Abstract
The ARTEMIS Global Antifungal Susceptibility Program (ARTEMIS Program) was initiated in 2001 to provide focused surveillance of the activities of fluconazole and voriconazole against Candida spp. isolated from blood and other normally sterile sites. A total of 1,586 episodes of infection were detected at 61 international study sites. Overall, 57.7% of the infections were due to Candida albicans, followed by C. glabrata (14.8%), C. parapsilosis (12.5%), C. tropicalis (9.4%), C. krusei (2.7%), and C. lusitaniae (1.5%). Isolates of C. albicans, C. parapsilosis, and C. tropicalis were all highly susceptible to fluconazole (for 99% of the isolates the MICs were ≤8 μg/ml). Likewise, 99 to 100% of these species were inhibited by ≤1 μg of voriconazole per ml. Voriconazole was also active against C. glabrata (93% of the isolates were susceptible [MICs ≤ 1 μg/ml]) and C. krusei (100% of the isolates were susceptible). The agar-based Etest and disk diffusion methods performed well for the testing of both fluconazole and voriconazole compared to the broth microdilution MIC reference method. These observations establish the continued importance of C. albicans as a pathogen and the sustained activity of fluconazole and the broad spectrum of activity of voriconazole and will serve as the first-year benchmark for the ARTEMIS Program. Continued surveillance and refinement of broth- and agar-based test methods will help to identify susceptibility trends and improve the laboratory capability for antifungal susceptibility testing.
Antimicrobial resistance surveillance serves many purposes. The most common is detection and tracking of resistance trends and emerging new resistance threats (10, 15, 16, 18). Clearly, this is very important and serves as the basis for both empirical treatment recommendations and as a means to assess interventional efforts (8, 10, 14, 16). In addition to these potentially far-reaching objectives, surveillance programs also serve as a means to monitor the prevalent pathogens causing serious infections (e.g., bloodstream infections [BSIs]). The isolates collected in those programs that use a central laboratory can be used to assess the activities of new antimicrobial agents and to conduct postmarketing surveillance of the activities of established agents and aid in the development and validation of new susceptibility testing methods (8-10, 16). In order to address effectively any of these secondary objectives, the availability of a geographically diverse collection of isolates from clinically important sites of infection (e.g., blood and normally sterile body fluids [NSBFs]) is essential (16, 18, 31).
Although there are numerous ongoing antibacterial resistance surveillance programs that address with various degrees of success the objectives noted above (10, 15, 16, 31), very few programs provide similar information for fungal infections and antifungal resistance (5, 21, 31). The ARTEMIS Global Antifungal Susceptibility Program (ARTEMIS Program) was initiated in 2001 in order to provide focused surveillance of the activities of fluconazole and voriconazole against Candida spp. causing invasive infections and to provide continuous development and validation of various broth- and agar-based antifungal susceptibility test systems. The ARTEMIS Program uses a central reference laboratory and an international network of 105 participating centers as sources of clinical isolates.
Fluconazole is a very widely used systemic antifungal agent with a broad therapeutic range and little toxicity (34). Concerns regarding the inappropriate usage of fluconazole (e.g., poor indications and inadequate dosing practices) abound (3, 20), coupled with concerns of increased resistance due to either the emergence of resistance in previously susceptible species or the emergence of species with primary resistance to fluconazole (3, 7, 28, 30, 36). Likewise, there is a need for broad and ongoing assessment of the potency and spectrum of activity of voriconazole both prior to and after its introduction into clinical practice. Assessment of susceptibility and resistance to these agents among Candida spp. is dependent upon the use of standardized antifungal susceptibility testing methods. For surveillance purposes the National Committee for Clinical Laboratory Standards (NCCLS) broth microdilution (BMD) method (19) is ideal, as it is standardized, provides quantitative MICs, and is well suited for use in a high-volume reference laboratory. The NCCLS method may not be optimal for use in many clinical laboratories, as there are few validated commercial MIC systems and many laboratories test only a few isolates per year. Thus, development of agar-based tests such as disk diffusion and Etest methods has been deemed desirable (27, 30). Both methods have been evaluated, and Etest appears to perform well in providing quantitative MICs of both fluconazole and voriconazole (22, 25). The disk diffusion method has only recently been shown to be useful for the testing of fluconazole (2, 11, 17, 19a), and the published data for voriconazole disk testing is limited (13). In the present study we report the first data from the ARTEMIS Program, including data on both the surveillance of antifungal resistance among Candida spp. and the continued development and validation of the disk diffusion and Etest methods for fluconazole and voriconazole.
