Abstract
A number of malarial blood-stage candidate vaccines are currently being tested in human clinical trials, but our understanding of the relationship between clinical immunity and data obtained from in vitro assays remains inadequate. An in vitro assay which could reliably predict protective immunity in vivo would facilitate vaccine development. Merozoite surface protein1 (MSP1) is a leading blood-stage malaria vaccine candidate, and anti-MSP1 antibodies from individuals that are clinically immune to malaria inhibit the invasion of Plasmodium merozoites into erythrocytes in vitro. Using expression in Escherichia coli and subsequent refolding, we have produced two allelic forms of MSP142 (FVO and 3D7). Aotus nancymai monkeys were immunized with MSP142-FVO, MSP142-3D7, or a combination of FVO and 3D7 allelic forms, (MSP142-C1) and were subsequently challenged with Plasmodium falciparum FVO parasites. Sera obtained prior to challenge were tested by standardized enzyme-linked immunosorbent assay (ELISA) to determine antibody titer, and immunoglobulin G (IgG) fractions were also obtained from the same sera; the IgG fractions were tested in an in vitro growth inhibition (GI) assay to evaluate biological activity of the antibodies. Regardless of the immunogen used, all monkeys that had >200,000 ELISA units against MSP142-FVO antigen before challenge controlled their infections. By contrast, all monkeys whose purified IgGs gave <60% inhibition activity in an in vitro GI assay with P. falciparum FVO required treatment for high parasitemia after challenge. There is a strong correlation between ELISA units (Spearman rank correlation of greater than 0.75) or GI activity (Spearman rank correlation of greater than 0.70) and protective immunity judged by various parameters (e.g., cumulative parasitemia or day of patency). These data indicate that, in this monkey model, the ELISA and GI assay values can significantly predict protective immunity induced by a blood-stage vaccine, and they support the use of these assays as part of evaluation of human clinical trials of MSP1-based vaccines.
Vaccination against Plasmodium falciparum has the potential to reduce malaria-associated severe morbidity and mortality in areas with the most intense transmission, and it may do so without completely preventing blood-stage infection (10, 20, 22, 23). Efforts are under way by various groups to test a number of blood-stage vaccine antigens and formulations in both animals and humans. However, at present there is no demonstrable in vitro assay which correlates with in vivo protective immunity. Such an assay would greatly accelerate the selection of antigens produced in different expression systems, as well as different formulations of these antigens. Choices could be made based on clinical vaccination studies with small numbers of volunteers as opposed to expensive and time-consuming efficacy studies with children living in areas where malaria is endemic. An in vitro assay which correlates with protective immunity in humans may eventually be identified retrospectively, once some clinical efficacy is shown with blood-stage antigens. Until some protection is achieved in humans, it is possible to evaluate selected assays by using immunization and challenge of nonhuman primates. Aotus monkeys of various species are some of the few nonhuman primates that are susceptible to infection with the human malarial parasite Plasmodium falciparum. Identification of laboratory assays that correlate with protection induced by vaccination in monkeys would serve as a basis for extending such assays to human clinical vaccine trials.
The P. falciparum merozoite surface protein 1 (MSP1) is a leading malarial vaccine candidate (10). The MSP1 gene encodes a 185- to 215-kDa protein that is cleaved into several polypeptides during merozoite maturation and red cell invasion (1-4). Previous studies with rodent malaria parasites and immunization challenge studies with P. falciparum in nonhuman primates indicated that vaccines based on the carboxy-terminal portion of MSP1—either the C-terminal MSP119 or the larger MSP142—can confer protection against challenge with virulent malaria parasites (5, 6, 11, 12, 29, 31). Thus, evaluating these constructs as vaccine candidates is a focus of several laboratories.
Genetic variation in the P. falciparum MSP1 gene has been extensively investigated, since it may represent an obstacle for the development of vaccines based on this molecule (26). The gene may be divided into 17 blocks according to levels of interallele sequence divergence (25). Amino acid sequences may be categorized into one of two allelic groups, known as K1 and MAD20 (24, 33, 34). There is considerable nucleotide substitution and length variation between the two groups but much less variation within each group (33). Amino acid polymorphisms appear for the most part when comparisons are made between the two allele groups, whereas amino acid, as well as synonymous, polymorphisms are very low within each allele group (1, 8, 9). Block 17 encodes MSP119, the 19-kDa C-terminal product of enzymatic processing of MSP1 that remains anchored to the merozoite surface at the time of erythrocyte invasion (1). The amino acid sequence of block 17 is highly conserved except for major variations at four positions. Since protective immunity has been induced both in rodent malarias and in nonhuman primates with this portion of MSP1 (27-29), successful immunization of humans might elicit cross-reactive protection against parasites carrying different allelic variants of MSP119.
Previous efforts to address this issue experimentally have been primarily limited to immunization and challenge studies with rodent malarias, where immunization with a recombinant C-terminal fragment of Plasmodium yoelii merozoite surface protein 1 protects mice against homologous but not against heterologous challenge (27, 28). Using recombinant MSP1 as an immunogen, studies of protection against homologous challenge have been carried out with Aotus monkeys (5, 18, 19); the effect of infection with heterologous parasites has not been studied. To address these questions, we have produced two allelic forms of MSP142, one based on the sequence of the Vietnam Oak Knoll (FVO) parasite clone (K1 type) and the second based on the sequence of the 3D7 clone (MAD 20 type). Using these sequences produced as clinical-grade recombinant MSP142 proteins, we immunized Aotus nancymai monkeys with MSP142-FVO, MSP142-3D7, or a mixture of FVO and 3D7 (designated MSP142-C1) and then challenged them with FVO parasites. Blood was obtained from the monkeys just prior to challenge, and serum samples were analyzed both by enzyme-linked immunosorbent assay (ELISA) and by an in vitro growth inhibition (GI) assay. The latter involves measuring the ability of antibodies to inhibit the invasion and growth of P. falciparum parasites in red cells in vitro. Both data sets were then compared with the in vivo data for protection against parasite challenge. This study shows that there are significant correlations between the two assays, i.e., ELISA titer and GI activity in vitro, and that both types of assays also correlate with in vivo protection against FVO parasite challenge, regardless of the immunogen used. These results provide a rationale for future evaluation of both of these candidates in human clinical trials of malaria blood-stage vaccines.
