Abstract
FhSAP-2 is a novel member of the Fasciola hepatica saposin-like protein family that induces protection in rabbits against a challenge infection. We investigated the presence of lineal B-cell epitopes within this protein using a set of overlapping synthetic peptides. Peptides were tested in enzyme-linked immunosorbent assays against sera from rabbits infected with F. hepatica. Two dominant epitopes were identified, which were also highly reactive with sera from mice infected with Schistosoma mansoni. These peptides may be suitable to incorporate into a polyepitope-based vaccine formulation against F. hepatica.
Fascioliasis, caused by Fasciola hepatica, represents a recognized unsolved agricultural problem responsible for economic losses estimated to be around 3 billion U.S. dollars per year to rural agricultural communities and commercial producers worldwide, including the United States (5). Recent reports indicate that F. hepatica is also a major human pathogen and is increasingly recognized as causing significant human diseases, with 2 to 4 million people infected with this parasite (2, 5). Liver fluke infections can be highly pathogenic and can lead to severe morbidity and even death of the host. The flukicide triclabendazole is the most effective drug for controlling Fasciola (19, 26); however, the prohibitive cost of treatment prevents its widespread use by rural producers in developing countries. Moreover, resistance to triclabendazole has been reported in sheep infected with F. hepatica (19), suggesting that selection of resistant parasites may eventually compromise the use of this drug. Vaccines represent the most attractive long-term alternative to invert this scenario.
Recently, we described the molecular cloning and expression of an F. hepatica antigen termed FhSAP-2, which is expressed at early stages of infection and which, by its structural homology with a F. hepatica NK-lysin (20), three amoebapores of Entamoeba histolytica (14), a porcine NK-lysin (1), and several other related proteins (3, 23), falls in the saposin-like/NK-lysin protein family of F. hepatica. It is an 11.5-kDa polypeptide that elicits a strong antibody response during active infection and exhibits a potent lytic activity against human erythrocytes and peripheral blood mononuclear cells (7), which stimulates specific cell-mediated and humoral immune responses (6). This novel antigen is highly reactive with sera from rabbits experimentally infected with F. hepatica, and it is recognized by more than 83% of sera from patients with chronic Fasciola infection (7). We have also found that FhSAP-2 is a potent immunogen, since rabbits vaccinated with FhSAP-2 and challenged with F. hepatica metacercariae had lower parasite burdens, fewer parasite eggs, and less liver damage than nonvaccinated controls (8). However, the immune mechanism that made this protection possible is not clear. Correct identification of epitopes in FhSAP-2 will greatly help in the characterization of targets for immunization-vaccination and may provide an antigen basis for designing diagnostics specific for F. hepatica. The aim of this study was to identify linear B-cell epitopes of FhSAP-2 by screening a synthetic peptide library based upon this protein with sera from rabbits immunized with FhSAP-2 and rabbits infected with F. hepatica using an enzyme-linked immunosorbent assay (ELISA) and a peptide ELISA inhibition assay. Similar studies have been carried out on other antigens associated with Fasciola and related parasites with success (4, 24, 30).
Recombinant protein and animal sera.
Recombinant FhSAP-2 was purified from transformed Escherichia coli TOP10 competent cell inclusion bodies, solubilized in 6 M guanidine hydrochloride, and then purified by affinity chromatography through a HiTrap Ni2+ chelating column (Amersham Biosciences Corp, New Jersey) as previously described (7). Purified protein was analyzed by conventional sodium dodecyl sulfate-polyacrylamide gel electrophoresis and identified by Western immunoblotting using a specific polyclonal antiserum (7). Eighteen peptides representing the entire 101-amino-acid sequence of FhSAP-2 were synthesized by standard 9-fluoroenyl-methoxicarbonyl polyamide solid-phase synthesis. The quality of peptides was assessed by reverse-phase chromatography on a Supelco Bio Wide Pore column and by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Peptides were synthesized as 15 mers, with adjacent peptides overlapping by 10 amino acids and termed sequentially as SAP-1 to SAP-18. The last C terminus peptide (SAP-18) was synthesized as a 16 mer.