MATERIALS AND METHODS
Study design.
The ARTEMIS Program was established in 2001 to monitor the species and antifungal susceptibility patterns of Candida spp. isolated from blood and other NSBFs via a broad network of 105 sentinel hospital sites in North America, Latin America, Europe, Africa, and Asia. Candida spp. causing invasive disease (BSIs and infections of NSBFs) were reported from 61 of 105 medical centers monitored in 2001 (January through December).
Each participant hospital contributed results (organism identification, date of isolation, and hospital location) for consecutive blood and NSBF culture isolates (one isolate per patient) of Candida spp. judged to be clinically significant by local criteria and detected in each calendar month during the study period. All isolates were saved on agar slants and were sent to the University of Iowa College of Medicine (Iowa City) for storage and further characterization by reference identification methods and susceptibility testing (19, 37). Isolates were also subjected to testing by the disk diffusion and Etest methods (2, 19a, 22, 25).
Organism identification.
All Candida sp. isolates were identified at the participating institutions by the routine method used in each laboratory. Upon receipt at the University of Iowa, the isolates were subcultured onto potato dextrose agar (Remel, Lenexa, Kans.) and CHROMagar Candida medium (Hardy Laboratories, Santa Maria, Calif.) to ensure viability and purity. Confirmation of species identification was performed with Vitek and API products (bioMerieux, St. Louis, Mo.), as recommended by the manufacturer or by conventional methods, as required (37). Isolates were stored as suspensions in water or on agar slants at ambient temperature until needed.
Susceptibility testing.
Reference antifungal susceptibility testing of Candida spp. was performed by the BMD method described by the NCCLS (19). Reference powders of fluconazole and voriconazole were obtained from Pfizer Pharmaceuticals (Groton, Conn.).
Etest strips for fluconazole and voriconazole were provided by AB BIODISK (Solna, Sweden). MICs were determined by Etest as described previously (22, 25) with RPMI agar with 2% glucose (Remel), an inoculum suspension adjusted to the turbidity of a 0.5 McFarland standard (∼106 cells/ml), and incubation at 35°C for 48 h. The MICs of both fluconazole and voriconazole were read as the lowest concentration at which the border of the elliptical inhibition zone intercepted the scale on the strip. Any growth, such as microcolonies, throughout a discernible inhibition ellipse was ignored.
Disk diffusion testing of fluconazole and voriconazole was performed as described by Barry et al. (2) and in NCCLS document M44-P (19a). Fluconazole (25 μg) and voriconazole (1 μg) disks were obtained from Becton Dickinson (Sparks, Md.). For disk diffusion testing, 150-mm-diameter plates containing Mueller-Hinton agar (Difco Laboratories) supplemented with 2% glucose and methylene blue (0.5 μg/ml) at a depth of 4.0 mm were used. The agar surface was inoculated by using a swab dipped in a cell suspension adjusted to the turbidity of a 0.5 McFarland standard. The plates were incubated in air at 35°C and read at 24 h. Zone diameter endpoints were read at 80% growth inhibition by using the BIOMIC image analysis plate reader system (version 5.9; Giles Scientific, Santa Barbara, Calif.) (17).
MIC interpretive criteria for fluconazole were those published by Rex et al. (29) and the NCCLS (19) and were as follows: susceptible, MIC ≤ 8 μg/ml; susceptible-dose dependent, MIC = 16 to 32 μg/ml; resistant, MIC ≥ 64 μg/ml. The interpretive criteria for the fluconazole disk test were those published by Barry et al. (2) and the NCCLS (19a): susceptible, zone diameter of ≥19 mm; susceptible-dose dependent, zone diameter of 15 to 18 mm; resistant, zone diameter of ≤14 mm. Interpretive breakpoints have not yet been established for voriconazole.
QC.