MATERIALS AND METHODS
Expression, refolding, and purification of MSP142.
The production and purification of two allelic forms of the P. falciparum MSP142 by using the Escherichia coli expression system will be described elsewhere (S. Singh et al., unpublished data). Briefly, recombinant MSP142 based on the protein sequence of the FVO parasite line (EcMSP142-FVO; lot WRAIR0997) was expressed, refolded, and purified at the Walter Reed Army Institute of Research Pilot Bioproduction Facility, Silver Spring, MD, under the current Good Manufacturing Practice conditions. The final purity (percentage in a single band run under reduced conditions on sodium dodecyl sulfate-polyacrylamide gel electrophoresis) was ∼95.0%, with a host cell protein contamination level of 0.036% and an endotoxin contamination level (as measured by a Limulus amoebocyte lysate gel clot assay) of 0.12 endotoxin unit/mg. The second allele of MSP142 (EcMSP142-3D7; lot WRAIR0984) was also expressed, refolded, and purified at the Walter Reed Army Institute of Research Pilot Bioproduction Facility, based on the protein sequence of the 3D7 parasite line. The final purity was ∼97.4% (percentage in a single band run under reduced conditions on sodium dodecyl sulfate-polyacrylamide gel electrophoresis), with a host cell protein contamination level of 0.096% and an endotoxin contamination level of 6.7 endotoxin units/mg.
Vaccination and challenge infection of malaria-naive A. nancymai monkeys.
A. nancymai monkeys were housed at the Primate Research Facility, National Institutes of Health (NIH), in compliance with a protocol (LPD-8E) approved by the NIH Animal Care and Use Committee. Twenty-eight monkeys were randomly assigned to four groups of seven. Group assignment was masked from the primary investigators who cared for or vaccinated the animals, read blood films, or determined when a monkey should be drug cured. The monkeys were immunized subcutaneously three times at 3-week intervals with the following antigens emulsified in complete Freund's adjuvant (Sigma Chemical Co., St. Louis, MO) (first vaccination) or Montanide ISA51 (SEPPIC Inc., Fairfield, N.J.) (subsequent vaccinations). Group 1 received 50 μg/dose of a recombinant form of the Plasmodium falciparum sexual-stage protein Pfs25H (21) as a control; group 2 received 50 μg/dose of EcMSP142-FVO, homologous to the challenge parasite; group 3 received 50 μg/dose of EcMSP142-3D7; and group 4 received an equal-mass mixture of EcMSP142-FVO and EcMSP142-3D7 (designated EcMSP142-C1) for a total of 100 μg/dose.
Seventeen days after the third immunization, animals were challenged by intravenous infusion of a freshly passaged preparation of 5 × 104 red blood cells (RBCs) infected with the highly virulent P. falciparum strain FVO according to our established protocol (30, 31). Hematocrits and Giemsa-stained thin films were made from blood collected by puncture of superficial veins in the dorsum of the calf. Parasitemia was monitored daily by inspection of Giemsa-stained thin blood smears until treatment and was calculated based on examination of approximately 2,000 RBCs; if no parasites were seen, then 40 more high-power fields were examined. Monkeys were drug treated when parasitemia reached 5% or when their hematocrit fell below 25%. All monkeys not treated previously were treated on day 28. Treatment consisted of mefloquine administered in a single dose of 25 mg/kg of body weight by intubation.
ELISA.
Ninety-six-well ELISA plates were coated with 100 ng/well of MSP142-FVO or MSP119-FVO protein at 4°C overnight. The MSP119 was produced in Saccharomyces cerevisiae as described previously (17). After the plates were blocked with 5% skim milk, diluted sera were added to antigen-coated wells in triplicate and incubated for 2 h at room temperature. After extensive washing, the plates were incubated with alkaline phosphatase-labeled goat anti-human immunoglobulin G (IgG) (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) for 2 h at room temperature. Bound antibodies were visualized by adding p-nitrophenyl phosphate Sigma 104 substrate (Sigma Chemical Co., St. Louis, MO). The absorbance at 405 nm was read using a SPECTRAmax 340PC microplate reader (Molecular Devices Co., Sunnyvale, CA).
An Aotus anti-MSP142 standard serum was prepared using sera from monkeys immunized with both MSP142 antigens and was stored at −80°C until use. Serially diluted standard sera were tested and assigned unit values as the reciprocal of the dilution giving an optical density at 405 nm of 1 for both plate antigens (MSP142 and MSP119). We assigned 55,000 units for MSP142-FVO-coated plates and 15,000 units for MSP119-coated plates. Duplicates of serially diluted standard sera were included on each test plate in order to generate a standard curve. A four-parameter hyperbolic curve was generated from the standard curve values, and this curve was used to convert the absorbance of individual test sera into antibody units (SOFTmax PRO version 3; Molecular Devices Co.).
In vitro GI assay.
The IgG fraction of an individual monkey serum taken on 17 days after the final immunization (just prior to parasite challenge) was obtained using Protein G PLUS columns (Pierce, Rockford, IL) with binding and elution buffers supplied by manufacturer (Pierce). The eluted IgGs were dialyzed against RPMI 1640, concentrated to 10 mg/ml, and subsequently sterilized with a 0.22-μm filter (Millipore, Billerica, MA). The purified IgGs were preadsorbed with uninfected human O+ RBCs (25 μl of RBCs per 1 ml of sample) to remove anti-human RBC immunoglobulins, and then the samples were aliquoted and frozen at −80°C until use. ELISA units of the purified IgGs were also determined using MSP142-FVO as outlined above. Because of technical limitations, the IgG was purified only from sera for which more than 800 μl was available.
