Abstract
PVC-211 murine leukemia virus (MuLV) is a neuropathogenic variant of Friend MuLV (F-MuLV) which causes a rapidly progressive spongiform neurodegenerative disease in rodents. The primary target of PVC-211 MuLV infection in the brain is the brain capillary endothelial cell (BCEC), which is resistant to F-MuLV infection. Previous studies have shown that changes in the envelope gene of PVC-211 MuLV confer BCEC tropism to the virus. However, little is known about how infection of BCECs by PVC-211 MuLV induces neurological disease. Previous results suggest that nitric oxide (NO), which has been implicated as a potential neurotoxin, is involved in PVC-211 MuLV-induced neurodegeneration. In this study, we show that expression of inducible nitric oxide synthase (iNOS), which produces NO from l-arginine, is induced in BCECs from PVC-211 MuLV-infected rats. Furthermore, elevated levels of a 32-kDa cellular protein modified by 3-nitrotyrosine, which is a hallmark of NO production, were observed in virus-infected BCECs. BCECs from rats infected with BCEC-tropic but nonneuropathogenic PVF-e5 MuLV, which is a chimeric virus between PVC-211 MuLV and F-MuLV, fail to induce either iNOS expression or elevation of tyrosine nitration of a 32-kDa protein. These results suggest that expression of iNOS and nitration of tyrosine residues of a 32-kDa protein in PVC-211 MuLV-infected BCECs may play an important role in neurological disease induction.
A number of murine leukemia viruses (MuLVs) have been shown to induce diseases of the central nervous system (CNS) that are characterized by progressive loss of neuronal function (35, 39). The major cell types within the CNS that are prominently infected with the MuLVs are glial and endothelial cells, with neurons being infrequently infected. The most commonly observed pathological changes are gliosis, neuronal loss, and demyelination. The mechanism(s) by which the MuLVs induce neurological diseases remains to be elucidated.
PVC-211 MuLV is a neuropathogenic variant of the leukemia-inducing Friend MuLV (F-MuLV) (21). Infection of susceptible rats with PVC-211 MuLV causes a rapidly progressive neurodegenerative disease characterized by tremor, spasticity, ataxia, and hind limb paralysis. Neuropathological changes include widespread perivascular gliosis, neuropil vacuolation without inflammation, and neuronal degeneration in the brain stem, cerebellum, and spinal cord (19, 28).
The primary target of PVC-211 MuLV infection in the CNS is the brain capillary endothelial cell (BCEC), which is resistant to F-MuLV infection (19). The determinant of the BCEC tropism of PVC-211 MuLV was mapped to two amino acids (G116 and K129) which lie within the putative receptor binding domain of the envelope surface glycoprotein (SU) (30). Within the CNS, reactive astrocytes and degenerating neurons showed no evidence of virus infection (19). BCEC tropism of the virus has been shown to be necessary for neuropathogenesis (29), suggesting that CNS injury is indirect and that molecular events in virus-infected BCECs play a very important role in neurological disease induction.
Nitric oxide (NO) is an important messenger and effector molecule involved in a number of biological functions (31). NO is synthesized from l-arginine by three isoforms of NO synthases (NOS). Endothelial cell NOS (eNOS) and neuronal NOS are constitutively expressed, and their activities are regulated by Ca2+. In contrast, inducible NOS (iNOS) is inducible and Ca2+ independent (13). In the CNS, NO may play important roles in neurotransmitter release, neurotransmitter reuptake, neurodevelopment, synaptic plasticity, and regulation of gene expression, although excessive production of NO can lead to neurotoxicity (9, 27). iNOS is an attractive candidate for mediating NO-associated neurotoxicities, because long bursts of large amounts of NO are produced by iNOS (7, 9, 32). Indeed, elevated iNOS expression has been demonstrated in such human neurological diseases as Alzheimer's disease (24) and Parkinson's disease (26).
Recently, the spongiform vacuolation observed in PVC-211 MuLV-infected brains was reported to be associated with oxidative damage as detected by increased immunoreactivity for 3-nitrotyrosine (NTyr) in infected brains (43). NTyr is widely used as an indicator of NO formation, because nitration of tyrosine is mediated by reactive nitrogen species derived from NO (2, 11, 15). Elevated expression of NTyr has also been reported in human neurodegenerative diseases such as familial amyotrophic lateral sclerosis (41), Alzheimer's disease (17), Parkinson's disease (12), and human immunodeficiency virus type 1 dementia complex (5). In this study, we examined expression of iNOS and elevated expression of NTyr in PVC-211 MuLV-infected BCECs to evaluate the contribution of NO produced by infected BCECs to the neuropathogenicity induced by PVC-211 MuLV infection.
MATERIALS AND METHODS
Viruses.
Neuropathogenic PVC-211 and PVF-e5 MuLV, a nonneuropathogenic variant of PVC-211, were grown in NIH 3T3 cells as described previously (30). The viral supernatants had titers of 105 to 106 PFU/ml as determined by an XC assay (40). Virus samples were stored at −80°C until use.
Animals.
Pregnant Fisher 344 (F344) rats were obtained from Charles River (Raleigh, N.C.) and housed in the Small Animal Facility at the Department of Veterans Affairs Medical Center (Baltimore, Md.). All experiments were performed in accordance with Public Health Service guidelines, using an IACUC-approved protocol (P. M. Hoffman). Two-day-old F344 rats were inoculated intracerebrally with 0.03 ml of supernatant from virus-producing NIH 3T3 cells.
Cells.