Quality control (QC) was performed for BMD and Etest in accordance with NCCLS document M27-A (19) by using Candida krusei ATTC 6258 and C. parapsilosis ATCC 22019 (1, 19). QC determinations made on each day of testing were within the control limits for fluconazole and voriconazole described by Barry et al. (1). QC for disk diffusion testing was performed by using C. albicans ATCC 90028 and C. parapsilosis ATCC 22019 (2, 17, 19a).
Analysis of results.
The Etest MICs of fluconazole and voriconazole read at 48 h were compared to the reference BMD MICs read at 48 h. The Etest MICs were rounded up to the next even log2 concentration in order to simplify analysis (2, 22, 25). Discrepancies of no more than 2 dilutions were used to calculate the percent agreement.
The diameters of the zones of inhibition (in millimeters) surrounding the fluconazole and voriconazole disks at 24 h of incubation were plotted against their respective BMD MICs read at 48 h (2). The method of least squares was used to calculate a regression line for each comparison. The interpretive breakpoints described by Barry et al. (2) and the NCCLS (19a) were used to determine the categorical agreement between the disk diffusion and BMD results for fluconazole. Major errors were identified as a classification of resistance by the disk diffusion test and susceptibility by BMD, very major errors were identified as a classification of susceptibility by the disk diffusion method and resistance by BMD, and minor errors occurred when the result of one of the tests was susceptibility or resistance and that of the other test was susceptible-dose dependent.
RESULTS AND DISCUSSION
During the 2001 study period, a total of 1,586 isolates of Candida spp. from blood and other NSBFs were submitted from 61 participating centers in North America (20 centers), Latin America (8 centers), Europe (19 centers), Africa (5 centers), and Asia (9 centers). The species identification provided from the submitting laboratory was confirmed for 92% of the isolates (range, 25% for C. guilliermondii to 97% for C. albicans). The frequencies of infections due to the various species of Candida identified by the central reference laboratory are presented in Table 1. As observed in other surveys (17, 26), 97% of serious candidal infections were due to C. albicans (57.7%), C. glabrata (14.8%), C. parapsilosis (12.5%), C. tropicalis (9.4%), and C. krusei (2.7%). Likewise, the rank order of these species is essentially identical to that reported by the SENTRY Program (26) and by Meis and colleagues for the Global Antifungal Surveillance Group (17). As noted previously (23, 24), these data further demonstrate the sustained importance of C. albicans as an etiologic agent of BSIs and infections of other normally sterile sites. Although the frequency of C. albicans as a cause of BSIs may vary among different institutions (26), these data indicate that the role of C. albicans as a major fungal pathogen had not diminished in 2001 and in fact may be increasing in some locations, despite the widespread use of fluconazole (4, 20, 26, 32, 33, 35).
TABLE 1.
Species distributions of Candida bloodstream and other normally sterile body fluid isolates in the ARTEMIS Program, 2001
| Species | No. of isolates | % of isolates |
|---|---|---|
| Candida albicans | 916 | 57.7 |
| Candida glabrata | 235 | 14.8 |
| Candida parapsilosis | 198 | 12.5 |
| Candida tropicalis | 150 | 9.4 |
| Candida krusei | 43 | 2.7 |
| Candida lusitaniae | 24 | 1.5 |
| Candida speciesa | 20 | 1.3 |
| Total | 1,586 | 100 |
Includes C. kefyr (n = 4), C. pelliculosa (n = 8), C. rugosa (n = 2), C. lipolytica (n = 2), C. guilliermondii (n = 2), C. famata (n = 1), and C. zeylanoides (n = 1).
In vitro susceptibility testing by both the reference BMD method and Etest demonstrated that resistance to fluconazole (MIC ≥ 64 μg/ml) remains rare among isolates of Candida from blood and NSBFs (Table 2). By using the BMD results, 99% of C. albicans, C. parapsilosis, and C. tropicalis isolates were susceptible (MIC ≤ 8 μg/ml) to fluconazole. Overall, 90.5% of isolates were susceptible and only 2.5% were resistant. As noted previously (26), C. glabrata and C. krusei were the least susceptible; however, only 7% of C. glabrata isolates demonstrated high-level resistance. Although the Etest MIC results tended to be slightly higher than the BMD MIC results, especially for C. glabrata, the overall agreement between Etest and BMD was 96.4%, consistent with that reported previously (22).