All experiments were performed with eight 3-month-old male New Zealand White rabbits (Harland Inc., Indianapolis, Ind.) and nine Swiss male mice (Biomedical Research Institute, Maryland) which were maintained in the animal care facility at the University of Puerto Rico, Medical Sciences Campus, and treated according to international regulations for the care of laboratory animals. Rabbits were orally infected with 25 F. hepatica metacercariae each (Baldwin Aquatics Inc., Monmouth, Oreg.) and necropsied 12 weeks after infection. Mice were infected with 150 S. mansoni cercariae by skin penetration and necropsied 9 weeks after infection. Rabbits and mice were bled before infection and then biweekly until necropsy for collection of serum. Sera from rabbits and mice were tested by ELISA against FhSAP-2 following a preestablished protocol (9). FhSAP-2 reacted strongly with sera from rabbits at 4 weeks (0.23 ± 0.06) to 12 weeks (1.76 ± 0.012) postinfection with F. hepatica, and it was also reactive with sera from mice at 4 weeks (0.18 ± 0.06) to 9 weeks (0.76 ± 0.015) postinfection with S. mansoni.
B-cell epitope prediction.
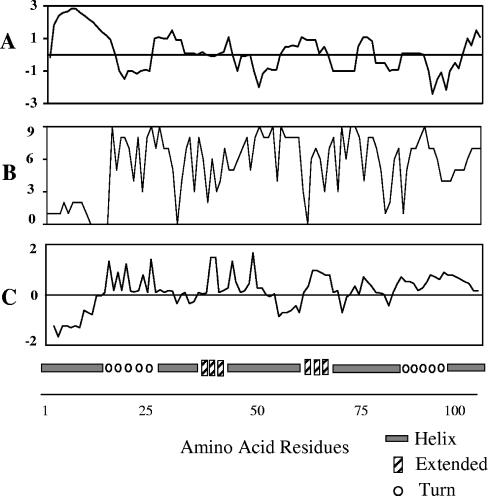
Because most, if not all, antigenic sites are located within surface-exposed regions of a protein, the presence of B-cell epitopes is often predicted by computer analysis based on measurement of hydrophobicity (17), hydrophilicity and surface accessibility (21), antigenic index (13), and predicted helix or turns (11). Using the deduced amino acid sequence of the cDNA encoding FhSAP-2, these predictions revealed the presence of several putative antigenic regions randomly distributed throughout the protein (Fig. 1A to C), with the exception of the first 15 amino acid residues at the N terminus. This region exhibits the highest hydrophobic index. It has been predicted that this region contains a string of hydrophobic and hydroxylic amino acid residues that were predicted to constitute an N-terminal signal sequence with a signal cleavage site between amino acids Ala15 and Ser16 (7). Therefore, it is possible that this region may be absent in the mature protein, which could eventually explain the lack of reactivity between peptides containing residues 1MNPLFVLMLAAVTFASFDVP20 and sera of infected animals. A prediction of the secondary structure of FhSAP-2 is shown in Fig. 1D.
FIG. 1.
(A) Prediction of hydrophobicity. >0, high hydrophobicity; <0, low hydrophobicity. (B) Solvent accessibility. 0, low accessibility; 9, high accessibility. (C) Antigenic index. Values of >0 indicate potential antigenic regions. Regions of low hydrophobicity have more solvent accessibility and a higher antigenic index. (D) Secondary structure predicted for the protein moiety of FhSAP-2 (GenBank accession no. AF286903).
Experimental identification of linear B-cell epitopes within FhSAP-2 protein.
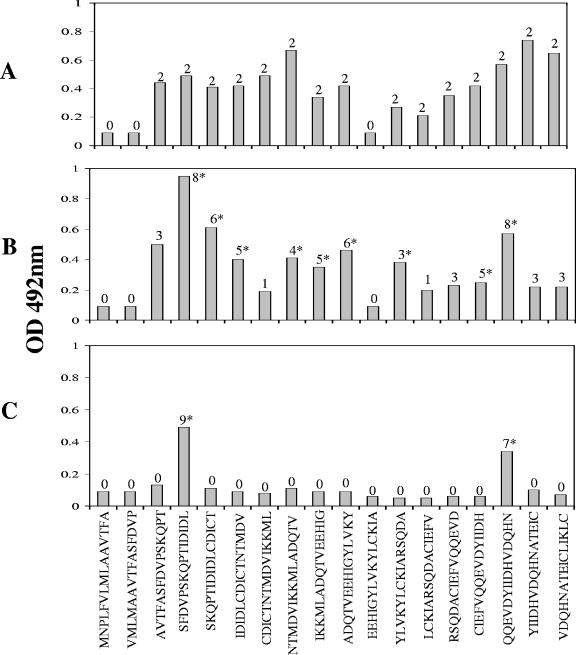
Two rabbit anti-FhSAP-2 sera were produced as described previously (7). The ELISA was standardized for each synthetic peptide derived from FhSAP-2 using an anti-FhSAP-2 serum. Plates were coated with different peptide concentrations (from 5 to 60 μg/ml) and tested against different antiserum dilutions (from 1:50 to 1:800) at different conjugate dilutions (from 1:2,000 to 1:10,000) to define the optimal dilution of all reagents that renders the best differentiation between a positive and a negative serum. The assay was performed essentially as previously described (7). Anti-FhSAP-2 sera, sera from rabbits infected with F. hepatica, and sera from mice infected with S. mansoni were tested in the ELISAs against each single peptide. As a result of this scanning, the presence of two areas of increased reactivity corresponding to amino acid residues 21 to 55 and 66 to 101 was demonstrated in the FhSAP-2 protein moiety. The rabbit anti-FhSAP-2 sera were capable of recognizing multiple peptides, thus indicating a complex polyclonal response to the protein moiety of FhSAP-2 (Fig. 2A). Two immunogenic domains within FhSAP-2 similar to those revealed by epitope mapping experiments using hyperimmune sera were identified when peptides were scanned using sera from rabbits with 12 weeks of Fasciola infection (Fig. 2B). Three peptides without reactivity with the anti-FhSAP-2 sera or infection sera were identified. The nonreactive peptides covered amino acid residues 1 to 20 (peptides SAP-1 and SAP-2) and residues 51 to 65 (SAP-11). When sera from mice infected with S. mansoni were individually tested against peptides, reactivity was observed only with peptides SAP-4 and SAP-16 (Fig. 2C).
