Abstract
The concept that circulating dendritic cells mediate neuroinvasion in transmissible spongiform encephalopathies received strong support from recent observations that prion protein is expressed in myeloid dendritic cells. We observed that prion protein fragment 106-126 is a chemoattractant for monocyte-derived immature but not mature dendritic cells. Signaling events in chemotaxis involved enzymes downstream of Gq protein and were inhibited by blockade of sphingosine kinase, suggesting transactivation of sphingosine-1-phosphate-dependent cell motility by prion protein.
Prion glycoproteins may become unprecedented infectious pathogens that cause a group of invariably fatal neurodegenerative diseases by novel mechanisms (27, 29). New-variant Creutzfeldt-Jakob disease [(v)CJD] and scrapie, known as transmissible spongiform encephalopathies, are typically initiated by exposure to the causative agent and early prion replication in lymphoid tissues (20). (v)CJD has raised concerns that bovine spongiform encephalopathies might be communicable to humans by dietary exposure (4, 28).
Gut M-cell-dependent transepithelial uptake of dietary prion protein is followed by transcytosis directly to intraepithelial pockets, where key players of the immune system, including dendritic cells (DCs), are located (11). DCs are also able to open the tight junctions between epithelial cells, send dendrites outside the epithelium, and directly sample pathogens in an M-cell-independent way (30). The details of the mechanism by which infective prions are transferred from the gastrointestinal tract to the nervous system are unknown. It is important to understand how central lymphoid organs and peripheral neurons become exposed to infective prion protein (PrPsc).
Evidence suggests that circulating blood cells may have a role in enteral prion infection. Results from animal models have emphasized the fact that infective material can be isolated from the cell fraction of spleen soon after the ingestion of PrPsc (19), whereas in mice, bone marrow-derived myeloid cells have been shown to be required for its propagation and spread (2). It was shown previously that cellular prion protein (PrPc) is strongly expressed in myeloid DCs, which may act as carrier cells for the spread and circulation of the abnormal isoform PrPsc (3). In the absence of prion disease, high levels of expression of PrPc in human spleen occur principally on myeloid DCs immediately adjacent to the white pulp, whereas follicular DCs do not strongly express PrPc; myeloid DCs are found in the red pulp of the spleen, and cells migrate into its lymphoid areas after receiving a maturation stimulus (3). Moreover, DCs can be found in the peripheral and central nervous system (9, 25). Here we report on the chemotaxis of immature DCs and arrest of mature DCs by a synthetic peptide corresponding to residues 106 to 126 of human PrP (PrP106-126). Signal transduction mechanisms that may be involved in directed migration of monocyte-derived DCs toward PrP106-126 are described.
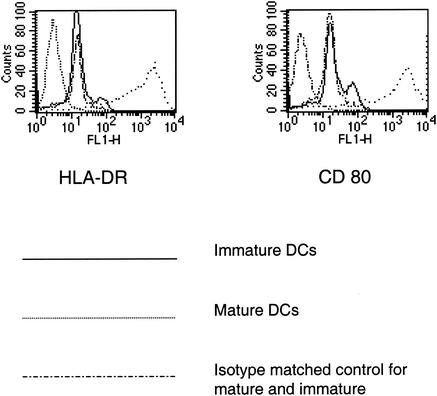
PrP106-126, which is toxic to neurons, increases chemotaxis, oxygen free radical release, and intracellular calcium concentration in neutrophils and monocytes (5). To determine whether PrP106-126 is a chemoattractant of monocyte-derived DCs (17), chemotaxis experiments in modified multiwell Boyden chambers (Neuroprobe, Gaithersburg, Md.) using nitrocellulose micropore filters (Sartorius, Göttingen, Germany) were performed as previously described (6). DCs were prepared as described previously (6, 7, 17, 18). Distinction between mature and immature DCs was made by fluorescence-activated cell sorting analyses (Fig. 1).
FIG. 1.
Cytofluorometric analysis of DC surface phenotype. A total of 5 × 105 DCs were washed in phosphate-buffered saline-2% fetal calf serum and resuspended in a solution containing 250 μg of human immunoglobulin G per ml, phosphate-buffered saline, and 2% fetal calf serum. After pelleting, DCs were incubated alternatively with 10 μg of anti-CD80 per ml or anti-HLA-DR monoclonal antibodies and the respective isotype-matched control immunoglobulins. After a washing in phosphate-buffered saline-2% fetal calf serum, a 1:40 dilution of fluorescein isothiocyanate-anti-mouse immunoglobulin G in phosphate-buffered saline-2% fetal calf serum was incubated for 30 min at 4°C. Cells were immediately analyzed on a FACScan. Analysis was performed with CellQuest software (BD Biosciences, Mountain View, Calif.).
