Abstract
The biological correlates of an effective immune response that could contain or prevent HIV infection remain elusive despite substantial scientific accomplishments in understanding the interactions among the virus, the individual and the community. The observation that some individuals appear to possess resistance to HIV infection or its consequences has generated a host of epidemiologic investigations to identify biological or behavioral characteristics of these individuals. These data might hold the keys to developing appropriate strategies for mimicking the effective responses of those who appear immune. In this paper we review genetic mechanisms including the role of chemokines and their receptors, cytokines, host genetic immune response to HIV infection, local immune response correlating with behavioral variables, co-infection and immune based mechanisms that have been elucidated so far. We offer suggestions for how to use these observations as platforms for future research to further understand natural resistance to HIV infection through cohort studies, population genotype sampling, mathematical modeling of virus–host interactions and behavioral analyses.
Keywords: Resistance, HIV infection, Susceptibility, Progression
Introduction
Since the reporting of the first cases of AIDS in 1981, and the discovery of its etiologic agent, human immunodeficiency virus type 1 (HIV-1), in 1983, there has been substantial scientific progress in the development of both effective antiretroviral therapy and the understanding of virus–host cellular interactions. Nonetheless, the correlates of effective immunity to HIV remain elusive, and the paucity of knowledge has hindered development of effective vaccines. One approach to understanding the immune response to HIV and why it fails in most people lies in examining those few hosts who appear to be resistant either to acquisition of the virus or to its devastation once acquired.
Two phenomena have indicated that natural resistance to HIV-1 infection, while rare, does exist. First, there are individuals who have been exposed to HIV, in some cases repeatedly and over long periods of time, who have remained HIV-uninfected. Such “exposed-uninfecteds” have been reported among commercial sex workers,1–4 individuals having unprotected sex with seropositive partners,5,6 infants born to HIV-infected mothers,7,8 health workers with accidental occupational exposure,9,10 intravenous drug users using contaminated needles,11 and hemophiliacs exposed to HIV-infected blood.12 Second, there are individuals who have become infected with HIV but whose disease has not progressed or has progressed very slowly compared to the average experience. Criteria defining these “long-term non-progressors” have varied among reports but usually include survival with HIV infection for ≥7 years with consistently low levels of HIV-1 RNA and little or no loss of the primary target of HIV, CD4+ T-cells. Long-term non-progressors have been identified among various groups, including homosexual men, women, injection drug users and children.13
Some of the same genetic mutations have been found in both exposed-uninfected populations and in long-term non-progressor populations, suggesting a unifying theory for both conditions, namely that host traits that prevent or hinder HIV-1 entry into cells will reduce the likelihood of infection and, should infection occur, slow or entirely eliminate the development of serious disease. Current HIV vaccine studies would be viewed as successful if they achieved either prevention of initial infection or amelioration of disease. As natural mechanisms appear capable of accomplishing both goals, it benefits us to study individuals in whom these mechanisms seem to be operating. Here we will review the evidence for HIV-1 resistance and discuss a research agenda for the field.
Factors Affecting Susceptibility and Disease Progression
Chemokine and Chemokine Receptor Polymorphisms
Chemokines are chemoattractant cytokines, which are small peptides that are secreted by cells and serve to regulate chemotaxis (the movement of cells), adhesion, and the activity but not the proliferation of immune responsive cells and tissues. Once secreted, chemokines attach to other cells via chemokine receptors present on the target cell surface. Eighteen chemokine receptors have been identified, each of which can accept more than one chemokine and often accept many.6 The binding characteristics of chemokine receptors are determined by a specific gene that codes for the receptor. Most people have two copies of the most common variant of the gene, denoted as “wildtype” (wt). Others may instead carry one or two copies of a mutated form of the gene coding for the receptor. In some cases, mutations (or polymorphisms) affect the binding characteristics of the receptor.
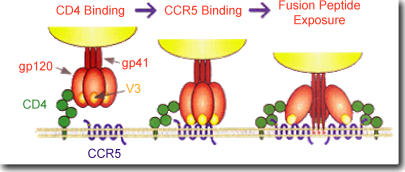
HIV-1 needs two receptors to gain entry into human cells: the CD4 receptor found on some cells of the immune system and the chemokine binding co-receptor. The chemokine co-receptor used by most circulating strains of HIV-1 is named CCR5. HIV strains that use this co-receptor are known as macrophage tropic (M-tropic) or R5 subtypes and account for more than 95% of incident HIV-1 infections.14,15 Other subtypes of HIV, called T-tropic or X4 viruses, require the slightly different CXCR4 co-receptor in addition to CD4.16 X4 viruses usually appear late in the course of HIV disease but have on rare occasions been detected close to the time of HIV acquisition, presumably as the result of direct or indirect transmission from an individual with advanced disease. Once HIV has bound to CD4 and a chemokine receptor, an area of the virus is exposed that can fuse with the human cell and permit entry of viral genetic material into the cell. A schematic of this entry process is shown in Fig. 1. The knowledge of this mechanism of HIV entry into cells has resulted in the development of a new class of HIV therapy called entry inhibitors, which blockade the CCR5 or CXCR4 co-receptors and prevent ongoing HIV infection of susceptible CD4+ cells.
Figure 1.
