Abstract
In cystic fibrosis (CF), the condition limiting the prognosis of affected children is the chronic obstructive lung disease accompanied by chronic and persistent infection with mostly mucoid strains of Pseudomonas aeruginosa. The majority of CF patients have antineutrophil cytoplasmic antibodies (ANCA) primarily directed against the bactericidal permeability-increasing protein (BPI) potentially interfering with antimicrobial effects of BPI. We analyzed the expression of BPI in the airways of patients with CF. In their sputum samples or bronchoalveolar lavage specimens, nearly all patients expressed BPI mRNA and protein, which were mainly products of neutrophil granulocytes as revealed by intracellular staining and subsequent flow cytometry. Repeated measurements revealed consistent individual BPI expression levels during several months quantitatively correlating with interleukin-8. In vitro, P. aeruginosa isolates from CF patients initiated the rapid release of BPI occurring independently of protein de novo syntheses. Furthermore, purified natural BPI as well as a 27-mer BPI-derived peptide displayed antimicrobial activity against even patient-derived mucoid P. aeruginosa strains and bacteria resistant against all antibiotics tested. Thus, BPI that is functionally active against mucoid P. aeruginosa strains is expressed in the airways of CF patients but may be hampered by autoantibodies, resulting in chronic infection.
Cystic fibrosis (CF) is the most common lethal hereditary disease in the Caucasian population (about 1:2,500). In the majority of CF patients, mutations in the cystic fibrosis transmembrane conductance regulator gene occur. These mutations result in a nonfunctional chloride channel on epithelial membranes, therefore leading to an imbalance of electrolyte transport on epithelia and subsequently to severe dysfunction in multiple organs (16).
The major cause for morbidity and mortality in CF patients is the neutrophil-dominated lung inflammation caused predominantly by chronic Pseudomonas aeruginosa airway infections (10). The events that lead to chronic lung infection in CF patients are still poorly understood.
Immune defense mechanisms and antibiotic therapy against P. aeruginosa infections in CF patients are partially ineffective because of the capacity of P. aeruginosa to develop mucoid phenotypes by producing an alginate capsule that enables biofilm formation. Bacteria in alginate biofilms are less accessible to endogenous antibacterials, phagocytes, and complement (12, 15). Furthermore, biofilms contribute tremendously to the resistance of P. aeruginosa to applied antibiotics, partially because of slow growth (11), but hypermutation also plays a key role in the development of multiple-antimicrobial resistance (5, 18), namely, to ticarcillin, ceftazidime, and gentamicin (19).
Antimicrobial peptides and proteins are important effector molecules in innate immunity to combat bacterial infections. The main producers of such molecules are neutrophil granulocytes that play a key role in CF lung inflammation. Bactericidal permeability-increasing protein (BPI) is abundantly expressed in neutrophils and is stored in acidic granules (8). In addition, it was shown that BPI is also expressed in epithelial cells (3). The 55-kDa BPI has restricted antimicrobial activity against gram-negative bacteria. Direct bactericidal activity and lipopolysaccharide neutralization are mediated by the N-terminal part of the protein, whereas the C-terminal region has been shown to opsonize bacteria (9). Recently, a mouse ortholog of human BPI was described, indicating the importance of this antimicrobial protein throughout evolution in different vertebrate species (7). Antineutrophil cytoplasmic autoantibodies against BPI (BPI-ANCA) have been found in up to 90% of CF patients (22, 28), and the presence of BPI-ANCA has been shown to correlate with chronic P. aeruginosa lung infection (1, 6) and Pseudomonas-induced lung damage (4). In vitro, BPI-ANCA have been shown to inhibit neutrophil-mediated killing of P. aeruginosa (24). This raises the possibility that neutralizing BPI-ANCA interfere with the bacterial killing of P. aeruginosa in the lungs of CF patients and thereby are a key factor for the bacteria to establish chronic infection.
However, there is no information on the expression and regulation of BPI in the airways of CF patients or on the interaction of BPI with mucoid as well as multiresistant P. aeruginosa strains. In this study, we show that BPI is present in the bacterially infected airways of CF patients. Furthermore, BPI is released from granulocytes after bacterial encounter with P. aeruginosa. Finally, BPI is effective against mucoid and drug-resistant strains of P. aeruginosa and kills these bacteria rapidly and efficiently. These results indicate that BPI may have potential as a new therapeutic agent in CF lung infections.
MATERIALS AND METHODS
Sputum samples.
Sputum samples and bronchoalveolar lavage specimens (BAL) were collected from CF patients for diagnostic purposes after informed consent. To extract the cells in sputum, fresh sputum samples were treated with 2 ml of sputolysin reagent (Calbiochem; Merck, Darmstadt, Germany) and 18 ml of phosphate-buffered saline (PBS) for 15 min at room temperature (RT) with occasional mixing. Samples were then filtered through a cell strainer (70 μm) (BD Falcon; BD Biosciences, Bedford, Mass.) and washed with PBS. After centrifugation, the cell pellet was resuspended in PBS and the cell concentration was determined.
Isolation of RNA.