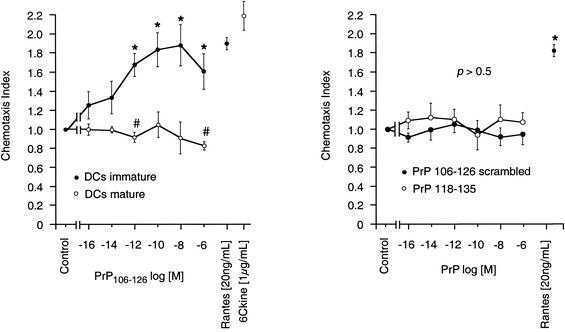
Immature DCs migrated for 4 h toward PrP106-126 (Bachem, Bubendorf, Switzerland) in a concentration-dependent manner, whereas PrP106-126 was not chemotactic for mature DCs (Fig. 2). Maximum chemotactic activity of PrP106-126 for immature DCs was seen at concentrations of 0.1 to 10 nmol/liter and was comparable in its potency to that of RANTES [20 ng/ml] (Peprotech, London, United Kingdom). As a control, chemotaxis toward scrambled PrP106-126 and PrP118-135 was monitored. Neither the scrambled form nor PrP118-135 exerted chemotactic effects on immature DCs (Fig. 2). Checkerboard analysis revealed that the migration of immature DCs toward PrP106-126 is true concentration gradient-dependent chemotaxis (Table 1). The influence of PrP106-126 on 6Ckine-induced chemotaxis of mature DCs was tested. Combination of 6Ckine (1 μg/ml) with PrP106-126 (10 fM to 1 μM) in the lower wells of the chemotaxis chamber deactivated mature DC migration. The mean distance of random migration was 45 ± 5.2 μm, the mean 6Ckine-induced migration was 100 ± 8.4 μm, and the mean when 6Ckine was combined with PrP106-126 was 78 ± 6.3 μm (P < 0.05). The effects of PrP106-126 and 6Ckine alone are shown in Fig. 1.
FIG. 2.
PrP induces chemotaxis in immature DCs and lacks an effect on mature DCs. DCs were allowed to migrate toward various concentrations of PrP106-126 for 4 h in modified multiwell Boyden chambers with micropore nitrocellulose filters. The mean random migration distances were 40 ± 6.2 μm for immature and 49 ± 8.3 μm for mature DCs, respectively. The chemotaxis index is the ratio between directed and random migration. Statistical analysis was done by the Mann-Whitney U test after Kruskal-Wallis analysis; *, P < 0.05, n = 6. Random migration of mature DCs was inhibited (#, P < 0.05, n = 6). RANTES and 6Ckine were used as positive controls for immature and mature DCs, respectively.
TABLE 1.
Checkerboard analysis of effects of concentration gradients of PrP106-126 on immature DC migrationa
| PrP106-126 concn (mol/liter) in lower compartment | Chemotaxis index (mean ± SEM)b for PrP106-126 concn (mol/liter) in upper compartment
|
|||
|---|---|---|---|---|
| Medium | 10−10 | 10−8 | 10−6 | |
| Medium | 1.000 ± 0.000 | 1.025 ± 0.053 | 1.084 ± 0.086 | 0.943 ± 0.192 |
| 10−10 | 1.884 ± 0.138∗ | 0.963 ± 0.052 | 1.036 ± 0.137 | 0.691 ± 0.099∗ |
| 10−8 | 2.211 ± 0.106∗ | 2.411 ± 0.132∗ | 0.951 ± 0.090 | 1.014 ± 0.142 |
| 10−6 | 2.613 ± 0.098∗ | 2.273 ± 0.175∗ | 1.712 ± 0.161∗ | 0.663 ± 0.081∗ |
Directed migration of DCs to PrP106-126 was tested by checkerboard analysis. Cells were allowed to migrate to various concentration gradients of PrP106-126 for 4 h. Migration depth was quantified by microscopy.
