Abstract
To study anti-inflammatory cytokine effects on RANKL+-T-cell-mediated osteoclastogenesis in vivo, we injected human interleukin-10 (hIL-10) into pathogen-infected HuPBL-NOD/SCID mice. The results show significantly decreased RANKL+ Th1-associated alveolar bone loss and coexpression of human gamma interferon (hIFN-γ) and human macrophage colony-stimulating factor, but not hIL-4, in RANKL+ Th cells compatible with those from successfully treated aggressive periodontitis subjects. Thus, there are critical cytokine interactions linking hIFN-γ+ Th1 cells to RANKL-RANK/OPG signaling for periodontal osteoclastogenesis in vivo.
The study of osteoimmunology has demonstrated that host immune responses, especially T-cell immunity, play a pivotal role in regulating osteoclastogenesis and bone remodeling (26, 32-35, 36). The recently identified tumor necrosis factor (TNF) family molecule receptor activator of NF-κB ligand (RANKL), its receptor RANK, and its natural antagonist, osteoprotegerin (OPG), have been shown to be the key regulators of bone remodeling involved in differentiation, activation, and survival of osteoclasts and osteoclast precursors (35). Activated CD4+ T cells express RANKL, which can directly trigger osteoclastogenesis and bone loss in periodontitis (29). Thus, blocking RANKL activity via OPG treatment results in significantly reduced bone loss in arthritis, periodontitis, osteoporosis, and cancer-related bone metastasis and enhanced alveolar bone loss in type 1 diabetes (4, 13, 14, 19, 29, 35). In particular, OPG injections yield a robust inhibition of alveolar bone loss of up to 80 to 90% in mouse periodontitis models, suggesting that the RANKL-RANK/OPG axis is the key pathway controlling periodontal osteoclastogenesis (19, 29). Although some studies suggest an inhibitory effect of gamma interferon (IFN-γ) on RANKL-associated osteoclastogenesis via proteasomal degradation of TRAF6 (8, 25), it has also been shown that RANKL+ Th cells expressing IFN-γ are associated with enhanced bone loss in periodontal and arthritic lesions (2, 5, 18, 21, 27). In addition, the existence of a TRAF6-independent pathway(s) and non-STAT1-family molecules involved in NF-κB and/or Jun N-terminal protein kinase pathways for osteoclastogenesis has been reported (16, 17, 22).
Interleukin-10 (IL-10), an anti-inflammatory Th2 cytokine, is known to down-regulate the functions of antigen-presenting cells (APC) and Th1 development, and IL-10 deficiency is associated with a higher susceptibility to alveolar bone loss after microbial infection in experimental animals (1, 23). To date, the exact contribution of IL-10 in modulating RANKL expression and bone resorption in human periodontitis in vivo remains unclear. To understand the potential interactions of anti-inflammatory cytokines with RANKL-mediated osteoclastogenesis during periodontal disease progression, we hypothesized that human IL-10 (hIL-10) negatively modulates RANKL+-Th-cell-mediated alveolar bone loss under inflammatory conditions in vivo. This issue was addressed by (i) injecting hIL-10 into Actinobacillus actinomycetemcomitans-infected HuPBL-NOD/SCID mice and then assessing the coexpression of human IFN-γ, IL-4, and macrophage colony-stimulating factor (M-CSF) with RANKL and alveolar bone loss in vivo; and (ii) analyzing human T-cell isolates extracted from periodontal tissues of successfully treated aggressive periodontitis (AgP) subjects. The results showed that higher hIL-10 levels are associated with less coexpression of hIFN-γ (and M-CSF), without significant changes on hIL-4, in RANKL+ Th cells from an A. actinomycetemcomitans-reactive Th-cell pool in vivo.
