Abstract
Enterocytozoon bieneusi, a microsporidian, is clinically one of the most significant opportunistic causes of diarrhea and wasting associated with profound human immunodeficiencies. The lack of an animal model for E. bieneusi hinders serious investigations and limits the availability of spores to individuals with severe human immunodeficiency virus/AIDS disease who are infected with E. bieneusi. The development of procedures for purification and concentration of spores from stools of infected humans has led to the production of immune reagents and provided a source of spores to conduct research, including attempts to develop and serially propagate E. bieneusi in rodent models. We have evaluated and successfully infected six different immunodeficient and/or immunosuppressed rodent models and have demonstrated persistent infections lasting at least 18 weeks in SCID mice and in nude rats. To enhance the intensity and duration of infection in these two models, animals were given anti-gamma interferon monoclonal antibody injections at regular intervals. Of the six models evaluated, nude rats and gerbils immunosuppressed with dexamethasone excreted the highest number of spores and for longer time periods. Four different E. bieneusi isolates were equally infectious, and one of them was serially propagated in nude rats six times over a period of 10 months. Typically, rats challenged orally with 104 spores yielded 2 × 107 to 6.3 × 107 spores per single fecal sample when the level of spores was measured 2 weeks later. Rodent models and a nonhuman source of fresh spores will considerably enhance future investigations on this important opportunistic pathogen, including the screening and evaluation of urgently needed chemotherapeutic agents.
Microsporidia are obligate intracellular eukaryotic fungal parasites found in most invertebrates and vertebrates. Microsporidia have emerged as important opportunistic pathogens of humans during the AIDS pandemic. Enterocytozoon bieneusi and Encephalitozoon intestinalis are the two species responsible for human gastrointestinal disease (5). Of the two species, E. bieneusi is clinically by far the most significant and is responsible for 15 to 50% of chronic cases of diarrhea and wasting in humans with immunodeficiencies, particularly individuals with human immunodeficiency virus (HIV)/AIDS (10). Microsporidiosis also occurs in HIV-negative immunodepressed transplant recipients (7, 15). E. bieneusi infection is linked to profound diarrhea and is localized primarily within the epithelium lining the intestinal and biliary tracts (3, 4, 13, 14). E. bieneusi infections respond poorly to currently available chemotherapy (10).
E. bieneusi has been experimentally transmitted from humans to rhesus macaques (6, 22) and, to a limited degree, to piglets (9, 12). Although it appears that the infection is widespread in mammals, attempts to infect and serially propagate this pathogen in laboratory animals have been unsuccessful. The inability to serially propagate E. bieneusi in cultured cells and/or in animal models has limited laboratory investigations and hindered research progress on this pathogen and the search for an effective therapy. Recent progress, including the development of purification and concentration procedures for spores from stools of infected humans (11, 16), has led to the production of immune reagents (2, 25) and to renewed attempts to propagate E. bieneusi in cell culture and in small laboratory animals. We report here the successful transmission and serial propagation of E. bieneusi obtained from humans in several rodent models.
MATERIALS AND METHODS
Parasite.
E. bieneusi spores were purified from fresh stools collected from infected adult humans (20, 21). For this study, spores from four different individuals were used (EBA206, EBA325, EBA327, and EBA337). The spores were purified as previously described (16). Briefly, the stool samples were washed with phosphate-buffered saline (PBS), passed through a sieve to remove large particulate material and concentrated by centrifugation. The spores were enriched by a salt flotation step, followed by a continuous sucrose gradient centrifugation step, which separated the spores from the fecal material, bacteria, and yeasts, as previously described. The purified spores were detected by an indirect fluorescent-antibody (IFA) assay using antibodies specific for E. bieneusi (16, 25) and were further characterized as E. bieneusi by PCR (21). The purity of each spore preparation was also evaluated by microbial culture and by transmission electron microscopy, as previously described (16). E. bieneusi spores from infected rodents were likewise purified and identified using the same protocol as described above.
Animals.
Six rodent models were evaluated for susceptibility to E. bieneusi infection. All rodents were housed in sterile microisolators. The first five rodent models were orally inoculated with purified E. bieneusi spores (106 in 100 μl) originally isolated from infected adult humans. The Mongolian gerbils were inoculated with 104 spores purified from nude rats (RNU). Because the number of available purified spores initially was limited, in some experiments the number of animals used per group was small. The six models evaluated (Table 1) were as follows.