Ratio between directed and undirected migration of cells. The mean distance of migration to medium in the upper and lower chambers was 36 ± 4.2 μm. Statistical analysis was done with the Mann-Whitney U test after Kruskal-Wallis analysis of variance (P < 0.001); n = 3. ∗, P < 0.05 compared with the value obtained for medium in the upper and lower chambers. Only groups within the matrix for which chemotaxis was extensive enough to distinguish between chemokinesis and chemotaxis were compared statistically by the Mann-Whitney U test.
Tyrphostin-23 (a tyrosine kinase inhibitor), bisindolylmaleimide (GFX; a protein kinase C inhibitor), wortmannin (WTN; a phospholipase 3 inhibitor), rolipram (a phosphodiesterase inhibitor; Sigma Chemical Co., St. Louis, Mo.), and dimethylsphingosine (DMS; a sphingosine kinase inhibitor) were used for blocking signaling enzymes; pertussis toxin (PTX) and cholera toxin (CTX; Sigma Chemical Co.) were used for testing involvement of Gi/G0 protein and Gs protein, respectively. Patterns of migration toward other DC attractants, including N-formyl-methionyl-phenylalanine (fMLP; Sigma Chemical Co.) and substance P (Neosystem, Strasbourg, France), were compared with that of PrP106-126. Signaling studies revealed that PrP106-126-induced chemotaxis is sensitive to GFX, tyrphostin-23, rolipram, WTN, and PTX. This signaling pattern mimicked that of substance P, another peptide attractant of DCs (8), but differed from that of fMLP. CTX did not affect chemotaxis to any of them (Table 2).
TABLE 2.
Effects of signaling enzyme blockers on DC chemotaxisa
| Treatment | Chemotaxis indexb (mean ± SEM)
|
||
|---|---|---|---|
| PrP106-126 (10 nmol/liter) | Substance P (1 nmol/liter) | fMLP (10 nmol/liter) | |
| Medium | 1.477 ± 0.208 | 1.529 ± 0.221 | 1.820 ± 0.048 |
| GFX (500 nmol/liter) | 1.112 ± 0.031∗ | 0.723 ± 0.092∗ | 1.654 ± 0.135 (NS) |
| Rolipram (10 μmol/liter) | 1.034 ± 0.180∗ | 1.029 ± 0.106∗ | 1.816 ± 0.064 (NS) |
| Tyr-23 (10 ng/ml) | 0.889 ± 0.209∗ | 1.058 ± 0.098∗ | 1.685 ± 0.028∗ |
| WTN (10 nmol/liter) | 1.111 ± 0.043∗ | 1.049 ± 0.243∗ | 1.859 ± 0.069 (NS) |
| CTX (1 nmol/liter) | 1.636 ± 0.244 (NS) | 1.729 ± 0.172 (NS) | 1.918 ± 0.064 (NS) |
| PTX (1 nmol/liter) | 1.074 ± 0.080∗ | 1.227 ± 0.033∗ | 1.268 ± 0.037∗ |
DCs were incubated with GFX, rolipram, tyrphostin-23 (Tyr-23), WTN, CTX, and PTX for 20 min. Chemotaxis toward PrP106-126, substance P, and fMLP was tested after a washing using modified multiwell Boyden chambers.
Ratio between directed and random migration. The mean random migration distance was 54 ± 0.05 μm. Statistical analysis was done by the Mann-Whitney U test after Kruskal-Wallis analysis. ∗, P < 0.05, n = 3. NS, not significant.
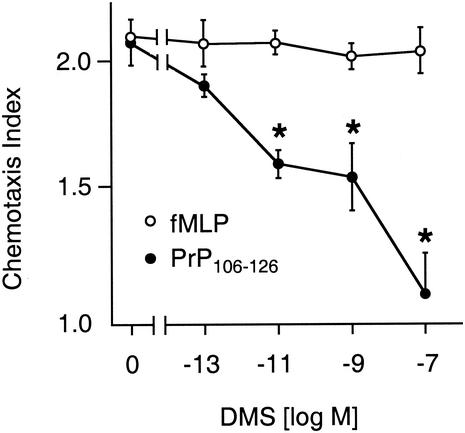
Activation of protein kinase C leads to phosphorylation of sphingosine kinase and sphingosine-1-phosphate production (24), which induces G-protein-dependent cell migration (16). Sphingosine-1-phosphate-induced chemotaxis of DCs has been reported recently (14). As PrP106-126-induced migration of DCs was inhibited by the inhibitor of protein kinase C and by blockade of Gi/G0 (Table 2), effects of the sphingosine kinase inhibitor DMS (Biomol, Plymouth Meeting, Pa.) on PrP106-126-induced chemotaxis were tested. Migration of DCs toward optimal concentrations of PrP106-126 was inhibited in a concentration-dependent manner by pretreatment with DMS, whereas chemotaxis of immature DCs toward fMLP was not inhibited (Fig. 3).