TABLE 2.
In vitro susceptibilities of Candida spp. to fluconazole determined by BMD and Etest methods, ARTEMIS Program, 2001
| Species (no. of isolates tested) | Test Method | Cumulative % susceptible at MIC (μg/ml) ofa:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | ||
| C. albicans (916) | BMD | 14 | 80 | 94 | 98 | 99 | 99 | 99 | 99 | 99 | 99 |
| Etest | 42 | 87 | 98 | 99 | 99 | 99 | 99 | 99 | 99 | 99 | |
| C. glabrata (235) | BMD | 0 | 0 | 0 | 1 | 6 | 25 | 57 | 89 | 93 | 95 |
| Etest | 0 | 0 | 0 | 1 | 6 | 15 | 32 | 66 | 86 | 91 | |
| C. parapsilosis (198) | BMD | 0 | 7 | 44 | 85 | 96 | 99 | 99 | 100 | ||
| Etest | 4 | 20 | 62 | 88 | 94 | 97 | 99 | 99 | 100 | ||
| C. tropicalis (150) | BMD | 0 | 5 | 25 | 57 | 93 | 99 | 99 | 99 | 99 | 100 |
| Etest | 0 | 13 | 69 | 92 | 97 | 99 | 100 | ||||
| C. krusei (43) | BMD | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 56 | 98 |
| Etest | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 37 | 58 | |
| C. lusitaniae (24) | BMD | 4 | 21 | 67 | 88 | 92 | 96 | 96 | 96 | 96 | 100 |
| Etest | 8 | 29 | 54 | 83 | 92 | 96 | 96 | 96 | 96 | 96 | |
MICs were determined according to NCCLS guidelines (19).
Voriconazole demonstrated excellent potency and broad-spectrum activity against all species (Table 3). Overall, 99% of isolates were inhibited by voriconazole at ≤1 μg/ml. As noted previously (26), C. albicans was the most susceptible (MIC at which 90% of isolates are inhibited [MIC90], 0.015 μg/ml) and C. glabrata was the least susceptible (MIC90, 1 μg/ml). All of the C. krusei isolates tested were inhibited by voriconazole at ≤1 μg/ml. Similar to the results obtained with fluconazole, the agreement between BMD and Etest results was good (98.1%), with Etest MICs tending to be slightly higher than those determined by BMD. Again, these results are consistent with those reported previously (25).
TABLE 3.
In vitro susceptibilities of Candida spp. to voriconazole determined by BMD and Etest methods, ARTEMIS Program, 2001
| Species (no. of isolates tested) | Test method | Cumulative % susceptible at MIC (μg/ml) ofa:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.007 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ||
| C. albicans (916) | BMD | 79 | 94 | 99 | 99 | 99 | 99 | 99 | 99 | 99 | 100 | |
| Etest | 73 | 98 | 99 | 99 | 99 | 99 | 99 | 99 | 99 | 100 | ||
| C. glabrata (235) | BMD | 0 | 0 | 1 | 6 | 29 | 74 | 88 | 93 | 97 | 99 | 100 |
| Etest | 0 | 1 | 3 | 12 | 30 | 66 | 86 | 92 | 95 | 96 | 97 | |
| C. parapsilosis (198) | BMD | 18 | 63 | 83 | 96 | 99 | 99 | 100 | ||||
| Etest | 25 | 64 | 85 | 97 | 99 | 99 | 100 | |||||
| C. tropicalis (150) | BMD | 4 | 11 | 41 | 87 | 98 | 100 | |||||
| Etest | 3 | 22 | 71 | 92 | 99 | 100 | ||||||
| C. krusei (43) | BMD | 0 | 0 | 0 | 0 | 16 | 65 | 93 | 100 | |||
| Etest | 0 | 0 | 0 | 0 | 7 | 72 | 95 | 100 | ||||
| C. lusitaniae (24) | BMD | 79 | 88 | 88 | 96 | 96 | 96 | 100 | ||||
| Etest | 54 | 92 | 92 | 96 | 96 | 96 | 100 | |||||
MICs were determined according to NCCLS guidelines (19).