The isolation of RNA from 2 × 105 to 4 × 106 sputum-derived cells as well as from isolated human granulocytes was performed with RNAqueous (Ambion, Austin, TX) following the manufacturer's instructions. When RNA isolation was not performed immediately, pelleted cells were resuspended in an adequate amount of lysis/binding solution (RNAqueous) and stored at −70°C until use. Possible contaminating genomic DNA was digested using DNA-free (Ambion).
PCR.
Reverse transcription of RNA was performed using SuperScript II RNase H-reverse transcriptase (Invitrogen, Carlsbad, CA), with a preincubation of 2 min at 42°C before SuperScript was added and run as follows: 42°C for 50 min, 70°C for 15 min, and 4°C. For qualitative analysis of BPI mRNA expression, the primers 5′-GCAGCCCACCGGCCTTACCTTCTACC-3′ (sense) and 5′-GTGCCCCACAGCCCATCAGGAACA-3′ (antisense) were used at an annealing temperature of 62°C. Real-time PCR was performed using a LightCycler (Roche, Mannheim, Germany) with the following primer pairs: 5′-ATGATGCTGCTTACATGTCTCGAT-3′ (sense) and 5′-AGCGTACTCCAAAGATTCAGGTT-3′ (antisense) for β2-microglobulin (annealing temperature, 52°C; 4 mM MgCl2) and 5′-GAGGTCAGCGCCGAGTCC-3′ (sense) and 5′-CCCCTGGTGCCTTCATTTAT-3′ (antisense) for BPI (annealing temperature, 60°C; 4 mM MgCl2). SYBR green was purchased from Invitrogen (Molecular Probes). All primers were purchased from ThermoElectron (Ulm, Germany).
ELISA.
BPI from isolated granulocytes was measured by a BPI-enzyme-linked immunosorbent assay (ELISA) purchased from HyCult Biotechnology (Uden, The Netherlands), and interleukin-8 (IL-8) protein was measured using DuoSet purchased from R&D Systems (Wiesbaden, Germany) following the manufacturer's protocols.
Western blot.
Cells extracted from sputum samples were lysed (lysis buffer, 50 mM Tris, 1% NP-40, 150 mM NaCl, 1 mM EDTA, pH 8.5, containing 1 μg/ml aprotinin, 1 μg/ml leupeptin and 1 μg/ml pepstatin A to inhibit digestion by proteases), 50 μg of total protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane, and BPI was detected by polyclonal rabbit immunoglobulin G1 (IgG1) anti-BPI antibody (HyCult Biotechnology) and a secondary horseradish peroxidase-conjugated donkey anti-rabbit IgG1 antibody (Dianova, Hamburg, Germany) and visualized by ECL Plus reagent (Amersham, Little Chalfont, England).
Bacteria.
For the stimulation of isolated granulocytes, P. aeruginosa strain ATCC 27853 was used. In addition, experiments for bacteriostatic and bactericidal activity of BPI and BPI peptide were performed with different mucoid and nonmucoid P. aeruginosa strains isolated from CF patients, including strains fully resistant to all applied antibiotics, namely, piperacillin, ceftazidime, cefepime, imipenem, meropenem, tobramycin, amikacin, and ciprofloxacin. Bacteria were cultivated in tryptic soy broth to exponential growth phase, harvested by centrifugation at 2,800 × g for 10 min, and washed twice with PBS before use.
Stimulation of human-isolated granulocytes.
Granulocytes were isolated from human whole blood by density gradient centrifugation using Percoll (Amersham Biosciences, Uppsala, Sweden) following sedimentation of erythrocytes through 3% dextran T70 (Roth, Karlsruhe, Germany) in PBS for 20 min to separate granulocytes from erythrocytes. Experiments were performed in RPMI medium (PAA, Pasching, Austria) with 10% fetal calf serum. A total of 4 × 106 granulocytes were stimulated with a multiplicity of infection (MOI) of 20 CFU with UV-inactivated P. aeruginosa for several time points without and with 10 μg/ml of cycloheximide (Sigma, Steinheim, Germany). Supernatants were collected and stored at −70°C until IL-8 and BPI measurement. Stimulated cells were harvested directly with lysis/binding solution (RNAqueous; Ambion) and stored at −70°C until RNA extraction.
Flow cytometry.
For the detection of granulocytes, fluorescein isothiocyanate-conjugated anti-CD66b monoclonal antibody (mouse IgM, clone G10F5; BD Pharmingen, BD Biosciences) and, for macrophages, allophycocyanin-conjugated anti-CD14 monoclonal antibody (mouse IgG1, clone M5E2; BD Pharmingen) were used at 5 μl/106 cells. BPI was detected using 500 ng of monoclonal mouse IgG1 anti-BPI antibody (clone HM2042; HyCult Biotechnology, Uden, The Netherlands) and was labeled with a secondary phycoerythrin-conjugated monoclonal rat anti-mouse IgG1 antibody (clone A85-I; BD Pharmingen). Nonconjugated mouse IgG1(κ) (clone MOPC-21; BD Pharmingen) was used as an isotype control. After Fc binding sites were blocked to prevent unspecific binding, antibodies were applied for 20 min at RT for surface staining. For intracellular staining of BPI, 100 μl of cells were incubated (10 min at RT) with 1 ml of fluorescence-activated cell sorter lysing solution (Becton Dickinson), followed by incubation (10 min at RT) with 500 μl fluorescence-activated cell sorter lysing solution with 0.2% saponin (Sigma). After the cells were washed, BPI antibody was applied for 30 min at RT. For the detection of oxidative burst, granulocytes were incubated with dihydrorhodamine (Sigma) at a final concentration of 5 μM for 15 min at 37°C and 2 × 106 cells were then stimulated with an MOI of 200 P. aeruginosa CFU for 15 min or 1 h at 37°C, respectively. After the cells were washed with PBS-1% fetal calf serum, analysis was performed by flow cytometry.
