Abstract
The Period 2 (Per2) gene is a key molecular component in controlling mammalian circadian rhythms at the levels of gene expression, physiology, and pathogenesis. Although many immune parameters, such as the number of different subtypes of circulating immune cells and the level of cytokine production in response to infection with bacteria and viruses, have been well documented to display a circadian pattern in mammals, the basic features of molecular clock components in the immune system and the role of clock genes in regulating host immune defenses remain uncharacterized. Previously, we have reported that circadian clock genes oscillate in human mononuclear cells. Here we report that Per2-deficient mice were more resistant to lipopolysaccharide (LPS)-induced endotoxic shock than control wild-type mice. We further demonstrate that the levels of the proinflammatory cytokines gamma interferon (IFN-γ) and interleukin-1β (IL-1β) in the serum were dramatically decreased in Per2−/− mice following LPS challenge, while production of tumor necrosis factor alpha, IL-6, and IL-10 was approximately normal, compared to that in control wild-type mice. Flow cytometric analyses confirmed that the cellularity of most of the immune cell subsets in the spleens of LPS-challenged mice was normal and that the impaired IFN-γ production in Per2−/− mice was attributable to defective NK and NKT cell function. Our data suggest that Per2 is an important regulator of NK cell function, therefore providing the first direct link between the circadian clock system and innate immune responses.
Circadian rhythms are daily oscillations of multiple biological processes driven by endogenous clocks. The master circadian clock in mammals resides in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus. The endogenous clock is further distinguished by its ability to be entrained to a new day/night regimen by environmental cues such as light and temperature cycles (13, 27, 29). It was originally thought that the mammalian circadian clock system was hierarchically organized; however, while the SCN is a master circadian clock tissue, generating self-sustained circadian oscillators which determine the pace and amplitude of the expression of the circadian clock genes in peripheral tissues through neuronal and hormonal signals, these peripheral clocks in turn control the output of circadian physiology and behavior. However, the details of how the SCN′s signaling pathway controls the peripheral clock still remain to be elucidated.
Circadian rhythms are known to influence the immune response of mammals through their effects on the circulation of the blood as related to diurnal sleeping/waking and activity cycles. Born and coworkers (6) demonstrated that in humans, blood cell compartmentalization, such as with peripheral cell counts of neutrophils, T-lymphocyte subsets, B lymphocytes, monocytes, and natural killer (NK) cells, displays a circadian fluctuation across the day. The peak of each subtype of cells in peripheral blood varies with time. The numbers of monocytes, B cells, and T cells reach maximum value during the sleep phase, whereas neutrophils, NK cells, and activated T cells peak during the waking phase. Generally, these phenomena have been attributed to neuroendocrine circuits involving hormonal mediators, such as cortisol, melatonin, and insulin-like growth factor. A similar oscillation has also been observed in rodents (14, 26). Thus, the circadian immunological parameters which affect activity in both humans and rodents are well conserved under baseline physiological conditions, indicating parallel clock control mechanisms for the human and mouse immune systems. Due to the absence of an appropriate model with which to examine circadian immunoregulation directly, the molecular mechanism(s) of action has not been determined.
The molecular apparatus governing circadian rhythms has been elucidated to comprise a transcription-translation feedback loop involving more than 12 genes, including Period2 (Per2) (1, 21, 28). The generation of the mutant mouse strain rendered deficient in Per2 (Per2−/−) (35) provides an ideal experimental model to elucidate the specific characteristics of molecular clock components in the immune system and to directly analyze the role of Per2 in immune regulatory function. Employing this animal model, we carried out a series of experiments designed to analyze the role of Per2 in immune functioning at the molecular and cellular levels. Our studies demonstrated that Per2 plays an important role in immune host response by modulating gamma interferon (IFN-γ) production in NK cells and interleukin-1β (IL-1β) production from macrophages and another unidentified cell type(s), therefore providing the first evidence for interaction between the clock and the immune system.
MATERIALS AND METHODS
Mice.
Per2−/− mice and age- and sex-matched wild-type control mice were housed to adapt to a cycle of 12 h of light and 12 h of dark for at least 2 weeks prior to the start of experimentation. The room was maintained at 23 ± 2°C and a constant humidity and was equipped with a white-noise generator (91-dB speaker power level; Lafayette Instruments, Lafayette, IN) to mask environmental sounds. All mice were housed in cages with filter tops in a laminar-flow hood and fed food and acid water ad libitum at Weill Medical College of Cornell University Animal Facilities, in accordance with the accepted principles of animal care (25a).