FIG. 3.
DMS inhibits PrP106-126-induced chemotaxis. DCs were incubated with DMS for 20 min. Chemotaxis toward PrP106-126 and fMLP was tested after a washing by using modified multiwell Boyden chambers. Mean random migration was 52 ± 7.5 μm. The chemotaxis index is the ratio between directed and random migration. Statistical analysis was done by the Mann-Whitney U test after Kruskal-Wallis analysis; *, P < 0.05, n = 3.
Results of this in vitro study demonstrate that PrP106-126 induces migration of monocyte-derived immature DCs in a dose-dependent manner. In tissues of animals infected by transmissible spongiform encephalopathy agent and in humans suffering from (v)CJD, PrPsc is particularly concentrated in follicular DCs, whereas high levels of PrPc are present in myeloid DCs (3), which are ontologically and functionally distinct from follicular DCs (15). Myeloid DCs are derived from bone marrow precursor cells or from monocytes and their precursors and are readily identified within circulating cell populations. In the spleen, myeloid DCs are found in the red pulp and immediately adjacent to the white pulp. The cells migrate into the lymphoid areas, where they are powerful mediators of T-cell activation. Given the close anatomic and functional connection of myeloid DCs with lymphoid follicles, these results raise the possibility that migration of myeloid DCs toward prion protein may play a role in the propagation of PrPsc in humans (3). It has been observed that injection of prion-infected DCs induced scrapie without accumulation of prions in the spleen in a model of RAG-1 knockout mice, indicating that DCs can propagate prions from the periphery to the central nervous system in the absence of any additional lymphoid element (1). Migratory bone marrow-derived DCs, entering the intestinal wall from blood, sample antigens from the gut lumen and carry them to the lymph nodes. Huang et al. showed that DCs acquire PrPsc in vitro and transport intestinally administered PrPsc directly into lymphoid tissue in vivo, suggesting that DCs are a cellular bridge between the gut lumen and the lymphoid transmissible spongiform encephalopathy replication machinery (13).
In the present study, it was observed that the prion protein fragment is able to induce a chemotactic response in immature DCs and to arrest mature DCs, suggesting that DC trafficking may directly depend on the infective agent. DCs might be attracted toward high concentrations of prion protein, as are found in the gut epithelial venules or splenic lymphoid follicles, where uptake and accumulation of pathogens take place as a prerequisite of prion spread. When stimuli are received as a result of pathogen uptake, maturation might occur and DCs might subsequently be arrested at the sites of prion uptake.
We further observed an inhibition of migration after pretreatment of cells with DMS, which was used as specific inhibitor of sphingosine kinase. Previously it was suggested for monocytes that PrP106-126 might use the formyl peptide-like receptor 1, a pattern recognition seven transmembrane receptor for chemotaxis also expressed in DCs (12). This receptor is coupled to G proteins and may be sufficient for migration induction (22). The observation that PrP106-126-induced chemotaxis is sphingosine kinase dependent suggests that transactivation of chemotaxis toward sphingosine-1-phosphate, a lysophospholipid that activates cytoskeletal remodeling and motility via endothelial-differentiation-gene receptors (EDG) (21), is also involved in prion protein fragment-dependent effects in DCs (23, 24, 26, 31). Immature and mature DCs express the mRNA for different sphingosine-1-phosphate receptors (EDG-1, -3, -5, and -6), where sphingosine-1-phosphate induces further chemotaxis (14). Activation of sphingosine kinase and production of sphingosine-1-phosphate increases intracellular Ca2+ concentrations, an effect known to take place in PrP106-126-induced apoptosis of several cell lines (10). These observations strengthen the concept that migration of antigen-presenting cells toward high concentrations of PrP106-126 involves sphingosine kinase.
Since in dietary prion disease, PrPsc is incorporated via the lymphatic system of the gut, migration of circulating DCs toward high concentrations of prion protein and their accumulation for prion uptake may be important pathophysiological mechanisms that involve a chemotactic lysophospholipid. Interference with sphingosine kinase-dependent pathways might become a novel pharmaceutical target for preventing prion-dependent DC trafficking and the associated spread of infection.
