling experiments with [3H]Man was consistent with previously published findings (48).
All of the [3H]mannosylated glycolipids (Fig. 4B) were also subjected to headgroup analysis. Neutral glycan headgroups were obtained from HPTLC-purified glycolipids by deacylation, deamination, reduction, and dephosphorylation. The desalted 2,5-anhydromannitol-containing glycan headgroups obtained from glycolipids I to IV were analyzed by Bio-Gel P4 gel filtration before and after the addition of various glycosidases, α-mannosidase, α-glucosidase, and β-N-acetylhexosaminidase (Table 1). The glycans obtained from both glycolipids III and VI had sizes of 5.8 to 6.0 GU and were sensitive to α-mannosidase and β-N-acetylhexosaminidase, giving glycan products of 4.4 and 4.2 GU, respectively, which corresponded to GalNAcβ1-4Manα1-4anhydromannitol and Manα1-2Manα1-6Manα1-4anhydromannitol, respectively. Thus, the original neutral glycan was Manα1-2Manα1-6(GalNAcβ1-4)Manα1-4anhydromannitol. The difference in Rf values of the glycolipids and the resistance of glycolipid III but sensitivity of glycolipid VI to α-mannosidase suggest that the former has an ethanolamine phosphate group on the terminal mannose; thus, glycolipid III has the structure EtNP-Man2-(GalNAc)ManGlcN-PI and glycolipid VI has the structure Man2-(GalNAc)ManGlcN-PI.
The glycans obtained from glycolipids I, II, IV, and V have sizes of 6.8 to 7.0 GU and were sensitive to α-mannosidase and α-glucosidase, giving glycan products of 4.9 and 5.9 GU, which corresponded to Glcα1-4GalNAcβ1-4Manα1-4anhydromannitol and Manα1-2Manα1-6(GalNAcβ1-4)Manα1-4anhydromannitol, respectively. Thus, the original neutral glycan obtained from glycolipids I, II, IV, and V corresponded to Manα1-2Manα1-6(Glcα1-4GalNAcβ1-4)Manα1-4anhydromannitol. The difference in Rf values of the glycolipids and the resistance of glycolipids I and II but sensitivity of glycolipids VI and V to α-mannosidase suggest that the former glycolipids have an ethanolamine phosphate group on the terminal mannose. However, this gives glycolipid I the same headgroup as glycolipid II and, likewise, glycolipid IV the same headgroup as glycolipid V, and thus the Rf difference between the glycolipids is probably due to a difference of an acyl chain. Since all of these glycolipids are sensitive to PI-PLC, the difference is not due to inositol acylation but is probably due to loss of an acyl chain from the glycerol, forming a lyso-GPI species. These glycolipid characterizations are in accordance with previously described Toxoplasma gondii GPI intermediates (48).
Taken together, these findings suggest that glycolipids (I to VI) synthesized in vitro have identical core glycans to those generated from the corresponding glycolipids synthesized in vivo. The difference in TLC migration between the two profiles (Fig. 4A and B) could possibly be due to differences in the hydrophobic ties of the lipids. This difference and the existence of lyso-species may be an indication of lipid remodeling, which has been observed in the GPI pathways of other organisms (see reference 9 and references therein), and this potential remodeling is presently being investigated (T. K. Smith, unpublished data). An alternative explanation could be that only GPI intermediates generated in vivo have an extra unknown modification on their glycan headgroups, which occurs prior to the formation of the least mature mannosylated GPI intermediate observed, i.e., Man2-(GalNAc)ManGlcN-PI. The lack of evidence for this glycan modification may be due to the methodologies previously employed to form and subsequently characterize the neutral glycan headgroup. This possibility is presently being investigated and will be published elsewhere (Smith, unpublished data).
Topology of T. gondii GPIs.
Although we did not provide direct experimental proof that GPI biosynthesis occurs at the ER, we concluded by analogy to other systems that this is also the case for T. gondii. We used hypotonically permeabilized cells to examine the orientation within the ER membrane of different GPI precursors. In order to achieve this goal, glycolipids were radiolabeled in vitro with different nucleotide sugar donors. After a period of labeling, the incubation mixtures were subjected to B. thuringiensis PI-PLC treatment. Treatment with the enzyme releases exclusively the hydrophilic glycans of the GPI precursors present at the cytoplasmic face of the ER (51, 52).
The early GPI biosynthetic intermediates, GlcN-PI and GlcNAc-PI, are found at the cytoplasmic side of the ER.