Endotoxic shock.
Mice were injected intraperitoneally (i.p.) with Escherichia coli lipopolysaccharide (LPS; 25 mg/kg of body weight; Sigma-Aldrich, St. Louis, Missouri). Mice in the survival study were monitored with the help of a mouse survival score (20) taken every 2 h up to 96 h after LPS injection. Score 1 was given to mice with percolated fur but no detectable behavior differences from untreated control mice, score 2 was assigned to mice with percolated fur and a huddle reflex but which responded to stimuli (such as a tap on their cage) and were just as active on handling as untreated control mice, score 3 was given to mice that exhibited a slower response to a tap on the cage and that were passive or docile when handled but still curious when alone in a new setting, score 4 was assigned to mice that exhibited lack of curiosity and little or no response to stimuli and that were quite immobile, score 5 was given to mice that had labored breathing and were unable or slow to right themselves after being rolled onto their backs (moribund), and score 6 was assigned to mice that died.
ELISA.
Sera were derived from the peripheral blood of wild-type and knockout mice at indicated times (see Fig. 2) after LPS (25 mg/kg) i.p. injection and stored at −20°C. Mouse cytokines, such as IFN-γ, IL-1β, IL-6, tumor necrosis factor alpha (TNF-α), IL-12 p70, and IL-10, were detected using 100 μl of derived serum from each mouse with the OPT-EIA enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences) according to the manufacturer's instructions. Concentrations were calculated by regression analysis of a standard curve.
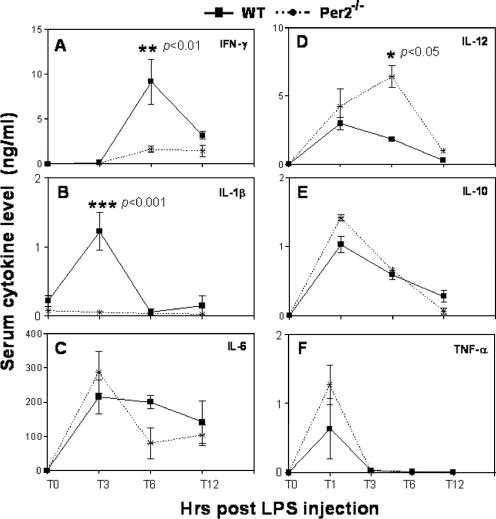
FIG. 2.
Serum cytokine levels after LPS injection in vivo. Wild-type (WT) and Per2−/− mice were injected i.p. with 25 mg/kg LPS. Five mice were sacrificed at each time point (1, 3, 6, and 12 h) after LPS challenge, and serum cytokine levels were measured from 100 μl serum by ELISA. ANOVA was used for multigroup comparisons, followed by Tukey's multiple-comparison test if ANOVA showed a significant difference. T, time (in hours).
Cells.
To obtain splenocytes for fluorescence-assisted cell sorting (FACS) analysis, the spleens were ground and filtered to make a single-cell suspension, and then the red blood cells were lysed with ammonium chloride for 5 min. The single-cell suspension of splenocytes was used for FACS analysis. To obtain adherent splenocytes, the spleens were ground and filtered to make a single-cell suspension, and then the red blood cells were lysed. The splenocytes were plated in 100-mm tissue culture dishes for 2 h to deplete the nonadherent cells. The adherent cells were treated with LPS (1 μg/ml) for different times.
Monoclonal antibodies and reagents.
We used the rat anti-mouse monoclonal antibodies IFN-γ-phycoerythrin (PE) (XMG1.2), CD49b/Pan-NK-fluorescein isothiocyanate (FITC) (DX5), CD45R/B220-FITC, F4/80-Alexa Fluor 488, hamster anti-mouse CD11C-PE (HL3), hamster anti-mouse CD3e-PE-Cy5 (145-2C11), and purified mouse anti-mouse-CD16/CD32. Immunoglobulin isotype controls used were rat immunoglobulin G1 (IgG1) (R3-34)-PE, rat IgG2a-Alexa Fluor 488, and rat IgM-FITC (R4-22). FACS lysing solution and FACS permeabilizing solution 2 were obtained from BD Pharmingen (San Diego, CA).
Cell surface antigen and intracellular cytokine staining.