REFERENCES
- 1.Aucouturier, P., F. Geissmann, D. Damotte, G. P. Saborio, H. C. Meeker, R. Kascsak, R. Kascsak, R. I. Carp, and T. Wisniewski. 2001. Infected splenic dendritic cells are sufficient for prion transmission to the CNS in mouse scrapie. J. Clin. Investig. 108:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blattler, T., S. Brandner, A. J. Raeber, M. A. Klein, T. Voigtlander, C. Weissmann, and A. Aguzzi. 1997. PrP-expressing tissue required for transfer of scrapie infectivity from spleen to brain. Nature 389:69-73. [DOI] [PubMed] [Google Scholar]
- 3.Burthem, J., B. Urban, A. Pain, and D. J. Roberts. 2001. The normal cellular prion protein is strongly expressed by myeloid dendritic cells. Blood 98:3733-3738. [DOI] [PubMed] [Google Scholar]
- 4.Chesebro, B. 1997. Human TSE disease—viral or protein only? Nat. Med. 3:491-492. [DOI] [PubMed] [Google Scholar]
- 5.Diomede, L., S. Sozzani, W. Luini, M. Algeri, L. De Gioia, R. Chiesa, P. M. Lievens, O. Bugiani, G. Forloni, F. Tagliavini, and M. Salmona. 1996. Activation effects of a prion protein fragment [PrP-(106-126)] on human leucocytes. Biochem. J. 320:563-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunzendorfer, S., A. Kaser, C. Meierhofer, H. Tilg, and C. J. Wiedermann. 2000. Dendritic cell migration in different micropore filter assays. Immunol. Lett. 71:5-11. [DOI] [PubMed] [Google Scholar]
- 7.Dunzendorfer, S., A. Kaser, C. Meierhofer, H. Tilg, and C. J. Wiedermann. 2001. Cutting edge: peripheral neuropeptides attract immature and arrest mature blood-derived dendritic cells. J. Immunol. 166:2167-2172. [DOI] [PubMed] [Google Scholar]
- 8.Dunzendorfer, S., and C. J. Wiedermann. 2001. Neuropeptides and the immune system: focus on dendritic cells. Crit. Rev. Immunol. 21:523-557. [PubMed] [Google Scholar]
- 9.Fischer, H. G., and G. Reichmann. 2001. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J. Immunol. 166:2717-2726. [DOI] [PubMed] [Google Scholar]
- 10.Florio, T., S. Thellung, C. Amico, M. Robello, M. Salmona, O. Bugiani, F. Tagliavini, G. Forloni, and G. Schettini. 1998. Prion protein fragment 106-126 induces apoptotic cell death and impairment of L-type voltage-sensitive calcium channel activity in the GH3 cell line. J. Neurosci. Res. 54:341-352. [DOI] [PubMed] [Google Scholar]
- 11.Heppner, F. L., A. D. Christ, M. A. Klein, M. Prinz, M. Fried, J. P. Kraehenbuhl, and A. Aguzzi. 2001. Transepithelial prion transport by M cells. Nat. Med. 7:976-977. [DOI] [PubMed] [Google Scholar]
- 12.Hollopeter, G., H. M. Jantzen, D. Vincent, G. Li, L. England, V. Ramakrishnan, R. B. Yang, P. Nurden, A. Nurden, D. Julius, and P. B. Conley. 2001. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409:202-207. [DOI] [PubMed] [Google Scholar]
- 13.Huang, F. P., C. F. Farquhar, N. A. Mabbott, M. E. Bruce, and G. G. MacPherson. 2002. Migrating intestinal dendritic cells transport PrPSc from the gut. J. Gen. Virol. 83:267-271. [DOI] [PubMed] [Google Scholar]
- 14.Idzko, M., E. Panther, S. Corinti, A. Morelli, D. Ferrari, Y. Herouy, S. Dichmann, M. Mockenhaupt, P. Gebicke-Haerter, F. Di Virgilio, G. Girolomoni, and J. Norgauer. 2002. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. FASEB J. 16:625-627. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez-Huete, A., P. M. Lievens, R. Vidal, P. Piccardo, B. Ghetti, F. Tagliavini, B. Frangione, and F. Prelli. 1998. Endogenous proteolytic cleavage of normal and disease-associated isoforms of the human prion protein in neural and non-neural tissues. Am. J. Pathol. 153:1561-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaneider, N., A. Djanani, R. Fischer-Colbrie, and C. Wiedermann. 2002. Sphingosine kinase-dependent directional migration of leukocytes in response to phorbol ester. Biochem. Biophys. Res. Commun. 297:806.. [DOI] [PubMed] [Google Scholar]