Primary rat BCECs were isolated from the brains of virus- or medium-inoculated 3-week-old F344 rats as described previously (6, 19) and grown for 2 weeks in minimum essential medium (MEM) with d-valine (Life Technologies, Inc., Gaithersburg, Md.) supplemented with 20% fetal calf serum, 2 mM l-glutamine, 50 U of penicillin/ml, 50 μg of streptomycin/ml, 1 mM MEM nonessential amino acids solution (Life Technologies), 1 mM vitamin solution (Life Technologies), 50 μg of endothelial mitogen (Biomedical Technology Inc., Stoughton, Mass.)/ml, and 16 U of heparin (Life Technologies)/ml at 37°C in a 5% CO2 humidified incubator for 2 weeks. Isolated BCEC populations were >95% pure as determined by immunohistochemistry with the endothelial cell marker factor VIII.
Immunohistochemistry on brain sections.
Brain tissue was collected from medium- or virus-inoculated rats following intracardiac perfusion. The tissues were then fixed, and serial sections were stained for expression of iNOS, eNOS, and MuLV SU (gp70). Briefly, sections were first incubated for 48 h with mouse anti-iNOS (610329 from BD Transduction Laboratories, Lexington, Ky.); mouse anti-eNOS (610296 from BD Transduction Laboratories); or goat anti-Rauscher MuLV (R-MuLV) gp70 (National Cancer Institute, Bethesda, Md.). After washing in Tris-buffered saline, sections were incubated for 1 h with the appropriate biotinylated secondary antibody (Southern Biotechnology, Birmingham, Ala.). Antibody complexes were visualized using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, Calif.). Sections were then dehydrated and coverslipped with Permount (Sigma, St. Louis, Mo.). Three to six sections per rat brain were analyzed. Images were collected using a Nikon Eclipse 600 equipped with a Nikon DMX 1200 digital camera.
RT- PCR analysis.
To examine expression of NOS isoforms, we obtained total cellular RNA from BCECs isolated from the brains of rats inoculated with medium, PVC-211 MuLV, or PVF-e5 MuLV by using the RNA-STAT60 reagent (TEL-TEST, Inc., Friendswood, Tex.). RNA from BCECs stimulated with lipopolysaccharide (LPS) was used as a positive control for iNOS expression. RNA (2 μg) was reverse transcribed in the presence of 50 mM random hexamers using 200 U of Superscript II reverse transcriptase (RT; Invitrogen, Carlsbad, Calif.) according to the protocol supplied by the manufacturer. PCR was performed to amplify iNOS, eNOS, or β-actin sequences. Specific primer pairs used for each amplification were as follows: iNOS sense, 5′-ATGGAACAGTATAAGGCAAACACC-3′; iNOS antisense, 5′-GTTTCTGGTCGATGTCATGAGCAAAGG-3′; eNOS sense, 5′-TACGGAGCAGCAAATCCAC-3′; eNOS antisense, 5′-GATCAAAGGACTGCAGCCTG-3′; β-actin sense, 5′-CGTAAAGACCTCTATGCCAA-3′; and β-actin antisense, 5′-AGCCATGCCAAATGTCTCAT-3′.
Each amplification reaction mixture contained 100 ng of cDNA, 400 ng of each specific primer, a 0.2 mM concentration of each deoxyribonucleoside triphosphate, 2.5 U of Taq polymerase (Invitrogen), and 10× reaction buffer. Amplification conditions were as follows: 95°C for 5 min for 1 cycle; 30 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min; and 72°C for 10 min for 1 cycle.
Western blot analysis.
To prepare cell lysates, cells were washed with cold phosphate-buffered saline and lysed with immunoprecipitation buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 10 mM EDTA, 10 μg of aprotinin/ml, and 1 mM phenylmethylsulfonyl fluoride). The protein samples were prepared in buffer containing 625 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, and 50 mM dithiothreitol (SDS-sample buffer) and boiled for 5 min. Then, SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was performed, and protein samples were electrophoretically transferred to nitrocellulose membranes (Invitrogen). The membranes were then incubated with goat antibody to R-MuLV gp70 or p30 (National Cancer Institute); rabbit antibody to iNOS (Santa Cruz Biotechnology, Santa Cruz, Calif.); rabbit antibody to NTyr (Upstate Biotechnology, Lake Placid, N.Y.); or mouse antibody to β-tubulin (Sigma). Bound antibodies were detected with peroxidase-labeled secondary antibodies (DAKO, Carpinteria, Calif.) by using the enhanced chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, N.J.). Blocking experiments for NTyr immunoreactivity were performed by preincubating (25°C, 30 min) the anti-NTyr antibody with 10 mM NTyr (Sigma) prior to incubation with the membrane. For reprobing the membrane, antibodies bound to the membrane were removed by incubating with buffer containing 62.5 mM Tris-HCl (pH 6.7), 2% SDS, and 100 mM 2-mercaptoethanol at 55°C for 30 min. After washing the membrane, subsequent procedures for the binding of antibodies were carried out as described above. A cell lysate from BCECs stimulated with LPS was used as a positive control for iNOS expression. Stimulation was performed by incubation with 100 ng of LPS/ml for 24 h.
Detection of iNOS enzymatic activity.
iNOS enzymatic activity was measured by production of l-[3H]citrulline from l-[3H]arginine (14) with the use of the NOSdetect kit (Stratagene, La Jolla, Calif.) according to the manufacturer's specifications. The reaction was performed in the presence of EGTA (1 mM) to determine the calcium-independent activity of the inducible enzyme. Boiled samples were used to determine the background level.