The in vitro biological activity of the purified IgG samples (25% [vol/vol] in the test well) was analyzed as described previously (20). Briefly, synchronized late-stage P. falciparum-parasitized erythrocytes were incubated with IgG fractions of Aotus sera for 40 h, and the growth of parasites was evaluated by biochemical assay of parasite lactate dehydrogenase.
Statistical methods. (i) Analysis of ELISA and GI assay data.
To compare antibody units in the prechallenge sera of the four groups, a one-way analysis of variance (ANOVA) and multiple comparisons with the t distribution were performed. To test the correlation between (i) anti-MSP142 ELISA units and anti-MSP119 ELISA units and (ii) anti-MSP142 ELISA units and GI assay data sets, a Spearman rank correlation was used. Statistical analyses were done with UNISTAT 5.0 (P-STAT Inc., Hopewell, NJ). Probability values of less than 0.05 are considered significant. Curve fitting analyses were performed using Sigma Plot (SPSS Inc., Chicago, IL).
(ii) Analysis of parasite challenge data.
Trial outcomes were measured with a primary statistical end point and several secondary end points. In the past, we have found in general that Aotus monkeys that control their parasitemia either self-cure or suffer anemia. Thus, monkeys that control their parasitemia but suffer anemia will, at some stage, require treatment for anemia. Previously we have used the day that the first monkey was treated for anemia (hematocrit of <25%) as the primary end point of Aotus immunization-challenge studies (29, 31, 32). At this point, it is impossible to say what would have occurred to such a monkey's parasite burden without treatment; the monkey may have self-cured or continued to control the parasite load, or it may have lost control and suffered acute parasitemia. Thus, for the primary end point in the present trial, we included data up until the first monkey was treated for low hematocrit rather than high parasitemia. On that day, all monkeys were ranked as follows. Monkeys treated for parasitemia were ranked according to the day of treatment. When two or more monkeys were treated for parasitemia on the same day, then monkeys were ranked in order of decreasing cumulative parasitemia at the time of treatment.
Secondary statistical comparisons were also made using two other end points: (i) day at which parasites first became patent in each monkey and (ii) final outcome. All monkeys were ranked using each end point, and the data were compared with anti-MSP142 ELISA units (before parasite challenge) or with GI assay data by using a Spearman rank correlation test. To compare the protection ranking between the groups, the one-way ANOVA and multiple comparisons with the t distribution were performed.
RESULTS
Study design modifications.
During the immunization, four animals (two from the Pfs25H group and two from the MSP142-FVO group) were excluded from the study for technical reasons as a deviation from the original design. Two animals died of cardiomyopathy (one in the Pfs25H group [vaccinated but removed from the study before challenge] and one in the MSP142-C1 group [1 day prior to the second vaccination]). Prior to parasite challenge, the health of all the enrolled Aotus monkeys was evaluated. Thus, the challenge study was reduced to 22 animals distributed as follows: 4 animals in the Pfs25H group, 5 animals in the MSP42-FVO group, 7 animals in the MSP42-3D7 group, and 6 animals in the MSP142-C1 group.
ELISA units before challenge.
Four groups of monkeys were immunized three times at 3-week intervals with 50 μg/dose of Pfs25H, 50 μg/dose of MSP142-FVO, 50 μg/dose of MSP142-3D7, or 100 μg/dose of MSP142-C1. Antiserum was obtained 17 days after the last immunization just before parasite challenge, and all sera were tested by ELISA with MSP142-FVO-coated plates. As shown in Table 1, the Pfs25H group showed less than 3,000 ELISA units to MSP142-FVO, and the geometric mean of ELISA units in the group was 1,019. The geometric means of the MSP142-FVO, MSP142-3D7, and MSP142-C1 groups were 84,840, 74,923, and 159,905 units, respectively. There is a significant difference between the four groups (one-way ANOVA, P = 0.007), and the value for the Pfs25H group is significantly lower than those for the other three groups; P values comparing Pfs25H with MSP142-FVO, MSP142-3D7, and MSP142-C1 are 0.004, 0.007, and 0.0001, respectively, by multiple comparisons with the t distribution. The MSP142-C1 group shows significantly higher antibody units than the MSP142-3D7 group (P = 0.033), but there is no significant difference between the MSP142-C1 and MSP142-FVO groups (P = 0.128) or between the MSP142-FVO and MSP142-3D7 groups (P = 0.591).
TABLE 1.
Course of infection in A. nancymai monkeys challenged with P. falciparum (FVO) parasites
| Vaccine group | Monkey | ELISA units
|
Days to patency | Days to treatmenta | GI assay activity (% inhibition) | Peak parasitemia (%) | Outcomeb | |
|---|---|---|---|---|---|---|---|---|
| MSP142 | MSP119 | |||||||
| Pfs25H | T1208 | 2,458 | 1,745 | 7 | 11 | 14 | 10.20 | Virulent |
| T1307 | 1,718 | 1,525 | 7 | 11 | NDc | 30.30 | Virulent | |
| T1378 | 138 | 460 | 7 | 11 | ND | 11.60 | Virulent | |
| T1418 | 1,858 | 460 | 7 | 15 | ND | 1.60 | Anemic | |
| MSP142-FVO | 2944 | 107,840 | 30,075 | 7 | 21 | 78 | 0.32 | Anemic |
| T1163 | 124,560 | 45,750 | 27 | 28 | 82 | 0.01 | Controlled | |
| T1332 | 40,480 | 14,405 | 5 | 18 | ND | 0.50 | Anemic | |
| T1373 | 149,920 | 34,000 | 9 | 21 | 87 | 0.21 | Anemic | |
| T1376 | 53,920 | 11,640 | 7 | 16 | 64 | 5.60 | Virulent | |
| MSP142-3D7 | 2969 | 32,430 | 16,080 | 6 | 11 | 62 | 6.05 | Virulent |
| T1309 | 50,160 | 18,100 | 7 | 24 | ND | 0.55 | Anemic | |
| T1352 | 469,260 | 111,850 | 25 | 28 | ND | 0.01 | Controlled | |
| T1366 | 23,700 | 6,535 | 5 | 21 | 69 | 0.29 | Anemic | |
| T1413 | 71,100 | 21,000 | 6 | 11 | ND | 7.90 | Virulent | |
| T1414 | 108,000 | 15,345 | 7 | 16 | ND | 5.10 | Virulent | |
| T923 | 95,400 | 34,150 | 7 | 14 | ND | 6.80 | Virulent | |
| MSP142-C1 | 2983 | 135,090 | 47,150 | 8 | 23 | 76 | 0.24 | Deadd |
| T1315 | 485,190 | 119,950 | NPe | 28 | 80 | NP | Controlled | |
| T1379 | 215,190 | 76,100 | 11 | 28 | ND | 0.04 | Controlled | |
| T1428 | 42,570 | 15,260 | 7 | 16 | ND | 5.10 | Virulent | |
| T1434 | 140,490 | 51,800 | 12 | 21 | 82 | 1.10 | Anemic | |
| T1442 | 198,180 | 76,450 | 10 | 28 | 83 | 0.03 | Controlled | |
If not already treated, all monkeys were treated on day 28.