By using experimental mouse periodontitis models and human samples and performing semiquantitative reverse transcription-PCR (sq-RT-PCR), we showed that (i) hIFN-γ is coexpressed with RANKL in pathogen-reactive CD4+ Th cells in vivo and (ii) increased hIFN-γ coexpression in pathogen-reactive RANKL+ Th cells is associated with elevated alveolar bone loss (19, 30, 32). In this study, to assess the effects of an anti-inflammatory cytokine on RANKL+ Th cells coexpressing hIFN-γ during periodontal pathogenesis, we injected hIL-10 (200 ng/mouse, administered intraperitoneally; 50 to 250 ng tested; BD Pharmingen) (32) or phosphate-buffered saline (sham control) into A. actinomycetemcomitans-infected HuPBL-NOD/SCID mice (five or six mice/human peripheral blood leukocyte [HuPBL] donor/group) twice a week for the first 3 weeks, as described previously (30, 32). Briefly, a total of 82 6-to-8-week-old female NOD/SCID mice were housed under specific-pathogen-free conditions at the University Animal Colony Facilities. Human blood and periodontal surgical discard samples were obtained from AgP subjects (n = 4; age, 21 ± 4 years) (30) and those having successful periodontal treatment without attachment loss (n = 3; age, 20.3 ± 1.1 years) (30, 32), respectively. Informed consent was obtained and approved by the Human Ethics and Animal Experimentation Committees at the University of Western Ontario, Ontario (where the study was initiated), and University of Rochester, Rochester, New York. The levels of individual HuPBL engraftment into NOD/SCID mice were comparable to those reported previously (≥30%) (28, 29). All mice were maintained for 8 weeks before being euthanized, followed by the study of hIFN-γ, hM-CSF, and hIL-4 coexpression in RANKL+ Th cells by sq-RT-PCR, the frequency of cytokine expression by limiting dilution analysis (LDA), and the mean alveolar bone loss via histomorphometry (28, 30, 32). The generation of defleshed jaw samples, A. actinomycetemcomitans-specific RANKL+ Th cells from HuPBL-NOD/SCID mice and human clinical samples, and their cDNAs were described previously (30, 32). The jaws harvested were defleshed and stained with methylene blue to define the area between the cementum-enamel junction and the alveolar bone crest (30, 32). The surface areas measured represent the total amounts of alveolar bone loss in the jaws (μm2), examined with a Leica MZ95 stereomicroscope and a Hamamatsu digital camera (29, 30). The jaw images were captured at a magnification of ×16, with the right and left maxillary first two molars (M1 and M2) perpendicular to the optical light source being scanned and quantified via a digital histomorphometry feature of OpenLab software (v.3.1.10; ImproVision). To ensure reproducibility and accuracy, jaw images were taken by two calibrated coworkers, who were blinded from sample identifications and from each other during examination, where the results showed consistent measurements of the exposed surfaces between the cementum-enaml junction and the alveolar bone crest. The resulting values were expressed as the means of surface areas ± standard errors (μm2) from each mouse in each group.
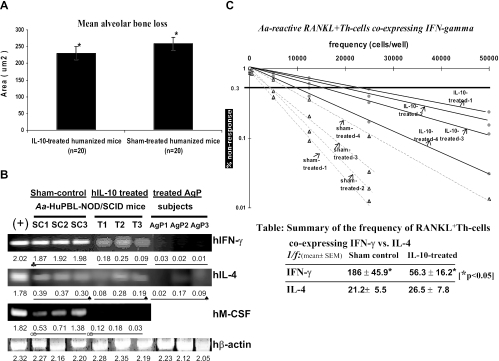
The results showed that hIL-10 injection led to significantly decreased alveolar bone loss in A. actinomycetemcomitans-infected HuPBL-NOD/SCID mice compared to that in the sham control group in vivo (P < 0.002) (Fig. 1A). Furthermore, hIL-10 administration yielded significantly less coexpressed hIFN-γ in A. actinomycetemcomitans-reactive RANKL+ Th cells in vivo, without significant changes in hIL-4 expression (P > 0.05), than that in sham control mice, based on sq-RT-PCR (Fig. 1B). Interestingly, hIFN-γ and hIL-4 expression in RANKL+ Th cells derived from successfully treated periodontal tissues from AgP subjects (n = 3 posttherapy maintenance patients undergoing surgeries for other dental reasons, where periodontal pockets were ≤4 mm without loss of periodontal attachment for ≥9 months) showed a cytokine expression pattern comparable to those from IL-10-treated human-mouse chimeras (Fig. 1B). Several studies reported significantly higher IL-10 expression that was often associated with nondiseased, treated, and healthy periodontal tissues or gingivitis (9, 10, 24). It is clear that in both cases, there was no alveolar bone loss or active disease progression in vivo; however, we cannot be certain whether the cytokine regulatory mechanisms in well-treated periodontal tissues are the same or similar to those in IL-10-treated mice. Nevertheless, the data support our hypothesis that anti-inflammatory hIL-10 can inhibit RANKL-mediated osteoclastogenesis in vivo in association with down-regulation of pathogen-specific RANKL+ Th cells coexpressing IFN-γ.