We used 1 × 106 splenocytes for each staining. For cell surface staining, cells were first blocked with 1 μg of anti-mouse CD16/32 and then stained on ice with optimal amounts of conjugated antibodies (0.1 μg to 0.5 μg/106 cells) diluted in staining medium (phosphate-buffered saline, 3% bovine serum albumin, 0.05% azide) for 30 min in the dark. The cells were washed twice with 2 ml of staining medium and spun at 400 × g at 4°C for 5 min. They were fixed with 1 ml of 1× FACS lysing solution for 10 min at room temperature and washed once. The cells were permeabilized using 500 μl of 1× permeabilizing solution 2 for 10 min at room temperature. The cells were then incubated with PE-conjugated rat anti-mouse IFN-γ or rat IgG1 isotype control for 30 min on ice in the dark. Cells were washed twice for 10 min at 4°C and were resuspended in 0.5 ml phosphate-buffered saline-3% bovine serum albumin-0.05% azide. Flow cytometric analysis was performed within 16 h. Stained splenocyte samples were analyzed with a FACSCalibur (Becton Dickinson, Palo Alto, CA), and 50,000 events were acquired.
Statistical analysis.
The standard deviation of the mean is shown unless otherwise indicated. Differences in survival between wild-type and Per2−/− mice after LPS challenge were determined by Kaplan-Meier analysis. Analysis of variance (ANOVA) was used for multigroup comparisons, followed by Tukey's multiple-comparison test if ANOVA showed a significant difference.
RESULTS
Deficiency in Per2 renders mice resistant to endotoxic shock.
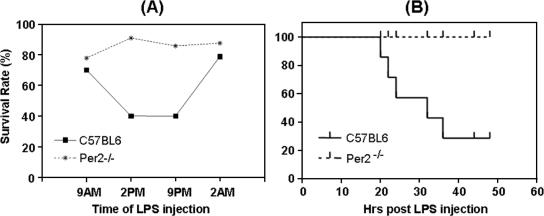
To determine if the clock gene Per2 plays a role in host response to bacterial challenge, a septic-shock mouse model was used. We conducted a study to examine the kinetics of the acute-phase inflammatory response in Per2−/− mice and normal control mice challenged with LPS at different times throughout the day. Animals were evaluated, by assigning a mouse survival score, for septic shock in the ensuing 96 h. Symptoms of sepsis, characterized by shock and difficulty in breathing, in all mice were observed at 4 h after the injection. The wild-type mice had a survival rate of 70% or 80% when given LPS at 9 a.m. or 2 a.m., respectively, in contrast to a 40% survival rate at 2 p.m. and 9 p.m. (Fig. 1A), clearly showing a well-defined circadian rhythm. The observed sickness score for Per2−/− mice was less severe overall than that for the control mice. These mice were globally more resistant to LPS-induced death, with survival rates of 80 to 95% at all times, showing a lack of circadian rhythm (Fig. 1A). In a separate, kinetic experiment to determine the time course of pathological development and shock-induced death, wild-type and Per2−/− mice were injected with LPS at 2 p.m., which is the time wild-type mice exhibited the greatest susceptibility. Wild-type mice started to display the lethal response around 20 h after the LPS injection, and by 50 h, all wild-type mice had died of shock, while all Per2−/− mice survived (Fig. 1B).
FIG. 1.
Survival of wild-type and Per2−/− mice injected with LPS. (A) Ten mice per group of each strain were kept under a cycle of 12 h of dark and 12 h of light for 2 weeks and were challenged with 25 mg/kg of LPS at 9 a.m., 2 p.m., 9 p.m., or 2 a.m. The mice were observed every 2 h continuously for up to 96 h, at which point the rate of survival was calculated. All surviving mice eventually recovered from shock induced by LPS injection. (B) Kinetics of LPS-induced shock/death in wild-type mice and Per2−/− mice following LPS injection at 2 p.m. Survival rates were calculated based on results for 10 mice per strain 48 h after LPS injection.
These data demonstrate that there is a circadian clock influence on host resistance to LPS-induced inflammatory death, called LPS-induced susceptibility rhythm, and that this rhythm is disrupted in Per2−/− mice but not in Per1−/− mice (data not shown), rendering the former much less susceptible to endotoxin-induced death.
Dramatically reduced levels of the proinflammatory cytokines IFN-γ and IL-1β in LPS-treated Per2−/− mice.