Inoculation of PVC-211 MuLV to primary BCECs in vitro.
One day before inoculation (day 0), primary rat BCECs (passage 2) were seeded at 104 per well into 24-well tissue culture plates (Costar, Cambridge, Mass.). The following day (day 1), PVC-211 MuLV (multiplicity of infection = 10) was incubated with cells in the presence of Polybrene (5 μg/ml) for 1 h at 37°C. Then the cells were washed with medium, and fresh culture medium was added. This inoculation step was performed once a day and completed by day 4. At day 14, cell extracts were prepared and analyzed for expression of viral protein and iNOS by Western blotting as described above.
RESULTS
Specific expression of iNOS in brains and BCECs from PVC-211 MuLV-infected rat brain.
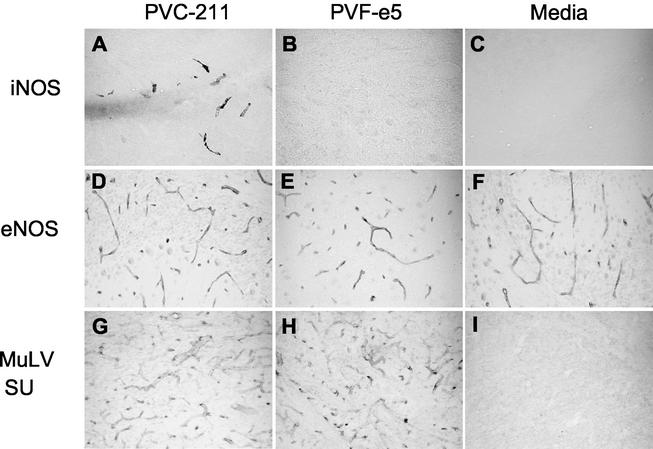
To evaluate the molecular events in PVC-211 MuLV-infected brains that may be contributing to neuropathogenicity, we examined brains from virus-infected rats for expression of iNOS. As a control, we used brains from rats infected with PVF-e5 MuLV, a chimeric virus between F-MuLV and PVC-211 MuLV that efficiently infects BCECs but fails to induce neurological disease (29, 43). We first used immunohistochemistry to examine brain sections for expression of iNOS. As shown in Fig. 1, iNOS could be detected only in brains from PVC-211 MuLV-infected rats, and the protein was expressed predominantly in BCECs (Fig. 1A). Although only one time point is shown (14 days postinfection), iNOS immunoreactivity was also observed at 21 and 28 days after PVC-211 MuLV inoculation (data not shown) and was not restricted to specific brain regions. Roughly 20 to 30% of all BCECs in microvessels exhibited iNOS immunoreactivity, compared with 90 to 100% of all BCECs in microvessels that express the viral SU gp70 glycoprotein (Fig. 1G). Other cell populations known to be activated following PVC-211 MuLV infection, either microglia (43) or astrocytes (19), failed to express iNOS immunoreactivity. In contrast to brains from PVC-211 MuLV-infected rats, iNOS immunoreactivity could not be detected in brains from rats infected with the nonneuropathogenic PVF-e5 MuLV at the day 14 time point shown (Fig. 1B) or at other time points examined (21 days and 16 weeks postinfection [data not shown]), despite comparable levels of expression of viral SU gp70 (compare Fig. 1G with H). As expected, brain sections from medium-inoculated rats exhibited essentially no iNOS immunoreactivity at any time point examined (Fig. 1C, 14 days postinfection). Neither PVC-211 MuLV (Fig. 1D) nor PVF-e5 MuLV (Fig. 1E) infection affected the expression of eNOS compared to that observed in brains from medium-inoculated rats (Fig. 1F) at the day 14 postinfection time point shown or at other time points examined (21 days and 16 weeks postinfection [data not shown]).
FIG. 1.
Expression of iNOS in PVC-211 MuLV-infected brains. Brain sections (cerebellum) from rats inoculated 14 days previously with PVC-211 MuLV (A, D, and G), PVF-e5 MuLV (B, E, and H) or medium (C, F, and I) were fixed and stained for iNOS (A to C), eNOS (D to F), or viral envelope proteins (G to I) using the ABC peroxidase technique, with diaminobenzidine as substrate. Panels A, B, C, G, H, and I show 40-μm-thick frozen fixed sections. Panels D, E, and F show 8-μm-thick paraffin sections. Final magnification for all panels is ×25.
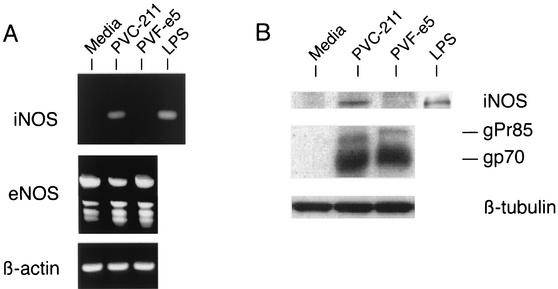
To further examine the expression of iNOS in virus-infected BCECs, we isolated primary BCECs from virus-infected rat brains and propagated them in vitro for 2 weeks, which is the minimum time required to obtain sufficient cell numbers for subsequent analysis. As a positive control, we used BCECs stimulated with LPS, a known inducer of iNOS. When RT-PCR analysis using specific primers was carried out on BCEC RNA from rats inoculated with medium, PVC-211 MuLV, or PVF-e5 MuLV, iNOS transcripts could be detected in BCECs stimulated with LPS as well as in BCECs from PVC-211 MuLV-infected rats, but not in BCECs from rats injected with PVF-e5 MuLV or medium (Fig. 2A). eNOS was expressed at equivalent levels in all samples (Fig. 2A). Consistent with this result, a 130-kDa iNOS protein was specifically detected in only LPS-stimulated BCECs or BCECs from PVC-211 MuLV-infected rats (Fig. 2B). The successful infection of BCECs with both PVC-211 MuLV and PVF-e5 MuLV was demonstrated by the expression of viral envelope SU proteins (gPr85 and gp70) (Fig. 2B). All samples expressed low levels of eNOS protein (data not shown).
