Abstract
The development of novel vaccine strategies supplementing Mycobacterium bovis BCG (BCG) constitutes an urgent research challenge. To identify potential subunit vaccine candidates, we have tested a series of eight recently identified Mycobacterium tuberculosis antigens in M. bovis-infected and BCG-vaccinated cattle. These antigens were characterized on the basis of their ability to induce in vitro gamma interferon responses in infected or BCG-vaccinated calves. We were able to establish a hierarchy of these antigens based on how frequently they were recognized in both groups of animals. In particular, we were able to prioritize frequently recognized proteins like Rv0287, Rv1174, and Rv1196 for future evaluation as subunit vaccines to be used in BCG-protein heterologous prime-boost vaccination scenarios. In addition, the antigen most dominantly recognized in M. bovis-infected cattle in this study, Rv3616c, was significantly less frequently recognized by BCG vaccinees and could be a target to improve BCG, for example, by increasing its secretion, in a recombinant BCG vaccine.
More than 50 million cattle are infected with Mycobacterium bovis, resulting in economic losses of approximately $3 billion annually (42). Current tuberculosis control in cattle is based on the tuberculin skin test to identify infected animals and the subsequent slaughter of tuberculin-positive animals. This has dramatically reduced tuberculosis in cattle in countries where such test-and-slaughter strategies have been implemented. However, attempts to eradicate the disease have not been equally and universally successful, especially in countries with M. bovis wildlife reservoirs, like Great Britain (29), Ireland, and New Zealand. The urgency for new and improved cattle vaccines and diagnostic reagents has been highlighted in an independent scientific review of the situation in Great Britain (29) and this conclusion has been confirmed in the report of a recent advisory study group (28). As cattle can be considered a large animal model for human tuberculosis, vaccination experiments with cattle will also be of direct interest to the human tuberculosis (TB) vaccine development program (23).
M. bovis bacillus Calmette-Guérin (BCG) has displayed variable efficacy both in humans and in cattle, and therefore improving its efficacy is a research priority (18, 23). For example, heterologous prime-boost strategies combining DNA vaccines, proteins, BCG, or live attenuated viruses have improved the efficacy of vaccination against TB (17, 30, 31). The results have been highly encouraging, both in augmenting and in modulating vaccine-induced immunity. Moreover, application of recombinant attenuated viruses such as modified vaccinia Ankara strain expressing the mycobacterial protective antigen Ag85A (Rv3804c) in a prime-boost scenario together with BCG has shown promise as a vaccine against human tuberculosis (22, 32). Recently, it was also shown that heterologous prime-boost protocols based on DNA (40) or protein subunit vaccination (47) of cattle in combination with BCG induced superior protection compared to BCG vaccination alone. However, only a limited number of protective protein subunit antigens (for example, Ag85A) that boost and complement BCG-induced immune responses have been defined for cattle (or humans), and there is a need to identify more such antigens. In particular, antigens that are recognized by BCG-vaccinated and M. bovis-infected cattle could constitute promising candidates for use as subunit vaccines to boost BCG. It is with this objective in mind that the present study was undertaken.
The antigens tested in this study consist of a set of antigens characterized by Corixa Corporation in a collaborative effort with GlaxoSmithKline Biologicals. Their strategy was to use peripheral blood mononuclear cells (PBMC) from healthy purified protein derivative-positive (PPD+) subjects with no history of TB, based on the working hypothesis that such donors were latently infected with Mycobacterium tuberculosis yet, due to active immune surveillance, were able to control infection. Thus, antigens recognized by T cells from this donor group could constitute protective antigens (11, 14, 15, 38, 39). Eight such antigens, found predominantly in bacterial cell lysates, culture filtrate, or both, were evaluated in the present study in BCG-vaccinated and M. bovis-infected cattle together with previously described antigens (ESAT-6, CFP-10, and Ag85B, i.e., Rv3875 and Rv1886c, respectively). A list of the antigens, including their Rv designations, functions, and characteristics, is found in Table 1. Although precise immune surrogates of protection await definition, experiments conducted over the last 5 to 10 years with cattle demonstrated that vaccinated animals were characterized by gamma interferon (IFN-γ) in trials where significant protection against M. bovis challenge was observed (23, 43). Therefore, we concentrated on assessing cellular immune responses and, in particular, IFN-γ production after in vitro antigen stimulation. By the nature of the antigens used in this study (recombinant proteins), we address mainly responses of CD4+ T cells, which constitute the main T-cell subpopulation during infection with M. bovis, although it has to be acknowledged that CD8+ T cells as well as δγ TCR+ cells also play a role in antituberculous immunity in cattle (35, 36). Our results have prioritized a number of the antigens tested for further evaluation as subunit vaccine candidates, in particular Rv0287 and Rv3616c.
TABLE 1.