- 17.Kaser, A., S. Dunzendorfer, F. A. Offner, O. Ludwiczek, B. Enrich, R. O. Koch, W. W. Cruikshank, C. J. Wiedermann, and H. Tilg. 2000. B lymphocyte-derived IL-16 attracts dendritic cells and Th cells. J. Immunol. 165:2474-2480. [DOI] [PubMed] [Google Scholar]
- 18.Kaser, A., S. Dunzendorfer, F. A. Offner, T. Ryan, A. Schwabegger, W. W. Cruikshank, C. J. Wiedermann, and H. Tilg. 1999. A role for IL-16 in the cross-talk between dendritic cells and T cells. J. Immunol. 163:3232-3238. [PubMed] [Google Scholar]
- 19.Kimberlin, R. H., and C. A. Walker. 1989. The role of the spleen in the neuroinvasion of scrapie in mice. Virus Res. 12:201-211. [DOI] [PubMed] [Google Scholar]
- 20.Klein, M. A., R. Frigg, A. J. Raeber, E. Flechsig, I. Hegyi, R. M. Zinkernagel, C. Weissmann, and A. Aguzzi. 1998. PrP expression in B lymphocytes is not required for prion neuroinvasion. Nat. Med. 4:1429-1433. [DOI] [PubMed] [Google Scholar]
- 21.Kluk, M. J., and T. Hla. 2001. Role of the sphingosine 1-phosphate receptor EDG-1 in vascular smooth muscle cell proliferation and migration. Circ. Res. 89:496-502. [DOI] [PubMed] [Google Scholar]
- 22.Le, Y., J. J. Oppenheim, and J. M. Wang. 2001. Pleiotropic roles of formyl peptide receptors. Cytokine Growth Factor Rev. 12:91-105. [DOI] [PubMed] [Google Scholar]
- 23.Lee, M. J., S. Thangada, J. H. Paik, G. P. Sapkota, N. Ancellin, S. S. Chae, M. Wu, M. Morales-Ruiz, W. C. Sessa, D. R. Alessi, and T. Hla. 2001. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol. Cell 8:693-704. [DOI] [PubMed] [Google Scholar]
- 24.Mazurek, N., T. Megidish, S. Hakomori, and Y. Igarashi. 1994. Regulatory effect of phorbol esters on sphingosine kinase in BALB/C 3T3 fibroblasts (variant A31): demonstration of cell type-specific response—a preliminary note. Biochem. Biophys. Res. Commun. 198:1-9. [DOI] [PubMed] [Google Scholar]
- 25.McWilliam, A. S., S. Napoli, A. M. Marsh, F. L. Pemper, D. J. Nelson, C. L. Pimm, P. A. Stumbles, T. N. Wells, and P. G. Holt. 1996. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J. Exp. Med. 184:2429-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paik, J. H., S. Chae, M. J. Lee, S. Thangada, and T. Hla. 2001. Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of αvβ3- and β1-containing integrins. J. Biol. Chem. 276:11830-11837. [DOI] [PubMed] [Google Scholar]
- 27.Peretz, D., R. A. Williamson, K. Kaneko, J. Vergara, E. Leclerc, G. Schmitt-Ulms, I. R. Mehlhorn, G. Legname, M. R. Wormald, P. M. Rudd, R. A. Dwek, D. R. Burton, and S. B. Prusiner. 2001. Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature 412:739-743. [DOI] [PubMed] [Google Scholar]
- 28.Priola, S. A. 1996. Similar protein signatures for BSE and vCJD. Nat. Med. 2:1303-1304. [DOI] [PubMed] [Google Scholar]
- 29.Prusiner, S. B. 1994. Inherited prion diseases. Proc. Natl. Acad. Sci. USA 91:4611-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J. P. Kraehenbuhl, and P. Ricciardi-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2:361-367. [DOI] [PubMed] [Google Scholar]
- 31.Rosenfeldt, H. M., J. P. Hobson, S. Milstien, and S. Spiegel. 2001. The sphingosine-1-phosphate receptor EDG-1 is essential for platelet-derived growth factor-induced cell motility. Biochem. Soc. Trans. 29:836-839. [DOI] [PubMed] [Google Scholar]