TABLE 1.
Summary of experimental inoculations of rodents fed ∼106 E. bieneusi spores of human origina
| Parameter | Value for the following groupb:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Germfree mice
|
GKO mice | Dex mice | SCID mice
|
RNU
|
Gerbils
|
||||||
| Ctrl | Infected | SCID/Ab | SCID* | RNU/Ab | RNU* | Ctrl | Infected | Ctrl | |||
| No. shedding/total | 0/3 | 2/3 | 2/3 | 5/6 | 20/20 | 5/5 | 5/5 | 5/5 | 0/2 | 12/12 | 0/2 |
| Spore excretionc at wk: | |||||||||||
| 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| 2 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 0 | 1 | 0 |
| 3 | 0 | 0 | 1 | 2 | 1 | 1 | 3 | 2 | 0 | 3 | 0 |
| 4 | 0 | 0 | 1 | 1 | 2 | 1 | 2 | 3 | 0 | 3 | 0 |
| 5 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 2 | 0 |
| 6 | 0 | 0 | 1 | 1 | 3 | 1 | 2 | 1 | 0 | 1 | 0 |
| 7 | 1 | 1 | 3 | 1 | 3 | 1 | 0 | 1 | 0 | ||
| 8 | 3 | 1 | 3 | 1 | |||||||
| 9 | 2 | 1 | 3 | 1 | |||||||
| 10 | 1 | 3 | 3 | 1 | |||||||
| 11 | 1 | 2 | 2 | 1 | |||||||
| 12 | 1 | 2 | 1 | 1 | |||||||
| 13 | 1 | 1 | 1 | 1 | |||||||
| 14 | 1 | 1 | 0 | 0 | |||||||
| 15 | 2 | 3 | 1 | 0 | |||||||
| 16 | 2 | 1 | |||||||||
| 17 | 1 | 1 | |||||||||
| 18 | 1 | 1 | |||||||||
Fecal spore excretion was monitored weekly for up to 18 weeks.
Dex, dexamethasone immunosuppressed; SCID/Ab, SCID mice injected with anti-IFN-γ MAb; SCID*, SCID mice with delayed antibody injection (see text); RNU/Ab, RNU injected with anti-IFN-γ MAb; RNU*, RNU with initial injections of anti-IFN-γ MAb (see text); Ctrl, control groups fed placebo (PBS).
Scores represent intensity of spore excretion in feces: 0, no spores were found in 30 microscopic HPF; 1, ≤10 spores identified in 30 HPF; 2, 10 to 100 spores per 30 HPF; 3, ≥100 spores per 30 HPF. The scores represent the average weekly shedding for the group.
(i) C57BL/6 dexamethasone-immunosuppressed mice.
Groups of 4-week-old mice (Charles River Laboratories, MA) were immunosuppressed with dexamethasone (1 mg/mouse; Sigma-Aldrich Chemical Co., St. Louis, MO) given subcutaneously four times every second day before oral inoculation and twice every second week thereafter. Nine mice were immunosuppressed and then randomized into three groups of three mice each. Two groups were inoculated with isolate EBA206 or EBA337, and the third immunosuppressed control group received a placebo.
(ii) C.B-17 SCID mice.
Twenty-five 4-week-old mice (Charles River Laboratories) were injected intraperitoneally (i.p.) with 1 mg/mouse of anti-gamma interferon (IFN-γ) monoclonal antibody (MAb) XMG1.2 at 2 h before oral challenge and randomly assigned to five groups of five mice each. Mice were orally infected with E. bieneusi spores of isolate EBA206, EBA325, EBA327, or EBA337 or given a placebo (PBS). All groups were given subsequent injections of 0.5 mg of anti-IFN-γ MAb i.p. every week thereafter until the end of the experiment. To determine the impact of anti-IFN-γ MAb on susceptibility to E. bieneusi infection, a group of five C.B-17 SCID mice received no antibody prior to oral inoculation with a mixture of spores from the four isolates. This group, however, was given anti-IFN-γ MAb 8 weeks after the oral challenge and every week thereafter until week 18, when the experiment was terminated.