FIG. 2.
Specific expression of iNOS in PVC-211 MuLV-infected BCECs. (A) RNA was isolated from BCECs cultured from the brains of rats inoculated with medium, PVC-211 MuLV, or PVF-e5 MuLV. RT-PCR was then carried out using specific primers to detect iNOS, eNOS, and β-actin. RNA from LPS-stimulated BCECs was used as a positive control for iNOS. (B) Protein extracts (20 μg) of BCECs cultured from the brains of rats inoculated with medium, PVC-211 MuLV, or PVF-e5 MuLV were separated by SDS-8% PAGE, and Western blot analysis was performed using anti-iNOS antibody. The anti-iNOS antibody was stripped from the membrane, and it was reprobed with goat anti-MuLV envelope SU (gp70) antibody. Viral envelope proteins, gp70 and its noncleaved precursor gPr85, were detected. The same membrane was reprobed with anti-β-tubulin antibody as an internal control. LPS-stimulated BCECs were analyzed separately as a positive control for iNOS.
Detection of iNOS enzymatic activity in PVC-211 MuLV-infected BCECs.
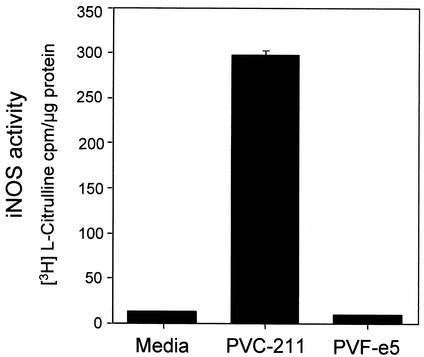
iNOS catalytic activity was also directly measured in extracts of BCECs from either virus-infected or medium-inoculated rats. iNOS enzymatic activity was measured by the conversion of l-arginine to NO and l-citrulline. Since it has been reported that the expression of ecotropic MuLV envelope proteins can affect the intracellular arginine concentration due to down-modulation of the cationic amino acid transporter CAT-1 (42), iNOS enzymatic activity was determined by measuring the conversion of radioactive l-arginine into l-citrulline in the presence of EGTA to inhibit Ca2+-dependent eNOS activity. As a positive control, we used a murine macrophage cell line, RAW264.7, which showed a sevenfold increase in iNOS activity after LPS stimulation (data not shown). As shown in Fig. 3, the iNOS activities in extracts of BCECs from both medium- and PVF-e5 MuLV-inoculated rats were almost undetectable. In contrast, a marked increase in iNOS activity (>20-fold) was detected in extracts of BCECs from PVC-211-infected rats, in agreement with the results obtained for the specific expression of iNOS mRNA and protein (Fig. 1 and 2). This difference is significant as determined by Student's t test (P < 0.00001).
FIG. 3.
Measurement of enzymatic iNOS activity. Cell extracts of BCECs from rats inoculated with medium or virus were tested for iNOS enzymatic activity as described in Materials and Methods. All measurements were determined in triplicate, and the data are shown as means ± standard deviations. iNOS activity detected in BCECs from rats inoculated with PVC-211 MuLV is significantly higher (P < 0.00001 by Student's t test) than that in BCECs from rats inoculated with medium or PVF-e5 MuLV.
Detection of tyrosine-nitrated protein in PVC-211 MuLV-infected BCECs.
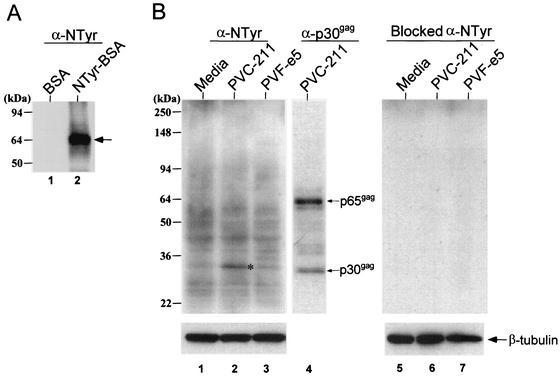
NTyr is a biomarker for the presence of NO. Since elevated immunoreactivity to NTyr was recently reported in brain sections from PVC-211 MuLV-infected rats (43), we tested more precisely for the presence of tyrosine-nitrated proteins in extracts of BCECs from PVC-211 MuLV-infected rats. The specificity of the anti-NTyr antibody was confirmed by the recognition of tyrosine-nitrated bovine serum albumin (NTyr-BSA) by Western blot analysis (Fig. 4A). This antiserum detected multiple bands with a wide range of molecular masses, mostly between 20 and 94 kDa, in all BCEC samples (Fig. 4B, lanes 1 to 3). Interestingly, a more intense immunoreactive band with a molecular mass of 32 kDa was found in PVC-211 MuLV-infected BCECs (lane 2). This 32-kDa protein appears to be a cellular protein, not a viral protein, because it migrates slower than MuLV p30gag (lane 4) and, unlike MuLV p30, it is not present in a 65-kDa precursor (p65gag). Specific binding of anti-NTyr was confirmed by blocking experiments using excess NTyr (lanes 5 to 7).