A representation of HIV-1 entry into target cells. Downloaded from http://www.aidsreagent.org/techlib/default.cfm?Action=HIVGraphics, 8/3/05
Variations in the genes encoding chemokines and chemokine receptors have been found to be important for both susceptibility to HIV infection and the rate of disease progression following HIV infection. To date, there are three well-studied variants of chemokine-related genes: CCR5-Δ32, CCR2-64I, and SDF1-3′A.
CCR5-Δ32 is a polymorphism in the gene encoding the CCR5 chemokine receptor in which a 32-base pair region has been deleted. The distribution of CCR5-Δ32 varies geographically, with the frequency of the allele being high in Northern Europe and decreasing towards the south. The frequency of the CCR5-Δ32 allele in European Caucasians is 5–15% whereas it is absent in Africans and East Asians.16 Individuals who have two copies of this mutation (i.e., CCR5-Δ32 homozygous or CCR5-Δ32/Δ32) have non-functional CCR5 receptors. This non-functionality renders CCR5-Δ32/Δ32 individuals immune to R5 strains of HIV. Approximately 1% of Caucasians are homozygous for CCR5-Δ32.17 There have been no clear indications of adverse health effects of the CCR5-Δ32/Δ32 genotype, although there may be beneficial effects, including a reduced risk of rheumatoid arthritis, an autoimmune illness.18 While CCR5-Δ32/Δ32 individuals are immune to infection with R5 strains of HIV, safer sex and injection practices are nonetheless imperative as these individuals are susceptible to infection with other pathogens spread by sex and needles. They also can be infected with strains of HIV that use receptor sites other than CCR5 to gain entry.
Individuals who possess one copy of CCR5-Δ32 and one copy of CCR5-wildtype (CCR5-Δ32 heterozygous or CCR5-Δ32/wt) may have altered chemokine receptor activity. Approximately 20% of Caucasians are heterozygous for CCR5-Δ32.17 The impact of the CCR5-Δ32/wt genotype on susceptibility to HIV infection has been somewhat controversial. Results from a meta-analysis of 11 published studies of children perinatally exposed to HIV did not indicate any significant difference in susceptibility for infants who were heterozygous for CCR5-Δ32 compared to those who were homozygous wildtype, CCR5-wt/wt.19 Nonetheless, there is strong evidence that heterozygosity for CCR5-Δ32 provides protection against sexual transmission of HIV infection both from male-to-male as well as from male-to-female.14,20 Transmission of X4 viruses is not affected by the CCR5-Δ32 mutation.
Although not commonly used by most HIV strains, CCR2 is another co-receptor for M-tropic virus,21 and a polymorphism at position 64 of the CCR2 gene (CCR2-64I) also has been shown to be important in the transmission and progression of HIV.22 Unlike CCR5-Δ32, the CCR2-64I allele is distributed throughout many different ethnicities. It is estimated that the allele frequency is 0.10 in European Caucasians, 0.23 in Africans, and 0.25 in East Asians.16 While epidemiological studies have been inconsistent, the data suggest that people who are homozygous for the CCR2-64I allele have some level of natural resistance to the sexual transmission of HIV.23 There also is evidence that people who are either homozygous or heterozygous for CCR2-64I experience delayed onset of AIDS though the results have been inconsistent between different populations.24 However, the polymorphism has not been shown to prolong time-to-death following onset of AIDS.25
Mutations in stromal cell-derived factor 1 (SDF-1), a relatively primitive and widely distributed chemokine, which is the main ligand (binding molecule) for CXCR4, also appear to play a role in HIV transmission. SDF1-3′A is a mutation in the SDF-1 gene,21 and individuals homozygous for SDF1-3′A exhibit reduced rates of progression to AIDS.16 The frequency of the SDF1-3′A allele is 0.21 in European Caucasians, 0.02 in Africans, and 0.26 in East Asians.16 An interaction effect also has been seen between homozygosity for SDF1-3′A and heterozygosity for either CCR5 or CCR2, resulting in a significant increase in protection against onset of AIDS.16
Cytokines
Like chemokines, cytokines are small proteins secreted by a variety of cell types that regulate the intensity and duration of the immune response and permit communication among cells. The role of cytokines in the modulation of HIV infection and the rate of disease progression remains to be fully understood. Evidence of strong epidemiological associations between cytokines and HIV disease progression has been limited and, in some cases, inconsistent across studies.
The cytokine interleukin-4 (IL-4) is known to differentially regulate the HIV co-receptors CCR5 and CXCR4. IL-4 decreases the levels of CCR5 on the surfaces of CD4-bearing cells and increases CXCR4 levels on the same or other cells. Decreased viral entry into host cells reduces replication of the R5 strain of HIV and correspondingly enhances replication of X4 virus.26 A polymorphism in the regulatory region of IL-4 (IL4-589T), initially identified among seropositive Japanese individuals, has been reported to have a protective effect against transmission of HIV through heterosexual contact.27 The authors hypothesized that this polymorphism was associated with increased IL-4 production, which led to decreased CCR5 levels and protection against R5 virus. A subsequent prospective study of a large cohort of HIV-infected European Caucasians revealed that IL4-589T also was associated with protection against disease progression.28 The IL4-589T allele frequency among HIV-infected Japanese individuals was 0.64 compared to 0.69 among HIV-uninfected individuals.27 The frequency of the T allele reported in the European Caucasian cohort of HIV-infected individuals, on the other hand, was 0.15.28 Two recent studies, however, one on HIV-infected homosexual men and the other on seropositive children, have reported no role for IL4-589T in disease progression.29,30
A second cytokine, interleukin-10 (IL-10), is known to inhibit HIV replication in vivo.31 A study by Shin et al.32 found that a polymorphism in the IL-10 regulatory region (IL10-5′-592A) was associated with significant acceleration in the rate of HIV disease progression. The frequency of the IL10-5′-592A allele was 0.24 among Caucasian Americans, 0.40 in African Americans, 0.33 among Hispanics, and 0.60 in Asians. The authors hypothesized that this polymorphism decreased IL-10 production and therefore most likely facilitated HIV replication and accelerated AIDS onset. It is the thesis of the present article that such a finding would imply that the IL10-5′-592A polymorphism also would increase not only progression of disease but also susceptibility to infection, although we are unaware of data confirming the latter at this time.