FIG. 1.
hIL-10 treatment is associated with decreased alveolar bone loss and less coexpression of hIFN-γ and M-CSF in A. actinomycetemcomitans-reactive RANKL+ Th cells in vivo. (A) There was significantly less alveolar bone loss in hIL-10-treated A. actinomycetemcomitans-infected HuPBL-NOD/SCID mice than in sham control mice at week 8 (where each autologous HuPBL sample constituted five mice each for four AgP donors, and thus, a total of 20 mice analyzed/group), as shown by statistical analysis of the difference between the two groups (means ± standard errors) (paired Student t test; P < 0.002 [*]). The data shown here are for one of two independent experiments with comparable results, for a total of 40 HuPBL-NOD/SCID mice (from four AgP donors). (B) sq-RT-PCR indicated more hIFN-γ and hM-CSF expression in A. actinomycetemcomitans-reactive RANKL+ Th cells from hIL-10-treated A. actinomycetemcomitans-infected HuPBL-NOD/SCID mice at week 8 (labeled T1 to -3) than in cells from sham control mice (labeled SC1 to -3) (n = 5 or 6 mice/HuPBL donor/group, with only three random PCR amplicons/group picked and shown here due to space limits). For hIFN-γ, P < 0.05 for SC1-3 being significantly different from the T1-3 and AgP1-3 groups; for hM-CSF, P < 0.05 for a significant difference between the SC1-3 and T1-3 groups. There was no difference in hIL-4 expression detected among the three groups (paired Student t test; P > 0.05). The positive control was concanavalin A-activated HuPBL-purified CD4+ T cells assayed at 72 h (30, 32). Extraction and purification of A. actinomycetemcomitans-reactive RANKL+ hCD4+ Th cells from periodontal tissues, sq-RT-PCR, cell preparation, and amplification of hIFN-γ and hIL-4 cDNAs were done as described previously (30, 32). For panels B and C, RANKL+ hCD4+ Th cells were purified from a total of 42 HuPBL-NOD/SCID mice, which were prepared from four AgP donors (five mice/AgP donor for three subjects and six mice for the fourth AgP donor; experiments were repeated twice). The primers used to amplify hM-CSF cDNA from study samples (labeled T1-3 and SC1-3) included the forward primer 5′-CAGATGGAGACCTCGTGCCAA-3′ and the reverse primer 5′-GCAGGCCTTGTCATGCTCTTC-3′. The PCR amplicon size for hM-CSF was 241 bp. Human β-actin was used as the internal control for PCR standardization and comparison as described previously (30, 32), with all PCR signals being quantified via LabWorks software, as reported previously (30, 32), and indicated below the gel images in panel B. The representative data shown are for one of two independent experiments with comparable results. (C) Decreased coexpression of hIFN-γ in A. actinomycetemcomitans-reactive RANKL+ Th cells after hIL-10 administration in vivo is manifested at the T-cell population level based on IF-LDA. IF-LDA was employed to assess the frequency of RANKL+ Th cells coexpressing hIFN-γ in the sham control versus IL-10-treated A. actinomycetemcomitans-infected HuPBL-NOD/SCID mice in vivo (hIL-4 results are not shown here). At weeks 7 to 8, periodontal and cervical lymph node-derived CD4+ Th cells from both groups of mice (total n = 21/group) were plated with APC and A. actinomycetemcomitans Ags for 72 h under LDA conditions as described previously (28) before being immunostained for cell surface expression of RANKL (FITC) and intracellular expression of IFN-γ (Texas Red) or IL-4 (Cy5.5) via a Leica DM-IRBE microscope with automated scanning capability and OpenLab software for quantitation of the IF signals detected (in pixels). The results showed that there was a significantly higher frequency (≅3.3×) of RANKL+ Th cells coexpressing hIFN-γ in the sham control mice than in the hIL-10-treated mice (P < 0.05; see the table in the figure for 1/f values). The resulting 1/f estimates for hIFN-γ versus hIL-4 coexpressed in FITC+ RANKL+ Th cells in both mouse groups are shown in the summary table, where asterisks depict statistical significance with P values of <0.05. The data shown are from three independent LDA experiments.