Recombinant antigens used in this study
| Rv designationa | Mb designationb | Synonym(s) | Size (aa) | Function/characteristicc | Rank ind:
|
Reference(s) | |
|---|---|---|---|---|---|---|---|
| Humans | Cattle | ||||||
| Rv0125 | Mb0130 | Mtb32a | 355 | Probable serine protease (C>L) | 8 | 6 | 38 |
| Rv0287 | Mb0295 | Mtb9.8, EsxG | 97 | ESAT-6-like protein (ND) | 1 | 1 | 1 |
| Rv0915c | Mb0939c | PPE14, Mtb41 | 423 | PPE family protein (ND) | 6 | 5 | 39 |
| Rv1174c | Mb1207c | Mtb8.4 | 110 | Secreted protein (C) | 7 | 3 | 11 |
| Rv1196 | Mb1228 | PPE18, Mtb39a | 391 | PPE family protein (L) | 3 | 2 | 15 |
| Rv1793 | Mb1821 | Mtb9.9A, EsxN | 94 | ESAT-6-like protein (L>C) | 5 | 7 | 2 |
| Rv1886c | Mb1918c | Ag85B, MPT59 | 325 | Secreted, mycolyl transferase (C>L) | 4 | 4 | 1, 3 |
| Rv2945c | Mb2970c | Lppx | 233 | Lipoprotein (ND) | NA | NA | 6 |
| Rv3616c | Mb3646c | 292 | A+G-rich protein, unknown function (ND) | 2 | 1 | 1, 19 | |
| Rv3874 | Mb3904 | CFP-10, EsxB | 100 | ESAT-6-like protein (C>L) | NA | NA | 3, 41 |
| Rv3875 | Mb3905 | ESAT-6, EsxA | 95 | ESAT-6-like protein (C>L) | NA | NA | 5 |
Designations of M. tuberculosis H37Rv proteins are according to those described by Cole et al. (10).
Corresponding designations of M. bovis AF2122/97 proteins are according to those described by Garnier et al. (21).
Protein function or functional characteristic, with subcellular location in parentheses: L, found in bacterial lysates; C, in culture filtrates; ND, not determined.
Immunogenicity ranking according to IFN-γ responder frequencies as described for M. tuberculosis-infected humans (1) or M. bovis-infected cattle (this study). 1= most frequently recognized. NA, not applicable.
MATERIALS AND METHODS
Experimental infection.
Approximately 6-month-old calves (Holstein or Holstein crosses, n = 21) were obtained from herds free of bovine tuberculosis and kept in the category 3 biosafety accommodation of the Animal Services Unit, Veterinary Laboratories Agency-Weybridge. Animals from different herds were used in this study. Calves were infected with an M. bovis field strain from Great Britain (AF 2122/97) by intratracheal instillation of between 1 × 102 and 1 × 103 CFU, as described previously (44). At 18 to 20 weeks postinfection, single intradermal comparative cervical tuberculin tests were performed as specified in European Economic Community (EEC) Directive 80/219EEC, amending directive 64/422/EEC, Annex B, and all animals tested positive for bovine tuberculosis. Their infection status was finally confirmed at postmortem examinations performed 20 to 22 weeks postinfection, where all presented with visible lesions typical of bovine tuberculosis, in the lymph nodes of the head and lung regions as well as in the lung itself. In addition, we were able to culture M. bovis from tissue samples collected during the postmortem (data not shown). Heparinized blood samples were obtained at 12 to 16 weeks after infection but before tuberculin skin tests were performed, when strong and sustained in vitro tuberculin responses were observed. In addition, five skin-test-positive naturally infected animals were also tested. Bovine tuberculosis in these cows was confirmed by culture of M. bovis from tissue samples.
BCG vaccination.
A group of 10 calves (ca. 6 months old, Holsteins) were vaccinated with BCG Pasteur by subcutaneous injection in the side of the neck of 106 CFU suspended in 1 ml of phosphate-buffered saline (44). Blood samples were taken at 6 to 8 weeks postvaccination, during the peak of tuberculin responses in vaccinated calves (44). Animals were skin tested with the single intradermal comparative cervical tuberculin test 8 weeks after the vaccine injection. The skin tests were performed as specified in EEC Directive 80/219EEC, amending directive 64/422/EEC, Annex B.
Environmentally sensitized cattle (PPD-A responders).
A group of eight calves (6 to 8 months old, Holsteins) that responded to stimulation with avian PPD (PPD-A) but did not respond or responded only weakly after stimulation with bovine PPD (PPD-B) were also tested. Responses biased to PPD-A are indicative of the sensitization of these animals to environmental mycobacteria.
Antigens and peptides.
Bovine (PPD-B) and avian (PPD-A) tuberculins were obtained from the Tuberculin Production Unit at the Veterinary Laboratories Agency-Weybridge and used in culture at 10 μg/ml. Recombinant ESAT-6 (Rv3875) and CFP-10 (Rv3874) were a donation from M. Singh (Gesellschaft fur Biotechnologische Forschung, Braunschweig, Germany).
The other nine recombinant proteins assessed in this study were prepared as described previously in the references listed in Table 1 (1). Briefly, the full-length open reading frames of the cloned genes were amplified by PCR followed by subcloning into the pET17b expression vectors to express the recombinant antigens, Escherichia coli BL-21 (pLysE) was transformed, and the recombinant proteins were purified by affinity chromatography. The yield of purified protein varied from 10 to 75 mg/liter of bacterial culture, with >98% purity. Endotoxin levels were typically <10 endotoxin units/mg protein. Recombinant proteins were used in the in vitro assays described below at a concentration of 5 μg/ml.