Course of infection: virulent, uncontrolled parasitemia requiring treatment (parasitemia of >5%); anemic, monkey required treatment for anemia (hematocrit of <20%); controlled, monkey controlled parasitemia (<0.05 %) without intervention through day 28.
ND, not determined.
Monkey 2983 was found dead on day 23. The hematocrit on day 21 was 39%; the parasitemia on day 22 was 0.19%.
NP, never patent.
To assess the specificity of the antibody responses, ELISAs were compared using the FVO allele of MSP142 or the MSP119 as plate antigens. For the MSP142-immunized groups, the MSP119/MSP142 ratio was constant regardless of immunogen; the mean of MSP119/MSP142 ratios in the MSP142-FVO, MSP142-3D7, and MSP142-C1 groups were 0.29, 0.31, and 0.34, respectively, and the anti-MSP142 ELISA units correlated significantly with the anti-MSP119 units (Spearman rank correlation, 0.946; 95% confidence interval [CI], 0.872 to 0.978; P < 0.0001).
Correlation between ELISA units and protection against P. falciparum challenge.
Three of the four monkeys in the control group vaccinated with Pfs25H were treated for uncontrolled parasitemia (Table 1 and Fig. 1). On the other hand, one of five in the MSP142-FVO group, four of seven in the MSP142-3D7 group, and one of six in the MSP142-C1 group required drug treatment before the study was terminated. All monkeys that had more than approximately 200,000 ELISA units measured against the MSP142-FVO protein controlled their infections, while all monkeys requiring treatment for uncontrolled parasitemia had less than 108,000 units against MSP142-FVO prior to parasite challenge.
FIG. 1.
Daily parasitemia in A. nancymai monkeys after challenge. Monkeys were vaccinated three times with 50 μg/dose of Pfs25H (a), 50 μg/dose of MSP142-FVO (b), 50 μg/dose of MSP142-3D7 (c), or 100 μg/dose of MSP142-C1 (d). Seventeen days after the final vaccination, they were challenged with 5 × 104 P. falciparum FVO parasitized red blood cells. Parasitemia was monitored daily by inspection of Giemsa-stained thin blood smears until treatment and was calculated based on examination of approximately 2,000 RBCs; if no parasites were seen, then 40 more high-power fields were examined. Monkeys were treated with a curative dose of mefloquine when parasitemia reached 5%, when their hematocrit fell below 25%, or at day 28. T, monkey was treated for high parasitemia; H, monkey was treated for anemia; C, monkey was treated at day 28. D, one monkey in the MSP142-C1 group was found dead on day 23; the hematocrit on day 21 was 39%, and the parasitemia on day 22 was 0.19%.
To express the level of protection in a more quantitative fashion, all monkeys were ranked by a number of outcome variables as described in Materials and Methods. There is a significant difference in the degree of protection among the four groups with all of the parameters tested, using a one-way ANOVA test (Table 2). With all the criteria there are consistent significant differences between the Pfs25H and MSP142-C1 groups, but the comparisons with the other groups show various statistical results depending on the protection parameter selected.
TABLE 2.
Statistical comparison between four groups of immunized and challenged A. nancymai monkeys by using different outcome parameters
| Rank of protection | One-way ANOVA |
P value for multiple comparison with t distribution
|
|||||
|---|---|---|---|---|---|---|---|
| Pfs25H vs MSP142-FVO | Pfs25H vs MSP142-3D7 | Pfs25H vs MSP142-C1 | MSP142-FVO vs MSP142-3D7 | MSP142-FVO vs MSP142-C1 | MSP142-3D7 vs MSP142-C1 | ||
| Parasitemiaa | 0.017 | 0.009 | NSd | 0.002 | NS | NS | 0.017 |
| Patencyb | 0.048 | NS | NS | 0.014 | NS | NS | 0.013 |
| Treatmentc | 0.040 | 0.016 | NS | 0.004 | NS | NS | NS |
The monkeys were ranked based on the cumulative parasitemia on the day that the first monkey was treated for anemia. Monkeys treated for high parasitemia prior to that day were ranked first, and then the other monkeys were ranked.
The monkeys were ranked based on the day that any parasite was first observed in the Giemsa-stained thin blood smears. If several monkeys showed parasites on the same day, they were ranked in the order of their percentage of parasitized erythrocytes.
Initially, the monkeys were grouped based on the reason for treatment. The monkeys treated for high parasitemia were grouped first, those treated for anemia were grouped second, and those not requiring treatment throughout the study were in the third group. In each group, monkeys were ranked in order of the day of treatment and then by cumulative parasitemia. Monkey 2983 was excluded from this analysis, since it had died on day 23 with 0.19% parasitemia and 39% hematocrit.