To determine whether the observed patterns of hIFN-γ and hIL-4 expression in RANKL+ Th cells occur at the single-cell or population level, we employed an immunofluorescence (IF)-based limiting dilution analysis (IF-LDA) (19, 28). Periodontal or cervical lymph node CD4+ Th cells from A. actinomycetemcomitans-infected HuPBL-NOD/SCID mice (five or six mice/HuPBL donor/group [see the figure legends]) were purified by positive panning (>95 to 97%) and then seeded into 96-well plates, using replicates of each dilution at 50, 500, 2,500, 12,500, and 50,000 cells/plate with irradiated autologous HuPBL-derived monocytes/macrophages (as APC feeder cells) and A. actinomycetemcomitans sonicate antigens (Ags) (JP2 strain; 10 μg/ml) (19, 28, 30, 32). Controls for LDA included (i) A. actinomycetemcomitans Ags plus feeder cells without T cells and (ii) A. actinomycetemcomitans Ags plus T cells without feeder cells and third-party control Porphyromonas gingivalis sonicate Ags. After 72 h, cells in the 96-well plates were first stained for cell membrane-bound RANKL expression by OPG-hFc (plus anti-Fc-fluorescein isothiocyanate [FITC]); later, cells were stained intracellularly with hIFN-γ (plus Texas Red) and hIL-4 (plus Cy5.5), using previously described standard protocols of fixation, permeabilization, and primary and secondary monoclonal antibody labeling (19). To quantify IF signals, CD4+-Th-cell-containing plates were mounted on a motorized stage attached to a Leica DM-IRBE fluorescence microscope (19). The signals were automatically captured via a digital camera to generate a series of scanned fields for each well under a magnification of 1.6 × 10. All of the optical parameters (e.g., exposure time, light source, shutter, focus, etc.) were kept constant. Quantification of the mean fluorescence intensity (FI; in pixels) was performed by enumerating the signal intensities detected on FITC (or Texas Red or Cy5.5)-expressing CD4+ Th cells in each well via the density slice features of OpenLab software. The sum FI was calculated by adding the total IF signals per well/plate and then subtracting the background intensity (≈530 ± 125 pixels). The results are shown as average FI values ± standard errors of the means, determined by enumerating counted CD4+ Th cells after 72 h of culture from three independent experiments. The cutoff was computed as the mean plus 3 standard deviations of the control values. Minimal estimates of the frequency of FITC+ RANKL-specific Th cells (coexpressing hIFN-γ/Texas Red or IL-4/Cy5.5) were calculated by using χ2 minimization as described previously (28). The analysis yielded a minimal frequency estimate and a χ2 estimate of probability. The frequency (1/f) was then expressed as the number of RANKL+ cells coexpressing IFN-γ (or IL-4) per 106 Th cells.