Although the antifungal susceptibility data presented in Tables 2 and 3 are very similar to those reported previously from other antifungal surveillance programs (17, 26), the manner of data presentation used in the ARTEMIS Program takes into account the suggestions and critiques of several expert individuals regarding the usefulness of antimicrobial resistance surveillance programs (10, 15, 18).
The ARTEMIS Program susceptibility data are generated by a standardized quantitative reference method (NCCLS BMD method) and a validated reference quality quantitative method (Etest) and are presented in a continuous fashion as the cumulative percentages of organisms susceptible at each dilution throughout the full dilution series. The presentation of susceptibility data generated by standardized methods in a continuous fashion has several important advantages (10, 18): (i) the data may be compared over time, and subtle changes in susceptibility (trends) can be detected before changes in MIC50s, MIC90s, or interpretive categories are observed; (ii) the data generated by the same methodology by different studies may be compared; (iii) the data may be more generalizable, as continuous data are not restricted by interpretive criteria that may vary by nation or by international consensus groups; and (iv) continuous data provide antimicrobial susceptibility information for organisms or agents for which breakpoints are not available. Thus, the MIC data for fluconazole and voriconazole presented in Tables 2 and 3 represent the initial benchmark results for the ARTEMIS Program. Testing of Candida spp. from defined sites of infection (blood and NSBFs) by a standardized reference method to generate full-scale MICs presented in a continuous fashion will ensure that the data from the ARTEMIS Program are truly international and not subject to the interpretive restrictions dictated by regulatory agencies, professional societies, or consensus standards organizations (10, 18). The longitudinal nature of this program will also ensure that any trends in the susceptibility of Candida spp. to fluconazole or voriconazole are readily detected.
Comparison of susceptibility test methods by use of a reference BMD method and disk diffusion testing of fluconazole and voriconazole is also an important aspect of the ARTEMIS Program. Figure 1 shows the correlation of the 25-μg fluconazole disk zone diameters read at 24 h compared to the BMD MIC results read at 48 h. The regression equation (y = 49.6 − 1.9x; R = 0.7) is similar to that of Barry et al. (2) (y = 55.4 − 2.7x; R = 0.8). The overall categorical agreement by use of the interpretive criteria of Barry et al. (2) and the NCCLS (19, 19a) was 93.1%, with 0.1% very major errors, 0.3% major errors, and 6.5% minor errors. These data support the findings of Meis et al. (17) and Barry et al. (2) and provide further validation of the disk diffusion test as a useful method for determining the in vitro susceptibilities of Candida spp. to fluconazole.
FIG. 1.
Zones of inhibition around 25-μg fluconazole disks tested on Mueller-Hinton agar supplemented with 2% glucose and methylene blue (0.5 μg/ml) plotted against the MICs at 48 h determined by the reference BMD method for 1,586 isolates of Candida spp. The method of least squares was used to calculate a regression line (y = 49.6 − 1.9x; R = 0.7).