Given the overall resistance of Per2−/− mice to LPS-induced death, unlike wild-type mice, we were interested in understanding the differences in the effector mechanisms underlying these differential responses. We postulated that there might be specific differences in the levels of production of proinflammatory cytokines that are well-defined mediators of LPS responses. We examined a number of proinflammatory cytokines that are well-known mediators of LPS-induced septic shock: TNF-α, IFN-γ, IL-1β, IL-6, IL-10, and IL-12 in LPS-treated wild-type and Per2−/− mice. At the indicated time points (Fig. 2), following LPS challenge, mice were sacrificed, peripheral blood was collected, and the plasma levels of the cytokines of interest were determined by ELISA. The levels of IFN-γ (Fig. 2A) and IL-1β (Fig. 2B) were dramatically lower at 6 h and 3 h, respectively, in Per2−/− mice than in wild-type mice, whereas IL-6 (Fig. 2C), IL-10 (Fig. 2E), and TNF-α (Fig. 2F) levels were not significantly different between the two strains. The IL-12 level was higher at 6 h in Per2−/− mice than in wild-type mice (Fig. 2D).
Analysis of immune subsets and their IFN-γ-production in LPS-challenged mice.
The striking differences in IFN-γ and IL-1β production between wild-type and Per2−/− mice prompted us to investigate further the underlying mechanisms. IFN-γ production in an innate immune response is primarily rendered by NK cells following stimulation by microbial infection. The dramatically lower IFN-γ production in Per2−/− mice could be due either to lower numbers of NK cells or to functional defects of NK cells. To discern between these possibilities, we analyzed the profiles of immune subsets in the spleens of wild-type and Per2−/− mice following LPS challenge. As shown in Table 1, most of the cellular subsets examined showed no major differences between wild-type and Per2−/− mice after LPS challenge, except for some minor differences in T and B lymphocytes and dendritic cells. There was no notable difference in the numbers of NK and NKT cells in non-LPS-challenged and LPS-challenged mice, suggesting that NK and NKT cell numbers are not the basis of the low levels of IFN-γ in LPS-challenged Per2−/− mice.
TABLE 1.
FACS analysis of the cellular compositions of Per2−/− mouse and control mouse spleensa
| Cell type | Mean cell composition ± SD (%) in:
|
|||
|---|---|---|---|---|
| Wild-type mice
|
Per2−/− mice
|
|||
| Unchallenged | LPS challenged | Unchallenged | LPS challenged | |
| T lymphocytes | 34.6 ± 0.6 | 25.7 ± 2.5 | 29.1 ± 2.5* | 27.3 ± 0.7 |
| B lymphocytes | 53.6 ± 0.4 | 62.5 ± 1.0 | 53.1 ± 1.0 | 53.4 ± 1.3** |
| NK cells | 2.8 ± 0.6 | 1.7 ± 0.3 | 2.7 ± 0.3 | 1.8 ± 0.2 |
| NKT cells | 1.0 ± 0.2 | 1.0 ± 0.1 | 1.2 ± 0.2 | 1.3 ± 0.1 |
| Macrophages | 4.4 ± 1.2 | 6.5 ± 1.4 | 6.2 ± 1.3 | 6.7 ± 1.4 |
| Dendritic cells | 1.5 ± 0.1 | 2.8 ± 0.4 | 2.0 ± 0.3 | 2.7 ± 0.2 |
Mice were challenged with LPS (25 mg/kg of body weight, intraperitoneally), and their spleens were harvested 6 h after the LPS injection. Splenocytes were subjected to FACS analysis as described in Materials and Methods. Three to eight mice of each strain were examined. ANOVA was used for multigroup comparison, followed by Tukey's multiple-comparison test if ANOVA showed a significant difference. Cell subsets that showed significant differences between wild-type and Per2−/− mice are in boldface. *, P < 0.05; **, P < 0.01.
