Abstract
Mason-Pfizer monkey virus (M-PMV) encodes a transmembrane (TM) glycoprotein with a 38-amino-acid-long cytoplasmic domain. After the release of the immature virus, a viral protease-mediated cleavage occurs within the cytoplasmic domain, resulting in the loss of 17 amino acids from the carboxy terminus. This maturational cleavage occurs between a histidine at position 21 and a tyrosine at position 22 in the cytoplasmic domain of the TM protein. We have demonstrated previously that a truncated TM glycoprotein with a 21-amino-acid-long cytoplasmic tail showed enhanced fusogenicity but could not be incorporated into virions. These results suggest that postassembly cleavage of the cytoplasmic domain removes a necessary incorporation signal and activates fusion activity. To investigate the contribution of tyrosine residues to the function of the glycoprotein complex and virus replication, we have introduced amino acid substitutions into two tyrosine residues found in the cytoplasmic domain. The effects of these mutations on glycoprotein biosynthesis and function, as well as on virus infectivity, have been examined. Mutation of tyrosine 34 to alanine had little effect on glycoprotein function. In contrast, substitutions at tyrosine 22 modulated fusion activity in either a positive or negative manner, depending on the substituting amino acid. Moreover, any nonaromatic substitution at this position blocked glycoprotein incorporation into virions and abolished infectivity. These results demonstrate that M-PMV employs a tyrosine signal for the selective incorporation of glycoprotein into budding virions. Antibody uptake studies show that tyrosine 22 is part of an efficient internalization signal in the cytoplasmic domain of the M-PMV glycoprotein that can also be positively and negatively influenced by changes at this site.
Mason-Pfizer monkey virus (M-PMV) belongs to the Betaretrovirus genus within the family Retroviridae and can be distinguished morphologically from other retroviruses. The betaretroviruses are characterized by the assembly of intracytoplasmic immature capsids, which are transported to the plasma membrane and are released by budding (39-41, 43, 50). The genomic organization of M-PMV is similar to that of most noncomplex retroviruses, with four genes in the order of 5′ long terminal repeat (LTR)-gag-pro-pol-env-3′ LTR (5-7).
The M-PMV glycoprotein is initially translated as a polyprotein precursor (Pr86) from a spliced envelope gene (env)-specific mRNA on the rough endoplasmic reticulum (6, 8-11). The glycosylated precursor is assembled into trimers in the endoplasmic reticulum and then cleaved by a cellular protease into two subunits, the surface (SU; gp70) and transmembrane (TM; gp22) proteins, in a late Golgi complex compartment (21, 25). The Env complexes are then transported to the cell surface, where they are incorporated into budding virions. The SU glycoprotein is responsible for cellular tropism for the virus, whereas the TM glycoprotein anchors the SU protein at the surface of infected cells or the viral membrane (25). The TM glycoprotein also mediates virus-cell membrane fusion during viral entry, as well as cell-cell fusion, via the action of an N-terminal fusion peptide and two heptad repeat motifs located in its extracellular domain (2, 20, 51; C. Song and E. Hunter, submitted for publication). The cytoplasmic domain of the TM protein in several retroviruses determines the rate of endocytosis of the glycoprotein at the plasma membrane through well-conserved tyrosine-based endocytosis motifs (4, 18, 29, 33, 45), mediates interactions with assembled Gag precursors (16, 22, 32), and plays a role in incorporation of glycoprotein into budding virions (12, 36, 44). Unlike lentiviral Env proteins that have very long cytoplasmic tails, the cytoplasmic domain of M-PMV is 38 amino acids long (10) and, following virus release, is cleaved by the virus-encoded protease. This results in conversion of gp22 into gp20 in the virus particle (10, 11, 47). Proteolytic cleavage of the cytoplasmic domain during virus maturation has also been observed in murine leukemia virus (MuLV) and equine infectious anemia virus (37, 38, 42). In the case of MuLV, the released C-terminal peptide has been termed the R peptide. In M-PMV this maturational cleavage results in the loss of 17 amino acids from the carboxy terminus and occurs between a histidine at position 21 and a tyrosine at position 22 in the cytoplasmic domain (I. Pichova, personal communication). We have demonstrated previously that truncation of the cytoplasmic domain to amino acid residue 22 enhanced fusogenicity dramatically and blocked incorporation of the mutant glycoprotein into virions, thereby abolishing virus infectivity (10). These results suggest that postassembly cleavage of the cytoplasmic domain removes a necessary incorporation signal and activates fusion activity.
In other retroviruses, the role of the cytoplasmic domain in Env incorporation varies. A Rous sarcoma virus Env protein lacking 22 amino acids from the cytoplasmic domain can be efficiently incorporated into virions with near-wild-type infectivity (35). In the case of MuLV, a majority of deletion mutations in the cytoplasmic domain of TM do not reduce incorporation but can affect infectivity (27).
There are two tyrosine residues within the cytoplasmic domain of the M-PMV TM protein. The membrane-proximal tyrosine (Y22) is located at position 22 from the predicted membrane-cytoplasm boundary. This Y22 residue is part of the maturational cleavage site and would be predicted to form part of a highly conserved tyrosine-based motif (YXXL) that frequently directs endocytosis from the membrane. The membrane-distal tyrosine (Y34) is also included in a potential YXXL endocytosis motif (3, 15), although the context of this tyrosine fits the canonical endocytosis sequence less well. In the studies described here, we have determined the effects of mutations in Y22 and Y34 on the synthesis, processing, transport, surface expression, and endocytosis of the glycoprotein. We also determined the effects of the mutations on fusogenicity, on the incorporation of the Env proteins into virions, and on virus infectivity. The results of this study indicate that Y22 substitutions can modulate the fusogenicity of the M-PMV Env protein, a function mediated by sequences in the ectodomain of the TM protein, as well as play a key role in a tyrosine-based endocytosis signal motif. Moreover, substitutions at the Y22 position dramatically reduced incorporation of M-PMV glycoprotein into virions and M-PMV infectivity.
MATERIALS AND METHODS
Cell culture and transfections.
African green monkey kidney cells (COS-1) were obtained from the American Type Culture Collection. The HOS-CD4/LTR-hGFP (GHOST) cell line, which expresses green fluorescence protein (GFP) under the control of a human immunodeficiency virus type 2 (HIV-2) LTR, was obtained through the AIDS Reference and Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, and was originally contributed by Vineet N. Kewal Ramani and Dan R. Littman. Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum. The HOS-CD4/LTR-hGFP cells were additionally maintained in medium containing hygromycin and G418 (Geneticin) as recommended by the contributor. Each mutant DNA was transfected into COS-1 cells by a modified calcium phosphate (CaPO4) technique (14).
DNAs and site-directed mutagenesis.