Proportions of the responses to Rv3616c were assessed using a pool of 15 peptides (Pepscan, Lelystad, The Netherlands; purity [>90%] and sequence fidelity were established by analytical high-pressure liquid chromatography and mass spectrometry, respectively) predicted to contain bovine BoLA-DR binding epitopes (46). Peptides were used in this pool at a concentration of 5 mg/ml for each peptide. A subset of eight of the M. bovis-infected, four of the BCG-vaccinated, and eight PPD-A responder animals were tested with this peptide pool. The responses observed with peptides were comparable to those observed with the recombinant protein. For example, the differences in responder frequencies between BCG-vaccinated and M. bovis-infected cattle were of the same magnitude when peptide- and protein-induced responses were compared (data not shown). Therefore, results obtained with proteins and peptides were combined in the analysis of responses. Peptide sequences were as follows: 3616.1, MSRAFIIDPTISAIDG; 3616.2, GLYDLLGIGIPNQGGI; 3616.3, GGILYSSLEYFEKALEEL; 3616.4, ADKYAGKNRNHVNFF; 3616.5, ISLIHDQANAVQTT; 3616.6, GLEFVRPVAVDLTYI; 3616.7, VDLTYIPVVGHALSAAFQ; 3616.10, AELVAAAIADIISDVADIIKG; 3616.11, LGEVWEFITNALNGL; 3616.12, SRGWSNLESFFAGV; 3616.13, TGLFGAAGLSASSG; 3616.14, SSGLAHADSLASSAS; 3616.15, SGFGGLPSLAQVHA; 3616.16, AEQVGGQSQLVSAQGSQGMG; and 3616.17, GGPVGMGGMHPSSGAS.
Ex vivo gamma interferon ELISPOT assay.
PBMC were isolated from heparinized blood by Histopaque-1077 (Sigma) gradient centrifugation and cultured immediately in RPMI 1640 tissue culture medium (Life Technologies, Paisley, Scotland, United Kingdom) supplemented with 5% controlled process serum replacement type 1 (Sigma-Aldrich, Poole, United Kingdom), nonessential amino acids (Sigma-Aldrich), 5 × 10−5 M 2-mercaptoethanol, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate. Ex vivo (direct) enzyme-linked immunospots (ELISPOTs) were enumerated as described earlier (44). Briefly, ELISPOT plates (Immobilon-P polyvinylidene difluoride membranes; Millipore, Molsheim, France) were coated overnight at 4°C with the bovine IFN-γ-specific monoclonal antibody 5D10 (BioSource, Wheatley, United Kingdom). Unbound antibody was removed by washing, and the wells were blocked with 10% fetal calf serum in RPMI 1640 medium. PBMC suspended in tissue culture medium (RPMI 1640 supplemented with 5% controlled process serum replacement type 1) were then added (2 × 105 PBMC/well) and cultured in the presence of PPD-B or recombinant proteins for 24 h at 37°C in a humidified 5% CO2 incubator. Spots were developed with rabbit serum specific for IFN-γ prepared at the Veterinary Laboratories Agency, followed by incubation with an alkaline phosphatase-conjugated monoclonal antibody specific for rabbit immunoglobulin G (Sigma-Aldrich). The spot-forming cells (SFC) were visualized with BCIP-NBT substrate (5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium; Sigma-Aldrich). A positive response was greater than the mean of the medium control plus 6.3137 standard errors of the mean (SEM) (90% confidence interval of the mean [33]).
Whole-blood IFN-γ assay (BOVIGAM assay).
Blood samples were collected from vaccinated or Mycobacterium bovis-infected cattle into heparinized Vacutainers (Becton Dickinson, Oxford, United Kingdom). The BOVIGAM assay (48) was performed according to the manufacturer's instructions (BIOCORE AH, Omaha, NE). Briefly, whole-blood cultures (0.2 ml) were performed in 96-well tissue culture plates. Cultures contained 0.10 ml heparinized blood and 0.1 ml RPMI 1640 (no antigen), tuberculin, or recombinant antigens. After 24 h culture at 37°C, plasma supernatants (100 μl/well) were transferred to a 96-well plate using a sterile pipette and stored at −20°C until tested by IFN-γ enzyme-linked immunosorbent assay (ELISA). BOVIGAM IFN-γ ELISA was performed according to the supplier's instructions. Color change in the ELISA reaction was measured as optical density at 450 nm.
Statistical analysis.
Statistical analysis was performed using Instat v 3.0a (GraphPad, San Diego, CA). Differences in the responder frequencies between the two animal groups were assessed by applying Fisher's exact test. Differences between the SFC/106 cells observed in samples from M. bovis-infected and BCG-vaccinated cattle or M. bovis-infected and PPD-A responder cattle were assessed with the two-tailed, unpaired Mann-Whitney test, with significance levels set at a P value of <0.05. Cutoff values for positivity were determined for each individual animal by calculating the 90% confidence interval of the mean IFN-γ responses (SFC/106 cells) using the following formula: mean of medium control + 6.3137 SEM (33).
RESULTS
Antigen-induced IFN-γ responses in M. bovis-infected and BCG-vaccinated cattle.