It is clear that IL-10 promotes Th2 while inhibiting Th1 development. As expected, the results of IF-LDA showed that hIL-10 treatment significantly reduced the frequency of A. actinomycetemcomitans-specific RANKL+ Th cells coexpressing IFN-γ in vivo, where changes were manifested at the mRNA and the whole-T-cell population levels (Fig. 1B and C) (for sham controls, 1/f = 186 ± 45.9; for IL-10 treatment, 1/f = 56.3 ± 16.2 RANKL+ Th cells coexpressing IFN-γ per 106 Th cells). In contrast to IL-10's inhibitory effect on APC functions (23), M-CSF was recently shown to increase the cytoplasmic turnover of major histocompatibility complex class I and II (MHC-I/II) molecules and to enhance MHC-restricted antigen processing and presentation to prime and activate T cells (3, 11). With this in mind, we sought to assess the levels of hM-CSF expressed by A. actinomycetemcomitans-reactive Th cells for their immune interactions with APC before and after hIL-10 treatment in vivo (3, 11). The results of sq-RT-PCR showed that hM-CSF was preferentially down-regulated in RANKL+ Th cells from IL-10-treated A. actinomycetemcomitans-infected HuPBL-NOD/SCID mice compared to those from sham control mice during disease progression in vivo (Fig. 1C and summary table). Thus, considering the complexity of APC-T-cell interactions and IL-10's effects described above, we favor the postulate that IFN-γ up-regulates the expression of MHC-II (or -I) and other accessory molecules on APC, leukocytes, and mesenchymal cells, which may further recruit other signaling molecules and/or immune effectors associated with bone destruction (7, 12, 20). Alternatively, IFN-γ may mediate its downstream effects independent of RANKL-induced osteoclastogenesis pathways under inflammatory conditions in vivo (e.g., the presence of TNF-α and IL-1) (26, 33, 34, 36). Thus, the fine balance between IFN-γ and RANKL-RANK signaling during inflammation, perhaps including the state of T-cell activation, contributes to determining the ultimate outcome of RANKL+ Th cells for osteoclastogenesis and bone remodeling in vivo (32-34, 36, 38).
IL-4 is a signature Th2 cytokine that inhibits Th1 (IFN-γ) development. Despite a slightly changed frequency of RANKL+ Th cells coexpressing IL-4, no statistical significance was observed (see the summary table below Fig. 1C) (P > 0.05; for sham controls, 1/f = 21.2 ± 5.5; for IL-10-treated cells, 1/f = 26.5 ± 7.8 RANKL+ Th cells coexpressing IL-4 per 106 Th cells). However, the reason that this was the case is not clear. It has been shown that the inhibitory effect of IL-4 on osteoclastogenesis is reversible, suggesting that other signaling pathways may influence its direct effects in vivo (37). It is interesting that in our separate study, hIL-4 injected into A. actinomycetemcomitans-infected HuPBL-NOD/SCID mice yielded a similar outcome where there was a concomitant lower frequency of RANKL+ Th cells coexpressing hIFN-γ associated with decreased alveolar bone loss in vivo but no significant changes regarding IL-4 expression on RANKL+ Th cells (data not shown). It is possible that there are auxiliary regulations mediated by non-T-cell sources (i.e., B and NK cells) (6, 15) or different cytokines (e.g., TNF-α, IL-1, and IL-12) (26, 31, 36) that may contribute, in part, to the above phenomenon. In addition, the physiological function of RANKL+ Th cells coexpressing hIL-4 for bone loss in vivo remains unclear and might be redundant, as other cytokines, such as type I IFNs, IL-7, IL-12, IL-18, etc., can inhibit RANKL-mediated osteoclastogenesis (26, 36).