The pTMT vector is a vector that expresses M-PMV Env and HIV-1 Tat. The pMTΤΔE vector is an M-PMV proviral expression vector in which the tat gene of HIV-1 has replaced the env gene of M-PMV (Song and Hunter, submitted).
Site-directed mutagenesis of the tyrosine at position 22 and tyrosine 34 within the cytoplasmic domain was carried out using the Altered Sites mutagenesis system (Promega), essentially as described previously (19). The KpnI-to-SphI env fragment of pSHRM15 was cloned into pSELECT for mutagenesis. Single tyrosine mutations at position 22 (A, I, V, T, H, and N) were generated using a mixed oligonucleotide (CCTATACAAGTCCATRYTCATCGCCTTGAAC; R = A+G; Y = C+T), the single tyrosine mutation at position 34 (A) was generated using a single mutagenic oligonucleotide (CAGTGGTGGCTCAGCTTTGAC), and the mutation encompassing both tyrosines (Y34A/Y22T) was produced using a mutagenic reaction where both mutagenic oligonucleotides were included.
The Y22F, Y22E, Y22R, and Y22W substitutions were performed using mega-primer PCR methods. A small fragment of the EcoRI-digested pTMT vector was purified and then used as a template for PCR mutagenesis. In the first PCR step, a reverse primer (5′-GCCAAGACATCATCCG) in the gp22 coding sequence was designed and used in combination with mutagenic primers for each corresponding site to amplify the DNA sequence. The sequences of the mutagenic oligonucleotides used were as follows: Y22F, 5′-CAAGTCCATTTTCATCGCCTTGAAC; Y22E, CAAGTCCATGAACATCGCCTTGAAC; Y22R, CAAGTCCATCGTCATCGCCTTGAAC; Y22W, CAAGTCCATTGGCATCGCCTT GAAC.
After the first PCR step, all the PCR products were purified. For the second PCR step, a primer (5′-CAGAGCAGGGAGGTATA) in the gp22 coding sequence was designed and used in combination with the first PCR products as a reverse primer. After the second PCR, the products were digested with BlpI and BspEI and cloned into the same unique sites in pTMT. The mutated sequences were excised from the pTMT vectors by digestion with EcoRI and BlpI, and the fragment was inserted into the proviral vector pSARM4, which has a unique EcoRI-BlpI site. All the mutations were confirmed by DNA sequencing.
Protein expression, radioactive labeling, and immunoprecipitation.
The env expression plasmid pTMT was transfected into COS-1 cells in 100-mm-diameter plates by a modified CaPO4 technique. At 36 h posttransfection, cells were divided into two sets of 60-mm-diameter plates, one for pulse and the other for pulse-chase labeling. At 72 h posttransfection, the cells were starved in leucine-free DMEM for 90 min and then pulse-labeled in leucine-free DMEM supplemented with [3H]leucine (1 mCi/ml; 0.25 ml/plate) for 30 min. At this point, one set of pulse-labeled cells was lysed with lysis buffer A on ice (1% Triton X-100, 15% sodium deoxycholate, 0.15 M NaCl, 0.05 M Tris; pH 7.5). The remaining set was then chased in complete DMEM for an additional 4 h prior to lysis of the cells. Using a goat anti-M-PMV antibody, viral proteins were immunoprecipitated from the cell lysates and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), as described previously (40).
Biotinylation of cell surface proteins.
Cell surface proteins were detected by a surface biotinylation assay based on the method of Lisanti et al. (31). Following pulse-chase radiolabeling as described above, the transfected COS-1 cells were placed on ice and washed three times with ice-cold PBS-C/M (phosphate-buffered saline containing 0.1 mM CaCl2 and 1 mM MgCl2), prior to incubation with PBS-C/M containing 0.5 mg of Sulfo-NHS-LC-biotin (Pierce) per ml for 30 min on ice. The cells were then washed twice with ice-cold PBS-C/M containing 50 mM NH4Cl and once with ice-cold PBS-C/M, and then lysed in lysis buffer A and divided into two equal aliquots. Both aliquots were immunoprecipitated using goat anti-M-PMV antiserum as described above, and then one of the immunoprecipitated samples was analyzed by SDS-12% PAGE. The other remaining aliquot was further treated with 10 μl of 10% SDS and heated at 95°C for 5 min to release biotinylated glycoprotein. The dissociated proteins were then dissolved in 1 ml of lysis buffer A and incubated with 30 μl of streptavidin-agarose beads (Pierce) at 4°C overnight. The bound biotinylated samples were washed two times with lysis buffer B (lysis buffer A with 0.1% SDS) and once with 20 mM Tris-HCl (pH 6.8) and then heated at 95°C for 5 min in the presence of gel loading buffer before analysis by SDS-12% PAGE.
Fusion assays of wild-type and mutant M-MPV Env proteins.
The fusion activity of M-PMV Env proteins was determined as described previously (Song and Hunter, submitted). Briefly, the glycoprotein expression vector pTMT containing either wild-type or mutant M-PMV env genes was transfected into COS-1 cells by a modified CaPO4 method for analysis in the cell fusion assay. At 36 h posttransfection, the COS-1 cells were trypsinized, mixed at a 1:2 ratio with untransfected HOS-CD4/LTRhGFP indicator cells, and replated in a six-well plate. After 24 to 30 h of incubation, cells were washed twice with deficient PBS (PBS without 0.1 mM CaCl2 and 1 mM MgCl2), and then 500 μl of PBS-1 mM EDTA was added to resuspend the cells. The resuspended cells were analyzed for GFP expression by flow cytometry. In control assays where increasing amounts (0.5, 1.0, 2.0, 3.0, and 4.0 μg) of wild-type pTMT DNA were transfected into COS-1 cells, the number of fluorescent cells increased in a linear manner up to 3 μg (data not shown).
Antibody uptake analysis.
All immunofluorescence-based antibody uptake analyses were done as described previously with modifications (33). The glycoprotein expression vector pTMT containing either wild-type or mutant Env was transfected into COS-1 cells grown on glass coverslips. To determine relative levels of Env surface expression, unfixed cells on the coverslips were incubated with goat anti-M-PMV for 30 min on ice, washed, fixed with methanol-acetic acid, and then stained with fluorescein isothiocyanate (FITC)-labeled rabbit anti-goat-immunoglobulin G as secondary antibody (Molecular Probes). In order to compare levels of internalization of wild-type and mutant Env, COS-1 cells grown on coverslips were washed in PBS at 48 h posttransfection. Cells were then incubated at 37°C with 100 μl of DMEM containing goat anti-M-PMV antibody (diluted 1:50) and 100 μM chloroquine (to prevent lysosomal degradation of endocytosed Env and antibodies) for 15 min or 2 h. Surface and endocytosed goat anti-M-PMV antibody was detected using FITC-labeled rabbit anti-goat immunoglobulin G as described above. All the samples were observed and photographed with an Olympus IX 70 microscope.