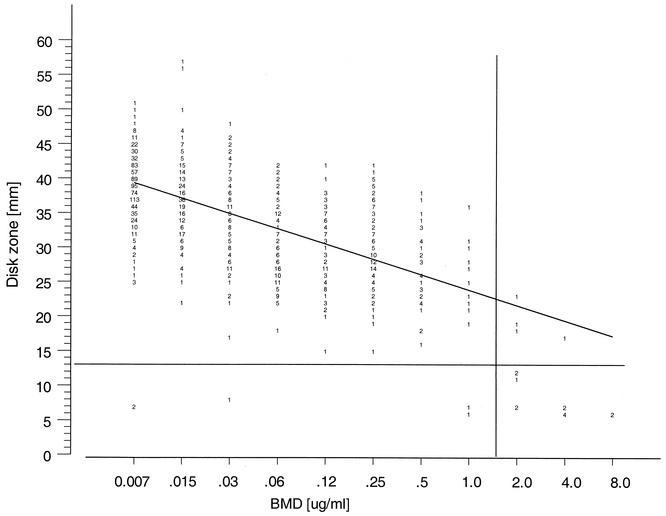
Although disk diffusion testing of voriconazole has not been fully developed (13), we used a 1-μg voriconazole disk and the same method for the testing of fluconazole to generate zone diameters and MIC correlates for voriconazole. Figure 2 shows the correlation of the 1-μg voriconazole disk zone diameters read at 24 h compared to the BMD MIC results read at 48 h. The regression statistics (y = 43.8 − 2.4x; R = 0.7) showed that there is a good level of agreement between the two methods. Although MIC breakpoints have not yet been established for voriconazole, the 17 isolates for which MICs were ≥2 μg/ml were either resistant (15 isolates) or susceptible-dose dependent (2 isolates) to fluconazole. Thirteen of these isolates were separated from the rest of the population by a zone diameter threshold of ≤13 mm. Although a single preliminary interpretive category of susceptible by use of an MIC threshold of ≤1 μg/ml and a zone diameter of >13 mm may be supported by pharmacokinetic and pharmacodynamic profiles (6, 12, 34), the establishment of clinical correlates is imperative before breakpoints can be used clinically (30). Despite these concerns, the overall categorical agreement of the results obtained by using these threshold values was 99.4% for the voriconazole disk method and the BMD MIC test. On the basis of these findings, it appears that the disk diffusion test is a useful method for testing of the activity of voriconazole against Candida spp. Continued application of this test in the context of the ARTEMIS Program will allow us to monitor the frequencies of clinical isolates surrounding these zone diameter and MIC thresholds. Depending on the frequency of occurrence and clinical response to therapy, more firmly established in vitro breakpoint criteria (susceptible, susceptible-dose dependent, and/or resistant) may be determined.
FIG. 2.
Zones of inhibition around 1-μg voriconazole disks plotted against the MICs determined at 48 h by the reference BMD method for 1,586 isolates of Candida spp. The method of least squares was used to calculate a regression line (y = 43.8 − 2.4x; R = 0.7).
The data reported herein provide additional support to those from the SENTRY Program (24, 26) and the Global Antifungal Surveillance Group (17) regarding the sustained activity of fluconazole against Candida spp. causing BSIs and other deeply invasive infections. The MIC data generated for both fluconazole and voriconazole in 2001 will serve as the benchmark for the ARTEMIS Program and the basis for future comparisons and the establishment of susceptibility trends. Continued monitoring of isolates from the international study sites by the BMD, disk diffusion, and Etest methods will provide useful quantitative and qualitative data and allow continued refinement of all methods with a robust collection of geographically diverse strains of Candida spp. Thus far, the studies appear to validate the usefulness of both Etest and the disk diffusion test for determination of the in vitro susceptibilities of Candida spp. to fluconazole and voriconazole.
Acknowledgments
This study was supported in part by research and educational grants from Pfizer Pharmaceuticals and Giles Scientific, Inc.
We thank Linda Elliott for secretarial assistance in the preparation of the manuscript. We appreciate the contributions of all ARTEMIS site participants. The following participants contributed isolates to the study: Hershey Medical Center, Hershey, Pa. (P. Appelbaum); Stanford Hospitals and Clinics, Stanford, Calif. (E. J. Baron); University of California Los Angeles Medical Center, Los Angeles (D. Brucker); New York State Department of Health, Albany (V. Chaturvedi); Wishard Health Services, Indianapolis, Ind. (T. Davis); Summa Health System, Akron, Ohio (J. DiPersio); Case Western Reserve University Hospital, Cleveland, Ohio (M. Ghannoum); Cleveland Clinic Foundation, Cleveland, Ohio (G. Hall); University of Rochester Medical Center, Rochester, N.Y. (D. Hardy); University of Virginia Health System, Charlottesville (K. Hazen); Veteran Affairs Medical Center, Ann Arbor, Mich. (C. Kauffman); and others, which are listed on the following website: http://www.medicine.uiowa.edu/pathology/path_folder/research/acknowledgements/artemis_participants.pdf.