FIG. 2.
Mapping analysis using peptides spanning the entire sequence of the protein the FhSAP-2. For this experiment ELISA plates were coated with 20 μg/ml per well. Rabbit and mouse sera were tested at dilutions of 1:200. To visualize specific peptide-antibody reactions, peroxidase-labeled anti-species immunoglobulin G (Bio-Rad Laboratories, Hercules, CA) conjugate, diluted 1:5,000, was used. Individual peptides were tested with two specific anti-FhSAP-2 sera (A), eight sera from rabbits at 12 weeks postinfection with Fasciola (B), and nine sera from mice at 9 weeks postinfection with S. mansoni (C). Serum was considered positive when its OD at 492 nm exceeded the previously established cutoff value (cutoff, 0.19), which was calculated as the mean absorbance plus two standard deviations of the negative rabbit or mouse sera. Numbers above columns indicate the number of sera reacting with a given peptide. An asterisk over the bar indicates statistical difference (P > 0.05) between mean absorbance values with infected sera and negative sera for each specific peptide.
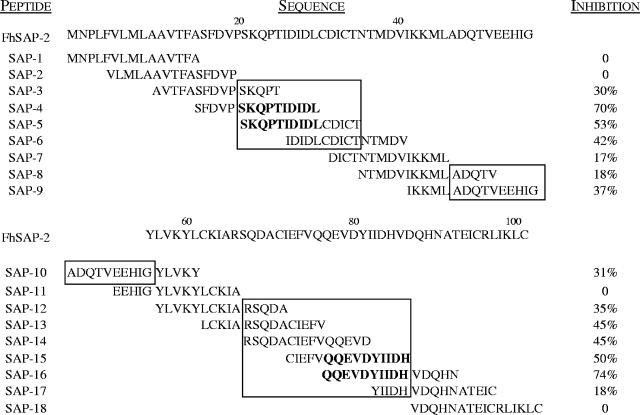
To define the fine localization of dominant immunoreactive regions, peptides were tested for their ability to inhibit the binding of rabbit antibody to a pool of peptides and FhSAP-2. A peptide inhibition assay was performed essentially as previously described (9). Peptides that inhibited the ELISA by more than 50% were considered as containing a dominant B-cell epitope. Comparison of the amino acid sequences of immunoreactive peptides revealed that FhSAP-2 possessed three main antigenic sites. The first antigenic site (site 1) was considered to be present in residues 21SKQPTIDIDLCDICT35 of peptides SAP-3, SAP-4, SAP-5, and SAP-6. All sera gave relatively high optical density (OD) values (between 0.6 to 1.2), with peptides SAP-4 and SAP-5 containing 21SKQPTIDIDL30 as part of their sequence, and these peptides inhibited the antibody binding to a peptide-coated plate by more than 50% (Fig. 3). Since peptide SAP-4 shared residues 21SKQPT25 with poorly immunoreactive peptide SAP-3 and since SAP-5 shared residues 26IDIDLCDICT35 with the less immunoreactive peptide SAP-6, we concluded that antigenic site 1 contained a dominant epitope present in residues 21SKQPTIDIDL30.
FIG. 3.