NS, no significant difference between the groups (P ≥ 0.05).
There are significant correlations between the prechallenge anti-MSP142-FVO antibody titers determined by ELISA and protection (Fig. 2 and Table 3). With three different outcome variables (parasitemia, patency, and treatment) used to rank protection, the Spearman rank correlation between protection and ELISA titers ranked from 0.75 to 0.85, all with P values of <0.001. Even when the monkeys were ranked based on other outcomes of infection (e.g., peak parasitemia during the study or average parasitemia until the monkey was treated for any reason), there were significant correlations between prechallenge ELISA titer and subsequent protection (Spearman rank correlation, approximately 0.7; P < 0.001).
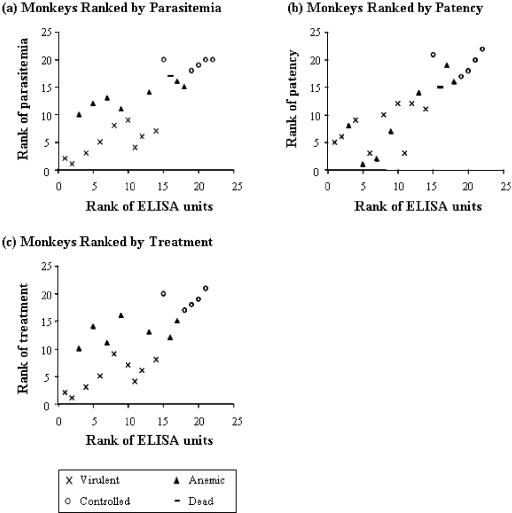
FIG. 2.
Correlation between ELISA units and protection data. Monkeys were vaccinated and challenged with P. falciparum as described in the legend to Fig. 1. The rank of protection data using various outcome measures is plotted against the rank of anti-MSP142-FVO ELISA units. (a) Ranking by parasitemia. Monkeys were ranked based on the parasitemia on the day that the first monkey was treated for anemia (15 days after challenge). There is a significant correlation between the ranks of ELISA and of parasitemia (Spearman rank correlation, 0.79; P < 0.001). (b) Ranking by day of patency. Monkeys were ranked based on the day that parasites were first observed in Giemsa-stained blood smears. There is a significant positive correlation between the rank of ELISA and of patency (Spearman rank correlation, 0.85; P < 0.001). (c) Ranking by treatment. Monkeys were ranked based on the day of treatment. There is a significant positive correlation between the rank of ELISA and day of treatment (Spearman rank correlation, 0.75; P < 0.001).
TABLE 3.
Outcome of challenge infection with P. falciparum FVO parasites in immunized A. nancymai monkeys correlated with in vitro ELISA and growth inhibition assay
| Variable used to rank protectiona | ELISA, MSP142-FVO
|
GIA, FVO parasites
|
||||
|---|---|---|---|---|---|---|
| SRCb | 95% CI | P | SRC | 95% CI | P | |
| Parasitemia | 0.79 | 0.54-0.91 | <0.001 | 0.78 | 0.29-0.94 | 0.004 |
| Patency | 0.85 | 0.66-0.93 | <0.001 | 0.75 | 0.26-0.93 | 0.004 |
| Treatment | 0.75 | 0.48-0.89 | <0.001 | 0.70 | 0.12-0.92 | 0.013 |
The monkeys were ranked based on the various parameters as described in the footnotes to Table 2.
SRC, Spearman rank correlation.
Correlation between ELISA units, growth inhibition assay data, and protection.
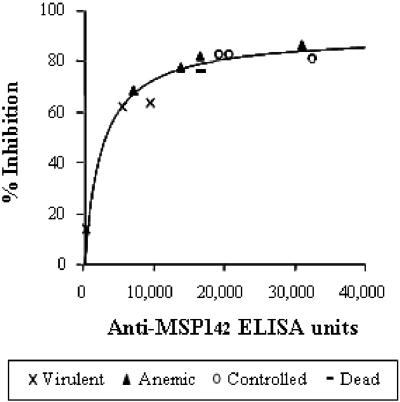
Because of the limitations of the blood volumes available, sera from 11 monkeys were used for IgG preparation (1 sample from the Pfs25H group, 4 from the MSP142-FVO group, 2 from the MSP142-3D7 group, and 4 from the MSP142-C1 group). The biological activities of the purified IgGs were then tested with P. falciparum FVO parasites in an in vitro GI assay involving a biochemical measurement of parasite lactate dehydrogenase as a readout of parasite growth. As shown in Fig. 3, there is a significant correlation between antibody units judged by ELISA and growth-inhibitory activity judged by GI assay against FVO parasites (Spearman rank correlation, 0.864; 95% CI, 0.547 to 0.964; P = 0.0003). A plot of the percent inhibition versus antibody concentration followed a hyperbolic curve, regardless of immunogen (r2 = 0.962; I = M − M/(1 + Ab/Ab50), where I is percent inhibition, M is maximum inhibition, Ab50 is the antibody ELISA unit that gives 50% inhibition, and Ab is the experimental concentration of antibodies in the GI well). All IgGs from monkeys requiring treatment for uncontrolled parasitemia showed less than 60% inhibition in vitro. The growth inhibition activities in vitro are also significantly correlated with protection data in vivo (Table 3).
FIG. 3.
Correlation between ELISA units and growth inhibition activity in vitro. From the individual monkey serum on 17 days after the final immunization (just before parasite challenge), IgG was purified using protein G columns. The antibody titers of the purified IgGs were determined by ELISA, and the GI activities were determined by a standardized GI assay with P. falciparum FVO parasites in vitro. The antibody units in the GI assay wells as determined by ELISA units against MSP142 FVO antigen were plotted on the abscissa and the percent inhibition in the GI assay on the ordinate. There is a significant positive correlation between the ELISA units and the percent inhibition by GI assay (Spearman rank correlation, 0.864; P = 0.0003). The line is the best fit for the hyperbolic function.