All flow cytometry measurements were performed with a FACSCalibur cytometer using CellQuest Pro software (BD Biosciences, Heidelberg, Germany).
Bacterial killing by BPI.
BPI purified from human blood was purchased from Wieslab AB (Lund, Sweden). BPI peptide (sequence, N-A-N-I-K-I-S-G-K-W-K-A-Q-K-R-F-L-K-M-S-G-N-F-D-L-S-I) was synthesized and high-pressure liquid chromatography purified by Peptron (Daejeon, Korea).
Assays for BPI activity against bacteria were performed in 96-well plates in a volume of 50 μl of PBS. A total of 104 bacteria/ml was incubated at 37°C with different concentrations of BPI or BPI peptide for the time points indicated and then plated on blood agar. After incubation at 37°C overnight, bacterial colonies were quantified.
Statistics.
Statistical significance was analyzed using Prism 3.0 GraphPad software. Linear regression was calculated for correlation of BPI and IL-8 (see Fig. 3). Results are expressed as means ± standard deviations (see Fig. 4B and C and 6). Significance was tested by one-way analysis of variance, followed by Dunnett's multiple comparison tests. Statistical significance was reached if P was <0.05.
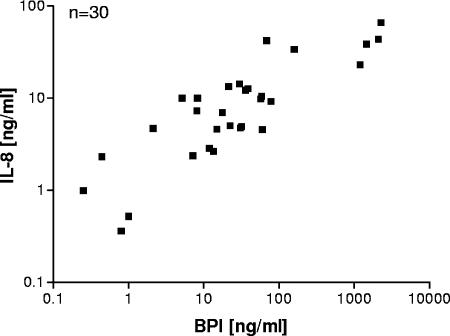
FIG. 3.
Positive correlation of BPI and IL-8 concentrations in cell-free lung fluid from CF patients. Supernatants of sputum samples from CF patients were collected after treatment with sputolysin and subsequent centrifugation. IL-8 and BPI levels were determined by ELISA. Linear regression shows the positive correlation r2 = 0.6785. The P value was <0.0001.
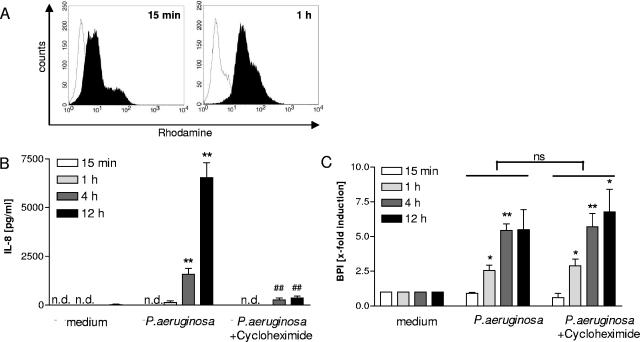
FIG. 4.
BPI is released from neutrophils after stimulation with P. aeruginosa. Human PMNs were isolated from whole blood from healthy donors by density gradient centrifugation. Oxidative burst was analyzed by flow cytometry of dihydrorhodamine-labeled cells stimulated with P. aeruginosa for the time periods indicated (A). Histograms are shown for stimulated cells (black) versus nonstimulated controls (white). The release of BPI and IL-8 was determined after stimulation with UV-inactivated bacteria (MOI, 20 CFU) in the presence or absence of cycloheximide, which was applied 15 min before the bacteria. Cell-free supernatants were collected after distinct time points, and IL-8 (B) and BPI (C) protein was measured by ELISA. Three individual experiments are shown for IL-8 and BPI measurements. Due to variances in the level of BPI from different blood donors, the results are given as induction levels (n-fold). *, P <0.05 for P. aeruginosa-stimulated cells versus median controls; **, P <0.01 for P. aeruginosa-stimulated cells versus median controls; ##, P <0.01 for P. aeruginosa-stimulated cells with cycloheximide versus P. aeruginosa-stimulated cells. n.d., not detected; ns, not significant. Error bars indicate standard deviations.