Human Leukocyte Antigens
Human leukocyte antigens (HLA) are the group of genes belonging to the human major histocompatibility complex (MHC), which help the immune system differentiate between ‘self’ and ‘non-self’ and play an important role in activating the immune system to respond to foreign substances. HLA genes fall into one of two classes. Class I HLA genes include HLA-A, -B, and -C and are involved in cytotoxic T cell-mediated immunity, which is thought to be the primary immune response against virus-infected cells. Class II is composed of the HLA-DR, -DP, and -DQ genes and plays a role in T-helper cell-mediated immunity, which activates an immune response against extracellular infection.33
An increasing body of evidence is emerging regarding the role of HLA in HIV transmission. Immune-mediated viral clearance has been reported to play a role in the resistance of some HIV-exposed-uninfecteds including commercial sex workers in Kenya and infants exposed to the virus perinatally.34–36 Differences in protective immune responses among HIV-exposed individuals have been the focus of many investigations, and the MHC has emerged as an important determinant of susceptibility (or resistance) to infection.
Concordance or discordance in HLA class I alleles between HIV transmission pairs has been shown to affect the likelihood of viral transmission. A study in Zambia of 104 persistently HIV-serodiscordant heterosexual couples and 125 initially serodiscordant couples in whom HIV infection occurred subsequently showed that sharing of HLA-B alleles resulted in a two-fold increase in the risk of virus transmission.37 The sharing of genes belonging to HLA Class I also has been shown to affect maternal-fetal transmission of HIV. A study of HIV-infected mothers and their children in Kenya found that viral transmission was more likely to occur with greater HLA class I concordance between mother and child.38
Possession of specific HLA alleles also has been implicated in HIV transmission. A prospective study of HIV-exposed-uninfected commercial sex workers in Kenya found that possession of the HLA gene group, A2/6802, was significantly associated with reduced risk of HIV seroconversion.39 A similar result was found in another study of a mother-infant cohort in Kenya.40 This association was noted independently of the protective effect of mother-infant HLA discordance.
Several explanations have been offered for the observed effects of HLA on HIV transmission. In the context of perinatal transmission, MacDonald et al.38 proposed that intense immune pressure can give rise to HIV variants that are not easily eliminated by the HLA-concordant recipient with a similar antiviral repertoire. This immune mediated viral mutational response has been termed “viral escape.” In relation to sexual transmission, a recent commentary by Ahuja and Catano41 suggests that HLA allele disparity between the donor and recipient might lead to immune responses that decrease the likelihood of virus transmission. Alternatively, in the case of HLA allele sharing, HIV escape mutants from the donor might be preferentially transmitted to a recipient who shares the same HLA type and therefore the same vulnerabilities to HIV strains that evolved under pressure from the donor HLA-determined immune system. Genetically determined HLA sharing is more likely to occur in closed homogeneous populations, thereby facilitating HIV transmission in such communities.
Repeated Low-Level Exposures: The Example of Kenyan Commercial Sex Workers
There are small populations of people who are resistant to infection with HIV despite repeated exposure to the virus. The reason for their decreased susceptibility to infection is still unclear although much work has been done to elucidate the mechanisms involved. The resistant populations that have been studied mainly include commercial sex workers from Africa and Thailand among whom the CCR5-Δ32 mutation has not been observed42 or is rare.
There is evidence that cytotoxic T-lymphocytes (CTLs) play a role in resistance to HIV infection although the resistance is believed to be dependent on persistent exposure to HIV.43 HIV-specific CTL responses have been found in occupationally exposed health care workers, babies born to infected mothers, regular sexual partners of HIV-infected individuals, and sex workers with high levels of exposure to HIV.44 Rowland-Jones et al.34 found that sex workers in Nairobi who were HIV-seronegative had CTL responses targeting epitopes defined by HIV clade B. Another study also looking at HIV-seronegative Kenyan sex workers found HIV-specific CD8+ T cells in the genital mucosa of the sex workers suggesting that CTL responses are protective against heterosexual transmission of HIV infection.45 Within a cohort of exposed-uninfected Kenyan sex workers, a subset of women who did seroconvert were found to have had lapses in sex work. This apparent interruption in HIV exposure was associated with a loss of HIV-specific CD8+ responses, suggesting that constant or frequent exposure to HIV is needed to maintain a protective CTL response.46 Despite the evidence for CTL involvement in resistance to HIV acquisition, the available data does not establish causation, leaving the possibility that CTLs are surrogate markers of some other mechanism.