DISCUSSION
In this study we have utilized the Aotus nancymai immunization-challenge model to demonstrate that monkeys immunized with either MSP142-FVO, MSP142-3D7, or a combination of the two (MSP142-C1) induced comparable levels of antibody and protective immunity against challenge with P. falciparum FVO parasites. We have also shown that the antibody titers determined by ELISA on MSP142-FVO plate antigen and the in vitro GI activity in prechallenge sera are significantly correlated with each other. More importantly, both sets of data also correlate with protective immunity against parasite challenge regardless of the immunogen used.
Immunization of Aotus monkeys with MSP142-3D7 induced anti-MSP142-FVO antibodies at the same level as in the monkeys immunized with MSP142-FVO (Table 1). Within MSP142, the MSP119 carboxy-terminal region has been proposed to be the most significant, since it can elicit protective immune responses in rodent and nonhuman primate models of malaria (6, 19). Moreover, genetically modified P. falciparum parasites have been used to develop evidence that a significant portion of the parasite-inhibitory activity in sera taken from those living in areas where malaria is endemic is directed to MSP119 (16, 25). Consequently, it is of interest that levels of antibodies to MSP119-FVO were also indistinguishable in the MSP142-FVO and MSP142-3D7 groups. This result is similar to the observation by other investigators that Aotus monkeys inoculated with P. falciparum FVO or FUP (Uganda Palo Alto strain), where the amino acid sequence of MSP142 is a recombinant between the 3D7 (33-kDa sequence) and FVO (19-kDa sequence), induced antibodies that did not discriminate between those two allelic types of MSP142 as judged by indirect ELISA and competitive ELISA (15). This suggests that humoral immune responses of Aotus monkeys recognize conserved epitopes in MSP119, even though there are polymorphisms in this polypeptide. The parasite challenge data also support this hypothesis, since there are no significant differences between MSP142-FVO and MSP142-3D7 groups observed using three different types of outcome criteria (Table 2). However, ELISA data and two of three different analyses using the protection data sets show significant differences between the MSP142-3D7 and MSP142-C1 groups, but no statistical differences were detected between the MSP142-FVO and MSP142-C1 groups in any data sets tested. Therefore, it may be possible that MSP142-3D7 induced slightly lower levels of anti-MSP142-FVO antibodies and consequently less protective activity against FVO parasite challenge. However, the small group sizes in this current Aotus challenge study do not provide a definitive test of significance using a nonparametric test.
While we and others have shown that the ELISA titer of MSP1-immunized Aotus correlates with protective immunity against homologous parasite challenge (7, 29, 31), there has not been a direct comparison between homologous and heterologous challenges in primates. Interestingly, in our study even when monkeys were immunized with MSP142-3D7, protective immunity against P. falciparum FVO parasite challenge correlated with anti-MSP142-FVO ELISA units, and the Spearman rank correlations were more than 0.7 when three different outcome parameters of challenge data were used. This suggests that the level of antibody against the relevant allelic protein is the critical factor for protection in this Aotus model for testing the efficacy of vaccine candidates. One might expect that there are cross-reactive and strain (allele)-specific antibodies and that the two types of antibodies have different biological activities. If this were the case, the protection rankings of the MSP142-FVO group and the MSP142-3D7 group should not be the same as the rankings of anti-MSP1-FVO antibody titers, since the MSP142-FVO-immunized group would be expected to produce both cross-reactive and strain-specific antibodies (both reactive to FVO ELISA coating antigens and FVO parasites), while only cross-reactive antibodies in the MSP142-3D7-immunized group would recognize FVO antigens and FVO parasites. However, our data suggest that this is not the case. They suggest that the important parameter is the amount of total antigen-specific antibody specifically to the conserved epitopes on the MSP119 portion of the recombinant antigens, as there are only four point mutations. It has been shown that antibody directed to the MSP119 portion in the anti-MSP142 antibody population is important to the biological activity (13, 18, 29). Since the rank order of the anti-MSP142 ELISA units tightly correlated with the anti-MSP119 units (Spearman rank test, 0.946), we cannot distinguish whether the rank order of anti-MSP142 or anti-MSP119 ELISA units is the primary determinant of the rank of protective immunity. However, this result is of importance in leading to the concept that those alleles of MSP142 which induce high antibody titers might be sufficient to induce protective immunity to homologous and heterologous strains. It appears that immunodominant epitopes are conserved and localized within the 19-kDa domain This hypothesis is supported by three pieces of evidence. (i) The first piece of evidence is antibody responses to recombinant MSP1; in the present study, the ELISA titer induced by homologous antigen reacted equally well with homologous and heterologous recombinant antigen. (ii) The second piece of evidence is antibody responses to parasite challenge in the animal model. Naive Aotus monkeys were infected by blood-stage challenge with either one of the two dimorphic MSP1 alleles represented by the FUP and FVO parasites. Sera collected after parasite clearance were analyzed by ELISAs. Monkeys infected with parasites carrying one allelic form of MSP1 had antibodies that were equally reactive with homologous or heterologous MSP1s (15). (iii) The third piece of evidence is natural immunity in the field against MSP1; surveys of antibody responses in both children and adults from areas of the Philippines where malaria is hyperendemic (Palawan District) as well as hypoendemic (Morong District) showed that the overwhelming majority (95 to 97%) of the serum samples recognized all of the MSP119 variants tested (14).
We had speculated that inclusion of MSP142-3D7 in a vaccine mixture might inhibit induction of antibodies to MSP142-FVO when the two proteins were used together as an immunogen. Therefore, we decided to use a total of 100 μg/dose of MSP142-C1 instead of a total of 50 μg/dose. Although it does not reach statistical significance, the geometric mean of anti-MSP142-FVO ELISA units in the MSP142-C1 group was twice that of the MSP142-FVO group; moreover, the MSP142-C1 group was more protected than the MSP142-FVO group as judged by the parameters of infection after the challenge. Overall, the presence of MSP142-3D7 in the vaccine formulation had an additive, not a competitive, effect on specific immunity to P. falciparum FVO parasites, suggesting that most of the protective epitopes in MSP142-FVO and MSP142-3D7 are conserved.