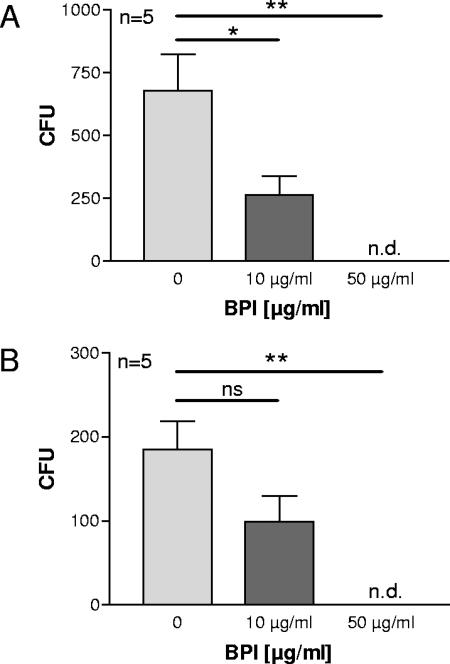
FIG. 6.
Mucoid and antibiotic-resistant clinical P. aeruginosa isolates are killed by BPI. Natural BPI isolated from human granulocytes was used at the indicated concentrations to determine bactericidal activity. The experiments were performed as described in the legend for Fig. 5. All bacteria shown are clinical P. aeruginosa isolates from CF patients. (A) P. aeruginosa strains with a mucoid phenotype, three of which have resistance to up to four antibiotics. (B) P. aeruginosa strains that are multiresistant to antibiotics. Two of the strains are fully resistant to all antibiotics applied. Statistically significant differences are given as P values (*, P <0.05; **, P <0.01). ns, not significant. Error bars indicate standard deviations.
RESULTS
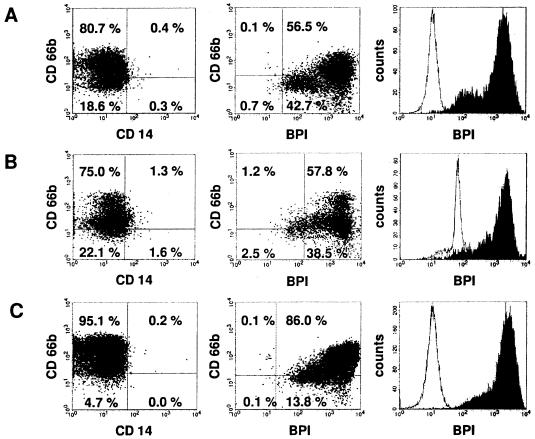
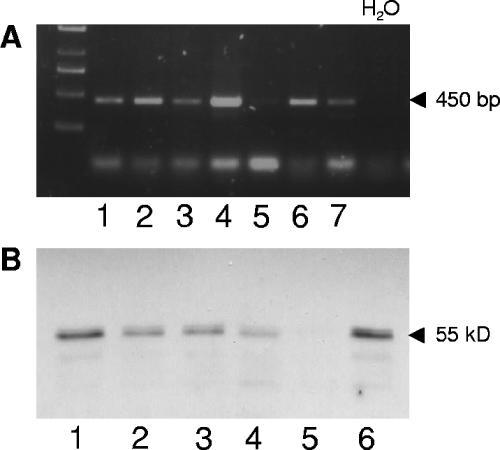
We were interested in whether BPI is detectable in the lungs of bacterially infected CF patients. To answer this question, cells contained in sputum samples of CF patients were isolated and prepared for mRNA and protein analyses of BPI. The cells extracted from sputum samples mainly consisted of neutrophil granulocytes (see Fig. 2), which are reported to form the large majority of infiltrating cells of the lung mucosa during CF (13). The vast majority (43 of 51 analyzed) of sputum samples were found positive for BPI mRNA as shown for seven patients in Fig. 1A. However, only low levels of BPI mRNA were detectable using a quantitative real-time PCR. This was also confirmed in repetitive samples isolated from patients chronically colonized with P. aeruginosa (data not shown). Furthermore, BPI could be detected in most samples on the protein level of whole-cell lysates by using a specific immunoblot assay (Fig. 1B). In order to determine which cells are the main producers of BPI in lung fluid from CF patients, we set up flow cytometry analysis. By this technique, we found that neutrophil granulocytes of sputum-derived cells express high levels of BPI intracellularly. In five of nine sputum samples analyzed, over 90% of CD66b-positive cells were also BPI positive, showing a high frequency of BPI-positive neutrophil granulocytes in CF lung fluid. In addition, a second cell population, which was CD66b/CD14 negative, also contained intracellular BPI. The expression of BPI in the CD66b/CD14-negative cell population was lower compared to that of the neutrophils (Fig. 2A and B). According to microscopic analysis of sputum samples, these cells have the morphological features of epithelial cells, although this could not be shown directly due to the lack of suitable marker molecules. Similar findings were observed in bronchoalveolar lavage specimens from CF patients (Fig. 2C). Therefore, we concluded that BPI is strongly expressed at the protein level in the infected lungs of CF patients and neutrophil granulocytes are the main source of BPI in lung fluid.
FIG. 2.
Neutrophils are the major source of BPI in lung fluid from CF patients. Isolated cells from sputum samples (A and B) or BAL (C) of CF patients were analyzed by flow cytometry. Cell types were distinguished by extracellular staining of CD66b for granulocytes and CD14 for monocytes. BPI was detected by intracellular staining. Percentages of the total amount of cells are given within the charts for each quadrant. The histograms to the right show BPI-positive cells (black) versus the isotype control (white).
FIG. 1.