Viral protein expression and glycoprotein incorporation.
COS-1 cells in 100-mm-diameter plates were transfected with 10 μg of the M-PMV molecular clone pSARM4 containing either the wild-type or mutant env genes, using the modified CaPO4 method. Three days after transfection, the cells were labeled with 500 μCi of [3H]leucine (NEN) in 0.8 ml of leucine-deficient DMEM. The cells were labeled for 90 min, at which time the label was removed, complete DMEM containing 10% fetal bovine serum was added, and incubation was continued for 6 h. The supernatant was collected and, following removal of the cell debris by filtering through a 0.45-μm-pore-size filter, the supernatant was loaded onto a 25% (wt/vol) sucrose cushion in PBS and centrifuged for 30 min in a TLA 100.3 rotor (Beckman) at 100,000 rpm. The pellet was resuspended in lysis buffer B, and M-PMV viral proteins were immunoprecipitated as described above with goat anti-M-PMV antiserum. Immunoprecipitates were analyzed by SDS-12% PAGE and fluorography.
Single-round virus infectivity assay.
The single-round infectivity of M-PMV molecular clones was determined as described elsewhere (Song and Hunter, submitted). Briefly, COS-1 cells in 100-mm-diameter plates were cotransfected with both the glycoprotein expression vector pTMT, containing either the wild-type or the mutant env genes, and pMTΔE, which encodes the tat gene of HIV-1 in place of env, by using a modified CaPO4 method. At 48 h after cotransfection, culture supernatants were collected and filtered through a 0.45-μm-pore-size filter. Relative levels of reverse transcriptase (RT) activity were determined for each sample as previously described (13), and the RT levels were normalized by dilution with complete medium. The normalized supernatants were used to infect HOS-CD4/LTRhGFP cells in duplicate. After another 48-h incubation, cells were washed twice with deficient PBS and 500 μl of PBS-1 mM EDTA was added to resuspend the cells. The resuspended cells were analyzed for the expression of GFP on a flow cytometer.
RESULTS
Construction of mutants in the tyrosine residues of the cytoplasmic domain of M-PMV env genes.
Our laboratory demonstrated previously that introduction of truncation mutations into the cytoplasmic domain of M-PMV TM changed viral infectivity, membrane fusion activity, and the level of Env incorporation into virions (10). In order to better understand the functional role of the cytoplasmic domain and especially the tyrosine residues in this region, we have introduced by PCR mutagenesis a series of amino acid substitutions into the two tyrosine residues located in the cytoplasmic domain of the TM protein (Fig. 1). All the mutations were confirmed by DNA sequencing to ensure that no additional mutations had been introduced. The mutant env genes were subsequently cloned into an M-PMV glycoprotein expression vector, pTMT, as well as into the infectious molecular clone pSARM4 (Song and Hunter, submitted).
FIG. 1.
Schematic diagram of M-PMV TM protein organization and the mutations introduced into the tyrosine residues of the cytoplasmic domain. The tyrosine residues in the 38-amino-acid-long cytoplasmic domain are shown in bold. Mutations are designated by the standard one-letter code of the wild-type amino acid followed by its numerical position within the cytoplasmic domain and then the resulting mutant amino acid.
Envelope protein synthesis and procession of the cytoplasmic domain mutants.
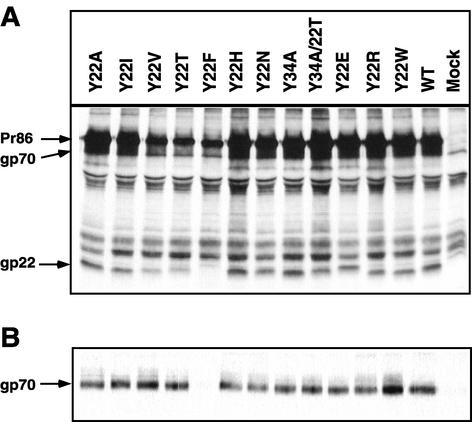
To study the effects of these mutations on the biosynthesis and processing of the M-PMV glycoprotein, the mutant env genes were inserted into the glycoprotein expression vector pTMT. This vector has been shown to direct the synthesis of high levels of glycoprotein when transfected into COS-1 cells (Song and Hunter, submitted). Three days after transfection of the wild-type or mutant env expression vectors, the transfected cells were starved in leucine-free medium for 90 min, labeled for 30 min with [3H]leucine, and then further incubated for 4 h in the presence of complete medium. The cells were lysed, and Env proteins were immunoprecipitated with goat anti-M-PMV polyclonal antibody from the lysates prior to SDS-PAGE analysis.
Each of the mutant Env proteins was expressed at a level similar to that of wild type in pulse-labeling experiments (Fig. 2A). After a 4-h chase, a fraction of the M-PMV glycoprotein precursor was processed into mature glycoproteins gp70 and gp22. The gp70 subunit migrated as a diffuse band because of terminal glycosylation of the protein. With the exception of Y22F, none of the substitutions at residues 22 or 34 had a significant effect on the biosynthesis and cleavage of the glycoprotein precursor (Fig. 2B). The gp22 of several of the mutants exhibited a modified mobility compared to the wild-type TM (e.g., Y22T, Y22H, Y22N, and Y22E). The change in mobility of these TM proteins is presumably due to the change to a polar or charged residue in place of Y22. In pulse-chase experiments with the Y22F mutant, significantly reduced amounts of gp70 and a faster-migrating TM protein were observed after the 4-h chase.
FIG. 2.
Synthesis and processing of mutant and wild-type glycoproteins. (A) COS-1 cells were transfected with the pTMT expression vector containing either the wild-type or mutant env genes; at 48 h posttransfection, the cells were labeled with [3H]leucine for 30 min and immunoprecipitated with goat anti-M-PMV serum as described in the text. The mutant designation is shown above each lane, and the position of the precursor glycoprotein, Pr86, is indicated to the left. (B) Processing of the Env polyprotein precursor protein. Following a 4-h chase in unlabeled medium, Env proteins were immunoprecipitated with goat anti-M-PMV serum and analyzed by SDS-12% PAGE. The positions of the precursor glycoprotein, Pr86, and the cleavage products, gp70 and gp22, are shown. (C) Secretion of SU into culture medium. Following pulse-chase, culture media were collected, immunoprecipitated with goat anti-M-PMV serum, and analyzed for the released SU proteins.
Since some of the gp70 SU protein of M-PMV is secreted into the culture medium when Env is expressed in COS-1 cells, we determined if any of the mutations in the cytoplasmic domain affected the secretion of gp70 into the culture medium after a 4-h chase. Similar amounts of gp70 were secreted into the culture medium for each of the mutants, including Y22F (Fig. 2C).