One of the major issues in the development of blood-stage vaccines for malaria is to identify a rapid, inexpensive way of evaluating protective immunity. As mentioned above, ELISA is such an assay to evaluate vaccine efficacy in general. However, since ELISA cannot distinguish antibodies that simply bind antigen as opposed to those with biological activity against parasites, an additional assay which reflects functional activity is needed. The GI assay is one such assay that measures the effect of immune IgG on parasite growth in vitro. Our data clearly show that there is a strong correlation (Spearman rank correlation, 0.7 or more) between the GI assay results and protective immunity in vivo (Table 3). Moreover, the growth-inhibitory activity of anti-MSP142 IgG on FVO parasites is directly proportional to the anti-MSP142-FVO ELISA units (Fig. 3). Because performing the ELISA or GI assay is much less time-consuming and labor-intensive than performing a parasite challenge study and because we have established that the data from these two assays correlate tightly with protective immunity against parasite challenge in the present system, these assays could be useful tools for MSP1-based malarial vaccine development for assessing biologically active antibodies induced by vaccination.
Using two distinct allelic forms of MSP142 produced as clinical-grade products, we have shown that in vivo protective immunity against P. falciparum FVO parasites strongly correlates with (i) the amount of specific antibody in the serum at the time of challenge as judged by ELISA and (ii) in vitro growth-inhibitory activity of the IgG purified from antiserum, regardless of immunogen. These results strongly support conducting similar assays in clinical trials of these blood-stage malaria proteins to assess possible correlations with protective immunity to malaria in humans.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Blackman, M. J., H. G. Heidrich, S. Donachie, J. S. McBride, and A. A. Holder. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 172:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackman, M. J., and A. A. Holder. 1992. Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP133 as a noncovalently associated complex with other fragments of the MSP1. Mol. Biochem. Parasitol. 50:307-315. [DOI] [PubMed] [Google Scholar]
- 3.Blackman, M. J., I. T. Ling, S. C. Nicholls, and A. A. Holder. 1991. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol. Biochem. Parasitol. 49:29-33. [DOI] [PubMed] [Google Scholar]
- 4.Blackman, M. J., H. Whittle, and A. A. Holder. 1991. Processing of the Plasmodium falciparum major merozoite surface protein-1: identification of a 33-kilodalton secondary processing product which is shed prior to erythrocyte invasion. Mol. Biochem. Parasitol. 49:35-44. [DOI] [PubMed] [Google Scholar]
- 5.Chang, S. P., S. E. Case, W. L. Gosnell, A. Hashimoto, K. J. Kramer, L. Q. Tam, C. Q. Hashiro, C. M. Nikaido, H. L. Gibson, C. T. Lee-Ng, P. J. Barr, B. T. Yokota, and G. S. Hut. 1996. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect. Immun. 64:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daly, T. M., and C. A. Long. 1995. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J. Immunol. 155:236-243. [PubMed] [Google Scholar]
- 7.Darko, C. A., E. Angov, W. E. Collins, E. S. Bergmann-Leitner, A. S. Girouard, S. L. Hitt, J. S. McBride, C. L. Diggs, A. A. Holder, C. A. Long, J. W. Barnwell, and J. A. Lyon. 2005. The clinical-grade 42-kilodalton fragment of merozoite surface protein 1 of Plasmodium falciparum strain FVO expressed in Escherichia coli protects Aotus nancymai against challenge with homologous erythrocytic-stage parasites. Infect. Immun. 73:287-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira, M. U., Q. Liu, M. Zhou, M. Kimura, O. Kaneko, H. Van Thien, S. Isomura, K. Tanabe, and F. Kawamoto. 1998. Stable patterns of allelic diversity at the merozoite surface protein-1 locus of Plasmodium falciparum in clinical isolates from southern Vietnam. J. Eukaryot. Microbiol. 45:131-136. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira, M. U., W. L. Ribeiro, A. P. Tonon, F. Kawamoto, and S. M. Rich. 2003. Sequence diversity and evolution of the malaria vaccine candidate merozoite surface protein-1 (MSP-1) of Plasmodium falciparum. Gene 304:65-75. [DOI] [PubMed] [Google Scholar]
- 10.Good, M. F. 2001. Towards a blood-stage vaccine for malaria: are we following all the leads? Nat. Rev. Immunol. 1:117-125. [DOI] [PubMed] [Google Scholar]
- 11.Hirunpetcharat, C., J. H. Tian, D. C. Kaslow, N. van Rooijen, S. Kumar, J. A. Berzofsky, L. H. Miller, and M. F. Good. 1997. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J. Immunol. 159:3400-3411. [PubMed] [Google Scholar]
- 12.Hirunpetcharat, C., P. Vukovic, X. Q. Liu, D. C. Kaslow, L. H. Miller, and M. F. Good. 1999. Absolute requirement for an active immune response involving B cells and Th cells in immunity to Plasmodium yoelii passively acquired with antibodies to the 19-kDa carboxyl-terminal fragment of merozoite surface protein-1. J. Immunol. 162:7309-7314. [PubMed] [Google Scholar]
- 13.Hogh, B., N. T. Marbiah, P. A. Burghaus, and P. K. Andersen. 1995. Relationship between maternally derived anti-Plasmodium falciparum antibodies and risk of infection and disease in infants living in an area of Liberia, West Africa, in which malaria is highly endemic. Infect. Immun. 63:4034-4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui, G. S. Unpublished data.