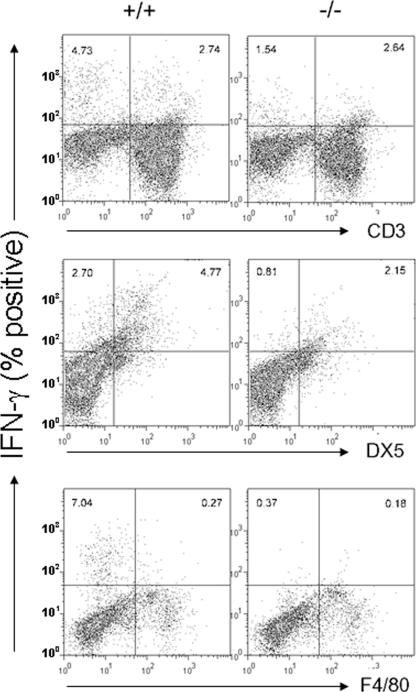
Next, we analyzed the capacity of each of the immune cell subsets to produce IFN-γ following in vivo LPS challenge by using intracellular staining and flow cytometry (Fig. 3). In wild-type mice, the largest IFN-γ+ fractions were produced from CD3−, non-F4/80−, and DX5+ (NK and NKT) cells. In Per2−/− mouse spleens, it was very clear that DX5+ cells were highly defective in IFN-γ production at the time of LPS injection. The numbers of IFN-γ+ CD3+ cells were very similar between Per2−/− mice and control mice, while the number of IFN-γ+ CD3− cells was much lower in Per2−/− mice than in control mice, again indicating that most IFN-γ+ cells were non-CD3+ cells. Similarly, most F4/80+ cells produced little IFN-γ, while most IFN-γ+ cells were nonmacrophages.
FIG. 3.
Characterization of IFN-γ-producing cells in the spleen after LPS challenge. Mice were challenged with LPS (25 mg/kg of body weight i.p.), and their spleens were harvested 6 h after LPS injection. The amounts of IFN-γ produced by DX5+ cells (NK and NKT cells), CD3+ cells (T cells) and F4/80+ cells (macrophages) were determined by flow cytometry. The plots shown are representative of eight mice from each strain. The data represent results from one of three experiments with similar results.
Taken together, these data demonstrate that Per2−/− NK and NKT cells, although apparently normal in cellularity, are highly defective in IFN-γ production.
DISCUSSION
Because the role of Per2 in the circadian clock regulatory loop is well understood at the central nervous system level (25, 33), the Per2−/− mouse model provides an excellent system for investigating the putative role of the clock gene in immune function occurring in peripheral tissues responding to conditional stress. The purpose of our study was to characterize the immune circadian clock and its role in innate immunity and host defense and to understand the mechanisms that underlie the interaction between the clock system and the immune apparatus.
Circadian rhythms are known to influence the immune response through their effects on the circulation of the blood as related to diurnal sleeping/waking and activity cycles (6). Generally these phenomena have been attributed to neuroendocrine circuits involving hormonal mediators, such as cortisol, melatonin, and insulin-like growth factor (8, 19, 22). Due to the absence of an appropriate model with which to examine circadian immunoregulation directly, the molecular mechanism(s) of action has not been determined. We have now developed an experimental model to examine the specific characteristics of the molecular clock function(s) in the immune system in mice genetically rendered deficient in the Per2 gene. Previous and current studies from others and us indicate that (i) the circadian clock machinery operates specifically in immune tissues, such as the spleen and peripheral blood mononuclear cells, in both humans and mice; (ii) the oscillation of circadian clock genes is disrupted in Per2−/− mice; (iii) as revealed by Halberg et al. (17), normal mice display a circadian rhythm in their response to LPS-induced shock and death and this rhythm is lost in Per2−/− mice, which are also generally resistant to endotoxic shock; and (iv) reduced production of IFN-γ by NK and NKT cells is likely to be a critical part of the underlying mechanism in the resistance to LPS-induced death in Per2−/− mice, which appear to develop normal immune cell subset numbers.
There were two reasons why we used only the Per2−/− homozygous mutant mice in our experiment: (i) heterozygous Per2 mutant mice did not show any phenotypic defect in circadian physiology, indicating that there is no gene-dosage effect, and (ii) the focus of our study was on the causative relationship between circadian defect and innate immune response. In fact, we also examined Per1−/− mice (Per1 is the prototypical member of the Per family, which is composed of three members) (34). The result was that Per1−/− mice did not exhibit any defects in response to LPS challenge (data not shown). These results demonstrate that Per2 plays a unique role in the regulation of innate immune function.
LPS is the principal activating signal in the pathogenesis of endotoxic shock. Endotoxic shock is a severe systemic inflammatory response triggered by the interaction of LPS with host cells, in particular with monocytes and macrophages (3). This interaction leads to the acute-phase response and a progressive release of cytokines and other inflammatory mediators, including IL-1, IL-6, TNF-α, IL-12, IFN-γ, and nitric oxide (NO) (9, 31). Plasma endotoxin levels are well correlated with clinical septic shock, acute respiratory distress syndrome, renal failure, multiple organ system failure, and death (18, 24, 30). The circadian feature of endotoxin in response to the LPS challenge indicates that the clock system may play an important role in immune function and host immune defense, though the mechanisms of how the clock system interacts with the immune system remain to be further characterized.