BPI is expressed in sputum samples of CF patients. BPI mRNA was detected with specific primers by reverse transcription-PCR (A) of sputum-derived cells from CF patients. BPI was detected by immunoblotting (B) whole-cell lysates of sputum-derived cells. BPI mRNA (panel A, lanes 1 to 7) and protein (panel B, lanes 1 to 6) are shown for the same CF patients.
Next, we were interested in whether BPI is also released by the BPI-expressing cells into lung fluid. Therefore, we analyzed cell-free sputum supernatants and were able to detect BPI as well as IL-8 protein in each sample. Furthermore, by comparison of IL-8 and BPI levels, a positive correlation (r2 = 0.6785) was found in supernatants of sputum samples (Fig. 3). Therefore, BPI and IL-8 seem to be released coordinately into lung fluid.
To determine the mechanism underlying the expression and release of BPI from neutrophil granulocytes in the presence of P. aeruginosa, in vitro experiments with isolated human granulocytes from healthy donors were performed. Polymorphonuclear leukocytes (PMNs) were isolated by density gradient centrifugation as described in Materials and Methods. Thereafter, PMNs were cultured in the presence of P. aeruginosa and oxidative burst was determined as an activation control (Fig. 4A). Both IL-8 and BPI were released from PMNs in a time-dependent manner following bacterial encounter (Fig. 4B and C). To analyze whether BPI is released solely from intracellular stores or in addition is transcriptionally activated, the protein de novo synthesis of the PMNs was inhibited during the P. aeruginosa stimulation period. We could show that the release of BPI induced by P. aeruginosa is completely independent of protein de novo synthesis and is solely mediated by release from, presumably, azurophil granules (Fig. 4C), whereas the levels of IL-8 could be significantly reduced by the inhibition of protein synthesis with cycloheximide (Fig. 4B). Similar results were obtained with the transcription inhibitor actinomycin D (data not shown). In accordance with these data on BPI inhibition and with the data obtained from neutrophils isolated from lung fluid, mRNA levels of BPI remained low in the neutrophil granulocytes throughout P. aeruginosa stimulation (data not shown). Therefore, it appears that BPI and IL-8 are released from PMNs in a coordinated fashion, but the release of BPI is mediated solely from intracellular stores, whereas IL-8 needs further synthesis during the exocytosis process.
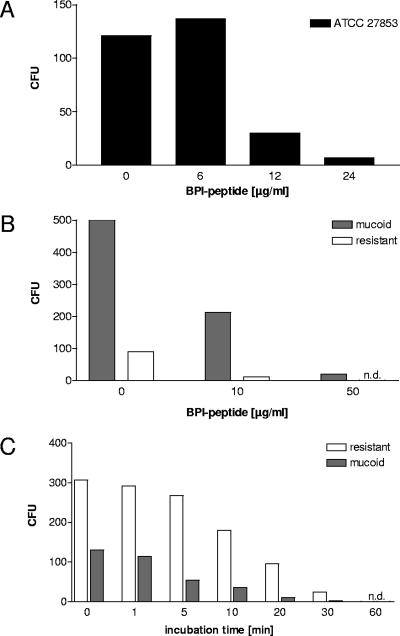
It has already been shown that BPI is able to kill wild-type P. aeruginosa (25). However, it is well documented that P. aeruginosa changes its phenotype during chronic and recurrent infection in the lungs of CF patients. One of the hallmarks of the phenotypic changes of P. aeruginosa is growth as a mucoid strain. Bacteria with a mucoid phenotype form alginate capsules which enable them to establish biofilms in the lung. Furthermore, these bacteria may already be resistant to antibiotics applied in the therapy of CF lung infections (11). Therefore, we were interested in whether BPI is biologically active against mucoid and antibiotic-resistant strains of P. aeruginosa. Natural BPI extracted from human neutrophils and a 27-amino-acid peptide of BPI were used in kill assays to determine the potency of BPI to act bactericidally against mucoid and antibiotic-resistant P. aeruginosa strains. The BPI peptide from the amino-terminal part of the protein has previously been shown to have bactericidal and lipopolysaccharide binding activity (17). As expected, the BPI peptide, like the full-length BPI, was able to kill P. aeruginosa (strain ATCC 27853) in a concentration-dependent fashion (Fig. 5A). More importantly, P. aeruginosa strains that were freshly isolated from CF lungs and growing with a mucoid phenotype were killed by BPI peptide and full-length BPI protein (Fig. 5B and C and 6A). The data shown for BPI peptide are representative for 12 clinical CF isolates tested, including 7 mucoid strains. Furthermore, we included clinical P. aeruginosa isolates derived from CF patients in the experiments; these isolates were either resistant to several antibiotics or were characterized as fully resistant to all antibiotics tested during routine diagnostics (piperacillin, ceftazidime, cefepime, imipenem, meropenem, tobramycin, amikacin, and ciprofloxacin). Nevertheless, full-length BPI and BPI peptide (data shown are representative for four resistant isolates tested) were both bactericidal against the antibiotic-resistant strains of P. aeruginosa in a concentration- and time-dependent manner (Fig. 5B and C and 6), similar to wild-type and nonresistant bacteria. Clinical isolates treated with 10 μg/ml of BPI, apart from the reduced number of CFU, showed dramatically reduced growth rates, observable by the reduced size of the colonies compared to nontreated controls. This finding is consistent with published data on bacteriostatic effects of BPI (2).