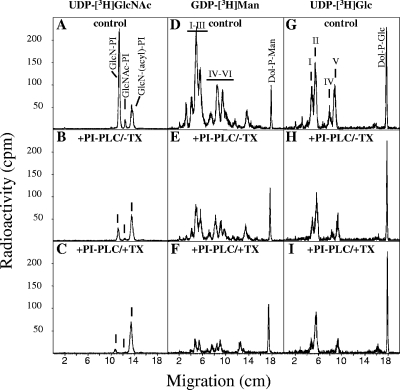
Hypotonically permeabilized cells were labeled with UDP-[6-3H]GlcNAc in buffer A containing 8 mM EDTA and no Mn2+ to prevent mannosylation of the early GPI intermediates (51). After a period of labeling (90 min, 37°C), PI-PLC treatment (30 min on ice) took place. Experiments were performed on ice to limit flipping activity, as previously reported by Vidugiriene and Menon (51, 52). Kinetic studies showed that maximal cleavage efficiency was observed after 20 min, without further cleavage at longer times (data not shown). PI-PLC treatments were performed in the absence and presence of 0.1% Triton X-100. As a negative control, parallel incubation was done in the absence of enzyme. Subsequently, glycolipids were extracted and subjected to butanol-water phase partitioning. An aliquot of each phase was first analyzed by scintillation counting to determine the extent of cleavage. The remainders of the butanol phases were analyzed by thin-layer chromatography. Figure 7A to C shows the TLC profiles of the results obtained for the early GPI intermediates. In the absence of mannosylated intermediates, we observed the accumulation of GlcN-PI, GlcNAc-PI, and a more hydrophobic unknown glycolipid (Fig. 7A). PI-PLC treatment cleaved GlcN-PI and GlcNAc-PI to a considerable extent (Fig. 7B). The high cleavage level of these two glycolipids suggests that they are accessible to the action of the phospholipase in intact ER membranes, indicating that they are oriented towards the cytoplasmic side of the ER. In the presence of detergent, allowing accessibility to both sides of the ER membrane, phospholipase cleavage was almost complete (Fig. 7C). The unknown hydrophobic glycolipid was insensitive to PI-PLC, even in the presence of detergent, suggesting that the inositol ring is acylated (36). Furthermore, its sensitivity to nitrous acid deamination and GPI-PLD and its dependence upon CoA and ATP for formation (Smith et al., unpublished) suggest that it is GlcN-(acyl)PI.
FIG. 7.
PI-PLC treatment of permeabilized T. gondii tachyzoites. Hypotonically permeabilized tachyzoites were labeled in vitro via UDP-[6-3H]GlcNAc in the presence of EDTA and absence of Mn2+ (A to C), GDP-[2-3H]Man (D to F), or UDP-[6-3H]Glc (G to I). Glycolipids were extracted and analyzed by TLC before (A, D, and G) and after B. thuringiensis PI-PLC treatment in the absence (B, E, and H) or presence (C, F, and I) of Triton X-100. Radioactivity was detected by a Berthold TLC scanner (LB 1842).
Trimannosylated GPI biosynthetic intermediates are also found at the cytoplasmic side of the ER.
To examine the distribution of mannosylated intermediates, labeling of hypotonically permeabilized parasites with GDP-[2-3H]mannose was performed (Fig. 7D to F). The incubations took place at 37°C in the presence of 5 mM Mg2+ and 5 mM Mn2+, which are needed for the optimum activity of the mannosyltransferases. After a period of incubation with radioactive nucleotide sugar donors, samples were placed on ice, and PI-PLC was added to the incubation mixtures in the absence or presence of Triton X-100. Glycolipids were subsequently extracted and analyzed by TLC before and after PI-PLC treatment (Fig. 7D to F). It is clear that the major portion of the mannosylated glycolipids (I to VI) in permeabilized cells is accessible to the PI-PLC (Fig. 7E), suggesting their presence on the cytoplasmic side of the ER. However, a portion of the glycolipids was protected from the action of PI-PLC, suggesting their localization on the luminal side of the ER. This pool became accessible only after the addition of Triton X-100 (Fig. 7F). Increasing the concentration of PI-PLC did not result in a higher cleavage efficiency (data not shown). The results are summarized in Table 2 and show that all of the mannosylated glycolipids were located predominantly on the cytoplasmic face of the ER, with proportions ranging from 60% (glycolipid VI) to 74% (glycolipid I).
TABLE 2.