The present findings are consistent with those from IL-10-deficient mice, which are prone to accelerated alveolar bone loss (1, 23). Together, these studies pinpoint the critical role of IFN-γ-expressing Th1 cells in modulating bone loss due to periodontitis in vivo. In summary, our data suggest an inhibitory effect of hIL-10 on RANKL+-Th-cell-mediated alveolar bone loss in an experimental periodontitis model which is correlated with the effects in successfully treated periodontal tissues in AgP. The present study illustrates and confirms the important role of gram-negative-pathogen-reactive RANKL+ Th cells coexpressing hIFN-γ, a signature Th1 cytokine, as a critical and positive cellular determinant in complex cytokine interactions and RANKL-RANK signaling for regulating osteoclastogenesis during periodontal disease progression in vivo (called RACIN [RANKL and cytokine interactions network] [34]). Thus, further understanding of these cellular and molecular interactions under pathological conditions may facilitate the development of novel therapeutic strategies and treatment protocols when dealing with inflammatory bone disorders such as human periodontitis in the future.
Acknowledgments
We thank D. Mahamed for her assistance with this work.
This work was supported in part by the Canadian Institute of Health Research (CIHR), Canada (MOP-37960), by the University of Rochester, and by the National Institutes of Health (DE12969).
Editor: J. B. Bliska
REFERENCES
- 1.Al-Rasheed, A., H. Scheerens, D. M. Rennick, H. M. Fletcher, and D. N. Tatakis. 2003. Accelerated alveolar bone loss in mice lacking interleukin-10. J. Dent. Res. 82:632-635. [DOI] [PubMed] [Google Scholar]
- 2.Baker, P. J., M. Dixon, R. T. Evans, L. Dufour, and D. C. Roopenian. 1999. CD4+ T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect. Immun. 67:2804-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron, C. L., G. Raposo, S. M. Scholl, H. Bausinger, D. Tenza, A. Bohbot, P. Pouillart, B. Goud, D. Hanau, and J. Salamero. 2005. Modulation of MHC class II transport and lysosome distribution by macrophage-colony stimulating factor in human dendritic cells derived from monocytes. J. Cell Sci. 114:999-1010. [DOI] [PubMed] [Google Scholar]
- 4.Brown, J. M., J. Zhang, and E. T. Keller. 2004. OPG, RANKL and RANK in cancer metastasis: expression and regulation. Cancer Treat. Res. 118:149-172. [DOI] [PubMed] [Google Scholar]
- 5.Canete, J. D., S. E. Martinez, J. Farres, R. Sanmarti, M. Blay, A. Gomez, G. Salvador, and J. Munoz-Gomez. 2000. Differential Th1/Th2 cytokine patterns in chronic arthritis: interferon gamma is highly expressed in synovium of rheumatoid arthritis compared with seronegative spondyloarthropathies. Ann. Rheum. Dis. 59:263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi, Y., K. M. Woo, S. H. Ko, Y. J. Lee, S. J. Park, H. M. Kim, and B. S. Kwon. 2001. Osteoclastogenesis is enhanced by activated B cells but suppressed by activated CD8(+) T cells. Eur. J. Immunol. 31:2179-2188. [DOI] [PubMed] [Google Scholar]
- 7.Ellis, T. N., and B. L. Beaman. 2004. Interferon-gamma activation of polymorphonuclear neutrophil function. Immunology 112:2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox, S. W., and T. J. Chambers. 2000. Interferon-γ directly inhibits TRANCE-induced osteoclastogenesis. Biochem. Biophys. Res. Commun. 276:868-872. [DOI] [PubMed] [Google Scholar]
- 9.Gorska, R., H. Gregorek, R. Kowalski, A. Laskus-Perendyk, M. Syczewska, and K. Madalinski. 2003. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J. Clin. Periodontol. 30:1046-1052. [DOI] [PubMed] [Google Scholar]