It is well documented that NK cells are critical components of the innate immune system, and they have been implicated in the pathogenesis of septic shock (4, 11, 32). NK cell-deficient mutant mice are more resistant to Streptococcus pyogenes-induced septic shock (16). In addition, depletion of NK cells leads to increased survival times and a slower development of disease that correlates with lower levels of circulating IFN-γ, as well as IL-12 and IL-6, suggesting that NK cells contribute to the amplification of the acute-phase response. In our study, IFN-γ and IL-1β exhibited expression patterns closely correlated with the relative susceptibility of wild-type mice to the lethal effects of systemic LPS; i.e., its serum expression was higher in animals given LPS at 2 p.m. than at 9 a.m. (data not shown), showing a circadian rhythm-dependent response that correlated positively with a higher mortality rate (Fig. 1A). Moreover, the production of IFN-γ in splenic NK/NKT cells was higher in wild-type mice than in Per2−/− mice given LPS at 9 a.m. or 2 p.m. (data not shown), again associated with higher lethality in wild-type mice. Thus, expression of IFN-γ in NK cells in response to LPS challenge in vivo is under the control of Per2 and is circadian rhythm dependent. The finding in this study is supported by the recent study of Arjona and Sarkar (2), who have reported the existence of circadian clock gene expression in the NK cells of the rat.
The basis for reduced IL-1β expression in response to LPS is clearly of relevance given the role of this cytokine in acute inflammatory responses. IL-1β is produced mainly by macrophages and the vascular endothelium in response to systemic endotoxin challenge. The FACS analysis indicated that the numbers of macrophages in the spleens of wild-type mice and Per2−/− mice before and after LPS challenge did not significantly differ (Table 1). To determine if the LPS response of splenic macrophages was affected by the Per2 deficiency, we treated the adherent splenocytes with LPS and collected the supernatant of the cells at several time points for IL-1β measurement by ELISA. The data showed that LPS-induced IL-1β production was decreased in Per2−/− mice by ∼30% compared to that in the wild-type control (data not shown), which cannot entirely account for the ∼95% decrease in serum IL-1β level in LPS-challenged Per2−/− mice (Fig. 2B). Thus, we reason that the Per2-deficient vascular endothelium, rather than monocytes/macrophages, may be the major cell source of IL-1β impairment in response to systemic endotoxin (5, 7, 12).
Recently it has been reported that Per2−/− mice are also prone to γ radiation-induced cancer, with a marked increase in tumor development and reduced apoptosis in thymocytes. This has been attributed to the role of Per2 in the regulation of the cell cycle, apoptosis, and DNA damage repair. Since both NK cells and IFN-γ have been shown to play critical roles in effector function against tumor metastasis (10), we surmise that impaired NK/NKT cell function and decreased IFN-γ production in Per2−/− mice may contribute to their greater susceptibility to tumorigenesis (15).
Understanding the nature of the circadian clock in the immune system and its role in immune regulation is critical for the advancement of our knowledge of immune function which can be used to benefit therapeutic efforts. Because cytokines that are produced by lymphocytes and macrophages are potent mediators of immune responses and the levels of individual cytokines can determine immune effector mechanisms, understanding immune-circadian clock control of specific immune mechanisms may have important clinical applications, such as optimization of the treatment of patients with septic shock, autoimmune diseases, and hematological diseases. As the toxicity and the antitumor efficiency of many traditional cytotoxic drugs in cancer therapy vary significantly with the time of day of drug administration (23), analysis of the circadian organization of physiological immune function not only might provide a better insight into the immune system in general, but also might offer a reduction in bone marrow toxicity and an increased intensity and improved efficiency of cytotoxic drugs for cancer patients.