Inhibition percentage obtained when peptides were individually added at 20 μg/ml to sera from rabbits at 12 weeks postinfection with F. hepatica. For this assay two pools of peptides were prepared. Pool 1 included the N terminus peptides (SAP-1 to SAP-9), and pool 2 included the C terminus peptides (SAP-10 to SAP-18). Microtiter plates were coated with 20 μg/ml of each pool of peptides. Serum diluted 1:200 was mixed with 20 μg/ml of a single peptide and incubated for 1 h at 37°C to favor peptide-antibody complex formation. After incubation, a serum-peptide mixture was added to the plates and incubated for 1 h at 37°C. Conjugate was then added, followed by the substrate solution as previously described (9). Percent inhibition (I) was calculated as follows: I = [1− (OD at 492 nm of serum-peptide mixture/OD at 492 nm of serum-PBS-Tween mixture)] × 100. Peptides that inhibited the binding capacity of antibodies to antigen-coated plates by >50% were considered distinctive of dominant B-cell epitopes. The three antigenic regions identified within the FhSAP-2 protein are enclosed in a box. The amino acid residues that were considered to contain dominant B-cell epitopes are in bold type.
The second antigenic site (site 2) appeared to be in residues 46ADQTVEEHIG55 of peptides SAP-8, SAP-9, and SAP-10 but not in the nonreactive peptide SAP-7 and only partially present in the nonreactive peptide SAP-11. However, because the OD values of the sera tested with these peptides were low (between 0.3 to 0.37) and none of these peptides significantly inhibited antibody binding to the peptide-coated plate, we concluded that this B-cell epitope is not dominant.
The last antigenic site (site 3) was considered to be in residues 66RSQDACIEFVQQEVDYIIDHVDQHNATEICRLIKLC101 of peptides SAP-12, SAP-13, SAP-14, SAP-15, SAP-16, SAP-17, and SAP-18. All sera gave low OD values (between 0.26 to 0.35) with peptides SAP-12, SAP-13, SAP-14, SAP-17, and SAP-18 but gave moderate to high OD values (between 0.43 to 0.8) with peptides SAP-15 and SAP-16, which contain the amino acid residues 76QQEVDYIIDH85 as part of their sequence. Peptide SAP-15 shared the residues 71CIEFV75 with the less immunoreactive peptide SAP-14, and peptide SAP-16 shared the residues 81YIIDHVDQHN90 and 86VDQHN90 with the poorly immunoreactive peptides SAP-17 and SAP-18, respectively. Because peptides SAP-15 and SAP-16 were the only peptides from this group that inhibited antibody binding to a peptide-coated plate by more than 50%, we concluded that the region spanned by amino acid residues 76QQEVDYIIDH85 is a dominant B-cell epitope (Fig. 3).
Quantitative comparison between both dominant B-cell epitopes demonstrates that sera were significantly (P < 0.05) more reactive with the dominant B-cell epitope of site 1 (N-terminal portion of protein) than with the dominant B-cell epitope of site 3 (C-terminal portion of protein). It is necessary to note that, unlike peptides SAP-4, SAP-5, (from the N-terminal region), and SAP-16 (from the C-terminal region) that were the only peptides that reacted with most sera, the other antigenic peptides from the antigenic site 1, site 2, and site 3 were not recognized by most of the positive sera tested (Fig. 2B). This suggested that it will not be possible to further define or shorten the epitopes SKQPTIDIDL, ADQTVEEHIG, and QQEVDYIIDH, since shortening of the peptide in these regions will probably result in decreasing the OD reading or elimination of the reactivity with some sera. Similar results have been reported by other investigators (27) and may occur because some antibodies in a particular serum react more avidly with certain amino acid residues than with others within the epitope. This would mean that a shortened peptide may react only with a portion of FhSAP-2 antibodies present in a particular serum, resulting in a weak-positive or false-negative reaction. Previous studies relating to the epitope mapping of Mycoplasma bovis surface lipoproteins have shown B-cell epitopes to be around three to seven amino acid residues long (22). However, other studies have shown reactive B-cell epitopes relating to fungal, bacterial, or parasitical antigens to be as long as 10 or even 15 residues (15, 27, 28, 29, 30), similar to the size of the 10-mer SKQPTIDIDL, ADQTVEEHIG, or QQEVDYIIDH described here.