Localization of GPI intermediates based on efficiency of cleavage by PI-PLC in permeabilized cellsa
| Glycolipid | Structure | % Glycolipid on cytoplasmic face of ER
|
||
|---|---|---|---|---|
| [3H]GlcN (n = 20) | [3H]Man (n = 4) | [3H]Glc (n = 5) | ||
| I | lyso-EtNP-Man2-(Glc-GalNAc)ManGlcN-PI | 74.3 ± 2.0 | 74.6 ± 5.8 | |
| II | EtNP-Man2-(Glc-GalNAc)ManGlcN-PI | 65.4 ± 7.3 | 64.0 ± 9.1 | |
| III | EtNP-Man2-(GalNAc)ManGlcN-PI | 66.2 ± 4.2 | ||
| IV | lyso-Man2-(Glc-GalNAc)ManGlcN-PI | 66.3 ± 9.2 | 63.3 ± 11.6 | |
| V | Man2-(Glc-GalNAc)ManGlcN-PI | 62.3 ± 9.0 | 60.8 ± 11.8 | |
| VI | Man2-(GalNAc)ManGlcN-PI | 59.2 ± 1.9 | ||
| GlcN-(acyl)PI | NDa | |||
| GlcN-PI | 82.2 ± 6.2 | |||
| GlcNAc-PI | 70.7 ± 6.2 | |||
Permeabilized cells were labeled with either UDP-[3H]GlcNAc, GDP-[3H]Man, or UDP-[3H]Glc and extracted before and after treatment with PI-PLC in the absence or presence of detergent (see Materials and Methods for further details). Data are means ± standard deviations.
ND, not determined because the glycolipid was insensitive to PI-PLC.
Topology of GPI biosynthetic intermediates following UDP-[6-3H]glucose labeling.
We also investigated the distribution of the T. gondii intermediates after labeling of hypotonically permeabilized parasites with UDP-[6-3H]glucose. Labeling was done under the same conditions as those for GDP-[2-3H]mannose labeling. Subsequently, glycolipids were extracted and analyzed by TLC before and after PI-PLC treatment (Fig. 7G to I). As expected, only glycolipids I, II, III, and IV were labeled with [3H]glucose, and for the [3H]mannose-labeled glycolipids, treatment with PI-PLC in the absence of detergent showed a cytosolic orientation which could be assigned to the majority of the [3H]glucose-labeled glycolipids. A portion of the material remained protected against cleavage with PI-PLC and became accessible only after the addition of detergent. The results (Table 2) show that the highly mature glucosylated GPI species are located predominantly on the cytoplasmic face of the ER, with proportions from 61% for glycolipid V to 75% for glycolipid I. These data for the [3H]Glc-labeled GPI intermediates correlate well with those for the [3H]Man-labeled intermediates, some of which are also glucosylated (glycolipids I, II, IV, and V).
DISCUSSION
GPI biosynthesis is a multistep process, in which some steps involve multiprotein complexes. The pathway involves a de-N-acetylase, at least four glycosyltransferases, and an ethanolaminephosphotransferase (for reviews, see references 10 and 19 to 21). Transfer of the preassembled GPI anchor to nascent proteins is a rapid process (1 to 5 min) accompanied by the release of a carboxy-terminal peptide containing a GPI signal sequence (10, 18). These early observations, along with additional data (4, 21, 51, 52), point to the fact that all of these processes take place in the endoplasmic reticulum. The orientation of the GlcNAc-PI and GlcN-PI intermediates is on the cytoplasmic face of the ER in microsomes of a Thy-1+ cell line (BW5147.3) permeabilized with streptolysin O (51). The orientation of the mannosylated intermediates in T. brucei microsomes showed that >60% of the mannosylated GPIs, i.e., Man1-GlcN-PI, Man2-GlcN-PI, and the mature ethanolaminephosphate-containing GPI anchor precursor (EtNP-Man3-GlcN-PI), were located on the outer (cytoplasmic) side of the microsomal membranes (52). Here we report the topological localization of early and late GPI anchor intermediates in the pathogen T. gondii, including for the first time those that have an additional side chain modification, i.e., Glc-GalNAc, to the core glycan. Hypotonically permeabilized tachyzoites were established as a model to test the orientation of GPI intermediates within the intact ER. The permeability of tachyzoites was confirmed by the release into the medium of β-galactosidase by T. gondii stably transfected with the lacZ gene (40). Since β-galactosidase (75 kDa) is able to escape from the permeabilized cells, the pores are of sufficient size to allow the penetration of PI-PLC (34 kDa) into the tachyzoites. Furthermore, protease protection assays showed the integrity and intactness of the ER membrane and the rhoptries.