- 10.Goutoudi, P., E. Diza, and M. Arvanitidou. 2004. Effects of periodontal therapy on crevicular fluid interleukin-1β and interleukin-10 levels in chronic periodontitis. J. Dent. 32:511-520. [DOI] [PubMed] [Google Scholar]
- 11.Han, S., Y. Song, Y.-H. Lee, Y.-R. Lee, C.-K. Lee, K. Cho, and K. Kim. 2005. Macrophage-colony stimulating factor enhances MHC-restricted presentation of exogenous antigen in dendritic cells. Cytokine 32:187-193. [DOI] [PubMed] [Google Scholar]
- 12.Herold, K. C. 2004. Achieving antigen-specific immune regulation. J. Clin. Investig. 113:346-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofbauer, L. C., and M. Schoppet. 2004. Clinical implication of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 28:490-495. [DOI] [PubMed] [Google Scholar]
- 14.Honore, P., N. M. Luger, M. A. C. Sabino, M. J. Schwei, S. D. Rogers, and D. B. Mach. 2000. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat. Med. 6:521-528. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi, T., C. L. Hahn, S. Tanaka, S. E. Barbour, H. A. Schenkein, and J. G. Tew. 2004. Dendritic cells stimulated with Actinobacillus actinomycetemcomitans elicit rapid gamma interferon responses by natural killer cells. Infect. Immun. 72:5089-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, N., Y. Kadono, M. Takami, J. Lee, S.-H. Lee, F. Okada, J. H. Kim, T. Kobayashi, P. R. Odgren, H. Nakano, W.-C. Yeh, S.-K. Lee, J. A. Lorenzo, and Y. Choi. 2005. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J. Exp. Med. 202:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koga, T., M. Inui, K. Inoue, S. Kim, A. Suematsu, E. Kobayashi, T. Iwata, H. Ohnishi, T. Matozaki, T. Kodama, T. Taniguchi, H. Takayanagi, and T. Takai. 2004. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428:758-763. [DOI] [PubMed] [Google Scholar]
- 18.Kotake, S., Y. Nanke, M. Mogi, M. Kawamoto, T. Furuya, T. Yago, T. Kobashigawa, and N. Kamatani. 2005. IFN-γ-producing human T cells directly induce osteoclastogenesis from human monocytes via the expression of RANKL. Eur. J. Immunol. 35:1-11. [DOI] [PubMed] [Google Scholar]
- 19.Mahamed, D. A., A. Marleau, M. Alnaeeli, B. Singh, X. Zhang, J. M. Penninger, and Y.-T. A. Teng. 2005. G(−) anaerobe-reactive CD4+ T cells trigger RANKL-mediated enhanced alveolar bone loss in diabetic NOD mice. Diabetes 54:1477-1486. [DOI] [PubMed] [Google Scholar]
- 20.Mochizuki, S., M. Kobayashi, T. Suzuki, A. Oikawa, T. Koseki, T. Nishihara, and K. Hasegawa. 2004. Gamma-interferon enhances expression of CD14/MyD88 and subsequent responsiveness to lipopolysaccharide from Actinobacillus actinomycetemcomitans in human gingival fibroblasts. J. Periodontal Res. 39:333-343. [DOI] [PubMed] [Google Scholar]
- 21.Park, S. H., D. J. Min, M. L. Cho, W. U. Kim, J. Youn, W. Park, et al. 2001. Shift toward T helper 1 cytokines by type II collagen-reactive T cells in patients with rheumatoid arthritis. Arthritis Rheum. 44:561-569. [DOI] [PubMed] [Google Scholar]
- 22.Romas, E., M. T. Gillespie, and T. J. Martin. 2002. Involvement of receptor activator of NFκB ligand and tumor necrosis factor-α in bone destruction in rheumatoid arthritis. Bone 30:340-346. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki, H., Y. Okamatsu, T. Kawai, R. Kent, M. Taubman, and P. Stashenko. 2004. The interleukin-10 knockout mouse is highly susceptible to Porphyromonas gingivalis-induced alveolar bone loss. J. Periodontal Res. 39:432-441. [DOI] [PubMed] [Google Scholar]