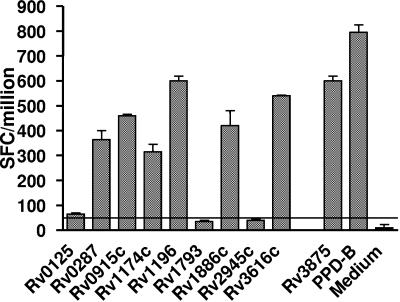
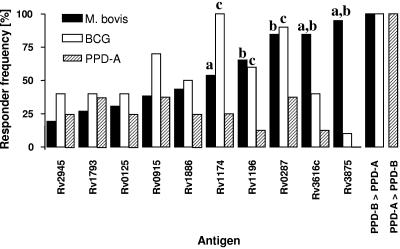
Three groups of cattle were included in this study: (i) 26 cattle infected with M. bovis (21 experimentally infected and 5 naturally infected field reactors), (ii) 10 cattle vaccinated with BCG, and (iii) 8 cattle from herds without a history of bovine TB, but naturally sensitized by environmental mycobacteria, as indicated by IFN-γ responses biased towards PPD-A (PPD-A responders). Disease was confirmed in all M. bovis-infected animals by the presence of gross and microscopic lesions as well as by culture of M. bovis from tissues collected at postmortem (data not shown). All 26 infected animals also presented as tuberculin skin test positive, with stronger PPD-B responses than PPD-A (data not shown). No significant differences in disease severity were observed between animals infected with different infective doses (M. bovis, 100 to 1,000 CFU/animal) (12). PBMC were prepared from these animals, and the in vitro responses after stimulation with the recombinant antigens listed in Table 1, as well as after stimulation with PPD-B, were determined by ex vivo ELISPOT analysis. Figure 1 gives the results of a representative M. bovis-infected animal. As shown in Fig. 1, PBMC from the infected calf responded strongly to Rv3616c, Rv1196, Rv1886c, Rv0287, Rv0915c, and Rv1174c, as well as to ESAT-6 (Rv3875) and bovine tuberculin PPD-B. Similar data were obtained from the other infected, vaccinated, or environmentally sensitized (PPD-A responder) animals that were analyzed identically. The results allowed us to group the antigens tested in a hierarchy based on the responses of M. bovis-infected cows (Fig. 2). The most frequently recognized antigens from the set of proteins prepared by Corixa by PBMC from M. bovis-infected animals were Rv3616c, Rv0287, Rv1174c, and Rv1196 (recognized by 84.6% [22/26], 84.6% [22/26], 65.4% [17/26], and 53.8% [14/26] of infected calves, respectively). As expected, ESAT-6 was also frequently recognized, by 95% of infected animals, while the other antigens were recognized by between 19.2% and 43.5% of the animals tested (Fig. 2). PBMC from BCG-vaccinated calves responded in a response hierarchy comparable to those infected with M. bovis, with Rv1174, Rv0287, Rv0915, and Rv1196 as the most frequently recognized antigens (recognized by 100% [10/10], 90% [9/10], 70% [7/10], and 60% [6/10], respectively). Interestingly, Rv1174 was more frequently recognized by T cells from BCG-vaccinated animals than from M. bovis-infected cattle (P = 0.015, Fisher's exact test). In contrast, Rv3616c was significantly less frequently recognized by BCG-vaccinated animals (40% [4/10], P = 0.04, Fisher's exact test) (Fig. 2) than infected cattle. Only 1/10 of BCG-vaccinated animals responded weakly to ESAT-6 (Rv3875), while all BCG-vaccinated or M. bovis-infected cattle responded more strongly to stimulation with PPD-B than to stimulation with PPD-A (Fig. 1). With the exception of ESAT-6, the antigens tested were also recognized by PPD-A responder animals tested, albeit at lower frequencies (12.5 to 37.5%) (Fig. 2). The differences in response frequencies of PPD-A responders reached statistical significance in the cases of Rv0287, Rv1174, and Rv1196 (P < 0.05, compared to BCG-vaccinated animals) and Rv1196 and Rv3616c (P < 0.05, compared to M. bovis-infected cattle) (Fig. 2). No responses to ESAT-6 were observed in PPD-A responder cattle, while all responded more strongly to PPD-A than to PPD-B (Fig. 2). These results were confirmed when a proportion of the animals was tested using the whole-blood BOVIGAM IFN-γ enzyme immunoassay test (data not shown).
FIG. 1.
Recognition of mycobacterial antigens by PBMC isolated from an M. bovis-infected calf. PBMC were cultured in the presence of recombinant proteins at 5 μg/ml and IFN-γ responses determined by ex vivo ELISPOT assay. Data are presented as SFC/106 PBMC ± SEM. Horizontal lines indicate cutoff values for positivity (mean SFC/106 with protein > mean SFC/106 of medium controls ± 6.3137 SEM [33]).
FIG. 2.
Response hierarchy of mycobacterial antigens when tested in M. bovis-infected or BCG-vaccinated calves. The antigens tested in this study were grouped according to their IFN-γ responder frequencies (proportion, in percent, of animals tested that responded to an antigen) established by ex vivo ELISPOT analysis of M. bovis-infected calves (n = 26; Rv1886c, n = 22), BCG-vaccinated calves (n = 10), and PPD-A responders (n = 8). Animals were defined as responders when mean SFC/106 with protein was greater than mean SFC/106 of medium controls ± 6.3137 (33). Statistical significance (P < 0.05, Fisher's exact test): a, M. bovis versus BCG; b, M. bovis versus PPD-A; c, BCG versus PPD-A responders.