Using PI-PLC as a tool, the cytosolic and lumen-facing GPI intermediates on the intact ER membranes could be distinguished, similar to previous studies of other organisms (51, 52). Unfortunately, other probes that bind GPI intermediates, e.g., concanavalin A or monoclonal antibodies, are unsuitable for use with T. gondii due to the large number of free GPIs on the cell surface. For example, immunofluorescence with the monoclonal antibody T33F12 against T. gondii GPIs shows intense staining of the plasma membrane, overshadowing any fluorescence inside the cell (data not shown).
Our results show for T. gondii that the early GPI intermediates GlcNAc-PI and GlcN-PI are situated on the cytoplasmic face of the ER membranes. This suggests that the catalytic domain (PIG-A) of the enzyme complex responsible for the first reaction (using GlcNAc transferase), which consists of at least five and six proteins in Saccharomyces cerevisiae and mammalian cells, respectively (19), is situated on the cytoplasmic face of the ER membranes. The presence of both GlcNAc-PI and GlcN-PI preferentially on the cytoplasmic face of the ER correlates well with the proposed cytosol-facing active site of the second biosynthetic step, involving GlcNAc-PI de-N-acetylase, as previously described for mammalian cells, T. brucei, and S. cerevisiae (58). The uncleavable pool of each species was due to incomplete digestion.
Since the earliest T. gondii mannosylated GPI intermediate observed during in vivo labeling experiments is Man2-(GalNAc)ManGlcN-PI, one can assume that the preceding mannosylated intermediates, Man1GlcN-PI, Man2GlcN-PI, and Man3GlcN-PI, are rapidly formed (i.e., are nonlimiting biosynthetic steps) on the luminal side of the ER. Also, the fact that the mannosyltransferases are Dol-P-Man dependent is an indication that the mannose donor and thus the orientation of their catalytic domains is on the luminal face of the ER. In the case of the α1-4-mannosyltransferase (the first mannosyltransferase), the enzyme is a multitransmembrane protein whose characteristic glycosyltransferase DXD motif, and thus presumably its active site, is detected at the luminal side of the ER (22). Comparative sequence analysis of the PIG-M genes from Homo sapiens, S. cerevisiae, and Caenorhabditis elegans with that from T. gondii showed a luminal orientation of the DXD motif, and thus we concluded that it has a conventional orientation. The same luminal localization of the catalytic domains of the other two mannosyltransferases has also been suggested (19). Therefore, we concluded that the intraluminal orientation of the active sites of all three mannosyltransferases is most probably conserved among species.
In the present work, we show for the first time the distribution of mannosylated GPI intermediates which have a side chain modification (GalNAc or Glc-GalNAc). The cytoplasmic portion of the polar T. gondii GPIs varies between 60 and 75%, presumably because the donors for the unknown GalNAc and Glc transferases are nucleotide sugars (47), i.e., UDP-GalNAc and UDP-Glc, which would be accessible on the cytoplasmic side of the ER. Thus, presumably Man3GlcN-PI must find a rapid and highly efficient means to go from the luminal to the cytoplasmic face of the ER to act as an acceptor substrate for the GalNAc transferase and subsequent Glc transferase. However, we do not have evidence for exclusive cytosolic localization of nucleotide sugars in T. gondii, and the possibility of intraluminal GalNAc exists (16). GDP-Man is usually not found in the lumen of the ER (16), but Dol-P-Man is present on both sides of the ER membrane.
However, the transamidation reaction, where the mature GPI anchors, i.e., EtNP-Man2-(GalNAc-Glc)ManGlcN-PI and EtNP-Man2-(GalNAc)ManGlcN-PI (glycolipids II and III, respectively), are attached to a nascent protein, e.g., SAG1, takes place by means of a protein complex whose catalytic component has been shown to be facing the lumen of the ER for several organisms (19). Thus, the large hydrophilic mature GPI anchors must cross the lipid bilayer from the cytoplasmic to the luminal side of the ER membrane, representing a great energy barrier and possibly requiring a flippase (28). This event is analogous to N-glycan biosynthesis, where an oligosaccharide Man5-GlcNAc2-P-P-Dol precursor flips to the luminal side of the ER for further processing before being added to the nascent protein (46).
The distribution of GPI intermediates does not necessarily give definitive information about which side of the ER is the location for the biosynthetic step, as flippases are postulated to transport assembling glycolipids back and forth across the ER membrane (3, 28, 32, 53). Recently, an ATP-independent protein-mediated flip-flop of early GPI intermediates was demonstrated (54).