- 24.Suarez, J. J., A. M. Ocampo, R. E. Duenas, and A. Rodriguez. 2004. Relative proportions of T-cell subpopulations and cytokines that mediate and regulate the adaptive immune response in patients with aggressive periodontitis. J. Periodontol. 75:1209-1215. [DOI] [PubMed] [Google Scholar]
- 25.Takayanagi, H., K. Ogasawara, S. Hida, T. Chiba, S. Murata, K. Sato, et al. 2000. T cell mediated regulation of osteoclastogenesis by signaling cross-talk between RANKL and IFN-γ. Nature 408:600-605. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka, S., K. Nakamura, N. Takahasi, and T. Suda. 2005. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANK-RANK signaling system. Immunol. Rev. 208:30-49. [DOI] [PubMed] [Google Scholar]
- 27.Taubman, M. A., and T. Kawai. 2001. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit. Rev. Oral Biol. Med. 12:125-135. [DOI] [PubMed] [Google Scholar]
- 28.Teng, Y.-T. A., H. Nguyen, A. Hassanloo, R. P. Ellen, H. Hozumi, and R. M. Gorczynski. 1999. Periodontal immune responses of human lymphocytes in Actinobacillus actinomycetemcomitans-inoculated NOD/SCID mice engrafted with peripheral blood leukocytes of periodontitis patients. J. Periodontal Res. 34:54-61. [DOI] [PubMed] [Google Scholar]
- 29.Teng, Y.-T. A., H. Nguyen, X. Gao, Y. Y. Kong, R. M. Gorczynski, B. Singh, R. P. Ellen, and J. M. Penninger. 2000. Functional human T-cell immunity and osteoprotegerin-ligand (OPG-L) control alveolar bone destruction in periodontal infection. J. Clin. Investig. 106:R59-R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teng, Y.-T. A. 2002. Mixed periodontal Th1-Th2 cytokine profile in Actinobacillus actinomycetemcomitans-specific osteoprotegerin ligand (or RANK-L)-mediated alveolar bone destruction in vivo. Infect. Immun. 70:5269-5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teng, Y.-T. A. 2003. The role of acquired immunity and periodontal disease progression. Crit. Rev. Oral Biol. Med. 14:237-252. [DOI] [PubMed] [Google Scholar]
- 32.Teng, Y.-T. A., D. Mahamed, and B. Singh. 2005. Gamma interferon positively modulates Actinobacillus actinomycetemcomitans-specific CD4+-T-cell-mediated alveolar bone destruction in vivo. Infect. Immun. 73:3453-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teng, Y.-T. A. 2006. Protective and destructive immunity in the periodontium. 1. Innate and humoral immunity and the periodontium. J. Dent. Res. 85:198-208. [DOI] [PubMed] [Google Scholar]
- 34.Teng, Y.-T. A. 2006. Protective and destructive immunity in the periodontium. 2. T-cell-mediated immunity in the periodontium. J. Dent. Res. 85:209-219. [DOI] [PubMed] [Google Scholar]
- 35.Theill, L. E., W. J. Boyle, and J. M. Penninger. 2002. RANK-L and RANK: T cell, bone loss and mammalian evolution. Annu. Rev. Immunol. 20:795-823. [DOI] [PubMed] [Google Scholar]
- 36.Walsh, M. C., N. Kim, Y. Kadono, J. Rho, S. Y. Lee, J. Lorenzo, and Y. Choi. 2006. Osteoimmunology: interplay between the immune system and bone metabolism. Annu. Rev. Immunol. 24:1-31. [DOI] [PubMed] [Google Scholar]
- 37.Wei, S., M. W. Wang, S. L. Teitelbaum, and F. P. Ross. 2002. Interleukin-4 reversibly inhibits osteoclastogenesis via inhibition of NF-kappa B and mitogen-activated protein kinase signaling. J. Biol. Chem. 277:6622-6630. [DOI] [PubMed] [Google Scholar]
- 38.Wyzga, N., S. Varghese, S. Wikel, E. Canalis, and F. A. Sylvester. 2004. Effects of activated T cells on osteoclastogenesis depend on how they are activated. Bone 35:614-620. [DOI] [PubMed] [Google Scholar]