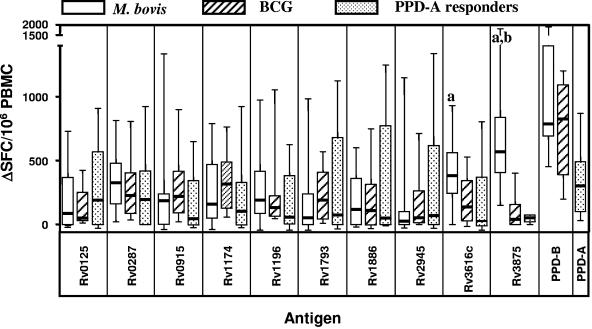
These observations were extended by assessing the strength of IFN-γ responses (represented as SFC/106 PBMC) (Fig. 3). Confirming the results discussed in the previous paragraph of the antigens obtained from Corixa, Rv3616c induced the strongest responses in M. bovis-infected cattle (median response, 385 SFC/106 PBMC), followed by Rv0287 (median response, 330 SFC/106 PBMC) and Rv1196 (median response, 195 SFC/106 PBMC). ESAT-6 (Rv3875) was also strongly recognized (median response, 570 SFC/106 PBMC) (Fig. 3). The responses of BCG-vaccinated animals were, for the majority of antigens tested, of similar magnitude compared to those determined with PBMC from M. bovis-infected animals, and no statistically significant differences in signal strengths were observed for any of the Corixa-derived antigens except Rv3616c (Fig. 3), although BCG responses to Rv1174 and Rv1793 gave higher median values than those observed in M. bovis-infected animals (e.g., for Rv1174: BCG, 320 SFC/106 PBMC; M. bovis, 160 SFC/106 PBMC; not significant) (Fig. 3). In contrast, statistically significantly fewer IFN-γ-producing cells were detected after stimulation of PBMC from BCG-vaccinated calves with Rv3616c than M. bovis-infected calves (median M. bovis, 385 SFC/106 PBMC; median BCG, 176 SFC/106 PBMC; P = 0.024) (Fig. 3), which confirmed the results shown in Fig. 2. As expected, ESAT-6-induced responses were significantly lower in BCG-vaccinated cows (median M. bovis, 570 SFC/106 PBMC; median BCG, 40 SFC/106 PBMC; P < 0.0001) (Fig. 3).
FIG. 3.
Strength of IFN-γ responses after antigen stimulation. The numbers of SFC/106 PBMC responding to antigenic stimulation are presented as a bar and whisker plot. The same animals described in the legend to Fig. 2 were assessed. Data are expressed as ΔSFC/106 PBMC (i.e., background values were subtracted). Statistical significance (unpaired, two-tailed t test, P < 0.025): a, M. bovis versus BCG; b, M. bovis versus PPD-A responders.
As predicted from the lower response frequencies observed in PPD-A responder animals to the antigens tested, we also observed lower numbers of SFC/106 PBMC following antigen stimulation than those observed in M. bovis-infected or BCG-vaccinated animals, with the exception of Rv0125, which presented with higher median values in PPD-A responders than both of the other groups (median PPD-A, 190 SFC/106 PBMC; M. bovis, 65 SFC/106 PBMC; BCG, 50 SFC/106 PBMC), although this difference was not statistically significant. No responses were observed after stimulation with ESAT-6 (Rv3875; median PPD-A, 55 SFC/106 PBMC compared to median M. bovis, 570 SFC/106 PBMC; P < 0.0001) (Fig. 3).
DISCUSSION
This study has prioritized a number of novel M. tuberculosis antigens for further evaluation as subunit vaccines against bovine TB in cattle. Efforts to improve on BCG have focused on two areas. First, heterologous prime-boost strategies applying BCG and subunit vaccines (e.g., DNA, protein subunits, or recombinant viruses like modified vaccinia virus Ankara or adenovirus) that are aimed to boost the strength of BCG-induced immunity are being considered (8, 31). Delivering the boosting subunit vaccine in a heterologous vector is aimed at not only increasing the quantity of the immune responses, but also modifying its quality. For example, administration of the mycobacterial protein Ag85A via recombinant modified vaccinia virus Ankara induced stronger CD8+ T-cell responses than BCG vaccination alone (22) and also increased the number of epitopes that were recognized by CD4+ T cells, thus significantly widening the T-cell repertoire (45). A prerequisite for the selection of such subunit antigens to function in this scenario is that the antigens are immunogenic not only after M. bovis infection, but also after BCG vaccination, so that the subunit is capable of boosting BCG-primed responses. This study has clearly highlighted the antigens Rv0287, Rv1174c, and Rv1196 as potential subunit vaccine candidates to be evaluated in such heterologous prime-boost scenarios. In a proof-of-principle experiment, we have recently shown that boosting BCG with protein subunits (in the form of a culture filtrate protein preparation) prepared in an adjuvant formulation containing bovine-optimized CpG oligonucleotides protected cattle better against bovine TB than BCG alone (47). The three antigens characterized in the present study could be used to move from a largely undefined antigen preparation toward a subunit vaccine containing defined antigen units.
We detected responses to the majority of these antigens also in cattle that were sensitized with environmental mycobacteria (PPD-A responders). As homologous sequence regions to most M. bovis proteins can be found readily in mycobacterial genomes not belonging to the M. tuberculosis complex (16), immunological cross-reactivity is not surprising. However, the antigens tested were generally recognized at lower frequencies and signal strengths than those observed in M. bovis-infected or BCG-vaccinated animals, indicating that infection or vaccination was able to induce immune responses to these antigens.
Rv0287 is an ESAT-6-like protein and, like other members of this protein family (34), has been shown in this study to be highly immunogenic. In contrast to ESAT-6 and CFP-10, its gene is present in the genome of BCG, and it is therefore not a suitable target for diagnosis of infection (4, 10). Rv0287 is therefore comparable to other protective antigens of the ESAT-6 family like Rv0288, which was recently shown to protect mice against M. tuberculosis when applied as a fusion protein with Ag85A (13).