As with all proteins belonging to the saposin-like/NK-lysin protein family, FhSAP-2 possesses six conserved cysteine residues as well as seven conserved hydrophobic residues, which are within five amphipathic α-helix domains (1, 18, 20, 23, 25). The number and the positioning of cysteine residues in this family strongly suggest a common structural organization among these proteins and indicate that a favorable tertiary structure that combines amphipathic α-helices with folding of a disulfide bond is conserved (7). An important observation of this study is that the immunoreactivity of the B-cell epitopes mapped on FhSAP-2 was found to be inversely related to the number of hydrophobic residues that include its sequence. That is, peptide SAP-7 (residues 32DICTNTMDVIKKML45), SAP-11 (residues 51EEHIGYLVKYLCKIA65), SAP-17 (81YIIDHVDQHNATEIC95) and SAP-18 (86VDQHNATEICRLIKLC101), which are the less immunoreactive peptides, span each one, three, or four conserved hydrophobic residues (underlined). Similarly, peptides SAP-8, SAP-9, and SAP-10 (36NTMDVIKKMLADQTV50, 41IKKMLADQTVEEHIG55, and 46ADQTVEEHIGYLVKY60, respectively), which cover the central part of the protein moiety and exhibit a moderate immunoreactivity, span two conserved hydrophobic regions. By contrast, peptides that were highly immunoreactive, specifically SAP-4 and SAP-5 (residues 16SFDVPSKQPTIDIDL30 and 21SKQPIDIDLCDICT35) do not span any hydrophobic conserved residues in their sequences. Nevertheless, the highly immunoreactive peptide SAP-16 (residues 76QQEVDYIIDHVDQHN90) includes a conserved hydrophobic residue (86V), but this residue is not included within the dominant epitope (76QQEVDYIIDH85). Another interesting observation is that, while the highly reactive N-terminal (residues 21SKQPTIDIDL30) dominant B-cell epitope is totally located in an extended region, the C-terminal dominant B-cell epitope (residues 76QQEVDYIIDH85), which is less reactive, is partially located in an α-helical region. These observations are consistent with the concept that extended regions have a stronger influence than helical regions on the outcome of the antibody recognition (28).
In our previous studies with FhSAP-2, we demonstrated that reduction of disulfide bonds with dithiothreitol (DTT) notably reduced the reactivity of protein with serum from infected animals (7). To determine if the antigenic regions described in the present study are reduction sensitive, DTT was added to single peptides following an established protocol (7). Then, sera from Fasciola- or Schistosoma-infected animals were tested by ELISA against peptides treated with DTT and untreated peptides. Interestingly, no diminution of reactivity against sera was observed even on high DTT concentrations (data not shown). This suggests that, in addition to the lineal epitopes described in this study, other conformational epitopes are necessarily formed in the native protein. Also, since FhSAP-2 is potentially a glycoprotein, as predicted from the deduced amino acid sequence (7), the carbohydrate portion of the molecule is likely to contain antigenic determinants that cannot be identified using this methodology. In our previously published study, we demonstrated that the lytic activity of FhSAP-2 is not a property ascribed to the conserved cysteine residues (7). Here, we found that neither of the hydrophobic conserved regions is immunoreactive. This experimental evidence supports the hypothesis that either the immunoreactivity with sera or the lytic activity of FhSAP-2 could be functions associated with the nonconserved residues present in the α-helix bundles themselves or, alternatively, with the unrelated regions connecting each helix rather than associated with the conserved regions.
Computer algorithms (10) applied to the primary structure of FhSAP-2 have predicted the existence of at least five potential major histocompatibility complex class II binding regions with their corresponding P1 anchor region contained in the α-helix regions, as well as regions that might form T-cell epitopes. Antigenic sites described in this study may interact with such helper T-cell epitopes, influencing the repertoire and specificity of antibody-producing cells. If this is the case in the antibody response to FhSAP-2, differences in the response elicited by the antigenic site may affect properties of other sites, which remain to be further investigated. It must be noted that the epitope mapping techniques used in the present study are limited to the identification of linear (continuous) epitopes defined by contiguous amino acid residues in the primary structure of the protein. In addition to the linear epitopes described herein, other discontinuous epitopes, defined by the interaction between amino acid residues that are distant in the protein sequence but brought together as a result of the natural folding of the protein, may be found on FhSAP-2 in its native conformation.
Immunogenicity of dominant B-cell epitopes.
To ascertain whether dominant epitopes are able to induce specific antibodies, peptide SAP-4, which contains the dominant epitope at residues 21SKQPTIDIDL30, and peptide SAP-16, which contains the dominant epitope at residues 76QQEVDYIIDH85, were coupled to keyhole limpet hemocyanin (Pierce, Rockford, Ill.) and used to immunize two BALB/c mice. The immunization schedule consisted of three subcutaneous injections at biweekly intervals of 70 μg each in phosphate-buffered saline (PBS), mixed with an equal volume of Freund's adjuvant (complete for initial dose; incomplete for booster injections). Two weeks after the last injection, animals were anesthetized, bled, and sacrificed to remove the spleen. Analysis of the resulting antisera revealed that both peptides were highly immunogenic and elicited titers of anti-peptide antibodies higher than 1:25,000. Anti-SAP-4 and anti-SAP-16 sera recognized the free peptides bound to the wells of microtiter plates in either a dose-dependent or saturable manner. The specificity of the reaction was demonstrated by the fact that each soluble peptide was an effective competitor of binding of the anti-peptide antiserum to the same peptide immobilized in the wells of a microtiter plate and was able to abolish binding at high concentrations (data not shown).