It is likely that no single immune response can explain the resistance to HIV infection seen in sex workers and other exposed-uninfected individuals. Immunoglobulin A (IgA) responses have been found in the cervicovaginal fluids of sex workers in Thailand as well as in other exposed-uninfected groups, suggesting that local mucosal immune responses may help to prevent HIV infection.47 It has also been demonstrated that purified IgA from HIV resistant sex workers can neutralize HIV isolates from different clades and inhibit HIV infection of susceptible cells in vitro.48 This provides further evidence that IgA may be an important part of the immune defense against HIV. Belec et al.49 also found evidence of elevated levels of IgA, IgG, IgM and RANTES (which stands for “regulated on activation normal T-cell expressed and secreted”), a chemokine known to suppress HIV, in the cervicovaginal secretions of a cohort of HIV-seronegative African sex workers, suggesting that the immune response may not be limited to just IgA. More research is needed in this area to establish causation.
Co-infection with GB Virus C
GB virus C (GBV-C) is a flavivirus that is primarily transmitted by contaminated needles and syringes but also can be transmitted sexually. It has no known pathogenic effects. GBV-C is present in populations throughout the world. GBV-C viremia has been found in 1.8% of blood donors in the United States, 4% of blood donors in France and 1.3% in Japan.50,51 GBV-C has been associated with reduced mortality and increased survival times among HIV-infected individuals.52 A study of injection drug users in Italy showed a near-significant (p=0.06) association with long-term non-progression of HIV disease.53 Among the long-term non-progressors, 15 individuals with GBV-C had a mean plasma HIV RNA by PCR of 26,701 copies/ml compared to 35,536 copies/ml among 25 individuals without GBV-C. In vitro studies have indicated that the secretion of the chemokines RANTES, macrophage inflammatory protein (MIP)-1α, MIP-1β, and SDF-1 were increased in GBV-C-infected cells compared to uninfected cells, and CCR5 expression on the surfaces of cells was significantly lower in GBV-C-infected cells.54 All of these factors have the potential to reduce susceptibility to HIV infection.
Other Biological Factors
The risk of HIV-1 infection is also influenced by a variety of other biological factors in the host.24,55 The stage of HIV infection has a substantial impact on risk of transmission, with the acute stage and the late stage being characterized by higher viral loads compared to the middle latent period, thereby increasing the likelihood of transmission during these two phases of infection. Infection with other viral or bacterial pathogens can influence HIV-1 transmission in some instances by facilitating viral replication and shedding.56,57 Damage of the genital mucosa and lack of male circumcision can also contribute to elevated risk of HIV acquisition.58,59 The route of HIV exposure, whether directly into the blood or through mucous membranes, for example, also may significantly affect the likelihood of infection.
Behavioral Issues Related to HIV Resistance
While numerous studies have delineated the relationships of various behaviors to HIV transmission, there has been little investigation of how transmission-related knowledge and beliefs affect HIV acquisition or transmission to others. Some research60,61 indicates that myths regarding HIV transmission, such as the belief that one cannot contract HIV from oral sex, may explain why some individuals place themselves at risk. Beliefs about genetic resistance to HIV may represent another set of cognitions that undermine safe sex practices. Some men who have sex with men (MSM) may believe that they are resistant to HIV infection because they have engaged in high risk behaviors with persons they thought or knew to be HIV infected without HIV-seroconverting. The attribution of continued HIV-seronegativity to genetically-conferred resistance may be incorrect, however, if their partners had low levels of viremia or if they failed to understand that the risk of HIV acquisition per sexual act seems to be quite low. Furthermore, dyad-specific issues, such as HLA discordance between donor and recipient as discussed above, may have lessened the risk of HIV acquisition from one partner but could have no bearing on the risk of HIV acquisition from others. False beliefs about HIV resistance thus may cause some individuals to abandon safe sex practices and may be responsible in part for recent increases in sexual risk-taking among men who have sex with men.
There is essentially no data on the health beliefs of women regarding natural resistance to HIV infection and the potential impact of such beliefs on safer sex practices. Among HIV-negative injection-drug users, a 1997 report asked how important subjects thought various factors had been to their remaining HIV-uninfected.62 Three percent of injection-drug users in Bangkok and 70% of injection-drug users in New York City attributed their HIV-uninfected status to “having a strong immune system.”
Fishbein63 argued that beliefs concerning the likelihood of consequences of behaviors influence attitudes towards self-protective actions, such as whether or not to use condoms during sex. Consistent with this hypothesis, Cochran et al.64 showed that MSM who believe they are at risk for HIV are more likely to use condoms during sexual encounters, and Bartholow et al.65 showed that persons taking part in an HIV vaccine study who thought they had been assigned to receive vaccine were more likely to engage in unprotected anal intercourse than were those who thought they had been assigned to receive placebo. Aspinwall et al.66 suggested that believing that one is not at risk predicts high-risk anonymous sex. Furthermore, Halkitis et al.67 showed that, among MSM, believing that a strong immune system would prevent HIV acquisition was related to sexual risk-taking with casual HIV-negative or HIV-unknown partners.