FIG. 4.
Expression of tyrosine-nitrated proteins in BCECs from PVC-211 MuLV-infected rats. (A) BSA (0.1 μg) (lane 1) or tyrosine-nitrated BSA (0.1 μg) (lane 2) was separated by SDS-4-to-20% PAGE, and Western blot analysis using anti-NTyr antibody was performed. The arrow represents the migration of BSA. (B) Protein extracts (20 μg) of cultured BCECs from medium- (lanes 1 and 5), PVC-211 MuLV- (lanes 2 and 6), or PVF-e5 MuLV- (lanes 3 and 7) inoculated rats were separated by SDS-4-to-20% PAGE, and Western blot analysis was performed using anti-NTyr antibody. The asterisk represents a 32-kDa protein. The anti-NTyr antibody was stripped from the membrane, and it was reprobed with goat anti-MuLV gag capsid (p30gag) antibody. Gag proteins, p30gag and its noncleaved precursor p65gag, were detected (lane 4). The right panel is the result of Western blot analysis performed using anti-NTyr antibody pretreated with 10 mM NTyr. The same membrane was reprobed with anti-β-tubulin antibody as an internal control.
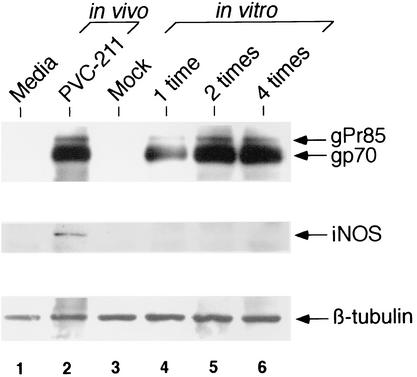
Infection of BCECs by PVC-211 MuLV in vitro fails to induce iNOS expression.
We next examined whether infection of primary BCECs by PVC-211 MuLV in vitro induces iNOS. In vivo, BCECs are repeatedly infected by circulating virus in the blood. To create a similar situation in vitro, BCECs were seeded on day 0 and inoculated with PVC-211 MuLV one time (on day 1), two times (on days 1 and 2), or four times (on days 1, 2, 3, and 4) (Fig. 5). Compared to a single inoculation, inoculation of BCECs on two or four separate occasions significantly improved the efficiency of virus infection (compare lane 4 to lanes 5 and 6). Almost equivalent levels of viral envelope proteins were detected between BCECs infected with PVC-211 MuLV in vivo and in vitro (compare lane 2 to lanes 5 and 6). The expression of iNOS protein, however, was not detected in BCECs infected with virus in vitro (lanes 4 to 6). This result suggests that an additional factor(s) induced in vivo after PVC-211 MuLV infection might be necessary to induce iNOS in BCECs.
FIG. 5.
Infection of BCECs by PVC-211 MuLV in vitro fails to induce iNOS. Primary BCECs (twice passaged) were inoculated in vitro with PVC-211 MuLV either one time, two times, or four times, 24 h apart. Fourteen days after the initial inoculation, cell extracts were prepared and compared with extracts of BCECs obtained from rats inoculated with PVC-211 MuLV in vivo. Lane 1, BCECs from rats inoculated in vivo with medium; lane 2, BCECs from rats inoculated in vivo with PVC-211 MuLV; lane 3, mock-infected BCECs; lane 4, BCECs infected in vitro with PVC-211 MuLV one time; lane 5, BCECs infected in vitro with PVC-211 MuLV two times; lane 6, BCECs infected in vitro with PVC-211 MuLV four times. Extracts (10 μg) were separated by SDS-4-to-20% PAGE, and Western blot analysis was performed using goat anti-MuLV envelope SU (gp70) antibody (upper panel). The anti-MuLV gp70 antibody was stripped from the membrane and reprobed with anti-iNOS antibody (middle panel). The same membrane was reprobed with anti-β-tubulin antibody as an internal control (bottom panel).
DISCUSSION
Our previous studies have shown that neurodegeneration induced by PVC-211 MuLV is indirect and occurs after the infection of BCECs by the virus. Since excessive production of NO can lead to neurotoxicity, we carried out studies to determine if infection of BCECs results in activation of iNOS or eNOS, which produce NO from l-arginine. Not only could we detect iNOS immunoreactivity localized to capillaries in brain sections from PVC-211 MuLV-infected rats, but also BCECs isolated from these brains expressed elevated levels of iNOS RNA, protein, and enzymatic activity compared to levels in BCECs from medium-inoculated rats or from rats infected with the nonneuropathogenic PVF-e5 MuLV. This is in contrast to eNOS, which continues to be expressed at a low, constitutive level after PVC-211 MuLV infection. Since NTyr is a biomarker for the presence of NO, we also examined infected BCECs for proteins modified by tyrosine nitration and detected elevated amounts of a 32-kDa tyrosine-nitrated cellular protein in BCECs from PVC-211 MuLV-infected rats. Thus, our data suggest that PVC-211 MuLV may be causing neuronal death indirectly by infecting BCECs and triggering, through iNOS activation, the production of large amounts of NO.