Specific B-cell receptor identification.
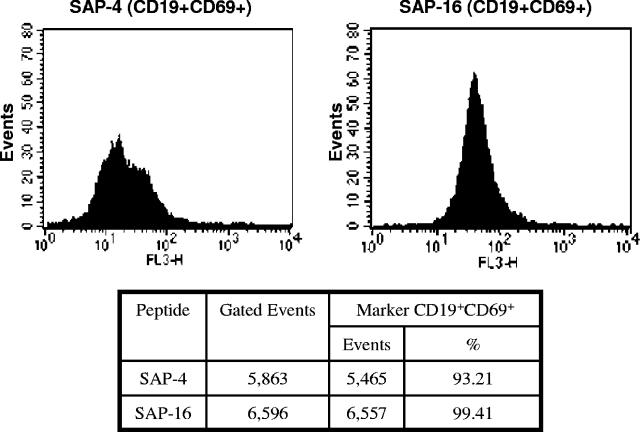
The spleens from immunized mice were washed and minced in PBS. After centrifugation for 10 min at 1,000 × g, red blood cells were removed by hypotonic lysis for 30 s, and the resulting suspensions were washed, centrifuged, and cultured for 2 days with optimum concentrations of each free peptide. Using standard protocols, stimulated cells were stained for fluorescence-activated cell sorter analysis with fluorescein isothiocyanate (FITC)-conjugated anti-CD19 or with phycoerythrin-Cy7 (PE-Cy7)-conjugated anti-CD69 (Pharmigen, San Diego, CA). As was expected, the vast majority of cells derived from animals immunized with peptides SAP-4 and SAP-16 stained specifically with anti-CD19, from which 93.2% and 99.41%, respectively, stained also with anti-CD69 (Fig. 4), which demonstrated that cells marked for CD19 were activated. Since CD19 is a characteristic B-cell marker expressed from the early stages of commitment to B-cell lineage and on all mature B cells, the large number of activated cells expressing this marker indicates that the sequences 21SKQPTIDIDL30 and 76QQEVDYIIDH85 from FhSAP-2 are actually B-cell epitopes.
FIG. 4.
Peptides SAP-4 and SAP-16 contain, respectively, the immunodominant B-cell epitopes 21SKQPTIDIDL30 and 76QQEVDYIIDH85. They were conjugated with keyhole limpet hemocyanin, emulsified in Freund's adjuvant, and injected separately into two mice. Splenocytes were isolated from each immunized animal and cultured in triplicate at 106 cells/well in 200 μl of RPMI-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin and stimulated with 5 μg/ml of each peptide. Cells were incubated for 48 h at 37°C under 5% CO2 atmosphere. After incubation, cells were centrifuged, washed with PBS, stained with anti-CD19-FITC or anti-CD69-PE-Cy7 (BD Pharmigen, San Diego, CA) monoclonal antibodies, and analyzed by fluorescence-activated cell sorting using a FACSort (Becton and Dickinson, San Jose, CA) instrument. Splenocytes were identified by their characteristic appearance on a dot plot of forward scatter versus side scatter. Splenocytes stained with FITC- or PE-Cy7-labeled antibodies were detected by FL1 or FL3, respectively. B-cells were gated on the basis of CD19 staining. The results were reported as the percentage of positive cells within this gate that appear activated.
It is interesting to recall that peptides SAP-4 and SAP-16 showed a strong reactivity with sera from mice infected with S. mansoni. Cross-reactivity between F. hepatica and S. mansoni antigens has been repeatedly demonstrated in serological assays (12, 16), suggesting the existence of ancestral genes conserved in the phylogeny of both species. The identification of these cross-reactive epitopes in the FhSAP-2 protein strongly suggests that the regions 21SKQPTIDIDL30 and 76QQEVDYIIDH85 of FhSAP-2 could be regions conserved between both species and could suggest the existence of a homolog of protein FhSAP-2 in S. mansoni. From a practical point of view, the design of diagnostic assays for F. hepatica based on synthetic peptides containing such sequences will have serious limitations; synthetic peptides should be used with caution, particularly in areas where fascioliasis and schistosomiasis are endemic. However, these polypeptides may be suitable for incorporation into a polyepitope-based vaccine formulation against these parasites.