Rv1196, a member of the PPE protein family (PPE18), (10) is of particular interest because it is part of the fusion protein vaccine Mtb72F that has been shown to protect mice and guinea pigs against aerosol challenge with M. tuberculosis (37). Furthermore, vaccination of guinea pigs as an adjunct to BCG vaccination, given at the same time as BCG or as a BCG-prime, heterologous protein boost protocol, significantly improved survival times after M. tuberculous infection (7). This fusion protein vaccine is currently in phase 1 clinical trials. It is therefore encouraging that Rv1196 was well recognized by T cells from infected or BCG-vaccinated cattle, and our study supports the assessment of Mtb72F in cattle. The other antigen that is part of Mtb72F, Rv0125, was less well recognized than Rv1196 in the present study, although about one-third of the infected cattle tested did respond.
Rv1174c, originally designated as Mtb8.4 by Coler et al. (11), is a secreted protein that induced significant Th1-type immune responses in the blood of healthy PPD+ human donors. Furthermore, it was part of a hexavalent protein vaccine that protected mice against M. tuberculosis infection (27).
Another strategy to improve BCG protective efficacy involves the complementation of BCG by overexpressing antigens not expressed or expressed only at low levels by wild-type BCG (24). rBCG30 is the prototype of this class of vaccines, which utilize BCG Tice as a host organism for expressing and secreting Mycobacterium tuberculosis proteins. rBCG30 expresses and secretes large amounts of an M. tuberculosis 30-kDa major secretory protein (Ag85B/Rv1886c) (25, 26). Variation in the frequency and strength at which antigens are recognized by T cells from M. bovis-infected cattle and BCG-vaccinated animals has been reported for a number of antigens. For example, antigens like MPB83 or MPB70 are expressed in some BCG strains (e.g., BCG Pasteur) at very low levels due to a point mutation in the start codon of sigK in all low-producing BCG strains (9).
In the present study we found Rv3616c to be the most strongly and frequently recognized protein tested in infected cattle, while it was significantly less strongly and less frequently recognized after BCG vaccination. Interestingly, a recent study (20) has provided a possible explanation for this reduced immunogenicity of Rv3616c. It demonstrated that the secretion of Rv3616c in H37Rv was crucially dependent on the RD1-encoded secretion system that is deleted from the genome of BCG. Although not formally proven for BCG, one could speculate that it also cannot be secreted by BCG, which could account for its reduced immunogenicity in BCG-vaccinated cattle compared to animals infected with M. bovis. Thus, one could further hypothesize that by preparing a recombinant BCG secreting large amounts of Rv3616c, for example, by the addition of a conventional secretion signal peptide, one could improve BCG efficacy.
The cattle model of TB has been considered a model for human tuberculosis (23) as the antigen specificities of humans and cattle after tuberculous infection displaying a high degree of overlap in the respective antigen repertoires become apparent. As the same set of novel antigens whose responses were assessed in this study in cattle were recently also tested in human TB patients (1), it was opportune to compare the responses in the two host species. When we ranked responder frequencies in humans and cattle (Table 1), we found good agreement between the response rankings. Our results therefore further support the notion that bovine TB in cattle can be considered a useful model to identify antigens relevant in human TB.
In conclusion, we have tested a series of recently identified antigens in M. bovis-infected and BCG-vaccinated cattle with the objective of prioritizing potential subunit vaccine candidates on the basis of their recognition in both groups of animals. We have found three such candidates, Rv0287, Rv1174c, and Rv1196. In addition, the most dominant antigen in M. bovis-infected cattle, Rv3616c, was significantly less frequently recognized by BCG vaccines and could be a target to improve BCG by increasing its secretion in a recombinant BCG vaccine.
Acknowledgments
The study was supported by Kuwait University Research Administration grant MI02/02 and by the Department for Environment, Food and Rural Affairs, United Kingdom.