FIG. 5.
Antimicrobial activity of a BPI-derived peptide and kinetics of BPI-mediated killing. A 27-amino-acid peptide from the amino-terminal region of BPI was synthesized and compared to natural BPI isolated from human granulocytes. BPI peptide was used at the indicated concentrations to determine bactericidal activity against P. aeruginosa. BPI peptide was coincubated with the bacteria for 1 h at increasing concentrations (A and B), whereas BPI was applied at 10 μg/ml for time-dependent killing of P. aeruginosa (C) and then plated on blood agar to quantify surviving bacteria (A, B, and C). In panel A, the ATCC 27853 strain was used. In panels B and C, the P. aeruginosa stains used were freshly isolated from the lungs of CF patients, one showing a mucoid phenotype (gray bars) and the other being fully resistant to common antibiotics applied in CF lung infection (white bars). n.d., not detected.
From these results, we conclude that BPI is bactericidal against P. aeruginosa even if the strains form alginate capsules or are resistant to a variety of antibiotics.
DISCUSSION
It has been suggested that BPI plays a crucial role during P. aeruginosa lung infections in CF patients (8, 23, 24, 22). Nevertheless, to date, there are no data available on the expression of BPI in the infected lungs of CF patients. Therefore, diverse samples originating from the lungs of CF patients were analyzed by different methods to determine the presence of BPI. Most of these patients suffer from chronic lung infection with P. aeruginosa. Both BPI mRNA and BPI could be detected from cells contained in sputum samples from CF patients (Fig. 1), and further analysis by flow cytometry showed that sputum-derived cells are mainly composed of neutrophil granulocytes (Fig. 2). In fact, in six of nine samples analyzed, the portion of CD66b-positive cells exceeded 70% and was even 85% in BALs, whereas only very low levels of monocytes could be detected. Although the main producers of BPI in the infected lungs of CF patients are neutrophils, another CD66b-negative cell type also carried BPI intracellularly. Recently, mucosal epithelia have been shown to be able to produce immune effector molecules, including BPI (3). In CF patients with chronic lung infection, the ongoing inflammation leads to the destruction of the lung tissue, which might explain the presence of epithelial cells in sputum samples, suggesting that the second BPI-positive cell population is epithelial cells. Microscopic analysis of these cells revealed that this population is very likely from epithelial origin. BPI could also be detected in cell-free lung fluid from CF patients (Fig. 3). This finding argues for a potent and ongoing stimulation of granulocyte degranulation in vivo. The high variance in the amount of BPI measured from sputum sample supernatants might be due to multiple reasons. The quality and amount of sputum samples vary greatly, and for technical reasons, cell numbers contained in different samples cannot be allowed for before processing. Due to the treatment, sputum samples already undergo a dilution of about 1:10 before BPI measurement, which further emphasizes the high concentration that can be reached in the lung. The finding that BPI levels correlate strongly with levels of IL-8 (r2 = 0.6785) suggests that both proteins are released in a coordinated fashion. BPI is known to be stored in azurophil (primary) granules of PMNs (2, 26). A possible scenario of the positive correlation of IL-8 with BPI in the lung fluid could be that IL-8 produced by other cells, like alveolar macrophages or epithelial cells, governs the amount of PMNs recruited to the lung and, therefore, the amount of BPI released in the lung fluid. However, BPI levels from sputum supernatants correlate only partially with cell numbers, and we could also show (Fig. 4) that BPI as well as IL-8 is released from PMNs after coincubation with P. aeruginosa. The release of BPI from neutrophils of CF patients was not altered in a limited number of samples tested so far (data not shown). In addition, the presence of very high concentrations of cell-free BPI in the airways of CF patients also argues against a defect of BPI release as a consequence of the chloride channel mutation.
BPI and IL-8 levels do not correlate with the P. aeruginosa status of the patients (nor with other pathogens often involved in CF lung infection, like Staphylococcus aureus or Candida albicans), suggesting that additional mechanisms triggering the release of effector proteins from granules are involved. BPI-specific ANCA, which are found in up to 90% of CF patients, might also play a role because ANCA are able to activate neutrophils, causing respiratory burst and degranulation (21, 24). It has also been shown that the release of superoxide is due to cross-linking of ANCA-antigens (14). It was previously reported that BPI mRNA occurs early in PMN development, consistent with the localization of BPI in primary (azurophil) granules (27). Our finding that the release of BPI, in contrast to IL-8, is independent of de novo protein synthesis, together with the fact that we were able to detect only low copy numbers of BPI mRNA, both in PMNs isolated from whole blood (Fig. 4) and in sputum-derived cells, suggests that the regulation of BPI on a transcriptional level after P. aeruginosa stimulation plays a minor role.
The ability to form a biofilm seems to be an important virulence factor for P. aeruginosa to establish chronic infection (11, 20). In CF, P. aeruginosa strains with mucoid phenotypes and the ability to form biofilms cause major therapeutic problems because of their lower susceptibilities to antibiotic treatment. Here we show that BPI is also able to kill P. aeruginosa strains that are encapsulated by alginate; moreover, BPI was also bactericidal against P. aeruginosa strains that were multiresistant or even completely resistant to all antibiotics used in P. aeruginosa treatment.