Acknowledgments
These studies were supported by the MBRS-SCORE Program of the University of Puerto Rico (grant S06-GM008224), the RCMI Program of the University of Puerto Rico (grant G12-RR-03051), and the NICHD (grant 1G11HD046326).
We thank Cynthia Rivera for her help in statistical analysis and Laura Bretaña for her excellent editorial assistance.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Andersson, M., H. Gunne, B. Agerberth, A. Boman, T. Bergman, R. Sillard, H. Jornvall, V. Mutt, B. Olsson, and H. Wigzell. 1995. NK-lysin, a novel effector peptide of cytotoxic T and NK cells. Structure and cDNA cloning of the porcine form, induction by interleukin 2, antibacterial and antitumour activity. EMBO J. 14:1615-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjorland, J., R. T. Bryan, W. Strauss, G. V. Hillyer, and J. B. McAuley. 1995. An outbreak of acute fascioliasis among Aymara Indians in the Bolivian Altiplano. Clin. Infect. Dis. 21:1228-1233. (Erratum, 23:423.) [DOI] [PubMed] [Google Scholar]
- 3.Bruno, M. A., R. J. Bohinski, J. E. Carter, K. A. Foss, and J. A. Whitsett. 1995. Structure and function of the mouse surfactant protein B gene. Am. J. Physiol. 268:L381-L389. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho-Queiroz, C., R. Cook, C. C. Wang, R. Correa-Oliveira, Bailey, N. A., N. K. Egilmez, E. Mathiowitz, and P. T. LoVerde. 2004. Cross-reactivity of Schistosoma mansoni cytosolic superoxide dismutase, a protective vaccine candidate, with host superoxide dismutase and identification of parasite-specific B epitopes. Infect. Immun. 72:2635-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, M. G., and K. E. Mott. 1990. Progress in assessment of morbidity due to Fasciola hepatica infection. A review of recent literature. Trop. Dis. Bull. 87:1-38. [Google Scholar]
- 6.Espino, A. M., A. Osuna, R. Gil, and G. V. Hillyer. 2005. Fasciola hepatica: humoral and cytokine responses to a member of the saposin-like protein family following delivery as a DNA vaccine in mice. Exp. Parasitol. 110:374-383. [DOI] [PubMed] [Google Scholar]
- 7.Espino, A. M., and G. V. Hillyer. 2003. Molecular cloning of a member of the Fasciola hepatica saposin-like protein family. J. Parasitol. 89:545-552. [DOI] [PubMed] [Google Scholar]
- 8.Espino, A. M., and G. V. Hillyer. 2004. A novel Fasciola hepatica saposin-like recombinant protein with immunoprophylactic potential. J. Parasitol. 90:876-879. [DOI] [PubMed] [Google Scholar]
- 9.Espino, A. M., J. R. Rodriguez-Medina, and G. V. Hillyer. 2001. Isolation and immunological characterization of fatty acid binding protein isoforms from Fasciola hepatica. J. Parasitol. 87:1028-1033. [DOI] [PubMed] [Google Scholar]
- 10.Fleckenstein, B., G. Jung, and K. H. Wiesmuller. 1997. Prediction and design of new MHC class II-ligands based on the activities pattern of a synthetic undecapeptide library vaccine, p 65-70. In F. Brown, D. Burtonm, P. Doherty, I. Mekalanos, and E. Norbay (ed.), Molecular approaches to the control of infectious diseases. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 11.Geourjon, C., and G. Deleage. 1994. SOPM: a self-optimized method for protein secondary structure prediction. Protein Eng. 7:157-164. [DOI] [PubMed] [Google Scholar]
- 12.Hillyer, G. V. 1995. Comparison of purified 12kDa and recombinant 15-kDa antigens related to Schistosoma mansoni fatty acid binding protein. Mem. Inst. Oswaldo Cruz 90:249-253. [DOI] [PubMed] [Google Scholar]
- 13.Hopp, T. P., and K. R. Woods. 1981. Prediction of protein antigenic determinants from amino acids sequences. Proc. Natl. Acad. Sci. USA 86:152-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leippe, M., J. Andra, R. Nickel, E. Tannich, and H. J. Muller-Eberhard. 1994. Amoebapores, a family of membranolytic peptides from cytoplasmic granules of Entamoeba histolytica: isolation, primary structure, and pore formation in bacterial cytoplasmic membranes. Mol. Microbiol. 14:895-904. [DOI] [PubMed] [Google Scholar]