Research opportunities
The field of natural resistance to HIV infection and disease thus appears to offer numerous opportunities. First, there are many inconsistencies in the literature that need to be resolved. The clearest data will come from cohort studies, as cross-sectional studies can be irretrievably biased by loss of rapid progressors in control groups with HIV-infection and lack of comparability in control groups without HIV infection. Historical cohort studies can be organized from records of physicians and clinics with large at-risk populations that existed at the time of onset of the HIV epidemic in the late 1970s and early 1980s. Additional studies could be conducted using archived samples from cohort studies and clinical trials. Stored samples from the HIVNET vaccine preparedness cohort, for example, might be mined to look at mechanisms of resistance other than CCR5-Δ32.14 Among questions that could be asked from the HIVNET samples are:
Is it possible that the lower HIV incidence rate observed among injection drug users compared to men who have sex with men in HIVNET might have been due in part to greater prevalence of infection with GBV-C among the drug users? The role of GBV-C in mediating the effects of HIV infection also raises the possibility of studies prospectively evaluating cohorts at risk of HIV-infection for pre-existing GBV-C infection and consideration for whether to incorporate some aspect of this apparently non-pathogenic virus into a vaccine model.
Have the associations of the chemokines and genotypes of interest been fully explored for their ability to explain HIV resistance in HIVNET and other cohort studies?
Is it possible that broad exploratory methods, such as the use of gene array chips, could identify novel genotype associations?
Another area of potential research is the mathematical modeling of the within-host dynamics of HIV replication and how these are determined by HIV receptor densities. Mathematical modeling of HIV replication dynamics within various CD4+ cell compartments has been well established by Ho et al.68 The finding that CCR5-Δ32 heterozygosity is associated with both reduced susceptibility to HIV infection and a reduced rate of disease progression following infection should be susceptible to mathematical modeling. It would seem conceptually straightforward to create a model that would predict the relative susceptibility of different individuals to HIV and their expected setpoint viral loads (that is, the “steady state” balance between HIV replication and the immune response in the untreated patient) once infection occurs on the basis of a modest number of variables, one of which would likely be the density of CCR5 receptors. A starting point for such modeling could be the mathematical model of HIV pathogenesis proposed by Wodarz and Nowak.69 This model could be further developed to explore the implications of variations in the density of CCR5 receptors, chemokine concentrations, and other host factors on the likelihood of HIV infection and rates of disease progression. This type of modeling also might be helpful in deciding whether viral setpoints achieved by participants in HIV vaccine clinical trials who become HIV-infected are affected by candidate vaccines.
The extent to which HIV-infected or susceptible lay people are aware of the concept of genetic resistance to HIV infection is also an area where little is known. Information with regard to these genetic variants has not been widely covered in the popular media. Though there appears to be some knowledge of this phenomenon in the gay male community of New York City, the extent to which this information is accurate is uncertain. Initial work in this area67 suggests that some knowledge does exist, but there is no clear relationship established between this cognition and sexual risk taking. Given the complexity of the biological systems (human and viral) and the complexity of the social systems in the various communities at risk, it will be important for behavioral studies to consider the impact of this information on belief and behavior, as has been seen in the impact of information about the uncertainty of HIV infection through oral intercourse.61
Future studies need to account for an evolving HIV epidemic, with an emphasis on the interplay between biological, sociological, and psychological phenomena, to understand how knowledge of genetic resistance interacts with knowledge of HIV treatment within the context of the community and sexual dyads.
Acknowledgement
This work was supported in part by grant AI057127 and Center for AIDS Research grant AI27742 from the National Institute on Allergy and Infectious Diseases, National Institutes of Health, and grant DA15303 from the National Institute on Drug Abuse, National Institutes of Health. S. Thomas is supported by NIH training grant 5T32 AI007382.
Financial arrangements: Dr. Marmor is principal investigator on several contracts from Merck & Co. Inc., to conduct clinical trials of HIV vaccines.
Footnotes
Marmor, Hertzmark and Thomas are with the Department of Environmental Medicine, New York University School of Medicine, 650 First Avenue, Room 560, New York, NY 10016, USA; Marmor is with the Department of Medicine, New York University School of Medicine, New York, NY, USA; Marmor, Hertzmark, Thomas, and Halkitis are with the The Center for AIDS Research, New York University School of Medicine, New York, NY, USA. Halkitis is with the Department of Applied Psychology, New York University Steinhardt School of Education, New York, NY, USA; Vogler is with the Division of International Medicine and Infectious Diseases, Weill Cornell College of Medicine, New York, NY, USA.
References
- 1.Fowke KR, Nagelkerke NJ, Kimani J, et al. Resistance to HIV-1 infection among persistently seronegative prostitutes in Nairobi, Kenya. Lancet. 1996;348(9038):1347–1351. [DOI] [PubMed]
- 2.Kaul R, Dong T, Plummer FA, et al. CD8(+) lymphocytes respond to different HIV epitopes in seronegative and infected subjects. J Clin Invest. 2001;107(10):1303–1310. [DOI] [PMC free article] [PubMed]
- 3.Kaul R, Rutherford J, Rowland-Jones SL, et al. HIV-1 Env-specific cytotoxic T-lymphocyte responses in exposed, uninfected Kenyan sex workers: a prospective analysis. AIDS. 2004;18(15):2087–2089. [DOI] [PubMed]
- 4.McNicholl JM, Promadej N. Insights into the role of host genetic and T-cell factors in resistance to HIV transmission from studies of highly HIV-exposed Thais. Immunol Res. 2004;29(1–3):161–174. [DOI] [PubMed]
- 5.Goh WC, Markee J, Akridge RE, et al. Protection against human immunodeficiency virus type 1 infection in persons with repeated exposure: evidence for T cell immunity in the absence of inherited CCR5 coreceptor defects. J Infect Dis. 1999;179(3):548–557. [DOI] [PubMed]
- 6.Hoffman R. Hematology: Basic Principles and Practice. 4th ed. Elsevier; 2005.