In contrast to our findings with several independent clinical isolates from CF patients in a previous study, other authors observed nearly no activity of BPI against P. aeruginosa (25). This may be due to (i) the preparation of BPI used in that study, (ii) the experimental setting of the killing assay, or (iii) the particular strain of Pseudomonas. Our data suggest that BPI, which is released from PMNs into lung fluid, should be able to eliminate invading P. aeruginosa. The concentrations that were reached in lung fluid from CF patients (Fig. 3) are in the range of those used in in vitro experiments (Fig. 5 and 6). The dose range of BPI-mediated Pseudomonas killing is similar to that reported for other gram-negative rods. However, ANCA specific for BPI may interfere with the antibiotic activity of BPI, as already shown in vitro (22, 24), leading to conditions that enable P. aeruginosa to form biofilms. Once a robust biofilm is established, the bacteria become very hard to treat with antibiotics and are additionally sheltered from attack by the immune system. Whether BPI is also able to efficiently kill these bacteria remains to be elucidated. Further analysis needs to be established to determine whether BPI or antimicrobial peptides derived from BPI are applicable in the treatment of P. aeruginosa lung infections during CF, which might open a new possibility in the management of this disease. In a proportion of CF patients, BPI-ANCA do recognize only the C-terminal portion of BPI (22), suggesting that the BPI peptide used in our experiments, which is part of the N-terminal portion of BPI, might not be recognized by BPI-ANCA of all patients. This indicates that small, BPI-derived peptides might be an additional therapeutic agent to treat P. aeruginosa lung infection in CF patients if therapy is applied as early as P. aeruginosa is detected and before biofilms can be established.
Acknowledgments
We thank Andrea Debus, Claudia Gießler, and Rimma Husch for excellent technical assistance.
This work was supported by IZKF Erlangen grant A2, DFG grant SCHN635/2, and the Mukoviszidose e.V.
Editor: J. N. Weiser
REFERENCES
- 1.Aebi, C., F. Theiler, C. C. Aebischer, and M. H. Schoeni. 2000. Autoantibodies directed against bactericidal/permeability-increasing protein in patients with cystic fibrosis: association with microbial respiratory tract colonization. Pediatr. Infect. Dis. J. 19:207-212. [DOI] [PubMed] [Google Scholar]
- 2.Calafat, J., H. Janssen, E. F. Knol, J. Malm, and A. Egesten. 2000. The bactericidal/permeability-increasing protein (BPI) is membrane-associated in azurophil granules of human neutrophils, and relocation occurs upon cellular activation. APMIS 108:201-208. [DOI] [PubMed] [Google Scholar]
- 3.Canny, G., O. Levy, G. T. Furuta, S. Narravula-Alipati, R. B. Sisson, C. N. Serhan, and S. P. Colgan. 2002. Lipid mediator-induced expression of bactericidal/permeability-increasing protein (BPI) in human mucosal epithelia. Proc. Natl. Acad. Sci. USA 99:3902-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlsson, M., L. Eriksson, I. Erwander, J. Wieslander, and M. Segelmark. 2003. Pseudomonas-induced lung damage in cystic fibrosis correlates to bactericidal-permeability increasing protein (BPI)-autoantibodies. Clin. Exp. Rheumatol. 21:S95-100. [PubMed] [Google Scholar]
- 5.Ciofu, O., B. Riis, T. Pressler, H. E. Poulsen, and N. Hoiby. 2005. Occurrence of hypermutable Pseudomonas aeruginosa in cystic fibrosis patients is associated with the oxidative stress caused by chronic lung inflammation. Antimicrob. Agents Chemother. 49:2276-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorlochter, L., M. Carlsson, E. J. Olafsdottir, O. D. Roksund, K. Rosendahl, and G. Fluge. 2004. Anti-neutrophil cytoplasmatic antibodies and lung disease in cystic fibrosis. J. Cyst. Fibros. 3:179-183. [DOI] [PubMed] [Google Scholar]
- 7.Eckert, M., I. Wittmann, M. Rollinghoff, A. Gessner, and M. Schnare. 2006. Endotoxin-induced expression of murine bactericidal permeability/increasing protein is mediated exclusively by Toll/IL-1 receptor domain-containing adaptor inducing IFN-β-dependent pathways. J. Immunol. 176:522-528. [DOI] [PubMed] [Google Scholar]
- 8.Elsbach, P. 1998. The bactericidal/permeability-increasing protein (BPI) in antibacterial host defense. J. Leukoc. Biol. 64:14-18. [DOI] [PubMed] [Google Scholar]
- 9.Elsbach, P., and J. Weiss. 1998. Role of the bactericidal/permeability-increasing protein in host defence. Curr. Opin. Immunol. 10:45-49. [DOI] [PubMed] [Google Scholar]