- 15.Lesenechal, M., L. Becquart, X. Lacoux, L. Ladaviere, R. C. Baida, G. Paranhos-Baccala, and J. F. da Silveira. 2005. Mapping of B-cell epitopes in a Trypanosoma cruzi immunodominant antigen expressed in natural infections. Clin. Diagn. Lab. Immunol. 12:329-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López-Aban, J., V. Ramajo, J. L. Perez Arellano, G. V. Hillyer, and A. Muro. 1999. A fatty acid binding protein from Fasciola hepatica induced protection in C57/BL mice from challenge infection with Schistosoma bovis. Vet. Parasitol. 83:107-121. [DOI] [PubMed] [Google Scholar]
- 17.Mitaku, S., T. Hirokawa, and T. Tsuji. 2002. Amphiphilicity index of polar amino acids as an aid in the characterization of amino acid preference at membrane-water interfaces. Bioinformatics 18:608-616. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien, J. S., and Y. Kishimoto. 1991. Saposin proteins: structure, function, and role in human lysosomal storage disorders. FASEB J. 5:301-308. [DOI] [PubMed] [Google Scholar]
- 19.Overend, D. J., and F. L. Bowen. 1995. Resistance of Fasciola hepatica to triclabendazole. Austr. Vet. J. 72:275-276. [DOI] [PubMed] [Google Scholar]
- 20.Reed, M. B., R. A. Strugnell, M. Panaccio, and T. W. Spithill. 2000. A novel member of the NK-lysin protein family is developmentally regulated and secreted by Fasciola hepatica. Mol. Biochem. Parasitol. 105:297-303. [DOI] [PubMed] [Google Scholar]
- 21.Rost, B., and C. Sander. 1994. PHDacc predicts per residue solvent accessibility from multiple sequence alignments. Proteins 20:216-226. [DOI] [PubMed] [Google Scholar]
- 22.Sachse, K., J. H. Helbig, I. Lysnyansky, C. Grajetzki, W. Muller, E. Jacobs, and D. Yogev. 2000. Epitope mapping of immunogenic and adhesive structures in repetitive domains of Mycoplasma bovis variable surface lipoproteins. Infect. Immun. 68:680-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sano, A., T. Mizuno, K. Kondoh, T. Hineno, S. Ueno, Y. Kakimoto, and N. Morita. 1992. Saposin-C from bovine spleen: complete amino acid sequence and relation between the structure and its biological activity. Biochim. Biophys. Acta 1120:75-80. [DOI] [PubMed] [Google Scholar]
- 24.Sexton, J. L., M. C. Wilce, T. Colin, G. L. Wijffels, L. Salvatore, S. Feil, M. W. Parker, T. W. Spithill, and C. A. Morrison. 1994. Vaccination of sheep against Fasciola hepatica with glutathione S-transferase. Identification and mapping of antibody epitopes on a three-dimensional model of the antigen. J. Immunol. 152:1861-1872. [PubMed] [Google Scholar]
- 25.Stenger, S., D. A. Hanson, R. Teitelbaum, P. Dewan, K. R. Niazi, C. J. Froelich, T. Ganz, S. Thoma-Uszynski, A. Melian, C. Bogdan, S. A. Porcelli, B. R. Bloom, A. M. Krensky, and R. L. Modlin. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282:121-125. [DOI] [PubMed] [Google Scholar]
- 26.Suhardono, S. Widjajanti, P. Stevenson, and I. H. Carmichael. 1991. Control of Fasciola gigantica with triclabendazole in Indonesian cattle. Trop. Anim. Health Prod. 23:217-220. [DOI] [PubMed] [Google Scholar]
- 27.Vanniasinkam, T., M. D. Barton, and M. W. Heuzenroeder. 2001. B-Cell epitope mapping of the VapA protein of Rhodococcus equi: implications for early detection of R. equi disease in foals. J. Clin. Microbiol. 39:1633-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vázquez-Talavera, J., C. F. Solis, E. Medina-Escutia, Z. M. Lopez, J. Proano, D. Correa, and J. P. Laclette. 2001. Human T and B cell epitope mapping of Taenia solium paramyosin. Parasite Immunol. 23:575-579. [DOI] [PubMed] [Google Scholar]
- 29.Viudes, A., S. Perea, and J. L. Lopez-Ribot. 2001. Identification of continuous B-cell epitopes on the protein moiety of the 58-kilodalton cell wall mannoprotein of Candida albicans belonging to a family of immunodominant fungal antigens. Infect. Immun. 69:2909-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waine, G. J., J. C. Scott, D. Mazzer, and D. P. McManus. 1998. Mapping of linear B-cell epitopes on the 14-kDa fatty-acid binding protein of Chinese Schistosoma japonicum. Int. J. Parasitol. 28:303-308. [DOI] [PubMed] [Google Scholar]