- 7.Farquhar C, Rowland-Jones S, Mbori-Ngacha D, et al. Human leukocyte antigen (HLA) B*18 and protection against mother-to-child HIV type 1 transmission. AIDS Res Hum Retrovir. 2004;20(7):692–697. [DOI] [PMC free article] [PubMed]
- 8.Kuhn L, Coutsoudis A, Moodley D, et al. T-helper cell responses to HIV envelope peptides in cord blood: protection against intrapartum and breast-feeding transmission. AIDS. 2001;15(1):1–9. [DOI] [PubMed]
- 9.Clerici M, Levin JM, Kessler HA, et al. HIV-specific T-helper activity in seronegative health care workers exposed to contaminated blood. JAMA. 1994;271(1):42–46. [DOI] [PubMed]
- 10.Pinto LA, Sullivan J, Berzofsky JA, et al. ENV-specific cytotoxic T lymphocyte responses in HIV seronegative health care workers occupationally exposed to HIV-contaminated body fluids. J Clin Invest. 1995;96(2):867–876. [DOI] [PMC free article] [PubMed]
- 11.Makedonas G, Bruneau J, Lin H, Sekaly RP, Lamothe F, Bernard NF. HIV-specific CD8 T-cell activity in uninfected injection drug users is associated with maintenance of seronegativity. AIDS. 2002;16(12):1595–1602. [DOI] [PubMed]
- 12.Barretina J, Blanco J, Gutierrez A, et al. Evaluation of the putative role of C–C chemokines as protective factors of HIV-1 infection in seronegative hemophiliacs exposed to contaminated hemoderivatives. Transfusion. 2000;40(4):461–467. [DOI] [PubMed]
- 13.Easterbrook PJ. Long-term non-progression in HIV infection: definitions and epidemiological issues. J Infect. 1999;38(2):71–73. [DOI] [PubMed]
- 14.Marmor M, Sheppard HW, Donnell D, et al. Homozygous and heterozygous CCR5-Delta32 genotypes are associated with resistance to HIV infection. J Acquir Immune Defic Syndr. 2001;27(5):472–481. [DOI] [PubMed]
- 15.Salkowitz JR, Bruse SE, Meyerson H, et al. CCR5 promoter polymorphism determines macrophage CCR5 density and magnitude of HIV-1 propagation in vitro. Clin Immunol. 2003;108(3):234–240. [DOI] [PMC free article] [PubMed]
- 16.O'Brien SJ, Moore JP. The effect of genetic variation in chemokines and their receptors on HIV transmission and progression to AIDS. Immunol Rev. 2000;177:99–111. [DOI] [PubMed]
- 17.de Silva E, Stumpf MP. HIV and the CCR5-Delta32 resistance allele. FEMS Microbiol Lett. 2004;241(1):1–12. [DOI] [PubMed]
- 18.Pokorny V, McQueen F, Yeoman S, et al. Evidence for negative association of the chemokine receptor CCR5 d32 polymorphism with rheumatoid arthritis. Ann Rheum Dis. 2005;64(3):487–490. [DOI] [PMC free article] [PubMed]
- 19.Contopoulos-Ioannidis DG, O'Brien TR, Goedert JJ, Rosenberg PS, Ioannidis JP. Effect of CCR5-delta32 heterozygosity on the risk of perinatal HIV-1 infection: a meta-analysis.[erratum appears in J Acquir Immune Defic Syndr. 2003 Apr 15;32(5):575]. J Acquir Immune Defic Syndr. 2003;32(1):70–76. [DOI] [PubMed]
- 20.Philpott S, Weiser B, Tarwater P, et al. CC chemokine receptor 5 genotype and susceptibility to transmission of human immunodeficiency virus type 1 in women. J Infect Dis. 2003;187(4):569–575. [DOI] [PMC free article] [PubMed]
- 21.Hogan CM, Hammer SM. Host determinants in HIV infection and disease. Part 2: genetic factors and implications for antiretroviral therapeutics. Ann Intern Med. 2001;134(10):978–996. [DOI] [PubMed]
- 22.Kostrikis LG. Impact of natural chemokine receptor polymorphisms on perinatal transmission of human immunodeficiency virus type 1. Teratology. 2000;61(5):387–390. [DOI] [PubMed]
- 23.Louisirirotchanakul S, Liu H, Roongpisuthipong A, et al. Genetic analysis of HIV-1 discordant couples in Thailand: association of CCR2 64I homozygosity with HIV-1-negative status. J Acquir Immune Defic Syndr. 2002;29(3):314–315. [DOI] [PubMed]
- 24.Kaslow RA, Dorak T, Tang JJ. Influence of host genetic variation on susceptibility to HIV type 1 infection. J Infect Dis. 2005;191(Suppl 1):S68–S77. [DOI] [PubMed]
- 25.Ioannidis JP, Rosenberg PS, Goedert JJ, et al. Effects of CCR5-Delta32, CCR2-64I, and SDF-1 3′A alleles on HIV-1 disease progression: an international meta-analysis of individual-patient data. Ann Intern Med. 2001;135(9):782–795. [DOI] [PubMed]