REFERENCES
- 1.Barry, A. L., M. A. Pfaller, S. D. Brown, A. Espinel-Ingroff, M. A. Ghannoum, C. Knapp, R. P. Rennie, J. H. Rex, and M. G. Rinaldi. 2000. Quality control limits for broth microdilution susceptibility tests of ten antifungal agents. J. Clin. Microbiol. 38:3457-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, A. L., M. A. Pfaller, R. P. Rennie, P. C. Fuchs, and S. D. Brown. 2002. Precision and accuracy of fluconazole susceptibility tests by broth microdilution, Etest and disk diffusion methods. Antimicrob. Agents Chemother. 46:1781-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berrouane, Y. F., L. A. Herwaldt, and M. A. Pfaller. 1999. Trends in antifungal use and epidemiology of nosocomial yeast infections in a university hospital. J. Clin. Microbiol. 37:531-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chryssanthou, E. 2001. Trends in antifungal susceptibility among Swedish Candida species bloodstream isolates from 1994 to 1998: comparison of the E-test and the Sensititre Yeast One colorimetric antifungal panel with the NCCLS M27-A reference method. J. Clin. Microbiol. 39:4181-4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis, D., D. Marriott, R. A. Hajjeh, D. Warnock, W. Meyer, and R. Barton. 2000. Epidemiology: surveillance of fungal infections. Med. Mycol. 38(Suppl. 1):173-182. [PubMed] [Google Scholar]
- 6.Ernst, E. J. 2001. Investigational antifungal agents. Pharmacotherapy 21(Pt. 2):165S-174S. [DOI] [PubMed]
- 7.Hudson, M. M. T. 2001. Antifungal resistance and over-the-counter availability in the UK: a current perspective. J. Antimicrob. Chemother. 48:345-350. [DOI] [PubMed] [Google Scholar]
- 8.Hunter, P. A., and D. S. Reeves. 2002. The current status of surveillance of resistance to antimicrobial agents: report on a meeting. J. Antimicrob. Chemother. 49:17-23. [DOI] [PubMed] [Google Scholar]
- 9.Jones, R. N. 1996. The emergent needs for basic research, education, and surveillance of antimicrobial resistance. Problems facing the report from the American Society for Microbiology Task Force on Antibiotic Resistance. Diagn. Microbiol. Infect. Dis. 25:53-61. [DOI] [PubMed] [Google Scholar]
- 10.Jones, R. N., and the MYSTIC Advisory Board. 2000. Detection of emerging resistance patterns within longitudinal surveillance systems: data sensitivity and microbial susceptibility. J. Antimicrob. Chemother. 46(Topic T2):1-8. [PubMed] [Google Scholar]
- 11.Kirkpatrick, W. R., T. M. Turner, A. W. Fothergill, D. I. McCarthy, S. W. Redding, M. G. Rinaldi, and T. F. Patterson. 1998. Fluconazole disk diffusion susceptibility testing of Candida species. J. Clin. Microbiol. 36:3429-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klepser, M. E., D. Malone, R. E. Lewis, E. J. Ernest, and M. A. Pfaller. 2002. Evaluation of voriconazole pharmacodynamics using time-kill methodology. Antimicrob. Agents Chemother. 44:1917-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kronvall, G., and I. Karlsson. 2001. Fluconazole and voriconazole multidisk testing of Candida species for disk calibration and MIC estimation. J. Clin. Microbiol. 39:1422-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy, S. B. 2002. Factors impacting the problem of antibiotic resistance. J. Antimicrob. Chemother. 49:25-30. [DOI] [PubMed] [Google Scholar]
- 15.Lewis, D. 2002. Antimicrobial resistance surveillance: methods will depend on objectives. J. Antimicrob. Chemother. 49:3-5. [DOI] [PubMed] [Google Scholar]
- 16.Masterton, R. G. 2000. Surveillance studies: how can they help the management of infection? J. Antimicrob. Chemother. 46(Topic T2):53-58. [PubMed] [Google Scholar]
- 17.Meis, J., M. Petrou, J. Bille, D. Ellis, D. Gibbs, and the Global Antifungal Surveillance Group. 2000. A global evaluation of the susceptibility of Candida species to fluconazole by disk diffusion. Diagn. Microbiol. Infect. Dis. 36:215-223. [DOI] [PubMed] [Google Scholar]