Surface expression of the mutant glycoprotein.
To investigate whether the mutations in the tyrosine motifs affect the ability of the Env complex to be transported to the plasma membrane, a surface biotinylation assay was performed. COS-1 cells were transfected with pTMT containing either wild-type or mutant env genes, and at 72 h posttransfection the cells were labeled with [3H]leucine for 30 min and then chased for 4 h. After the chase, labeled glycoprotein that had been transported to and was resident in the plasma membrane was tagged with the membrane-impermeable Sulfo-NHS-LC-biotin as described in Materials and Methods. The entire complement of labeled viral proteins within the lysates of biotinylated cells were first immunoprecipitated with goat anti-M-PMV antibody (Fig. 3A), and then biotinylated proteins present in one aliquot of the immunoprecipitates were selected following reprecipitation with streptavidin-agarose beads (Fig. 3B).
FIG. 3.
Biotinylation of envelope glycoproteins expressed on the cell surface. Two sets of cells expressing radiolabeled glycoprotein were biotinylated and immunoprecipitated with goat anti-M-PMV serum. (A) One set of immunoprecipitated samples was analyzed by SDS-PAGE. The mutant designation is shown above each lane, and the positions of the viral bands are indicated to the left. (B) The other set of immunoprecipitated samples was boiled in SDS, and streptavidin-agarose beads were added to isolated biotinylated Env proteins. The biotinylated Env proteins were then analyzed by SDS-PAGE. The positions of the viral bands are indicated to the left.
The results of a representative experiment are shown in Fig. 3. When lysates of the pulse-chase-labeled cells were immunoprecipitated with goat anti-M-PMV antibody, similar patterns of proteins were observed (Fig. 3A). Except for the Y22F mutant, each of the mutant Env proteins was transported and expressed on the plasma membrane at levels similar to that of the wild type, indicating that the bulk of mutations introduced into residues 22 and 34 in the cytoplasmic domain do not significantly affect transport of the glycoprotein to the cell surface (Fig. 3B).
Effect of the mutations in the tyrosine residues on endocytosis of the M-PMV Env protein.
To corroborate the surface biotinylation data indicating that the viral glycoproteins are expressed on the cell surface and to determine whether the tyrosine residues in the cytoplasmic domain play a role in the endocytosis of the M-PMV Env protein, we employed an antibody uptake assay. COS-1 cells grown on glass coverslips were transfected with pTMT vectors encoding either the wild-type or mutant env genes (Y22A, Y22F, or Y34A). Unfixed COS-1 cells were incubated with goat anti-M-PMV antiserum, either on ice to detect steady-state Env expression on the cell surface or at 37°C in the presence of chloroquine to measure internalization of surface-bound antibodies (Fig. 4).
FIG. 4.
Antibody uptake mediated by wild-type (WT) and tyrosine mutant glycoproteins in COS-1 cells. Steady-state surface expression of wild-type and mutant env gene products was detected after incubation of the pTMT vector-transfected unfixed COS-1 cells on ice with goat anti-M-PMV antibody followed by methanol-acetic acid fixation and secondary antibody incubation (Surface IF). Internalization of wild-type and mutant Env proteins from the plasma membrane to the prelysosomal vesicles was visualized by incubating Env-expressing cells with goat anti-M-PMV antibody in the presence of chloroquine at 37°C for either 15 min or for 2 h; the cells were then fixed and stained as for surface IF (Antibody Uptake).
In the steady-state surface staining, the wild-type and Y22A mutant glycoproteins showed a similar surface staining pattern. Even though we could not detect the Y22F mutant glycoproteins on the cell surface in the surface biotinylation assay, we were able to demonstrate significant levels of surface immunofluorescence, indicating that the mutant glycoproteins are on the surface of the plasma membrane. An analysis of the uptake of the surface-bound goat anti-M-PMV antibody showed dramatic differences in the manner by which the different glycoproteins were internalized. After a 15-min period of antibody uptake, the wild-type Env showed reduced surface immunofluorescence and the appearance of brightly staining vesicular structures (Fig. 4, WT). After 2 h, the bulk of the immunofluorescent staining was associated with intracellular vesicular structures. Cells expressing the Y22F Env protein showed very little surface labeling after 15 min and an increased accumulation of Env in the endocytic vesicles. As with the wild-type protein, a majority of the staining was concentrated in these vesicular structures after 2 h. In contrast, Y22A showed extensive plasma membrane staining and only a few fluorescent endocytic vesicles, even after 2 h of incubation at 37°C. The Y34 Env protein showed similar patterns of surface staining and internalization as the wild-type (data not shown). These data indicate that the tyrosine residue at position 22 of the cytoplasmic domain of M-PMV Env also plays a key role in the endocytosis of the glycoprotein.
Effect of the mutations in the tyrosine residues on the cell fusion activity of the M-PMV Env protein.
To test whether the substitutions in the tyrosine residues (22 and 34) of the cytoplasmic domain affect the fusogenicity of the M-PMV Env protein, COS-1 cells were transfected with the Env expression vector pTMT encoding either the wild-type or mutant env genes. As a negative control, COS-1 cells were transfected with a pTMTΔEnv vector that encodes only HIV-1 Tat. GHOST cells that have the cellular receptor for M-PMV glycoprotein and encode a GFP gene under the control of the HIV-2 LTR promoter were used as indicator cells. The pTMT vector can express high levels of both the HIV-1 Tat protein under the control of the simian virus 40 early promoter and M-PMV Env in the transfected COS-1 cells. Thus, fusion of COS-1 cells transfected with the pTMT vector with a GHOST cell results in activation of GFP expression. At 36 h posttransfection, the COS-1 cells were trypsinized and mixed with GHOST cells at a ratio of 1:2. After 24 to 36 h, the cells were resuspended and analyzed for the number of GFP-positive fused cells by gating on larger-than-single cells using flow cytometry (Song and Hunter, submitted). This allowed a quantitative analysis of fusogenicity for each of the mutants.
The relative fusion efficiency of each of the mutants compared to that of the wild type was averaged from three independent experiments and is shown in Fig. 5. For these calculations, background fusion observed with the negative control (pTMTΔEnv) was deducted from each experimental value. In each case, this represented less than 5% of the wild-type value (data not shown). Substitution of the Y22 residue with alanine, threonine, asparagine, or tryptophan had little effect on the fusogenicity of Env, and similar numbers of GFP-expressing cells were observed. This was also the case where Y34 was replaced by alanine. In contrast, substitution of isoleucine, valine, or phenylalanine for Y22 had a dramatic negative effect on the fusion activity of the glycoprotein. Y22I consistently yielded background levels of fusion. On the other hand, Y22H, Y22E, and Y22R mutants yielded significantly higher levels of fusion than the wild type. Because each of these mutants has a similar level of surface-expressed glycoprotein, the difference in fusion activity observed is presumably due to a functional change of the TM protein caused by substitution of the Y22 residue.