(iii) Nude rats.
Seven 3-week-old nude rats (Charles River Laboratories) were each given an i.p. injection of 5 mg/rat of anti-IFN-γ MAb XMG1.2 at 2 h before oral inoculation with E. bieneusi spores, followed by additional injections of 2.5 mg/rat every 2 weeks. These rats were randomized into two groups, of which the first group of five was orally inoculated with EBA325 spores and the remaining two animals received a placebo. Again, in order to study the effect of anti-IFN-γ MAb on susceptibility, a third group of five RNU received no antibody prior to inoculation with isolate EBA325. This group, however, received anti-IFN-γ MAb at weeks 1, 3, and 5 after the oral challenge.
(iv) Germfree mice.
Six 4-week-old mice (C57BL/6 background; North Carolina State University, Raleigh, NC) were randomized into two groups of three mice each. One group was inoculated with isolate EBA206, and the second received a placebo.
(v) GKO mice.
Six 4-week old BALB/c mice (B6.129S7 ifntm1Ts, IFN-γ knockout [GKO]; Jackson Laboratories, Bar Harbor, ME), were randomized into two groups of three mice each. One group was inoculated with the EBA337 isolate, and the second group received a placebo.
(vi) Mongolian gerbils.
Fourteen 4-week-old gerbils (Charles River Laboratories) were immunosuppressed with dexamethasone (0.8 mg/each; Sigma-Aldrich Chemical Co., St. Louis, MO) given intramuscularly every other day four times before inoculation and twice every week thereafter. The gerbils were orally inoculated with 104 spores purified from feces of experimentally infected rats. Two gerbils were given a placebo.
Serial passage of E. bieneusi spores in vivo.
Purified EBA325 spores (103/animal) from the nude rats described in Table 1 were serially passaged from group to group six times (Table 2). In the group, each of the 3-week-old SCID mice received 1 mg/mouse of anti-IFN-γ MAb i.p. 2 h prior to inoculation and a second injection of 0.5 mg/mouse 1 week later. Mice were monitored three times weekly for excretion of spores and euthanatized 2 weeks after oral challenge. Spores (104) purified from the gut contents and feces were then fed to nude rats which had received 5 mg/rat of anti-IFN-γ MAb i.p. 2 h prior to inoculation and 2.5 mg/rat 1 week later. Rats were monitored for spore excretion and euthanatized 2 weeks after challenge. Spores purified from the gut contents and feces from this group were fed to a second group of rats, and spores from the second group were fed to the third, etc. This process was repeated six times (Table 2).
TABLE 2.
Summary of six serial propagations of E. bieneusi in rodentsa
| Rodents | Passage no. | No. of animals | Inoculum (no. of spores) | No. of spores recovered fromb:
|
|
|---|---|---|---|---|---|
| Feces | Gut contents | ||||
| SCID mice | 1 | 5 | 1 × 103 | (3.2-8.8) × 104 | (0.5-2.0) × 106 |
| RNU | 2 | 4 | 1 × 104 | NDc | ND |
| 3 | 5 | 1 × 104 | (0.2-1.0) × 107 | (0.8-4.0) × 107 | |
| 4 | 4 | 1 × 104 | ND | (2.0-4.7) × 107 | |
| 5 | 5 | 1 × 104 | (2.0-4.6) × 107 | (1.8-6.3) × 107 | |
| 6 | 5 | 1 × 104 | ND | ND | |
| Control RNUd | 1 | 5 | 0 | 0 | 0 |
Mice were given 1 mg and rats 5 mg of anti-IFN-γ MAb intraperitoneally at challenge and 1 week later and were euthnatized on day 14.
Spores were recovered either from feces at 2 weeks after oral challenge or from gut contents at euthnasia 2 weeks after infection.
ND, not determined.
Gut contents of an uninfected control RNU from Table 1 were used to inoculate control RNU.
IFAT.