- 10.Govan, J. R., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoiby, N. 2002. Understanding bacterial biofilms in patients with cystic fibrosis: current and innovative approaches to potential therapies. J. Cyst. Fibros. 1:249-254. [DOI] [PubMed] [Google Scholar]
- 12.Hoiby, N., H. Krogh Johansen, C. Moser, Z. Song, O. Ciofu, and A. Kharazmi. 2001. Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect. 3:23-35. [DOI] [PubMed] [Google Scholar]
- 13.Hubeau, C., M. Lorenzato, J. P. Couetil, D. Hubert, D. Dusser, E. Puchelle, and D. Gaillard. 2001. Quantitative analysis of inflammatory cells infiltrating the cystic fibrosis airway mucosa. Clin. Exp. Immunol. 124:69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kettritz, R., J. C. Jennette, and R. J. Falk. 1997. Crosslinking of ANCA-antigens stimulates superoxide release by human neutrophils. J. Am. Soc. Nephrol. 8:386-394. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi, H. 2005. Airway biofilms: implications for pathogenesis and therapy of respiratory tract infections. Treat. Respir. Med. 4:241-253. [DOI] [PubMed] [Google Scholar]
- 16.Krauss, R. D., and T. A. Rado. 1989. Current approaches to the molecular and physiological basis of cystic fibrosis. Am. J. Med. Sci. 298:334-341. [DOI] [PubMed] [Google Scholar]
- 17.Little, R. G., D. N. Kelner, E. Lim, D. J. Burke, and P. J. Conlon. 1994. Functional domains of recombinant bactericidal/permeability increasing protein (rBPI23). J. Biol. Chem. 269:1865-1872. [PubMed] [Google Scholar]
- 18.Macia, M. D., D. Blanquer, B. Togores, J. Sauleda, J. L. Perez, and A. Oliver. 2005. Hypermutation is a key factor in development of multiple-antimicrobial resistance in Pseudomonas aeruginosa strains causing chronic lung infections. Antimicrob. Agents Chemother. 49:3382-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliver, A., R. Canton, P. Campo, F. Baquero, and J. Blazquez. 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288:1251-1254. [DOI] [PubMed] [Google Scholar]
- 20.Rogan, M. P., C. C. Taggart, C. M. Greene, P. G. Murphy, S. J. O'Neill, and N. G. McElvaney. 2004. Loss of microbicidal activity and increased formation of biofilm due to decreased lactoferrin activity in patients with cystic fibrosis. J. Infect. Dis. 190:1245-1253. [DOI] [PubMed] [Google Scholar]
- 21.Schultz, H., E. Csernok, A. Schuster, T. S. Schmitz, M. Ernst, and W. L. Gross. 2000. Anti-neutrophil cytoplasmic antibodies directed against the bactericidal/permeability-increasing protein (BPI) in pediatric cystic fibrosis patients do not recognize N-terminal regions important for the anti-microbial and lipopolysaccharide-binding activity of BPI. Pediatr. Allergy Immunol. 11:64-70. [DOI] [PubMed] [Google Scholar]
- 22.Schultz, H., S. Schinke, K. Mosler, K. Herlyn, A. Schuster, and W. L. Gross. 2004. BPI-ANCA of pediatric cystic fibrosis patients can impair BPI-mediated killing of E. coli DH5α in vitro. Pediatr. Pulmonol. 37:158-164. [DOI] [PubMed] [Google Scholar]
- 23.Schultz, H., J. Weiss, S. F. Carroll, and W. L. Gross. 2001. The endotoxin-binding bactericidal/permeability-increasing protein (BPI): a target antigen of autoantibodies. J. Leukoc. Biol. 69:505-512. [PubMed] [Google Scholar]
- 24.Sediva, A., J. Bartunkova, J. Bartosova, C. Jennette, R. J. Falk, and H. S. Jethwa. 2003. Antineutrophil cytoplasmic antibodies directed against bactericidal/permeability-increasing protein detected in children with cystic fibrosis inhibit neutrophil-mediated killing of Pseudomonas aeruginosa. Microbes Infect. 5:27-30. [DOI] [PubMed] [Google Scholar]
- 25.Weiss, J., P. Elsbach, C. Shu, J. Castillo, L. Grinna, A. Horwitz, and G. Theofan. 1992. Human bactericidal/permeability-increasing protein and a recombinant NH2-terminal fragment cause killing of serum-resistant gram-negative bacteria in whole blood and inhibit tumor necrosis factor release induced by the bacteria. J. Clin. Investig. 90:1122-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss, J., and I. Olsson. 1987. Cellular and subcellular localization of the bactericidal/permeability-increasing protein of neutrophils. Blood 69:652-659. [PubMed] [Google Scholar]
- 27.Zarember, K., P. Elsbach, K. Shin-Kim, and J. Weiss. 1997. p15s (15-kD antimicrobial proteins) are stored in the secondary granules of rabbit granulocytes: implications for antibacterial synergy with the bactericidal/permeability-increasing protein in inflammatory fluids. Blood 89:672-679. [PubMed] [Google Scholar]
- 28.Zhao, M. H., D. R. Jayne, L. G. Ardiles, F. Culley, M. E. Hodson, and C. M. Lockwood. 1996. Autoantibodies against bactericidal/permeability-increasing protein in patients with cystic fibrosis. QJM 89:259-265. [DOI] [PubMed] [Google Scholar]