The precise mechanism by which PVC-211 MuLV induces the expression of iNOS in BCECs remains to be elucidated. One possibility is that a viral protein expressed within BCECs is initiating a cascade of events culminating in activation of iNOS. However, we failed to detect elevated iNOS protein in BCECs after in vitro infection with PVC-211 MuLV, suggesting that expression of a viral protein(s) in these cells is not sufficient to activate iNOS. Recently, Wilt et al. (43) reported the appearance of activated microglia adjacent to PVC-211 MuLV-infected BCECs before neuronal damage. Activation of microglia appears to be important for disease induction, since activated microglia could not be detected in brains from rats infected with the nonneuropathogenic PVF-e5 MuLV (43). Activated microglia release a variety of inflammatory molecules which are known mediators of iNOS induction (16). If such cytokines or chemokines are released from the activated microglia in the brains of PVC-211 MuLV-infected rats, they may bind to receptors on BCECs and induce the expression of iNOS. It is unclear how microglia become activated in the brains of PVC-211 MuLV-infected rats. Virus cannot be detected in the microglia (19), but the virus or a viral protein could be interacting with a transmembrane receptor on these cells that results in their activation. An attractive candidate is TLR4, the transmembrane component of the receptor complex mediating the cellular response to LPS (3). It was recently reported that the envelope protein of Moloney MuLV, a retrovirus highly related to PVC-211 MuLV, can bind to TLR4 (36) and that TLR4 is expressed on rat microglia (25). Thus, activation of microglia in the brains of PVC-211 MuLV-infected rats could be due to interaction of the viral envelope protein with TLR4 on these cells. BCECs may also express TLR4 that can be activated by the viral envelope protein. This could lead to direct activation of iNOS in the BCEC, since one of the signals activated by TLR4 binding is NF-κB, a transcriptional activator of iNOS (45). The failure of PVF-e5 MuLV to cause neurological disease could be due to the inability of its envelope glycoprotein, which differs from that of PVC-211 MuLV, to interact with TLR4 on microglia or BCECs. Experiments to test these hypotheses are in progress. Interestingly, C3H/HeJ mice, which do not express TLR4 due to a missense mutation in the gene (34), are resistant to PVC-211 MuLV-induced neurological disease (19), suggesting that TLR4 may indeed play a role in PVC-211 MuLV disease induction.
The exact pathways by which excessive NO production by PVC-211 MuLV-infected BCECs causes neuronal death are not known. Reactive nitrogen species potentially generated from NO by multiple pathways (8, 11, 15, 27) can modify proteins by nitration of tyrosine residues (1, 2). Tyrosine nitration may alter a protein's conformation, structure, catalytic activity, and/or susceptibility to protease digestion (23, 38, 46) and has been shown to disrupt protein tyrosine kinase-related signal transduction (10, 18, 22). In this study, we showed increased anti-NTyr immunoreactivity of a 32-kDa cellular protein in PVC-211 MuLV-infected BCECs. It is possible that the modification of this protein by nitration might alter its physiological properties and affect the function of the BCECs. Since BCECs are a component of the blood-brain barrier and play an important role in maintaining the ion homeostasis of the CNS (33), disruption of their function by NO could indirectly lead to the death of neurons. Alternatively, NO released from PVC-211 MuLV-infected BCECs may move to the site of neurons where it generates reactive nitrogen species that directly damage the neurons. Although neurons are spatially separated from BCECs, models of potential diffusion of NO indicate that it can diffuse as far as 300 μm from its site of origin, which could include as many as 2 million synapses (44).
In addition to affecting BCECs and neurons, NO produced by PVC-211 MuLV-infected BCECs may also have other effects in the brain. NO generated by iNOS in human microvascular endothelial cells has been shown to inhibit the rolling and adhesion of leukocytes (4), and NO has been shown to inhibit proliferation of T cells that invade the CNS (20). Since PVC-211 MuLV-induced neurodegeneration is not associated with either inflammation or infiltration of leukocytes (19, 21), NO produced in PVC-211 MuLV-infected BCECs may be inhibiting adhesion and infiltration of leukocytes into the brain parenchyma or proliferation of T cells that do infiltrate. In addition, NO produced in PVC-211 MuLV-infected BCECs may also inhibit virus replication. Data from a number of laboratories using both RNA and DNA viruses suggest that NO may inhibit an early stage in viral replication (37). The virus titer in brains recovered from rats infected with PVC-211 MuLV is always significantly lower than that in the spleen (19, 21, 43). In addition, microglia or neurons adjacent to the infected BCECs show no evidence of PVC-211 MuLV infection despite the release of virions into the basement membrane of infected BCECs. Thus, it is possible that NO released from PVC-211 MuLV-infected BCECs inhibits replication and spread of the virus in the brain.
In summary, our results show that infection of BCECs with PVC-211 MuLV in vivo induces functionally active iNOS protein and elevates tyrosine nitration of a 32-kDa cellular protein. Our failure to detect iNOS expression in BCECs after in vivo infection with the nonneuropathogenic but BCEC-tropic PVF-e5 MuLV suggests that iNOS activation plays a crucial role in the development of neurological disease induced by PVC-211 MuLV. The use of iNOS inhibitors and the generation of iNOS-deficient rats should help to clarify how iNOS is involved in the pathological process.
Acknowledgments
We thank Takashi Yugawa, Karen Rulli, and Joan Cmarik for helpful advice.