FIG. 5.
Cell-cell fusion assay. COS-1 cells were transfected with the pTMT expression vector encoding either the mutant or wild-type env gene. COS-1 cells expressing glycoproteins were mixed at a 1:2 ratio with HOS-CD4/LTR-hGFP cells and replated. The cells were analyzed for expression of GFP and quantitated by fluorescence-activated cell sorting 24 h later as described in Materials and Methods. The mean (± standard deviation) percentage of GFP-expressing cells relative to wild type from three independent experiments is shown for each of the mutants.
Virion glycoprotein incorporation.
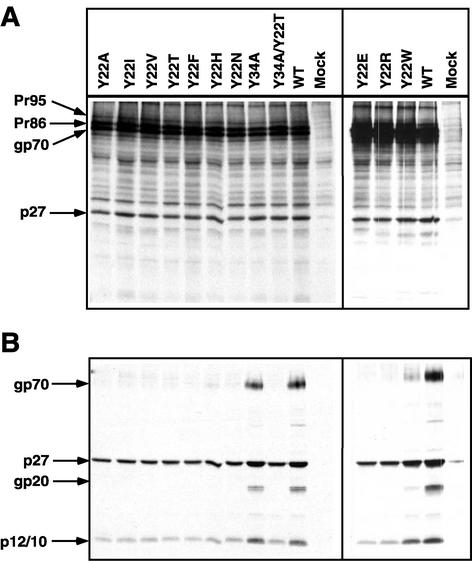
Previously we demonstrated that truncation of the M-PMV TM cytoplasmic domain to 21 amino acids blocked incorporation of glycoprotein into virions (10). To test whether the substitutions introduced into the tyrosine residues affected glycoprotein incorporation, the mutant env genes were cloned into the infectious molecular clone pSARM4. Each mutant clone was transfected into COS-1 cells and, at 72 h posttransfection, the cells were labeled with [3H]leucine for 30 min. Following a 6-h chase in complete medium, virus particles in the supernatants were filtered through a 0.45-μm filter, pelleted through a 25% sucrose cushion, and then immunoprecipitated as described in Materials and Methods.
The pattern of the viral Env and Gag proteins expressed in wild-type pSASRM4-transfected cells was determined (Fig. 6A, lane WT). After the pulse-chase labeling, the Env precursor, Pr86, and gag products, Pr95, Pr78, and p27, can be observed in the cell lysate. A similar pattern of viral proteins was observed when the mutant pSARM4 constructs were analyzed (Fig. 6A).
FIG. 6.
Expression of envelope glycoprotein mutants in the context of the pSARM4 provirus and incorporation of mutant glycoprotein into virions. The proviruses were transfected into COS-1 cells, and viral proteins were metabolically labeled as described in Materials and Methods. (A) The cell lysates were immunoprecipitated with goat anti-M-PMV serum and analyzed by SDS-PAGE. The mutant designation is shown above each lane, and the positions of the viral proteins are indicated to the left. (B) Virus-containing supernatants from metabolically labeled COS-1 cells transfected with either wild-type or mutant pSARM4 constructs were centrifuged through a 25% sucrose cushion. The virus pellets were recovered, immunoprecipitated with goat anti-M-PMV serum, and analyzed by SDS-PAGE. The positions of the viral bands are indicated to the left.
Viral proteins immunoprecipitated from the pelleted supernatants collected from the cells transfected with the wild-type pSARM4 are shown in Fig. 6B (lane WT). The viral glycoproteins gp70 and gp20, as well as the viral gag gene products p27 and p10/p12, can be observed in these particles. While virus particles were released from each of the mutant provirus-transfected cells, a majority of these, with the exception of Y22H and Y22W, were devoid of gp70 and gp20. The level of Env incorporation into Y22H particles was very low, but detectable, while that of Y22W was approximately 15% that of wild type. A similar pattern of viral proteins to that of the wild type was observed for the Y34A mutant species, although quantitation of the bands showed that Env incorporation was reduced to 60 to 65% of wild type. As might be expected, Env incorporation into Y34A/Y22T virions was reduced below detectable levels, confirming the importance of Y22 for incorporation of glycoprotein into virions. Interestingly for each of the mutants where Env incorporation was inhibited, we observed a significant decrease in the release of p27 and p12/10 into the medium (Fig. 6B), even though intracellular levels of Gag and Env were comparable (Fig. 6A).
Analysis of single-round infectivity by GFP expressions.
To analyze the effect of the mutations introduced into the tyrosine residues (22 and 34) on virus infectivity, a complementation assay was used. In this single-round assay, the mutants were analyzed for their ability to complement the pMTΔE vector that contains the tat gene of HIV-1 in place of the M-PMV env gene. The pTMT and pMTΔE vectors were cotransfected into COS-1 cells and, 2 days after transfection, the culture supernatants were assayed for RT activity as described previously (13). Equivalent amounts of RT-containing supernatant were used to infect GHOST cells, and 2 days postinfection the cells were analyzed by flow cytometry for the number of GFP-positive cells (Song and Hunter, submitted). As a negative control, the assay was also performed by transfecting COS-1 cells with either the pMTΔE vector or pTMTΔEnv vector alone to measure the background GFP expression level in the GHOST cells.
In general, wild-type infection yielded 3 × 103 to 4 × 103 GFP-positive cells/3 × 104 cells analyzed, while negative controls yielded 1 × 102 to 2 × 102 GFP-positive cells/3 × 104 cells analyzed. Each of the Y22 mutants yielded substantially fewer GFP-positive cells, which in several cases were near background levels. However, both the Y22H and Y22W mutants, for which we had shown decreased, but detectable, levels of incorporated Env (Fig. 6B), demonstrated infectivity that was 10 to 20% that of wild type. Similarly, the Y34A mutant Env, which is incorporated quite efficiently into virions, mediated infection at levels approximately 60% that of wild type (Fig. 7). These data indicate that mutations into Y22 result in a dramatic decrease of viral infectivity as measured by the single-round infectivity assay, which reflects the reduction of glycoprotein incorporation into virion.
FIG. 7.
Single-round infectivity assay. COS-1 cells were cotransfected with pTMT and pMTΤΔE expression vectors as described in Materials and Methods. At 48 h posttransfection, culture medium from cells expressing virus was filtered and normalized for RT activity. Normalized medium was used to infect HOS-CD4/LTR-hGFP cells. Infectivity was quantitated by fluorescence-activated cell sorter analysis from the number of GFP-expressing cells. The mean (± standard deviation) percentage of GFP-expressing cells relative to wild type from three independent experiments is shown for each of the mutants.