Editor: J. L. Flynn
REFERENCES
- 1.Al-Attiyah, R., A. S. Mustafa, A. T. Abal, A. S. El-Shamy, W. Dalemans, and Y. A. Skeiky. 2004. In vitro cellular immune responses to complex and newly defined recombinant antigens of Mycobacterium tuberculosis. Clin. Exp. Immunol. 138:139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderson, M. R., T. Bement, C. H. Day, L. Zhu, D. Molesh, Y. A. Skeiky, R. Coler, D. M. Lewinsohn, S. G. Reed, and D. C. Dillon. 2000. Expression cloning of an immunodominant family of Mycobacterium tuberculosis antigens using human CD4(+) T cells. J. Exp. Med. 191:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen, P., A. B. Andersen, A. L. Sorensen, and S. Nagai. 1995. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J. Immunol. 154:3359-3372. [PubMed] [Google Scholar]
- 4.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 5.Berthet, F. X., P. B. Rasmussen, I. Rosenkrands, P. Andersen, and B. Gicquel. 1998. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology 144:3195-3203. [DOI] [PubMed] [Google Scholar]
- 6.Betts, J. C., P. T. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717-731. [DOI] [PubMed] [Google Scholar]
- 7.Brandt, L., Y. A. W. Skeiky, M. R. Alderson, Y. Lobet, W. Dalemans, O. C. Turner, R. J. Basaraba, A. A. Izzo, T. M. Lasco, P. L. Chapman, S. G. Reed, and I. M. Orme. 2004. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect. Immun. 72:6622-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buddle, B. M., M. A. Skinner, D. N. Wedlock, G. W. de Lisle, H. M. Vordermeier, and R. G. Hewinson. 2005. Cattle as a model for development of vaccines against human tuberculosis. Tuberculosis 85:19-24. [DOI] [PubMed] [Google Scholar]
- 9.Charlet, D., S. Mostowy, D. Alexander, L. Sit, H. G. Wiker, and M. A. Behr. 2005. Reduced expression of antigenic proteins MPB70 and MPB83 in Mycobacterium bovis BCG strains due to a start codon mutation in sigK. Mol. Microbiol. 56:1302-1313. [DOI] [PubMed] [Google Scholar]
- 10.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 11.Coler, R. N., Y. A. Skeiky, T. Vedvick, T. Bement, P. Ovendale, A. Campos-Neto, M. R. Alderson, and S. G. Reed. 1998. Molecular cloning and immunologic reactivity of a novel low molecular mass antigen of Mycobacterium tuberculosis. J. Immunol. 161:2356-2364. [PubMed] [Google Scholar]
- 12.Dean, G. S., S. G. Rhodes, M. Coad, A. O. Whelan, P. J. Cockle, D. J. Clifford, R. G. Hewinson, and H. M. Vordermeier. 2005. Minimum infective dose of Mycobacterium bovis in cattle. Infect. Immun. 73:6467-6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietrich, J., C. Aagaard, R. Leah, A. W. Olsen, A. Stryhn, T. M. Doherty, and P. Andersen. 2005. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J. Immunol. 174:6332-6339. [DOI] [PubMed] [Google Scholar]
- 14.Dillon, D. C., M. R. Alderson, C. H. Day, T. Bement, A. Campos-Neto, Y. A. Skeiky, T. Vedvick, R. Badaro, S. G. Reed, and R. Houghton. 2000. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J. Clin. Microbiol. 38:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dillon, D. C., M. R. Alderson, C. H. Day, D. M. Lewinsohn, R. Coler, T. Bement, A. Campos-Neto, Y. A. Skeiky, I. M. Orme, A. Roberts, S. Steen, W. Dalemans, R. Badaro, and S. G. Reed. 1999. Molecular characterization and human T-cell responses to a member of a novel Mycobacterium tuberculosis mtb39 gene family. Infect. Immun. 67:2941-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ewer, K., P. Cockle, S. Gordon, H. Mansoor, M. Govaerts, K. Walravens, S. Marche, G. Hewinson, and M. Vordermeier. 2006. Antigen mining with iterative genome screens identifies novel diagnostics for the Mycobacterium tuberculosis complex. Clin. Vaccine Immunol. 13:90-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng, C. G., U. Palendira, C. Demangel, J. M. Spratt, A. S. Malin, and W. J. Britton. 2001. Priming by DNA immunization augments protective efficacy of Mycobacterium bovis bacille Calmette-Guerin against tuberculosis. Infect. Immun. 69:4174-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 19.Fisher, M. A., B. B. Plikaytis, and T. M. Shinnick. 2002. Microarray analysis of the Mycobacterium tuberculosis transcriptional response to the acidic conditions found in phagosomes. J. Bacteriol. 184:4025-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortune, S. M., A. Jaeger, D. A. Sarracino, M. R. Chase, C. M. Sassetti, D. R. Sherman, B. R. Bloom, and E. J. Rubin. 2005. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc. Natl. Acad. Sci. USA 102:10676-10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garnier, T., K. Eiglmeier, J. C. Camus, N. Medina, H. Mansoor, M. Pryor, S. Duthoy, S. Grondin, C. Lacroix, C. Monsempe, S. Simon, B. Harris, R. Atkin, J. Doggett, R. Mayes, L. Keating, P. R. Wheeler, J. Parkhill, B. G. Barrell, S. T. Cole, S. V. Gordon, and R. G. Hewinson. 2003. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. USA 100:7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goonetilleke, N. P., H. McShane, C. M. Hannan, R. J. Anderson, R. H. Brookes, and A. V. Hill. 2003. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J. Immunol. 171:1602-1609. [DOI] [PubMed] [Google Scholar]
- 23.Hewinson, R. G., H. M. Vordermeier, and B. M. Buddle. 2003. Use of the bovine model of tuberculosis for the development of improved vaccines and diagnostics. Tuberculosis 83:119-130. [DOI] [PubMed] [Google Scholar]
- 24.Horwitz, M. A. 2005. Recombinant BCG expressing Mycobacterium tuberculosis major extracellular proteins. Microbes Infect. 7:947-954. [DOI] [PubMed] [Google Scholar]
- 25.Horwitz, M. A., and G. Harth. 2003. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect. Immun. 71:1672-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz, M. A., G. Harth, B. J. Dillon, and S. Maslesa-Galic. 2000. Recombinant bacillus Calmette-Guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. USA 97:13853-13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hovav, A.-H., Y. Fishman, and H. Bercovier. 2005. Gamma interferon and monophosphoryl lipid A-trehalose dicorynomycolate are efficient adjuvants for Mycobacterium tuberculosis multivalent acellular vaccine. Infect. Immun. 73:250-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Independent Scientific Group on Cattle TB Vaccine Scoping Sub-Committee. 2003. Development of vaccines for bovine tuberculosis. Department for Environment, Food and Rural Affairs, London, United Kingdom.