By labeling of tachyzoites with UDP-[3H]GlcNAc in the presence of ATP and CoA, for the first time an inositol-acylated species was observed in the T. gondii biosynthetic pathway. In contrast to the case in T. brucei, where acylation of the inositol takes place after the transfer of the first mannose, T. gondii inositol acylation precedes the mannosylation steps, forming GlcN-(acyl)PI, as is the case with P. falciparum, S. cerevisiae, and mammalian cells (13, 34). However, in T. gondii, neither mature GPI precursors nor GPI anchor proteins (nor any mannosylated GPI intermediates) have been observed as inositol-acylated species, which may suggest a transient acylation. We speculate that the acylation/deacylation of inositol could play a role in regulating the correct and rapid sequential processing of GPI intermediates in T. gondii, similar to that observed in the T. brucei GPI biosynthetic pathway (17, 43).
Acknowledgments
This work was supported by Deutsche Forschungsgesellschaft, Hessisches Ministerium für Kultur und Wissenschaft, Fonds der Chemischen Industrie, Stiftung P. E. Kempkes, and Fondation pour la Recherche Médicale (to J.-F.D.). J.K. thanks the Hessische Graduiertenförderung for a fellowship. T.K.S. is funded by Wellcome Trust Senior Research Fellowship grant 067441.
We thank James Bangs, University of Wisconsin-Madison, for anti-BiP antibodies. We acknowledge helpful discussions with Boris Striepen and Hosam Shams-Eldin. We thank Jörg Schmidt, Marlies Trampe, and Ulrike Bieker for cell culture.
REFERENCES
- 1.Azzouz, N., P. Gerold, J. Schmidt, Y. Capdeville, and R. T. Schwarz. 2000. Transient N-acetylgalactosaminylation of mannosyl phosphate side chain in Paramecium primaurelia glycosylphosphatidylinositols. Eur. J. Biochem. 267:3385-3392. [DOI] [PubMed] [Google Scholar]
- 2.Carruthers, V. B. 2002. Host cell invasion by the opportunistic pathogen Toxoplasma gondii. Acta Trop. 81:111-122. [DOI] [PubMed] [Google Scholar]
- 3.Chang, Q. L., S. N. Gummadi, and A. K. Menon. 2004. Chemical modification identifies two populations of glycerophospholipid flippase in rat liver ER. Biochemistry 43:10710-10718. [DOI] [PubMed] [Google Scholar]
- 4.Conzelmann, A., H. Riezman, C. Desponds, and C. Bron. 1988. A major 125-kd membrane glycoprotein of Saccharomyces cerevisiae is attached to the lipid bilayer through an inositol-containing phospholipid. EMBO J. 7:2233-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debierre-Grockiego, F., N. Azzouz, J. Schmidt, J. F. Dubremetz, H. Geyer, R. Geyer, R. Weingart, R. R. Schmidt, and R. T. Schwarz. 2003. Roles of glycosylphosphatidylinositols of Toxoplasma gondii. Induction of tumor necrosis factor-alpha production in macrophages. J. Biol. Chem. 278:32987-32993. [DOI] [PubMed] [Google Scholar]
- 6.Dowse, T., and D. Soldati. 2004. Host cell invasion by the apicomplexans: the significance of microneme protein proteolysis. Curr. Opin. Microbiol. 7:388-396. [DOI] [PubMed] [Google Scholar]
- 7.Eckert, V., P. Gerold, and R. T. Schwarz. 1996. GPI anchor: structure and function, p. 223-243. In H. J. Gabius and S. Gabius (ed.), Glycosciences: status and perspectives. Chapmann & Hall, Weinheim, Germany.
- 8.Englund, P. T. 1993. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu. Rev. Biochem. 62:121-138. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson, M. A. 1999. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J. Cell Sci. 112:2799-2809. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson, M. A., and A. F. Williams. 1988. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu. Rev. Biochem. 57:285-320. [DOI] [PubMed] [Google Scholar]
- 11.Field, M. C., and A. K. Menon. 1992. Biosynthesis of glycosyl-phosphatidylinositol membrane protein anchors, p. 155-190. In N. M. Hooper and A. J. Turner (ed.), Lipid modification of proteins. Oxford University Press, Oxford, United Kingdom.