Editor: A. D. O'Brien
REFERENCES
- 1.Albrecht, U. 2004. The mammalian circadian clock: a network of gene expression. Front. Biosci. 9:48-55. [DOI] [PubMed] [Google Scholar]
- 2.Arjona, A., and D. K. Sarkar. 2005. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J. Immunol. 174:7618-7624. [DOI] [PubMed] [Google Scholar]
- 3.Ayala, A., and I. H. Chaudry. 1996. Immune dysfunction in murine polymicrobial sepsis: mediators, macrophages, lymphocytes and apoptosis. Shock 6(Suppl. 1):S27-S38. [PubMed] [Google Scholar]
- 4.Badgwell, B., R. Parihar, C. Magro, J. Dierksheide, T. Russo, and W. E. Carson III. 2002. Natural killer cells contribute to the lethality of a murine model of Escherichia coli infection. Surgery 132:205-212. [DOI] [PubMed] [Google Scholar]
- 5.Borish, L., M. S. King, J. J. Mascali, S. Johnson, B. Coll, and L. J. Rosenwasser. 1992. Transthyretin is an inhibitor of monocyte and endothelial cell interleukin-1 production. Inflammation 16:471-484. [DOI] [PubMed] [Google Scholar]
- 6.Born, J., T. Lange, K. Hansen, M. Molle, and H. L. Fehm. 1997. Effects of sleep and circadian rhythm on human circulating immune cells. J. Immunol. 158:4454-4464. [PubMed] [Google Scholar]
- 7.Bourdoulous, S., A. Bensaid, D. Martinez, C. Sheikboudou, I. Trap, A. D. Strosberg, and P. O. Couraud. 1995. Infection of bovine brain microvessel endothelial cells with Cowdria ruminantium elicits IL-1 beta, -6, and -8 mRNA production and expression of an unusual MHC class II DQ alpha transcript. J. Immunol. 154:4032-4038. [PubMed] [Google Scholar]
- 8.Chen, C. C., and C. R. Parker, Jr. 2004. Adrenal androgens and the immune system. Semin. Reprod. Med. 22:369-377. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello, C. A. 1992. The role of interleukin-1 in host responses to infectious diseases. Infect. Agents Dis. 1:227-236. [PubMed] [Google Scholar]
- 10.Dunn, G. P., L. J. Old, and R. D. Schreiber. 2004. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21:137-148. [DOI] [PubMed] [Google Scholar]
- 11.Emoto, M., M. Miyamoto, I. Yoshizawa, Y. Emoto, U. E. Schaible, E. Kita, and S. H. Kaufmann. 2002. Critical role of NK cells rather than V alpha 14(+)NKT cells in lipopolysaccharide-induced lethal shock in mice. J. Immunol. 169:1426-1432. [DOI] [PubMed] [Google Scholar]
- 12.Fabry, Z., K. M. Fitzsimmons, J. A. Herlein, T. O. Moninger, M. B. Dobbs, and M. N. Hart. 1993. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J. Neuroimmunol. 47:23-34. [DOI] [PubMed] [Google Scholar]
- 13.Florez, J. C., and J. S. Takahashi. 1995. The circadian clock: from molecules to behaviour. Ann. Med. 27:481-490. [DOI] [PubMed] [Google Scholar]
- 14.Floyd, R. A., and J. M. Krueger. 1997. Diurnal variation of TNF alpha in the rat brain. Neuroreport 8:915-918. [DOI] [PubMed] [Google Scholar]
- 15.Fu, L., H. Pelicano, J. Liu, P. Huang, and C. Lee. 2002. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111:41-50. [DOI] [PubMed] [Google Scholar]
- 16.Goldmann, O., G. S. Chhatwal, and E. Medina. 2005. Contribution of natural killer cells to the pathogenesis of septic shock induced by Streptococcus pyogenes in mice. J. Infect. Dis. 191:1280-1286. [DOI] [PubMed] [Google Scholar]
- 17.Halberg, F., E. A. Johnson, B. W. Brown, and J. J. Bittner. 1960. Susceptibility rhythm to E. coli endotoxin and bioassay. Proc. Soc. Exp. Biol. Med. 103:142-144. [DOI] [PubMed] [Google Scholar]
- 18.Hesse, D. G., K. J. Tracey, Y. Fong, K. R. Manogue, M. A. Palladino, Jr., A. Cerami, G. T. Shires, and S. F. Lowry. 1988. Cytokine appearance in human endotoxemia and primate bacteremia. Surg. Gynecol. Obstet. 166:147-153. [PubMed] [Google Scholar]