The presence of E. bieneusi spores in fecal samples was monitored by IFA assay (16). The spores were detected using a rabbit anti-E. bieneusi polyclonal antibody (1:1,000 dilution) and a goat anti-rabbit immunoglobulin G (IgG) conjugated with Alexa 488 secondary antibody (1:1,000 dilution; Molecular Probes, Eugene, OR). The slides were examined by fluorescence microscopy (BX40; Olympus Optical Pvt. Ltd.) at a magnification of ×400, and the number of spores per 30 high-power fields (HPF) was counted. The IFA test (IFAT) was also used to enumerate the number of spores for oral inoculation, as described below. Fecal excretion of spores was monitored by sampling each animal three times a week and examining the samples by IFAT. Fecal pellets were stored at 4°C in PBS. To estimate the total number of spores excreted per animal, fecal pellets or gut contents were washed with PBS, and after a salt flotation step, the number of spores in a 1-μl drop on a slide was counted; multiple drops per slide were fixed and stained using IFA assay.
Histology and immunohistochemistry.
Sections from the small and large intestines and liver were removed from euthanatized mice and rats, fixed in 10% buffered formalin, and sectioned for histology. To determine the location of E. bieneusi in the tissue, immunohistochemistry was performed on the gut and liver tissues of infected rodents. Tissue sections were cut at 5 μm and immunostained using an avidin-biotin-horseradish peroxidase complex technique with diaminobenzidine (DAB) chromogen as described previously (24). The tissue sections were also subjected to a microwave pretreatment protocol for antigen retrieval (8). The sections were then identified as E. bieneusi positive by staining with a specific MAb (2G4, IgM; 1:4 dilution of supernatant) or a rabbit anti-E. bieneusi polyclonal antibody (17). An irrelevant rabbit IgG antibody was included in the assay as a negative control. Briefly, sections were deparaffinized and rehydrated. Endogenous peroxidase was inactivated by incubation for 40 min in a solution of 0.3% hydrogen peroxide in H2O. Sections were blocked with normal horse serum for 30 min at room temperature and then incubated overnight (4°C) with the E. bieneusi-specific antibody, followed by a biotinylated horse anti-mouse immunoglobulin antibody (Vector Laboratories, Burlingame, CA; dilution, 1:1,000) or biotinylated goat anti-rabbit immunoglobulin antibody and ABC Elite (Vector Laboratories; dilution, 1:50) for 30 min each at room temperature. The slides were developed using the DAB substrate kit (Vector Laboratories) and then counterstained with Mayer's hematoxylin. Positive controls included liver sections from a macaque infected with E. bieneusi (17). Negative controls included E. bieneusi-infected tissue sections in which the primary antibody was replaced by an irrelevant antibody or blocking buffer.
RESULTS
Evaluation of E. bieneusi infection in the different rodent models.
Spores purified from four infected human adults were confirmed to be E. bieneusi by amplification and sequencing of their rRNA internal transcribed spacer genes (16). The spores were then used to evaluate the intensity of infection and pattern of spore excretion in six different rodent models. All six rodent models became infected with E. bieneusi, but the duration and the intensity of spore excretion differed significantly among the various models. Excretion was regular or intermittent and often tended to fluctuate within and between groups (Table 1). No spores were detected in the placebo control groups. No clinical signs of diarrhea, other disease symptoms, or weight loss were observed in any of the infected rodents, presumably because of the relatively low-grade infection.
(i) C57BL/6 dexamethasone-immunosuppressed mouse model.
Two groups of three mice which were immunosuppressed with dexamethasone were inoculated with spores from two E bieneusi isolates (EBA206 and EBA337). No differences in the spore shedding pattern between the EBA206 and EBA337 groups were observed. The mice began to shed spores on day 8 after challenge and reached a peak (≤100 spores/30 HPF) 3 weeks after challenge, after which the mice continued to shed low levels (≤10 spores/30 HPF) until the end of the experiment (Table 1).
(ii) C.B-17 SCID mouse model.
All four E. bieneusi isolates were tested in C.B-17 SCID mice, and no differences in the pattern of spore excretion among the different isolates were observed. The mice began to shed spores on day 5 after challenge and continued to shed intermittently for 18 weeks, when the experiment was terminated. Peaks in spore excretion (≤500/30 HPF) often followed i.p. injections of anti-IFN-γ MAb (Table 1). One additional group of mice, which did not receive anti-IFN-γ MAb prior to inoculation, shed fewer spores (≤10/30 HPF) over an observation period of 9 weeks, after which the group received weekly anti-IFN-γ MAb injections, which led to an intensification of spore excretion (100 to 500/30 HPF), illustrating the benefit of anti-IFN-γ antibody (Table 1).