- 26.Valentin A, Lu W, Rosati M, et al. Dual effect of interleukin 4 on HIV-1 expression: implications for viral phenotypic switch and disease progression. Proc Natl Acad Sci USA. 1998;95(15):8886–8891. [DOI] [PMC free article] [PubMed]
- 27.Nakayama EE, Hoshino Y, Xin X, et al. Polymorphism in the interleukin-4 promoter affects acquisition of human immunodeficiency virus type 1 syncytium-inducing phenotype. J Virol. 2000;74(12):5452–5459. [DOI] [PMC free article] [PubMed]
- 28.Nakayama EE, Meyer L, Iwamoto A, et al. Protective effect of interleukin-4 -589T polymorphism on human immunodeficiency virus type 1 disease progression: relationship with virus load. J Infect Dis. 2002;185(8):1183–1186. [DOI] [PubMed]
- 29.Kwa D, van Rij RP, Boeser-Nunnink B, Vingerhoed J, Schuitemaker H. Association between an interleukin-4 promoter polymorphism and the acquisition of CXCR4 using HIV-1 variants. AIDS. 2003;17(7):981–985. [DOI] [PubMed]
- 30.Singh KK, Hughes MD, Chen J, Spector SA. Lack of protective effects of interleukin-4-589-C/T polymorphism against HIV-1-related disease progression and central nervous system impairment, in children. J Infect Dis. 2004;189(4):587–592. [DOI] [PubMed]
- 31.Kollmann TR, Pettoello-Mantovani M, Katopodis NF, et al. Inhibition of acute in vivo human immunodeficiency virus infection by human interleukin 10 treatment of SCID mice implanted with human fetal thymus and liver. Proc Natl Acad Sci USA. 1996;93(7):3126–3131. [DOI] [PMC free article] [PubMed]
- 32.Shin HD, Winkler C, Stephens JC, et al. Genetic restriction of HIV-1 pathogenesis to AIDS by promoter alleles of IL10. Proc Natl Acad Sci USA. 2000;97(26):14467–14472. [DOI] [PMC free article] [PubMed]
- 33.Carrington M, O'Brien SJ. The influence of HLA genotype on AIDS. Annu Rev Med. 2003;54:535–551. [DOI] [PubMed]
- 34.Rowland-Jones SL, Dong T, Fowke KR, et al. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J Clin Invest. 1998;102(9):1758–1765. [DOI] [PMC free article] [PubMed]
- 35.Rowland-Jones SL, McMichael A. Immune responses in HIV-exposed seronegatives: have they repelled the virus? Curr Opin Immunol. 1995;7(4):448–455. [DOI] [PubMed]
- 36.Rowland-Jones SL, Nixon DF, Aldhous MC, et al. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet. 1993;341(8849):860–861. [DOI] [PubMed]
- 37.Dorak MT, Tang J, Penman-Aguilar A, et al. Transmission of HIV-1 and HLA-B allele-sharing within serodiscordant heterosexual Zambian couples. Lancet. 2004;363(9427):2137–2139. [DOI] [PubMed]
- 38.MacDonald KS, Embree J, Njenga S, et al. Mother–child class I HLA concordance increases perinatal human immunodeficiency virus type 1 transmission. J Infect Dis. 1998;177(3):551–556. [DOI] [PubMed]
- 39.MacDonald KS, Fowke KR, Kimani J, et al. Influence of HLA supertypes on susceptibility and resistance to human immunodeficiency virus type 1 infection. J Infect Dis. 2000;181(5):1581–1589. [DOI] [PubMed]
- 40.MacDonald KS, Embree JE, Nagelkerke NJ, et al. The HLA A2/6802 supertype is associated with reduced risk of perinatal human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;183(3):503–506. [DOI] [PubMed]
- 41.Ahuja SK, Catano G. Sharing is caring, except when it comes to HLA-class-I alleles in HIV-1 transmission. Lancet. 2004;363(9427):2103–2104. [DOI] [PubMed]
- 42.Yang C, Li M, Limpakarnjanarat K, et al. Polymorphisms in the CCR5 coding and noncoding regions among HIV type 1-exposed, persistently seronegative female sex-workers from Thailand. AIDS Res Human Retrovir. 2003;19(8):661–665. [DOI] [PubMed]
- 43.Kaul R, Rowland-Jones SL, Kimani J, et al. New insights into HIV-1 specific cytotoxic T-lymphocyte responses in exposed, persistently seronegative Kenyan sex workers. Immunol Lett. 2001;79(1–2):3–13. [DOI] [PubMed]
- 44.Rowland-Jones SL, Pinheiro S, Kaul R, et al. How important is the ‘quality’ of the cytotoxic T lymphocyte (CTL) response in protection against HIV infection? Immunol Lett. 2001;79:15–20. [DOI] [PubMed]
- 45.Kaul R, Plummer FA, Kimani J, et al. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J Immunol. 2000;164(3):1602–1611. [DOI] [PubMed]
- 46.Kaul R, Rowland-Jones SL, Kimani J, et al. Late seroconversion in HIV-resistant Nairobi prostitutes despite pre-existing HIV-specific CD8+ responses.[comment]. J Clin Invest. 2001;107(3):341–349. [DOI] [PMC free article] [PubMed]