- 19.Jackson, B. F., A. Blumsohn, A. E. Goodship, A. M. Wilson, and J. S. Price. 2003. Circadian variation in biochemical markers of bone cell activity and insulin-like growth factor-I in two-year-old horses. J. Anim. Sci. 81:2804-2810. [DOI] [PubMed] [Google Scholar]
- 20.Khan, N. A., A. Khan, H. F. Savelkoul, and R. Benner. 2002. Inhibition of septic shock in mice by an oligopeptide from the beta-chain of human chorionic gonadotrophin hormone. Hum. Immunol. 63:1-7. [DOI] [PubMed] [Google Scholar]
- 21.King, D. P., and J. S. Takahashi. 2000. Molecular genetics of circadian rhythms in mammals. Annu. Rev. Neurosci. 23:713-742. [DOI] [PubMed] [Google Scholar]
- 22.Kwiatkowski, F., C. Abrial, F. Gachon, R. Chevrier, H. Cure, and P. Chollet. 2005. Stress, cancer and circadian rhythm of melatonin. Pathol. Biol. 53:269-272. (In French.) [DOI] [PubMed] [Google Scholar]
- 23.Lis, C. G., J. F. Grutsch, P. Wood, M. You, I. Rich, and W. J. Hrushesky. 2003. Circadian timing in cancer treatment: the biological foundation for an integrative approach. Integr. Cancer Ther. 2:105-111. [DOI] [PubMed] [Google Scholar]
- 24.Marano, M. A., Y. Fong, L. L. Moldawer, H. Wei, S. E. Calvano, K. J. Tracey, P. S. Barie, K. Manogue, A. Cerami, G. T. Shires, et al. 1990. Serum cachectin/tumor necrosis factor in critically ill patients with burns correlates with infection and mortality. Surg. Gynecol. Obstet. 170:32-38. [PubMed] [Google Scholar]
- 25.Maywood, E. S., J. A. O'Brien, and M. H. Hastings. 2003. Expression of mCLOCK and other circadian clock-relevant proteins in the mouse suprachiasmatic nuclei. J. Neuroendocrinol. 15:329-334. [DOI] [PubMed] [Google Scholar]
- 25a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 26.Panteleeva, N. G., N. I. Gryazeva, L. V. Verbitskaya, A. V. Shurlygina, V. A. Trufakin, V. G. Kolpakov, T. A. Alekhina, and N. N. Barykina. 2004. Diurnal variations in lymphocyte subpopulations in lymphoid organs of rats with genetic catalepsy and Wistar rats. Bull. Exp. Biol. Med. 137:288-290. [DOI] [PubMed] [Google Scholar]
- 27.Pittendrigh, C. S. 1993. Temporal organization: reflections of a Darwinian clock-watcher. Annu. Rev. Physiol. 55:16-54. [DOI] [PubMed] [Google Scholar]
- 28.Reppert, S. M., and D. R. Weaver. 2002. Coordination of circadian timing in mammals. Nature 418:935-941. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi, J. S. 1995. Molecular neurobiology and genetics of circadian rhythms in mammals. Annu. Rev. Neurosci. 18:531-553. [DOI] [PubMed] [Google Scholar]
- 30.Tracey, K. J., Y. Fong, D. G. Hesse, K. R. Manogue, A. T. Lee, G. C. Kuo, S. F. Lowry, and A. Cerami. 1987. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330:662-664. [DOI] [PubMed] [Google Scholar]
- 31.Tracey, K. J., S. F. Lowry, T. J. Fahey III, J. D. Albert, Y. Fong, D. Hesse, B. Beutler, K. R. Manogue, S. Calvano, H. Wei, et al. 1987. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg. Gynecol. Obstet. 164:415-422. [PubMed] [Google Scholar]
- 32.Vinay, D. S., B. K. Choi, J. S. Bae, W. Y. Kim, B. M. Gebhardt, and B. S. Kwon. 2004. CD137-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharide-induced shock syndromes, and have lower IL-4 responses. J. Immunol. 173:4218-4229. [DOI] [PubMed] [Google Scholar]
- 33.Yan, L., and H. Okamura. 2002. Gradients in the circadian expression of Per1 and Per2 genes in the rat suprachiasmatic nucleus. Eur. J. Neurosci. 15:1153-1162. [DOI] [PubMed] [Google Scholar]
- 34.Zheng, B., U. Albrecht, K. Kaasik, M. Sage, W. Lu, S. Vaishnav, Q. Li, Z. S. Sun, G. Eichele, A. Bradley, and C. C. Lee. 2001. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 105:683-694. [DOI] [PubMed] [Google Scholar]
- 35.Zheng, B., D. W. Larkin, U. Albrecht, Z. S. Sun, M. Sage, G. Eichele, C. C. Lee, and A. Bradley. 1999. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 400:169-173. [DOI] [PubMed] [Google Scholar]