(iii) RNU.
Of the seven rats given 5 mg/rat of anti-IFN-γ MAb i.p., five were inoculated with E. bieneusi spores (EBA325). The rats were given additional MAb every other week thereafter. The rats started to excrete spores during week 1, with all animals shedding spores by week 2 (Table 1). They continued to excrete variable amounts of spores over the next 18 weeks, with peaks occurring at weeks 3 and at weeks 8 to 10.
A third group of five nude rats received anti-IFN-γ MAb prior to inoculation and then at weeks 1, 3, and 5 only. These rats began shedding within the first week, and shedding reached a peak at week 4. Shedding declined after the MAb treatment was discontinued, and no further peak was observed. This is in contrast to the case for the nude rats which received antibody every other week (Table 1).
(vi) Germfree mouse model.
Of the three mice orally inoculated with spores of isolate EBA325, two excreted spores (≤10/30 HPF) for the first 2 weeks only. The experiment was terminated at week 7.
(v) GKO mouse model.
Of the three mice orally inoculated with isolate EBA337, two excreted ≤10/30 HPF from week 2 until the end of the experiment 7 weeks later (Table 1).
(vi) Mongolian gerbils.
All 12 immunosuppressed gerbils inoculated with spores of isolate EBA325 purified from the nude rats began excreting spores 2 weeks after challenge (Table 1), with some excreting ≤500 spores/30 HPF at weeks 3 and 4, and then gradually dropped to intermittent excretion by week 6.
Consecutive passage of E. bieneusi spores in nude rats.
We passaged spores purified from one group of animals to the next to establish a rat-propagated E. bieneusi line. Nude rats were serially inoculated with 104 spores (Table 2). The SCID mice (group 1) were inoculated with spores isolated from the infected rats described in Table 1. Nude rats began shedding within the first week, and shedding peaked by week 2 after challenge, when the rats were euthanatized and the gut contents collected for a subsequent passage. Serial passage in rats was repeated several times, with similar spore excretion patterns observed at each passage (Table 2). Transmission of spores from one group of rats to another was successfully repeated six times, indicating that rat-adapted parasites can now be maintained in rodents under laboratory conditions, permitting continuous availability of infectious E. bieneusi spores for laboratory investigations. Spores from fecal pellets and gut contents were purified by a flotation step and the recovery estimated by IFAT (Fig. 1). The estimated total number of spores recovered from feces/gut contents was 0.5 × 106 to 2 × 106 per mouse and 2 × 107 to 6.3 × 107 per rat (Table 2). Spore counts in gerbils were similar to those in rats.
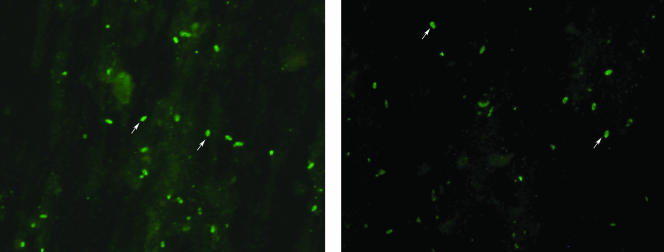
FIG. 1.
E. bieneusi spores excreted by experimentally infected rodents, identified by IFA assay using E. bieneusi-specific antibody. Fecal smears from a SCID mouse (left) and nude rat (right) are shown, with arrows indicating representative spores.
Immunohistochemistry.
No gross or histological abnormalities were observed in the tissues collected after euthanasia from any of the rodents. Immunohistochemistry using specific MAb or polyclonal antibodies identified E. bieneusi within enterocytes and epithelial cells lining the bile ducts and the gallbladders of infected animals (Fig. 2). No such staining was observed in sections stained without the primary antibody or with irrelevant primary antibody or in control animals.