DISCUSSION
Maturational cleavage of the M-PMV TM cytoplasmic domain, which appears to remove a necessary incorporation signal and enhance fusogenicity, takes place at a site that includes the tyrosine residue Y22 (I. Pichova, personal communication). In other viruses, tyrosine residues play important roles in the intracellular trafficking of the viral glycoproteins, their incorporation into virus, and the cell-to-cell transmission of virus (1, 4, 17, 18, 24, 26, 33, 34, 49, 53).
In this study we have explored the possibility that the tyrosine residue at position 22 of the cytoplasmic domain is critical for the process of maturational cleavage as well as the process of intracellular transport and endocytosis. The tyrosine residue at this position is part of a well-conserved tyrosine-based endocytosis motif (3, 15). Using site-directed mutagenesis, we have constructed a series of point mutations into Y22. We also introduced an additional alanine substitution into a second tyrosine residue found at position 34.
In order to investigate the effects of the mutation on the synthesis, plasma membrane expression, endocytosis, and biological function of TM, the mutated env genes were cloned into the pTMT vector. The results of these experiments showed that, except for the phenylalanine substitution (Y22F), all the mutants exhibit levels of synthesis, processing, and transport to the plasma membrane equivalent to the wild-type levels. Interestingly, while the Y22F mutant Env precursor was expressed at a level comparable to that of the wild type and could be detected on the cell surface in a steady-state immunofluorescent staining assay, the level of Y22F mutant Env proteins was dramatically reduced in the surface biotinylation assay. It is possible that the Y22F mutation creates a strong endocytosis signal that results in rapid trafficking from the surface. This would be consistent with the rapid internalization of the mutant glycoprotein observed in the immunofluorescence assay and the levels of soluble gp70 equivalent to wild type found in the culture supernatants. Phenylalanine can substitute for tyrosine in the YXXL motif, as has been reported for the influenza virus hemagglutinin (HA) (28, 48). Native HA protein is not endocytosed, because information in the TM domain has been shown to exclude it from incorporation into coated pits. However, mutation of the cytoplasmic domain to include an FTSL motif overcomes the negative signal in the TM domain, and the chimeric HA protein (HA-FTSL) can be efficiently endocytosed. In the case of HA-FTSL, substitution of tyrosine for phenylalanine (HA-YTSL) increased the efficiency of endocytosis (28), while in M-PMV the opposite appears to be the case.
It is also possible that substitution of phenylalanine for tyrosine changes the intracellular trafficking of the mutant Env protein, so that recycling of Env to the surface is reduced and degradation in lysosomes is increased. Consistent with this is the low level of mature cleavage products found in pulse-chase experiments with Y22F. It is of interest that the substitution of alanine for the Y22 residue significantly reduced the extent of internalization from that of the wild-type glycoprotein in indirect immunofluorescence experiments. These data suggest that the tyrosine residue at position 22 in the cytoplasmic domain functions as an active endocytosis signal in the context of the M-PMV glycoprotein. Experiments are currently under way to further characterize the role and function of these tyrosine residues in endocytosis.
In this study, we have used a very sensitive fusion assay that enables us to accurately quantitate the effect of the mutations on the fusogenicity of the mutant Env proteins (Song and Hunter, submitted). This is necessary since M-PMV Env is poorly fusogenic in a cell-cell fusion assay and yields small (two to three nuclei) syncytia. The cell fusion studies showed that substitutions of hydrophobic amino acids for the membrane-proximal tyrosine (Y22I, Y22V) significantly reduced the fusogenicity of the TM protein without decreasing cell surface expression. In the case of Y22I, fusion was reduced to the level of the negative control. In contrast to the hydrophobic amino acid substitutions, substitution of the Y22 with a charged amino acid (Y22H, Y22E, or Y22R) resulted in an increase in the fusogenicity of the glycoprotein. Thus, single amino acid changes in the cytoplasmic domain of the TM protein can have dramatic effects (both positive and negative) on the process of cell fusion that are primarily dependent on the conformation of the ectodomain of the glycoprotein complex. We therefore hypothesize that substitutions into the Y22 of the TM protein induce changes in the structure of the glycoprotein that either inhibit or facilitate the conformational changes that occur in the TM ectodomain in order to bring the membranes together and initiate lipid bilayer fusion.
A Chou-Fasman analysis of the M-PMV membrane-spanning and cytoplasmic domain sequences predicts that these regions would preferentially fold into an α-helical structure. Substitution of hydrophobic residues for Y22 could stimulate the formation of a stable α-helical domain, while introduction of charged residues might maintain a less stable structure. Thus, it is tempting to speculate that the overall flexibility of Env oligomer might be regulated by α-helical interactions in the cytoplasmic domain. It is interesting that insertion mutations in the cytoplasmic tail of the MuLV glycoprotein could suppress the inhibitory effect of the R peptide and that the suppression of the inhibitory effect may occur by breaking the rigidity of an α-helical structure in the cytoplasmic domain and thereby interfering with the communication between the R peptide and ectodomain of the TM glycoprotein (30, 52).
The results of the incorporation assays yielded the surprising result that, with the exception of Y22W, any substitutions of Y22 abolished incorporation of glycoprotein into virions. These data provide new insights into the role of Y22 as an active incorporation signal. It is interesting that in Semliki Forest virus a tyrosine residue in the cytoplasmic domain of the E2 transmembrane spike glycoprotein is absolutely necessary for budding and that the tyrosine can only be partially replaced with aromatic residues such as tryptophan (23, 53). Through molecular modeling, it has been demonstrated that the tyrosine residue in the Semliki Forest virus spike protein can engage, through aromatic interactions, with a cavity created by tyrosine and tryptophan residues in the nucleocapsid protein (46). Since a tryptophan substitution into Y22 allowed partial incorporation of M-PMV Env into virions, it will be of interest to determine whether similar hydrophobic pocket interactions are involved in Env incorporation in this system.
Infectivity assays confirmed that mutations which block glycoprotein incorporation have a very low level of infectivity, while the Y22W mutant has an intermediate level of infectivity compared to the wild type. Similarly, the Y34A mutant virions that showed approximately 65% incorporation of Env exhibited approximately 60% of the infectivity of the wild type. The remarkable correlation between extent of Env incorporation and virus infectivity in a single-cycle assay for these two mutants (Y22W and Y34A), as well as Y22H, argues strongly that there is a requirement for a specific number of Env oligomers per virion in order for virus entry to occur efficiently. This is consistent with our laboratory's previous study, in which we demonstrated that a truncation mutation (TL34) of M-PMV Env exhibited both reduced levels of glycoprotein and delayed infectivity (10).