- 18.Morris, A. K., and R. G. Masterton. 2002. Antibiotic resistance surveillance: action for international studies. J. Antimicrob. Chemother. 49:7-10. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 19a.National Committee for Clinical Laboratory Standards. 2003. Method for antifungal disk diffusion susceptibility testing of yeasts: proposed guideline M44-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.Natsch, S., M. H. M. Steeghs, Y. A. Hekster, J. F. G. M. Meis, J. W. M. van der Mier, and B. J. Kullberg. 2001. Use of fluconazole in daily practice: still room for improvement. J. Antimicrob. Chemother. 48:303-310. [DOI] [PubMed] [Google Scholar]
- 21.Pfaller, M. A. 1998. The epidemiology of invasive mycoses: narrowing the gap. Clin. Infect. Dis. 27:1148-1150. [DOI] [PubMed] [Google Scholar]
- 22.Pfaller, M. A., S. A. Messer, Å. Karlsson, and A. Bolmström. 1998. Evaluation of the Etest method for determining fluconazole susceptibilities of 402 clinical yeast isolates by using three different agar media. J. Clin. Microbiol. 36:2586-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, G. V. Doern, M. E. Brandt, and R. A. Hajjeh. 1999. Trends in species distribution and susceptibility to fluconazole among bloodstream isolates of Candida species in the United States. Diagn. Microbiol. Infect. Dis. 33:217-222. [DOI] [PubMed] [Google Scholar]
- 24.Pfaller, M. A., R. N. Jones, G. V. Doern, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, R. J. Hollis, and the SENTRY Participant Group. 2000. Bloodstream infections due to Candida species: SENTRY Antimicrobial Surveillance Program in North America and Latin America, 1997-1998. Antimicrob. Agents Chemother. 44:747-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaller, M. A., S. A. Messer, A. Houston, K. Mills, A. Bolmström, and R. N. Jones. 2000. Evaluation of the Etest method for determining voriconazole susceptibilities of 312 clinical isolates of Candida species by using three different media. J. Clin. Microbiol. 38:3715-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, S. A. Messer, and the SENTRY Participant Group. 2001. International surveillance of blood stream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY Antimicrobial Surveillance Program. J. Clin. Microbiol. 39:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfaller, M. A., and W. L. Yu. 2001. Antifungal susceptibility testing: new technology and clinical applications. Infect. Dis. Clin. N. Am. 15:1227-1261. [DOI] [PubMed] [Google Scholar]
- 28.Rex, J. H., M. G. Rinaldi, and M. A. Pfaller. 1995. Resistance of Candida species to fluconazole. Antimicrob. Agents Chemother. 39:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, and A. L. Barry for the Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole and itraconazole. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 30.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richet, H. M. 2001. Better antimicrobial resistance surveillance efforts are needed. ASM News 67:304-309. [Google Scholar]
- 32.Ruchel, R. 1997. Recurrence of Candida albicans? ISHAM Mycoses Newsl. 71:3. [Google Scholar]
- 33.Sandven, P., L. Bevangen, A. Digranes, P. Gaustad, H. H. Haukland, M. Steinbakk, and the Norwegian Yeast Study Group. 1998. Constant low rate of fungemia in Norway, 1991 to 1996. J. Clin. Microbiol. 36:3455-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheehan, D. J., C. A. Hitchcock, and C. M. Sibley. 1999. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12:40-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.St. Germain, G., M. Laverdiere, R. Pelletier, A.-M. Bourgault, M. Libman, C. Lemieux, and G. Noel. 2001. Prevalence and antifungal susceptibility of 442 Candida isolates from blood and other normally sterile sites: results of a2-year (1996 to 1998) multicenter surveillance study in Quebec, Canada. J. Clin. Microbiol. 39:949-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vazquez, J. A., G. Peng, J. D. Sobel, L. Steele-Moore, P. Schuman, W. Holloway, and J. D. Neaton for the Terry Beirr Community Programs for Clinical Research on AIDS (CPCRA). 2001. Evolution of antifungal susceptibility among Candida species isolates recovered from human immunodeficiency virus-infected women receiving fluconazole prophylaxis. Clin. Infect. Dis. 33:1069-1075. [DOI] [PubMed] [Google Scholar]
- 37.Warren, N. G., and K. C. Hazen. 1999. Candida, Cryptococcus, and other yeasts of medical importance, p. 1184-1199. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.