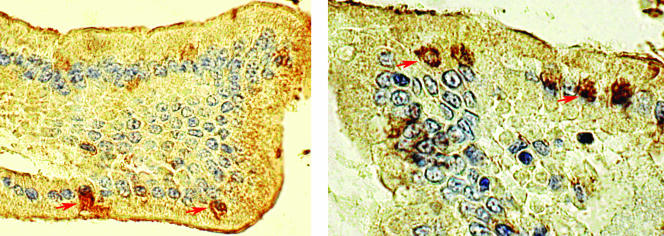
FIG. 2.
Immunohistochemistry performed on small intestinal sections of a SCID mouse (left) and a nude rat (right) using E. bieneusi-specific antibody, showing the presence of E. bieneusi forms within infected cells. The animals were inoculated with 106 spores each and were euthanized week 8 (left) and week 12 (right) after challenge (ABC method, DAB chromogen, hematoxylin counterstain; magnification, ×400). Arrows indicate infected cells.
DISCUSSION
The successful transmission of E. bieneusi from an infected human with HIV/AIDS to simian immunodeficiency virus-infected macaques, establishing a chronic infection, was achieved almost a decade ago (22). Further attempts to develop small-animal models for E. bieneusi have met with only limited success (1). While E. bieneusi infection has been recognized in humans since 1985, little progress has been made over the last 2 decades on the biology and pathogenesis of E. bieneusi because of a lack of laboratory tools and reagents and the inability to propagate the parasite in cell culture or in animals. The lack of immune reagents and a spore size similar to that of most bacteria have made identification, concentration, and purification difficult. Consequently, many investigators have resorted to using clinically less significant surrogate species of microsporidia. Since E. bieneusi infection, which appears to be widespread in most mammals and in some birds, is asymptomatic in the immunologically competent host, its emergence with HIV/AIDS in the mid 1980s is not surprising. Observations of patients with HIV/AIDS indicate that the disease, which is manifested as profound debilitating diarrhea and wasting, occurs when blood CD4 lymphocytes drop to a very low level, indicating the link between appearance of symptoms and severe immunodeficiency.
As with humans, immunodeficiency and/or immunosuppression was a requirement for establishing an apparent infection in the present study. Based on earlier studies to optimize a rodent model for cryptosporidiosis, which demonstrated that an injection of 1 mg/mouse of anti-IFN-γ MAb considerably enhanced the susceptibility of SCID mice to the infection (18, 19, 23), the administration of anti-IFN-γ MAb also increased the susceptibility of SCID mice and nude rats to E. bieneusi, contributing to the establishment of persistent infections lasting several months in these models. The impact of anti-IFN-γ MAb was clearly demonstrated by a sharp rise in spore excretion following each injection. Unlike in cryptosporidiosis, however, in which the absence of IFN-γ was key to complete susceptibility (19), for E. bieneusi the absence of T and/or B cells, as in SCID mice and RNU, appears to be a contributing factor.
The inclusion of several E. bieneusi isolates of human origin demonstrated that the ability to infect rodents is a characteristic common to presumably most, if not all, E. bieneusi isolates, possibly including isolates from other mammalian species. Although they are genetically very slightly different, we have successfully infected mice and rats with an isolate obtained from a naturally infected rhesus macaque (data not shown).
Curiously, immunosuppressed gerbils were equally susceptible to infection as were nude rats, which will provide an animal model for spore production, since gerbils are considerably less expensive. We have compared three different immunosuppression regimens in gerbils (cyclosporine, dexamethasone, and a combination of the two) and found that injectable dexamethasone was superior (data not shown). However, unlike the case for nude rats, in which infections can be maintained for months with repeated injections of anti-IFN-γ MAb, the infection in gerbils lasted 6 weeks, despite repeated administration of immunosuppressive drugs.
Whereas the spore yield from the two superior models (nude rats and gerbils) is not high, it should provide spores for serial propagation and laboratory maintenance of well-defined strains. The relatively small number of spores found in the feces also means that much effort is required to purify them. It is nevertheless a major step forward, and it is hoped that further optimization of these or additional models will provide even better prospects. Despite the limitations, the availability of parasites and rodent models will allow the screening and evaluation of urgently needed chemotherapeutic agents. Studies on some basic biological, biochemical, and genetic characteristics of E. bieneusi should also be possible and indeed are under way. Some aspects of the host-parasite interface and the nature of the immune dysfunction, the hallmark of this infection in humans, can now be investigated. The availability of fresh spores will greatly facilitate attempts to cultivate E. bieneusi in vitro.