The studies presented here, taken together with our previous observations that truncation mutation in the cytoplasmic domain of the M-PMV TM protein can modulate the fusogenicity of the glycoprotein, inhibit incorporation in virus particle, and play a role in endocytosis, argue strongly for a multifunctional role for the cytoplasmic domain. Our identification of the tyrosine residue at position 22 as a key element of the Env incorporation signal in the M-PMV TM protein points to necessary protein-protein interactions between Env and the matrix domain of the preassembled M-PMV capsid and suggests that it will provide a powerful model for this process in other retroviruses.
Acknowledgments
We thank Christina Ochsenbauer-Jambor for her valuable advice on antibody uptake experiments and Tshana Thomas for excellent technical assistance.
This work was supported by grant CA-27834 from the National Institutes of Health to E.H. Immunofluorescence analysis was performed at the High Resolution Image Facility. FACS analysis was performed at the Flow Cytometry Core Facilities of the University of Alabama at Birmingham Center for AIDS Research (supported by NIH grant P30-AI-27767).
REFERENCES
- 1.Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 73:1350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein, H. B., S. P. Tucker, S. R. Kar, S. A. McPherson, D. T. McPherson, J. W. Dubay, J. Lebowitz, R. W. Compans, and E. Hunter. 1995. Oligomerization of the hydrophobic heptad repeat of gp41. J. Virol. 69:2745-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boll, W., H. Ohno, Z. Songyang, I. Rapoport, L. C. Cantley, J. S. Bonifacino, and T. Kirchhausen. 1996. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 15:5789-5795. [PMC free article] [PubMed] [Google Scholar]
- 4.Bowers, K., A. Pelchen-Matthews, S. Honing, P. J. Vance, L. Creary, B. S. Haggarty, J. Romano, W. Ballensiefen, J. A. Hoxie, and M. Marsh. 2000. The simian immunodeficiency virus envelope glycoprotein contains multiple signals that regulate its cell surface expression and endocytosis. Traffic 1:661-674. [DOI] [PubMed] [Google Scholar]
- 5.Bradac, J., and E. Hunter. 1984. Polypeptides of Mason-Pfizer monkey virus. I. Synthesis and processing of the gag gene products. Virology 138:260-275. [DOI] [PubMed] [Google Scholar]
- 6.Bradac, J., and E. Hunter. 1986. Polypeptides of Mason-Pfizer monkey virus. II. Synthesis and processing of the env gene products. Virology 150:491-502. [DOI] [PubMed] [Google Scholar]
- 7.Bradac, J. A., and E. Hunter. 1986. Polypeptides of Mason-Pfizer monkey virus. III. Translational order of proteins on the gag and env gene specified precursor polypeptides. Virology 150:503-508. [DOI] [PubMed] [Google Scholar]
- 8.Brody, B. A., and E. Hunter. 1992. Mutations within the env gene of Mason-Pfizer monkey virus: effects on protein transport and SU-TM association. J. Virol. 66:3466-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brody, B. A., M. G. Kimball, and E. Hunter. 1994. Mutations within the transmembrane glycoprotein of Mason-Pfizer monkey virus: loss of SU-TM association and effects on infectivity. Virology 202:673-683. [DOI] [PubMed] [Google Scholar]
- 10.Brody, B. A., S. S. Rhee, and E. Hunter. 1994. Postassembly cleavage of a retroviral glycoprotein cytoplasmic domain removes a necessary incorporation signal and activates fusion activity. J. Virol. 68:4620-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brody, B. A., S. S. Rhee, M. A. Sommerfelt, and E. Hunter. 1992. A viral protease-mediated cleavage of the transmembrane glycoprotein of Mason-Pfizer monkey virus can be suppressed by mutations within the matrix protein. Proc. Natl. Acad. Sci. USA 89:3443-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celma, C. C., J. M. Manrique, J. L. Affranchino, E. Hunter, and S. A. Gonzalez. 2001. Domains in the simian immunodeficiency virus gp41 cytoplasmic tail required for envelope incorporation into particles. Virology 283:253-261. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee, S., J. Bradac, and E. Hunter. 1985. A rapid screening procedure for the isolation of nonconditional replication mutants of Mason-Pfizer monkey virus: identification of a mutant defective in pol. Virology 141:65-76. [DOI] [PubMed] [Google Scholar]
- 14.Chen, C., and H. Okayama. 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7:2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collawn, J. F., M. Stangel, L. A. Kuhn, V. Esekogwu, S. Q. Jing, I. S. Trowbridge, and J. A. Tainer. 1990. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell 63:1061-1072. [DOI] [PubMed] [Google Scholar]
- 16.Cosson, P. 1996. Direct interaction between the envelope and matrix proteins of HIV-1. EMBO J. 15:5783-5788. [PMC free article] [PubMed] [Google Scholar]
- 17.Delamarre, L., C. Pique, A. R. Rosenberg, V. Blot, M. P. Grange, I. Le Blanc, and M. C. Dokhelar. 1999. The Y-S-L-I tyrosine-based motif in the cytoplasmic domain of the human T-cell leukemia virus type 1 envelope is essential for cell-to-cell transmission. J. Virol. 73:9659-9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deschambeault, J., J. P. Lalonde, G. Cervantes-Acosta, R. Lodge, E. A. Cohen, and G. Lemay. 1999. Polarized human immunodeficiency virus budding in lymphocytes involves a tyrosine-based signal and favors cell-to-cell viral transmission. J. Virol. 73:5010-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubay, J. W., S. J. Roberts, B. Brody, and E. Hunter. 1992. Mutations in the leucine zipper of the human immunodeficiency virus type 1 transmembrane glycoprotein affect fusion and infectivity. J. Virol. 66:4748-4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubay, J. W., S. J. Roberts, B. H. Hahn, and E. Hunter. 1992. Truncation of the human immunodeficiency virus type 1 transmembrane glycoprotein cytoplasmic domain blocks virus infectivity. J. Virol. 66:6616-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Einfeld, D., and E. Hunter. 1988. Oligomeric structure of a prototype retrovirus glycoprotein. Proc. Natl. Acad. Sci. USA 85:8688-8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freed, E. O., and M. A. Martin. 1996. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J. Virol. 70:341-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garoff, H., R. Hewson, and D. J. Opstelten. 1998. Virus maturation by budding. Microbiol. Mol. Biol. Rev. 62:1171-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heineman, T. C., and S. L. Hall. 2001. VZV gB endocytosis and Golgi localization are mediated by YXXphi motifs in its cytoplasmic domain. Virology 285:42-49. [DOI] [PubMed] [Google Scholar]
- 25.Hunter, E., and R. Swanstrom. 1990. Retrovirus envelope glycoproteins. Curr. Top. Microbiol. Immunol. 157:187-253. [DOI] [PubMed] [Google Scholar]