- 29.Krebs, J. R. 1997. Bovine tuberculosis in cattle and badgers. Ministry of Agriculture, Fisheries and Food Publications, London, United Kingdom.
- 30.McShane, H., R. Brookes, S. C. Gilbert, and A. V. S. Hill. 2001. Enhanced immunogenicity of CD4+ T-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect. Immun. 69:681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McShane, H., A. A. Pathan, C. R. Sander, N. P. Goonetilleke, H. A. Fletcher, and A. V. Hill. 2005. Boosting BCG with MVA85A: the first candidate subunit vaccine for tuberculosis in clinical trials. Tuberculosis 85:47-52. [DOI] [PubMed] [Google Scholar]
- 32.McShane, H., A. A. Pathan, C. R. Sander, S. M. Keating, S. C. Gilbert, K. Huygen, H. A. Fletcher, and A. V. Hill. 2004. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 10:1240-1244. [DOI] [PubMed] [Google Scholar]
- 33.Motulsky, H. 1995. Intuitive biostatistics. Oxford University Press, Oxford, United Kingdom.
- 34.Pallen, M. J. 2002. The ESAT-6/WXG100 superfamily—and a new Gram-positive secretion system? Trends Microbiol. 10:209-212. [DOI] [PubMed] [Google Scholar]
- 35.Pollock, J. M., D. A. Pollock, D. G. Campbell, R. M. Girvin, A. D. Crockard, S. D. Neill, and D. P. Mackie. 1996. Dynamic changes in circulating and antigen-responsive T-cell subpopulations post-Mycobacterium bovis infection in cattle. Immunology 87:236-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhodes, S. G., R. G. Hewinson, and H. M. Vordermeier. 2001. Antigen recognition and immunomodulation by gamma delta T cells in bovine tuberculosis. J. Immunol. 166:5604-5610. [DOI] [PubMed] [Google Scholar]
- 37.Skeiky, Y. A., M. R. Alderson, P. J. Ovendale, J. A. Guderian, L. Brandt, D. C. Dillon, A. Campos-Neto, Y. Lobet, W. Dalemans, I. M. Orme, and S. G. Reed. 2004. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J. Immunol. 172:7618-7628. [DOI] [PubMed] [Google Scholar]
- 38.Skeiky, Y. A., M. J. Lodes, J. A. Guderian, R. Mohamath, T. Bement, M. R. Alderson, and S. G. Reed. 1999. Cloning, expression, and immunological evaluation of two putative secreted serine protease antigens of Mycobacterium tuberculosis. Infect. Immun. 67:3998-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skeiky, Y. A., P. J. Ovendale, S. Jen, M. R. Alderson, D. C. Dillon, S. Smith, C. B. Wilson, I. M. Orme, S. G. Reed, and A. Campos-Neto. 2000. T cell expression cloning of a Mycobacterium tuberculosis gene encoding a protective antigen associated with the early control of infection. J. Immunol. 165:7140-7149. [DOI] [PubMed] [Google Scholar]
- 40.Skinner, M., B. M. Buddle, N. Wedlock, D. Keen, G. W. de Lisle, R. E. Tascon, J. C. Ferraz, D. B. Lowrie, P. J. Cockle, H. M. Vordermeier, and R. G. Hewinson. 2003. A DNA prime-Mycobacterium bovis BCG boost vaccination strategy in cattle induces protection against bovine tuberculosis. Infect. Immun. 71:4901-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorensen, A. L., S. Nagai, G. Houen, P. Andersen, and A. B. Andersen. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steele, J. H. 1995. Regional and country status report, p. 169-172. In C. O. Thoen (ed.), Mycobacterium bovis infection in animals and humans. Iowa State University Press, Ames, Iowa.
- 43.Vordermeier, H. M., M. A. Chambers, B. M. Buddle, J. M. Pollock, and R. G. Hewinson. 2006. Progress in the development of vaccines and diagnostic reagents to control tuberculosis in cattle. Vet. J. 171:229-244. [DOI] [PubMed] [Google Scholar]
- 44.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Simmons, and R. G. Hewinson. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vordermeier, H. M., S. G. Rhodes, G. Dean, N. Goonetilleke, K. Huygen, A. V. Hill, R. G. Hewinson, and S. C. Gilbert. 2004. Cellular immune responses induced in cattle by heterologous prime-boost vaccination using recombinant viruses and bacille Calmette-Guerin. Immunology 112:461-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vordermeier, M., A. O. Whelan, and R. G. Hewinson. 2003. Recognition of mycobacterial epitopes by T cells across mammalian species and use of a program that predicts human HLA-DR binding peptides to predict bovine epitopes. Infect. Immun. 71:1980-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wedlock, D. N., M. Denis, M. A. Skinner, J. Koach, G. W. de Lisle, H. M. Vordermeier, R. G. Hewinson, S. van Drunen Littel-van den Hurk, L. A. Babiuk, R. Hecker, and B. M. Buddle. 2005. Vaccination of cattle with a CpG oligodeoxynucleotide-formulated mycobacterial protein vaccine and Mycobacterium bovis BCG induces levels of protection against bovine tuberculosis superior to those induced by vaccination with BCG alone. Infect. Immun. 73:3540-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood, P. R., and J. S. Rothel. 1994. In vitro immunodiagnostic assays for bovine tuberculosis. Vet. Microbiol. 40:125-135. [DOI] [PubMed] [Google Scholar]