Acknowledgments
This work was supported by grants R01 AI43196 and in part by grants R21 AI064118 and P01 DK55510.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Accoceberry, I., J. Carriere, M. Thellier, S. Biligui, M. Danis, and A. Datry. 1997. Rat model for the human intestinal microsporidian Enterocytozoon bieneusi. J. Eukaryot Microbiol. 44:83S. [DOI] [PubMed] [Google Scholar]
- 2.Accoceberry, I., M. Thellier, I. Desportes-Livage, A. Achbarou, S. Biligui, M. Danis, and A. Datry. 1999. Production of monoclonal antibodies directed against the microsporidium Enterocytozoon bieneusi. J. Clin. Microbiol. 37:4107-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalifoux, L. V., J. MacKey, A. Carville, D. Shvetz, K. C. Lin, A. Lackner, and K. G. Mansfield. 1998. Ultrastructural morphology of Enterocytozoon bieneusi in biliary epithelium of rhesus macaques (Macaca mulatta). Vet. Pathol. 35:292-296. [DOI] [PubMed] [Google Scholar]
- 4.Deplazes, P., A. Mathis, C. Muller, and R. Weber. 1996. Molecular epidemiology of Encephalitozoon cuniculi and first detection of Enterocytozoon bieneusi in faecal samples of pigs. J. Eukaryot. Microbiol. 43:93S. [DOI] [PubMed] [Google Scholar]
- 5.Desportes-Livage, I. 2000. Biology of microsporidia. Contrib. Microbiol. 6:140-165. [DOI] [PubMed] [Google Scholar]
- 6.Green, L. C., P. J. Didier, L. C. Bowers, and E. S. Didier. 2004. Natural and experimental infection of immunocompromised rhesus macaques (Macaca mulatta) with the microsporidian Enterocytozoon bieneusi genotype D. Microbes Infect. 6:996-1002. [DOI] [PubMed] [Google Scholar]
- 7.Guerard, A., M. Rabodonirina, L. Cotte, O. Liguory, M. A. Piens, S. Daoud, S. Picot, and J. L. Touraine. 1999. Intestinal microsporidiosis occurring in two renal transplant recipients treated with mycophenolate mofetil. Transplantation 68:699-707. [DOI] [PubMed] [Google Scholar]
- 8.Hendricks, E. E., E. Ludlage, S. Bussell, K. George, F. H. Wegner, and K. G. Mansfield. 2004. Wasting syndrome and disruption of the somatotropic axis in simian immunodeficiency virus-infected macaques with Mycobacterium avium complex infection. J. Infect. Dis. 190:2187-2194. [DOI] [PubMed] [Google Scholar]
- 9.Kondova, I., K. Mansfield, M. A. Buckholt, B. Stein, G. Widmer, A. Carville, A. Lackner, and S. Tzipori. 1998. Transmission and serial propagation of Enterocytozoon bieneusi from humans and rhesus macaques in gnotobiotic piglets. Infect. Immun. 66:5515-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotler, D. P., and J. M. Orenstein. 1998. Clinical syndromes associated with microsporidiosis. Adv. Parasitol. 40:321-349. [DOI] [PubMed] [Google Scholar]
- 11.Kucerova, Z., H. Moura, G. J. Leitch, R. Sriram, C. Bern, V. Kawai, D. Vargas, R. H. Gilman, E. Ticona, A. Vivar, and G. S. Visvesvara. 2004. Purification of Enterocytozoon bieneusi spores from stool specimens by gradient and cell sorting techniques. J. Clin. Microbiol. 42:3256-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, J. H. 2000. Using pig biliary system, in vivo propagation of Enterocytozoon bieneusi, an AIDS-related zoonotic pathogen. J. Vet. Sci. 1:105-111. [PubMed] [Google Scholar]
- 13.Mansfield, K. G., A. Carville, D. Hebert, L. Chalifoux, D. Shvetz, K. C. Lin, S. Tzipori, and A. A. Lackner. 1998. Localization of persistent Enterocytozoon bieneusi infection in normal rhesus macaques (Macaca mulatta) to the hepatobiliary tree. J. Clin. Microbiol. 36:2336-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansfield, K. G., A. Carville, D. Shvetz, J. MacKey, S. Tzipori, and A. A. Lackner. 1997. Identification of an Enterocytozoon bieneusi-like microsporidian parasite in simian-immunodeficiency-virus-inoculated macaques with hepatobiliary disease. Am. J. Pathol. 150:1395-1405. [PMC free article] [PubMed] [Google Scholar]