- 26.Inabe, K., M. Nishizawa, S. Tajima, K. Ikuta, and Y. Aida. 1999. The YXXL sequences of a transmembrane protein of bovine leukemia virus are required for viral entry and incorporation of viral envelope protein into virions. J. Virol. 73:1293-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Januszeski, M. M., P. M. Cannon, D. Chen, Y. Rozenberg, and W. F. Anderson. 1997. Functional analysis of the cytoplasmic tail of Moloney murine leukemia virus envelope protein. J. Virol. 71:3613-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazarovits, J., H. Y. Naim, A. C. Rodriguez, R. H. Wang, E. Fire, C. Bird, Y. I. Henis, and M. G. Roth. 1996. Endocytosis of chimeric influenza virus hemagglutinin proteins that lack a cytoplasmic recognition feature for coated pits. J. Cell Biol. 134:339-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, S., Y. Zhao, and W. F. Anderson. 1999. Receptor-mediated Moloney murine leukemia virus entry can occur independently of the clathrin-coated pit-mediated endocytic pathway. J. Virol. 73:5994-6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, M., C. Yang, and R. W. Compans. 2001. Mutations in the cytoplasmic tail of murine leukemia virus envelope protein suppress fusion inhibition by R peptide. J. Virol. 75:2337-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lisanti, M. P., I. W. Caras, T. Gilbert, D. Hanzel, and E. Rodriguez-Boulan. 1990. Vectorial apical delivery and slow endocytosis of a glycolipid-anchored fusion protein in transfected MDCK cells. Proc. Natl. Acad. Sci. USA 87:7419-7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murakami, T., and E. O. Freed. 2000. Genetic evidence for an interaction between human immunodeficiency virus type 1 matrix and alpha-helix 2 of the gp41 cytoplasmic tail. J. Virol. 74:3548-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ochsenbauer, C., S. R. Dubay, and E. Hunter. 2000. The Rous sarcoma virus Env glycoprotein contains a highly conserved motif homologous to tyrosine-based endocytosis signals and displays an unusual internalization phenotype. Mol. Cell. Biol. 20:249-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson, J. K., and C. Grose. 1997. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J. Virol. 71:4042-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez, L. G., G. L. Davis, and E. Hunter. 1987. Mutants of the Rous sarcoma virus envelope glycoprotein that lack the transmembrane anchor and cytoplasmic domains: analysis of intracellular transport and assembly into virions. J. Virol. 61:2981-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piller, S. C., J. W. Dubay, C. A. Derdeyn, and E. Hunter. 2000. Mutational analysis of conserved domains within the cytoplasmic tail of gp41 from human immunodeficiency virus type 1: effects on glycoprotein incorporation and infectivity. J. Virol. 74:11717-11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ragheb, J. A., and W. F. Anderson. 1994. pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: implications for the role of the R peptide and p12E TM in viral entry. J. Virol. 68:3220-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rein, A., J. Mirro, J. G. Haynes, S. M. Ernst, and K. Nagashima. 1994. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J. Virol. 68:1773-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhee, S. S., H. X. Hui, and E. Hunter. 1990. Preassembled capsids of type D retroviruses contain a signal sufficient for targeting specifically to the plasma membrane. J. Virol. 64:3844-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhee, S. S., and E. Hunter. 1987. Myristylation is required for intracellular transport but not for assembly of D-type retrovirus capsids. J. Virol. 61:1045-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhee, S. S., and E. Hunter. 1990. Structural role of the matrix protein of type D retroviruses in gag polyprotein stability and capsid assembly. J. Virol. 64:4383-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rice, N. R., L. E. Henderson, R. C. Sowder, T. D. Copeland, S. Oroszlan, and J. F. Edwards. 1990. Synthesis and processing of the transmembrane envelope protein of equine infectious anemia virus. J. Virol. 64:3770-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakalian, M., and E. Hunter. 1998. Molecular events in the assembly of retrovirus particles. Adv. Exp. Med. Biol. 440:329-339. [DOI] [PubMed] [Google Scholar]
- 44.Salzwedel, K., J. T. West, and E. Hunter. 1999. A conserved tryptophan-rich motif in the membrane-proximal region of the human immunodeficiency virus type 1 gp41 ectodomain is important for Env-mediated fusion and virus infectivity. J. Virol. 73:2469-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sauter, M. M., A. Pelchen-Matthews, R. Bron, M. Marsh, C. C. LaBranche, P. J. Vance, J. Romano, B. S. Haggarty, T. K. Hart, W. M. Lee, and J. A. Hoxie. 1996. An internalization signal in the simian immunodeficiency virus transmembrane protein cytoplasmic domain modulates expression of envelope glycoproteins on the cell surface. J. Cell Biol. 132:795-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skoging, U., M. Vihinen, L. Nilsson, and P. Liljestrom. 1996. Aromatic interactions define the binding of the alphavirus spike to its nucleocapsid. Structure 4:519-529. [DOI] [PubMed] [Google Scholar]
- 47.Sommerfelt, M. A., S. R. Petteway, Jr., G. B. Dreyer, and E. Hunter. 1992. Effect of retroviral proteinase inhibitors on Mason-Pfizer monkey virus maturation and transmembrane glycoprotein cleavage. J. Virol. 66:4220-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teuchert, M., A. Maisner, and G. Herrler. 1999. Importance of the carboxyl-terminal FTSL motif of membrane cofactor protein for basolateral sorting and endocytosis. Positive and negative modulation by signals inside and outside the cytoplasmic tail. J. Biol. Chem. 274:19979-19984. [DOI] [PubMed] [Google Scholar]
- 49.Tirabassi, R. S., and L. W. Enquist. 1999. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J. Virol. 73:2717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weldon, R. A., Jr., and E. Hunter. 1996. Molecular requirements for retrovirus assembly, p. 381-410. In W. Chiu, R. M. Burnnett, and R. Garcea (ed.), Structural biology of viruses. Oxford University Press, New York, N.Y.
- 51.Wild, C., J. W. Dubay, T. Greenwell, T. Baird, Jr., T. G. Oas, C. McDanal, E. Hunter, and T. Matthews. 1994. Propensity for a leucine zipper-like domain of human immunodeficiency virus type 1 gp41 to form oligomers correlates with a role in virus-induced fusion rather than assembly of the glycoprotein complex. Proc. Natl. Acad. Sci. USA 91:12676-12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang, C., and R. W. Compans. 1997. Analysis of the murine leukemia virus R peptide: delineation of the molecular determinants which are important for its fusion inhibition activity. J. Virol. 71:8490-8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao, H., B. Lindqvist, H. Garoff, C. H. von Bonsdorff, and P. Liljestrom. 1994. A tyrosine-based motif in the cytoplasmic domain of the alphavirus envelope protein is essential for budding. EMBO J. 13:4204-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]