REFERENCES
- 1.Beckman, J. S., T. W. Beckman, J. Chen, P. A. Marshall, and B. A. Freeman. 1990. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 87:1620-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckman, J. S. 1996. Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol. 9:836-844. [DOI] [PubMed] [Google Scholar]
- 3.Beutler, B. 2000. TLR4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12:20-26. [DOI] [PubMed] [Google Scholar]
- 4.Binion, D. G., S. Fu, K. S. Ramanujam, Y. C. Chai, R. A. Dweik, J. A. Drazba, J. G. Wade, N. P. Ziats, S. C. Erzurum, and K. T. Wilson. 1998. iNOS expression in human intestinal microvascular endothelial cells inhibits leukocyte adhesion. Am. J. Physiol. 275:G592-G603. [DOI] [PubMed] [Google Scholar]
- 5.Boven, L. A., L. Gomes, C. Herry, F. Gray, J. Verhof, P. Portegies, M. Tardieu, and H. S. Nottet. 1999. Increased peroxynitrite activity in AIDS dementia complex: implications for the neuropathogenesis of HIV-1 infection. J. Immunol. 162:4319-4327. [PubMed] [Google Scholar]
- 6.Bowman, P. D., A. L. Betz, J. S. Wolinsky, J. B. Penny, R. R. Shivers, and G. W. Goldstein. 1981. Primary culture of capillary endothelium from rat brain. In Vitro 17:353-362. [DOI] [PubMed] [Google Scholar]
- 7.Bredit, D. S., and S. H. Synder. 1994. Nitric oxide: a physiologic messenger molecule. Annu. Rev. Biochem. 63:175-195. [DOI] [PubMed] [Google Scholar]
- 8.Brennan, M.-L., W. Wu, X. Fu, Z. Shen, W. Song, H. Frost, C. Vadseth, L. Narine, E. Lenkiewicz, M. T. Borchers, A. J. Lusis, and S. L. Hazen. 2002. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generating reactive nitrogen species. J. Biol. Chem. 277:17415-17427. [DOI] [PubMed] [Google Scholar]
- 9.Dawson, T. M., and V. L. Dawson. 1998. Nitric oxide in neurodegeneration. Prog. Brain Res. 118:215-229. [DOI] [PubMed] [Google Scholar]
- 10.Di Stasi, A. M., C. Mallozzi, G. Macchia, T. C. Petrucci, and M. Minetti. 1999. Peroxynitrite induces tyrosine nitration and modulates tyrosine phosphorylation of synaptic proteins. J. Neurochem. 73:727-735. [DOI] [PubMed] [Google Scholar]
- 11.Eiserich, J. P., C. E. Cross, A. D. Jones, B. Halliwell, and A. van der Vliet. 1996. Formation of nitrating and chlorinating species by reaction of nitrite with hypochlorous acid. J. Biol. Chem. 271:19199-19208. [DOI] [PubMed] [Google Scholar]
- 12.Ferrante, R. J., P. Hantray, E. Brouillet, and M. F. Beal. 1999. Increased nitrotyrosine immunoreactivity in substantia nigra neurons in MPTP treated baboons is blocked by inhibition of neuronal nitric oxide synthase. Brain Res. 823:177-182. [DOI] [PubMed] [Google Scholar]
- 13.Forstemann, U., E. I. Closs, J. S. Pollock, M. Nakane, P. Schwarz, I. Gath, and H. Kleinert. 1994. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 23:1121-1131. [DOI] [PubMed] [Google Scholar]
- 14.Galea, E., D. L. Feinstein, and D. L. Reis. 1992. Induction of calcium-independent nitric oxide synthase activity in primary rat glial cultures. Proc. Natl. Acad. Sci. USA 89:10945-10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaut, J. P., J. Byun, H. D. Tran, W. M. Lauber, J. A. Carroll, R. S. Hotchkiss, A. Belaaouaj, and J. W. Heinecke. 2002. Myeloperoxidase produces nitrating oxidants in vivo. J. Clin. Investig. 109:1311-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gehrmann, J., Y. Matsumoto, and G. W. Kreutzberg. 1995. Microglia: intrinsic immuno-effector cell of brain. Brain Res. Rev. 20:269-287. [DOI] [PubMed] [Google Scholar]
- 17.Good, P. F., P. Werner, A. Hsu, C. W. Olanow, and D. P. Perl. 1996. Evidence for neuronal oxidative damage in Alzheimer's disease. Am. J. Pathol. 49:21-27. [PMC free article] [PubMed] [Google Scholar]
- 18.Hevel, J. M., K. A. White, and M. A. Marletta. 1991. Purification of the inducible murine macrophage nitric oxide synthase. Identification as a flavoprotein. J. Biol. Chem. 266:22789-22791. [PubMed] [Google Scholar]
- 19.Hoffman, P. M., E. H. Cimino, D. S. Robbins, R. D. Broadwell, J. M. Powers, and S. K. Ruscetti. 1992. Cellular tropism and localization in the rodent nervous system of a neuropathogenic variant of Friend murine leukemia virus. Lab. Investig. 67:314-321. [PubMed] [Google Scholar]
- 20.Juedes, A. E., and N. H. Ruddle. 2001. Resident and infiltrating central nervous system APCs regulate the emergence and resolution of experimental autoimmune encephalomyelitis. J. Immunol. 166:5168-5175. [DOI] [PubMed] [Google Scholar]
- 21.Kai, K., and T. Furuta. 1984. Isolation of paralysis-inducing murine leukemia viruses from Friend virus passaged in rats. J. Virol. 50:970-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong, S. K., M. B. Yim, E. R. Stadtman, and P. B. Chock. 1996. Peroxynitrite disables the tyrosine phosphorylation regulatory mechanism: lymphocyte-specific tyrosine kinase fails to phosphorylate nitrated cdc2(6-20)NH2 peptide. Proc. Natl. Acad. Sci. USA 93:3377-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo, W. N., J. M. Kreahling, V. P. Shanbhag, P. P. Shanbhag, and M. Mewar. 2000. Protein nitration. Mol. Cell. Biochem. 214:121-129. [DOI] [PubMed] [Google Scholar]