- 15.Metge, S., J. T. Van Nhieu, D. Dahmane, P. Grimbert, F. Foulet, C. Sarfati, and S. Bretagne. 2000. A case of Enterocytozoon bieneusi infection in an HIV-negative renal transplant recipient. Eur. J. Clin. Microbiol. Infect. Dis. 19:221-223. [DOI] [PubMed] [Google Scholar]
- 16.Sheoran, A. S., X. Feng, S. Kitaka, L. Green, C. Pearson, E. S. Didier, S. Chapman, J. K. Tumwine, and S. Tzipori. 2005. Purification of Enterocytozoon bieneusi from stools and production of specific antibodies. J. Clin. Microbiol. 43:387-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheoran, A. S., X. Feng, I. Singh, S. Chapman-Bonofiglio, S. Kitaka, J. Hanawalt, J. Nunnari, K. Mansfield, J. K. Tumwine, and S. Tzipori. 2005. Monoclonal antibodies against Enterocytozoon bieneusi of human origin. Clin. Diagn. Lab. Immunol. 12:1109-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theodos, C. M., J. K. Griffiths, J. D'Onfro, A. Fairfield, and S. Tzipori. 1998. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob. Agents Chemother. 42:1959-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theodos, C. M., K. L. Sullivan, J. K. Griffiths, and S. Tzipori. 1997. Profiles of healing and nonhealing Cryptosporidium parvum infection in C57BL/6 mice with functional B and T lymphocytes: the extent of gamma interferon modulation determines the outcome of infection. Infect. Immun. 65:4761-4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tumwine, J. K., A. Kekitiinwa, S. Bakeera-Kitaka, G. Ndeezi, R. Downing, X. Feng, D. E. Akiyoshi, and S. Tzipori. 2005. Cryptosporidiosis and microsporidiosis in Ugandan children with persistent diarrhea with and without concurrent infection with the human immunodeficiency virus. Am. J. Trop. Med. Hyg. 73:921-925. [PubMed] [Google Scholar]
- 21.Tumwine, J. K., A. Kekitiinwa, N. Nabukeera, D. E. Akiyoshi, M. A. Buckholt, and S. Tzipori. 2003. Enterocytozoon bieneusi among children with diarrhea attending Mulago Hospital in Uganda. Am. J. Trop. Med. Hyg. 67:299-303. [DOI] [PubMed] [Google Scholar]
- 22.Tzipori, S., A. Carville, G. Widmer, D. Kotler, K. Mansfield, and A. Lackner. 1997. Transmission and establishment of a persistent infection of Enterocytozoon bieneusi, derived from a human with AIDS, in simian immunodeficiency virus-infected rhesus monkeys. J. Infect. Dis. 175:1016-1020. [DOI] [PubMed] [Google Scholar]
- 23.Tzipori, S., W. Rand, and C. Theodos. 1995. Evaluation of a two-phase scid mouse model preconditioned with anti-interferon-gamma monoclonal antibody for drug testing against Cryptosporidium parvum. J. Infect. Dis. 172:1160-1164. [DOI] [PubMed] [Google Scholar]
- 24.Veazey, R. S., M. Rosenzweig, D. E. Shvetz, D. R. Pauley, M. DeMaria, L. V. Chalifoux, R. P. Johnson, and A. A. Lackner. 1997. Characterization of gut-associated lymphoid tissue (GALT) of normal rhesus macaques. Clin. Immunol. Immunopathol. 82:230-242. [DOI] [PubMed] [Google Scholar]
- 25.Zhang, Q., I. Singh, A. Sheoran, X. Feng, J. Nunnari, A. Carville, and S. Tzipori. 2005. Production and characterization of monoclonal antibodies against Enterocytozoon bieneusi purified from rhesus macaques. Infect. Immun. 73:5166-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]