- 12.Gerold, P., A. Dieckmann-Schuppert, and R. T. Schwarz. 1994. Glycosylphosphatidylinositols synthesized by asexual erythrocytic stages of the malarial parasite, Plasmodium falciparum. Candidates for plasmodial glycosylphosphatidylinositol membrane anchor precursors and pathogenicity factors. J. Biol. Chem. 269:2597-2606. [PubMed] [Google Scholar]
- 13.Gerold, P., N. Jung, N. Azzouz, N. Freiberg, S. Kobe, and R. T. Schwarz. 1999. Biosynthesis of glycosylphosphatidylinositols of Plasmodium falciparum in a cell-free incubation system: inositol acylation is needed for mannosylation of glycosylphosphatidylinositols. Biochem. J. 344:731-738. [PMC free article] [PubMed] [Google Scholar]
- 14.Gerold, P., L. Schofield, M. J. Blackman, A. A. Holder, and R. T. Schwarz. 1996. Structural analysis of the glycosyl-phosphatidylinositol membrane anchor of the merozoite surface proteins-1 and -2 of Plasmodium falciparum. Mol. Biochem. Parasitol. 75:131-143. [DOI] [PubMed] [Google Scholar]
- 15.Güther, M. L., and M. A. Ferguson. 1995. The role of inositol acylation and inositol deacylation in GPI biosynthesis in Trypanosoma brucei. EMBO J. 14:3080-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirschberg, C. B., P. W. Robbins, and C. Abeijon. 1998. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu. Rev. Biochem. 67:49-69. [DOI] [PubMed] [Google Scholar]
- 17.Homans, S. W., C. J. Edge, M. A. Ferguson, R. A. Dwek, and T. W. Rademacher. 1989. Solution structure of the glycosylphosphatidylinositol membrane anchor glycan of Trypanosoma brucei variant surface glycoprotein. Biochemistry 28:2881-2887. [DOI] [PubMed] [Google Scholar]
- 18.Ikezawa, H. 2002. Glycosylphosphatidylinositol (GPI)-anchored proteins. Biol. Pharm. Bull. 25:409-417. [DOI] [PubMed] [Google Scholar]
- 19.Kinoshita, T., and N. Inoue. 2000. Dissecting and manipulating the pathway for glycosylphosphatidylinositol-anchor biosynthesis. Curr. Opin. Chem. Biol. 4:632-638. [DOI] [PubMed] [Google Scholar]
- 20.Kinoshita, T., K. Ohishi, and J. Takeda. 1997. GPI-anchor synthesis in mammalian cells: genes, their products, and a deficiency. J. Biochem. (Tokyo) 122:251-257. [DOI] [PubMed] [Google Scholar]
- 21.Kodukula, K., R. Amthauer, D. Cines, E. T. Yeh, L. Brink, L. J. Thomas, and S. Udenfriend. 1992. Biosynthesis of phosphatidylinositol-glycan (PI-G)-anchored membrane proteins in cell-free systems: PI-G is an obligatory cosubstrate for COOH-terminal processing of nascent proteins. Proc. Natl. Acad. Sci. USA 89:4982-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda, Y., R. Watanabe, C. L. Harris, Y. Hong, K. Ohishi, K. Kinoshita, and T. Kinoshita. 2001. PIG-M transfers the first mannose to glycosylphosphatidylinositol on the lumenal side of the ER. EMBO J. 20:250-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masterson, W. J., T. L. Doering, G. W. Hart, and P. T. Englund. 1989. A novel pathway for glycan assembly: biosynthesis of the glycosyl-phosphatidylinositol anchor of the trypanosome variant surface glycoprotein. Cell 56:793-800. [DOI] [PubMed] [Google Scholar]
- 24.Mayor, S., and A. K. Menon. 1990. Structural analysis of glycosylinositol phospholipid anchors of membrane proteins. Methods 1:297-305. [Google Scholar]
- 25.McConville, M. J., and M. A. Ferguson. 1993. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem. J. 294:305-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McConville, M. J., and A. K. Menon. 2000. Recent developments in the cell biology and biochemistry of glycosylphosphatidylinositol lipids. Mol. Membr. Biol. 17:1-16. [DOI] [PubMed] [Google Scholar]
- 27.McDowell, W., and R. T. Schwarz. 1988. Lipid-mediated protein glycosylation: assembly of lipid-linked oligosaccharides and post-translational oligosaccharide trimming, p. 99-118. In U. Brodbeck and C. Bordier (ed.), Post-translational modifications of proteins by lipids. Springer-Verlag, Berlin, Germany.