- 15.Hui, G. S., C. Nikaido, C. Hashiro, D. C. Kaslow, and W. E. Collins. 1996. Dominance of conserved B-cell epitopes of the Plasmodium falciparum merozoite surface protein, MSP1, in blood-stage infections of naive Aotus monkeys. Infect. Immun. 64:1502-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.John, C. C., R. A. O'Donnell, P. O. Sumba, A. M. Moormann, T. F. de Koning-Ward, C. L. King, J. W. Kazura, and B. S. Crabb. 2004. Evidence that invasion-inhibitory antibodies specific for the 19-kDa fragment of merozoite surface protein-1 (MSP-1 19) can play a protective role against blood-stage Plasmodium falciparum infection in individuals in a malaria endemic area of Africa. J. Immunol. 173:666-672. [DOI] [PubMed] [Google Scholar]
- 17.Kaslow, D. C., G. Hui, and S. Kumar. 1994. Expression and antigenicity of Plasmodium falciparum major merozoite surface protein (MSP1 (19)) variants secreted from Saccharomyces cerevisiae. Mol. Biochem. Parasitol. 63:283-289. [DOI] [PubMed] [Google Scholar]
- 18.Kumar, S., W. Collins, A. Egan, A. Yadava, O. Garraud, M. J. Blackman, J. A. Guevara Patino, C. Diggs, and D. C. Kaslow. 2000. Immunogenicity and efficacy in Aotus monkeys of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C terminus of merozoite surface protein 1. Infect. Immun. 68:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar, S., A. Yadava, D. B. Keister, J. H. Tian, M. Ohl, K. A. Perdue-Greenfield, L. H. Miller, and D. C. Kaslow. 1995. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol. Med. 1:325-332. [PMC free article] [PubMed] [Google Scholar]
- 20.Malkin, E. M., D. J. Diemert, J. H. McArthur, J. R. Perreault, A. P. Miles, B. K. Giersing, G. E. Mullen, A. Orcutt, O. Muratova, M. Awkal, H. Zhou, J. Wang, A. Stowers, C. A. Long, S. Mahanty, L. H. Miller, A. Saul, and A. P. Durbin. 2005. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect. Immun. 73:3677-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miles, A. P., Y. Zhang, A. Saul, and A. W. Stowers. 2002. Large-scale purification and characterization of malaria vaccine candidate antigen Pvs25H for use in clinical trials. Protein Expr. Purif. 25:87-96. [DOI] [PubMed] [Google Scholar]
- 22.Miller, L. H., M. F. Good, and D. C. Kaslow. 1998. Vaccines against the blood stages of falciparum malaria. Adv. Exp. Med. Biol. 452:193-205. [DOI] [PubMed] [Google Scholar]
- 23.Miller, L. H., and S. L. Hoffman. 1998. Research toward vaccines against malaria. Nat. Med. 4:520-524. [DOI] [PubMed] [Google Scholar]
- 24.Miller, L. H., T. Roberts, M. Shahabuddin, and T. F. McCutchan. 1993. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1). Mol. Biochem. Parasitol. 59:1-14. [DOI] [PubMed] [Google Scholar]
- 25.O'Donnell, R. A., T. F. de Koning-Ward, R. A. Burt, M. Bockarie, J. C. Reeder, A. F. Cowman, and B. S. Crabb. 2001. Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 193:1403-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qari, S. H., Y. P. Shi, I. F. Goldman, B. L. Nahlen, M. Tibayrenc, and A. A. Lal. 1998. Predicted and observed alleles of Plasmodium falciparum merozoite surface protein-1 (MSP-1), a potential malaria vaccine antigen. Mol. Biochem. Parasitol. 92:241-252. [DOI] [PubMed] [Google Scholar]
- 27.Renia, L., I. T. Ling, M. Marussig, F. Miltgen, A. A. Holder, and D. Mazier. 1997. Immunization with a recombinant C-terminal fragment of Plasmodium yoelii merozoite surface protein 1 protects mice against homologous but not heterologous P. yoelii sporozoite challenge. Infect. Immun. 65:4419-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotman, H. L., T. M. Daly, and C. A. Long. 1999. Plasmodium: immunization with carboxyl-terminal regions of MSP-1 protects against homologous but not heterologous blood-stage parasite challenge. Exp. Parasitol. 91:78-85. [DOI] [PubMed] [Google Scholar]
- 29.Singh, S., M. C. Kennedy, C. A. Long, A. J. Saul, L. H. Miller, and A. W. Stowers. 2003. Biochemical and immunological characterization of bacterially expressed and refolded Plasmodium falciparum 42-kilodalton C-terminal merozoite surface protein 1. Infect. Immun. 71:6766-6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stowers, A. W., L. H. Chen Lh, Y. Zhang, M. C. Kennedy, L. Zou, L. Lambert, T. J. Rice, D. C. Kaslow, A. Saul, C. A. Long, H. Meade, and L. H. Miller. 2002. A recombinant vaccine expressed in the milk of transgenic mice protects Aotus monkeys from a lethal challenge with Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 99:339-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stowers, A. W., V. Cioce, R. L. Shimp, M. Lawson, G. Hui, O. Muratova, D. C. Kaslow, R. Robinson, C. A. Long, and L. H. Miller. 2001. Efficacy of two alternate vaccines based on Plasmodium falciparum merozoite surface protein 1 in an Aotus challenge trial. Infect. Immun. 69:1536-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stowers, A. W., M. C. Kennedy, B. P. Keegan, A. Saul, C. A. Long, and L. H. Miller. 2002. Vaccination of monkeys with recombinant Plasmodium falciparum apical membrane antigen 1 confers protection against blood-stage malaria. Infect. Immun. 70:6961-6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanabe, K., N. Sakihama, and A. Kaneko. 2004. Stable SNPs in malaria antigen genes in isolated populations. Science 303:493. [DOI] [PubMed] [Google Scholar]
- 34.Tanabe, K., N. Sakihama, Y. Nakamura, O. Kaneko, M. Kimura, M. U. Ferreira, and K. Hirayama. 2000. Selection and genetic drift of polymorphisms within the merozoite surface protein-1 gene of Plasmodium falciparum. Gene 241:325-331. [DOI] [PubMed] [Google Scholar]