- 24.Lee, S. C., M. L. Zhao, A. Hirano, and D. W. Dickson. 1999. Inducible nitric oxide synthase immunoreactivity in the Alzheimer disease hippocampus: association with Hirano bodies, neurofibrillary tangles, and senile plaques. J. Neuropathol. Exp. Neurol. 58:1163-1169. [DOI] [PubMed] [Google Scholar]
- 25.Lehnardt, S., C. Lachance, S. Patrizi, S. Lefebvre, P. L. O. Follett, F. E. Jensen, P. A. Rosenberg, J. J. Volpe, and T. Vartanian. 2002. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J. Neurosci. 22:2478-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liberatore, G. T., V. Jackson-Lewies, S. Vukosavic, M. Vila, W. G. McAuliffe, T. M. Dawson, and S. Przedorski. 1999. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 5:1403-1409. [DOI] [PubMed] [Google Scholar]
- 27.Lipton, S. A. 1999. Neuronal protection and destruction by NO. Cell Death Differ. 6:943-951. [DOI] [PubMed] [Google Scholar]
- 28.Masuda, M., M. P. Remington, P. M. Hoffman, and S. K. Ruscetti. 1992. Molecular characterization of a neuropathogenic and nonerythroleukemic variant of Friend murine leukemia virus PVC-211. J. Virol. 66:2798-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masuda, M., P. M. Hoffman, and S. K. Ruscetti. 1993. Viral determinants that control neuropathogenicity of PVC-211 murine leukemia virus in vivo determine brain capillary endothelial cell tropism of the virus in vitro. J. Virol. 67:4580-4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masuda, M., C. A. Hanson, W. G. Alvord, P. M. Hoffman, S. K. Ruscetti, and M. Masuda. 1996. Effect of subtle changes in the SU protein of ecotropic murine leukemia virus on its brain capillary endothelial cell tropism and interference properties. Virology 215:142-151. [DOI] [PubMed] [Google Scholar]
- 31.Moncada, S., R. M. Palmer, and E. A. Higgs. 1991. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 43:109-142. [PubMed] [Google Scholar]
- 32.Nathan, C., and Q.-W. Xie. 1994. Nitric oxide synthases: roles, tolls, and controls. Cell 78:915-918. [DOI] [PubMed] [Google Scholar]
- 33.Pardridge, W. M. 1999. Blood-brain barrier biology and methodology. J. Neurovirol. 5:556-569. [DOI] [PubMed] [Google Scholar]
- 34.Poltorak, A., X. He, I. Smirnova, M.-Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 35.Power, C. 2001. Retroviral diseases of the nervous system: pathogenic host response or viral gene-mediated neurovirulence? Trends Neurosci. 24:162-169. [DOI] [PubMed] [Google Scholar]
- 36.Rassa, J. C., J. L. Meyers, Y. Zhang, R. Kudaravalli, and S. R. Ross. 2002. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc. Natl. Acad. Sci. USA 99:2281-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiss, C. S., and T. Komatsu. 1998. Does nitric oxide play a critical role in viral infections? J. Virol. 72:4547-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts, E. S., H. L. Lin, J. R. Crowley, J. L. Vuletich, Y. Osawa, and P. F. Hollenberg. 1998. Peroxynitrite-mediated nitration of tyrosine and inactivation of the catalytic activity of cytochrome P450 2B1. Chem. Res. Toxicol. 11:1067-1074. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg, N., and P. Jolicoeur. 1997. Retroviral pathogenesis, p. 475-586. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 40.Rowe, W. P., W. E. Pugh, and J. W. Hartley. 1970. Plaque assay techniques for murine leukemia viruses. Virology 42:136-138. [DOI] [PubMed] [Google Scholar]
- 41.Sasaki, S., N. Shibata, T. Komori, and M. Iwata. 2000. iNOS and nitrotyrosine immunoreactivity in amyotrophic lateral sclerosis. Neurosci. Lett. 291:44-48. [DOI] [PubMed] [Google Scholar]
- 42.Wang, H., E. Dechant, M. Kavanaugh, R. A. North, and D. Kabat. 1982. Effects of ecotropic murine retroviruses on the dual-function cell surface receptor/basic amino acid transporter. J. Biol. Chem. 267:23617-23624. [PubMed] [Google Scholar]
- 43.Wilt, S. G., N. V. Dugger, N. D. Hitt, and P. M. Hoffman. 2000. Evidence for oxidative damage in a murine leukemia virus-induced neurodegeneration. J. Neurosci. Res. 62:440-450. [DOI] [PubMed] [Google Scholar]
- 44.Wood, J., and J. Garthwaite. 1994. Models of the diffusional spread of nitric oxide: implications for neural nitric oxide signalling and its pharmacological properties. Neuropharmacology 33:1235-1244. [DOI] [PubMed] [Google Scholar]
- 45.Xie, Q. W., Y. Kashiwabara, and C. Nathan. 1994. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 269:4705-4708. [PubMed] [Google Scholar]
- 46.Yamakura, F., H. Taka, T. Fujimura, and K. Murayama. 1998. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J. Biol. Chem. 273:14085-14089. [DOI] [PubMed] [Google Scholar]