- 28.Menon, A. K. 1995. Flippases. Trends Cell Biol. 5:355-360. [DOI] [PubMed] [Google Scholar]
- 29.Menon, A. K. 2004. Glycosylphosphatidylinositol (GPI) anchors, p. 308-311. In W. J. Lennarz and M. D. Lane (ed.), Encyclopedia of biological chemistry, vol. 2. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 30.Menon, A. K., M. Eppinger, S. Mayor, and R. T. Schwarz. 1993. Phosphatidylethanolamine is the donor of the terminal phosphoethanolamine group in trypanosome glycosylphosphatidylinositols. EMBO J. 12:1907-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menon, A. K., S. Mayor, and R. T. Schwarz. 1990. Biosynthesis of glycosyl-phosphatidylinositol lipids in Trypanosoma brucei: involvement of mannosyl-phosphoryldolichol as the mannose donor. EMBO J. 9:4249-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menon, A. K., and J. Vidugiriene. 1994. Topology of GPI biosynthesis in the endoplasmic reticulum. Braz. J. Med. Biol. Res. 27:167-175. [PubMed] [Google Scholar]
- 33.Mineo, J. R., and L. H. Kasper. 1994. Attachment of Toxoplasma gondii to host cells involves major surface protein, SAG-1 (P30). Exp. Parasitol. 79:11-20. [DOI] [PubMed] [Google Scholar]
- 34.Morita, Y. S., K. S. Paul, and P. T. Englund. 2000. Specialized fatty acid synthesis in African trypanosomes: myristate for GPI anchors. Science 288:140-143. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura, N., N. Inoue, R. Watanabe, M. Takahashi, J. Takeda, V. L. Stevens, and T. Kinoshita. 1997. Expression cloning of PIG-L, a candidate N-acetylglucosaminyl-phosphatidylinositol deacetylase. J. Biol. Chem. 272:15834-15840. [DOI] [PubMed] [Google Scholar]
- 36.Roberts, W. L., J. J. Myher, A. Kuksis, M. G. Low, and T. L. Rosenberry. 1988. Lipid analysis of the glycoinositol phospholipid membrane anchor of human erythrocyte acetylcholinesterase. Palmitoylation of inositol results in resistance to phosphatidylinositol-specific phospholipase C. J. Biol. Chem. 263:18766-18775. [PubMed] [Google Scholar]
- 37.Robinson, S. A., J. E. Smith, and P. A. Millner. 2004. Toxoplasma gondii major surface antigen (SAG1): in vitro analysis of host cell binding. Parasitology 128:391-396. [DOI] [PubMed] [Google Scholar]
- 38.Schneider, P., M. A. Ferguson, M. J. McConville, A. Mehlert, S. W. Homans, and C. Bordier. 1990. Structure of the glycosyl-phosphatidylinositol membrane anchor of the Leishmania major promastigote surface protease. J. Biol. Chem. 265:16955-16964. [PubMed] [Google Scholar]
- 39.Schutzbach, J. S., and J. W. Zimmerman. 1992. Yeast dolichyl-phosphomannose synthase: reconstitution of enzyme activity with phospholipids. Biochem. Cell Biol. 70:460-465. [DOI] [PubMed] [Google Scholar]
- 40.Seeber, F., and J. C. Boothroyd. 1996. Escherichia coli beta-galactosidase as an in vitro and in vivo reporter enzyme and stable transfection marker in the intracellular protozoan parasite Toxoplasma gondii. Gene 169:39-45. [DOI] [PubMed] [Google Scholar]
- 41.Sharma, S. D., J. Mullenax, F. G. Araujo, H. A. Erlich, and J. S. Remington. 1983. Western blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J. Immunol. 131:977-983. [PubMed] [Google Scholar]
- 42.Sibley, L. D. 2004. Intracellular parasite invasion strategies. Science 304:248-253. [DOI] [PubMed] [Google Scholar]
- 43.Smith, T. K., S. Cottaz, J. S. Brimacombe, and M. A. Ferguson. 1996. Substrate specificity of the dolichol phosphate mannose: glucosaminyl phosphatidylinositol α1-4 mannosyltransferase of the glycosyl-phosphatidylinositol biosynthetic pathway of African trypanosomes. J. Biol. Chem. 271:6476-6482. [DOI] [PubMed] [Google Scholar]