- 47.Beyrer C, Artenstein AW, Rugpao S, et al. Epidemiologiv and biologic characterization of a cohort of human immunodeficiency virus type 1 highly exposed, persistently seronegative female sex workers in Northern Thailand. J Infect Dis. 1999;179:59–67. [DOI] [PubMed]
- 48.Broliden K, Hinkula J, Devito C, et al. Functional HIV-1 specific IgA antibodies in HIV-1 exposed, persistently IgG seronegative female sex workers. Immunol Lett. 2001;79:29–36. [DOI] [PubMed]
- 49.Belec L, Ghys PD, Hocini H, et al. Cervicovaginal secretory antibodies to human immunodeficiency virus type 1 (HIV-1) that block viral transcytosis through tight epithelial barriers in highly exposed HIV-1-seronegative African women. J Infect Dis. 2001;184(11):1412–1422. [DOI] [PubMed]
- 50.Polgreen PM, Xiang J, Chang Q, Stapleton JT. GB virus type C/hepatitis G virus: a non-pathogenic flavivirus associated with prolonged survival in HIV-infected individuals. Microbes Infect. 2003;5(13):1255–1261. [DOI] [PubMed]
- 51.Williams CF, Klinzman D, Yamashita TE, et al. Persistent GB virus C infection and survival in HIV-infected men. N Engl J Med. 2004;350(10):981–990. [DOI] [PubMed]
- 52.Xiang J, Wunschmann S, Diekema DJ, et al. Effect of coinfection with GB virus C on survival among patients with HIV infection. N Engl J Med. 2001;345(10):707–714. [DOI] [PubMed]
- 53.Pomerantz RJ, Nunnari G. HIV and GB virus C—can two viruses be better than one? N Engl J Med 2004;350(10):963–965. [DOI] [PubMed]
- 54.Canducci F, Uberti Foppa C, Boeri E, et al. Characterization of GBV-C infection in HIV-1 infected patients. J Biol Regul Homeost Agents. 2003;17(2):191–194. [PubMed]
- 55.Royce RA, Sena A, Cates W Jr., Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336(15):1072–1078. [DOI] [PubMed]
- 56.Sha BE, Zariffard MR, Wang QJ, et al. Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J Infect Dis. 2005;191(1):25–32. [DOI] [PubMed]
- 57.Celum CL, Robinson NJ, Cohen MS. Potential effect of HIV type 1 antiretroviral and herpes simplex virus type 2 antiviral therapy on transmission and acquisition of HIV type 1 infection. J Infect Dis. 2005;191(Suppl 1):S107–S114. [DOI] [PubMed]
- 58.Seed J, Allen S, Mertens T, et al. Male circumcision, sexually transmitted disease, and risk of HIV. J Acquir Immune Defic Syndr Human Retrovirol. 1995;8(1):83–90. [PubMed]
- 59.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 trial. PLoS Med. 2005;2(11):e298. [DOI] [PMC free article] [PubMed]
- 60.Lowy E, Ross MW. “It'll never happen to me”: gay men's beliefs, perceptions and folk constructions of sexual risk. AIDS Educ Prev. 1994;6(6):467–482. [PubMed]
- 61.Halkitis PN, Parsons JT. Oral sex and HIV risk reduction: perceived risk, behavior and strategies among young HIV negative gay men. J Psychol Human Sex. 2000;11(4):1–24. [DOI]
- 62.Des Jarlais DC, Vanichseni S, Marmor M, et al. “Why I am not infected with HIV”: implications for long-term HIV risk reduction and HIV vaccine trials. J Acquir Immune Defic Syndr Human Retrovirol. 1997;16(5):393–399. [DOI] [PubMed]
- 63.Fishbein M. The role of theory in HIV prevention. AIDS Care. 2000;12(3):273–278. [DOI] [PubMed]
- 64.Cochran SD, Keidan J, Kalechstein A. Sexually transmitted diseases and acquired immunodeficiency syndrome (AIDS). Changes in risk reduction behaviors among young adults. Sex Transm Dis. 1990;17(2):80–86. [DOI] [PubMed]
- 65.Bartholow BN, Buchbinder S, Celum C, et al. HIV sexual risk behavior over 36 months of follow-up in the world's first HIV vaccine efficacy trial. J Acquir Immune Defic Syndr. 2005;39(1):90–101. [DOI] [PubMed]
- 66.Aspinwall LG, Kemeny ME, Taylor SE, Schneider SG, Dudley JP. Psychosocial predictors of gay men's AIDS risk-reduction behavior. Health Psychol. 1991;10(6):432–444. [DOI] [PubMed]
- 67.Halkitis PN, Zade DD, Shrem M, Marmor M. Beliefs about HIV non-infection and risky sexual behavior among MSM. AIDS Educ Prev. 2004;16(5):448–458. [DOI] [PubMed]
- 68.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373(6510):123–126. [DOI] [PubMed]
- 69.Wodarz D, Nowak MA. Mathematical models of HIV pathogenesis and treatment. BioEssays. 2002;24(12):1178–1187. [DOI] [PubMed]