- 44.Smith, T. K., D. K. Sharma, A. Crossman, J. S. Brimacombe, and M. A. Ferguson. 1999. Selective inhibitors of the glycosylphosphatidylinositol biosynthetic pathway of Trypanosoma brucei. EMBO J. 18:5922-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith, T. K., D. K. Sharma, A. Crossman, A. Dix, J. S. Brimacombe, and M. A. Ferguson. 1997. Parasite and mammalian GPI biosynthetic pathways can be distinguished using synthetic substrate analogues. EMBO J. 16:6667-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snider, M. D., and P. W. Robbins. 1982. Transmembrane organization of protein glycosylation. Mature oligosaccharide-lipid is located on the luminal side of microsomes from Chinese hamster ovary cells. J. Biol. Chem. 257:6796-6801. [PubMed] [Google Scholar]
- 47.Striepen, B., J. F. Dubremetz, and R. T. Schwarz. 1999. Glucosylation of glycosylphosphatidylinositol membrane anchors: identification of uridine diphosphate-glucose as the direct donor for side chain modification in Toxoplasma gondii using carbohydrate analogues. Biochemistry 38:1478-1487. [DOI] [PubMed] [Google Scholar]
- 48.Striepen, B., C. F. Zinecker, J. B. Damm, P. A. Melgers, G. J. Gerwig, M. Koolen, J. F. Vliegenthart, J. F. Dubremetz, and R. T. Schwarz. 1997. Molecular structure of the “low molecular weight antigen” of Toxoplasma gondii: a glucose alpha 1-4 N-acetylgalactosamine makes free glycosyl-phosphatidylinositols highly immunogenic. J. Mol. Biol. 266:797-813. [DOI] [PubMed] [Google Scholar]
- 49.Tomavo, S., J. F. Dubremetz, and R. T. Schwarz. 1992. Biosynthesis of glycolipid precursors for glycosylphosphatidylinositol membrane anchors in a Toxoplasma gondii cell-free system. J. Biol. Chem. 267:21446-21458. [PubMed] [Google Scholar]
- 50.Udenfriend, S., and K. Kodukula. 1995. How glycosylphosphatidylinositol-anchored membrane proteins are made. Annu. Rev. Biochem. 64:563-591. [DOI] [PubMed] [Google Scholar]
- 51.Vidugiriene, J., and A. K. Menon. 1993. Early lipid intermediates in glycosyl-phosphatidylinositol anchor assembly are synthesized in the ER and located in the cytoplasmic leaflet of the ER membrane bilayer. J. Cell Biol. 121:987-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vidugiriene, J., and A. K. Menon. 1994. The GPI anchor of cell-surface proteins is synthesized on the cytoplasmic face of the endoplasmic reticulum. J. Cell Biol. 127:333-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vidugiriene, J., D. K. Sharma, T. K. Smith, N. A. Baumann, and A. K. Menon. 1999. Segregation of glycosylphosphatidylinositol biosynthetic reactions in a subcompartment of the endoplasmic reticulum. J. Biol. Chem. 274:15203-15212. [DOI] [PubMed] [Google Scholar]
- 54.Vishwakarma, R. A., and A. K. Menon. 2005. Flip-flop of glycosylphosphatidylinositols (GPI's) across the ER. Chem. Commun. (Cambridge) 2005:453-455. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe, R., N. Inoue, B. Westfall, C. H. Taron, P. Orlean, J. Takeda, and T. Kinoshita. 1998. The first step of glycosylphosphatidylinositol biosynthesis is mediated by a complex of PIG-A, PIG-H, PIG-C and GPI1. EMBO J. 17:877-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe, R., T. Kinoshita, R. Masaki, A. Yamamoto, J. Takeda, and N. Inoue. 1996. PIG-A and PIG-H, which participate in glycosylphosphatidylinositol anchor biosynthesis, form a protein complex in the endoplasmic reticulum. J. Biol. Chem. 271:26868-26875. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe, R., Y. Murakami, M. D. Marmor, N. Inoue, Y. Maeda, J. Hino, K. Kangawa, M. Julius, and T. Kinoshita. 2000. Initial enzyme for glycosylphosphatidylinositol biosynthesis requires PIG-P and is regulated by DPM2. EMBO J. 19:4402-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watanabe, R., K. Ohishi, Y. Maeda, N. Nakamura, and T. Kinoshita. 1999. Mammalian PIG-L and its yeast homologue Gpi12p are N-acetylglucosaminylphosphatidylinositol de-N-acetylases essential in glycosylphosphatidylinositol biosynthesis. Biochem. J. 339:185-192. [PMC free article] [PubMed] [Google Scholar]
- 59.Zinecker, C. F., B. Striepen, H. Geyer, R. Geyer, J. F. Dubremetz, and R. T. Schwarz. 2001. Two glycoforms are present in the GPI-membrane anchor of the surface antigen 1 (P30) of Toxoplasma gondii. Mol. Biochem. Parasitol. 116:127-135. [DOI] [PubMed] [Google Scholar]