Abstract
Immune responses against monocytotropic ehrlichiosis during infection with a strain of Ehrlichia from Ixodes ovatus (IOE) were evaluated using a model that closely reproduces the pathology and immunity associated with tick-transmitted human monocytotropic ehrlichiosis. C57BL/6 mice were inoculated intradermally or intraperitoneally with high-dose highly virulent IOE or intraperitoneally with mildly virulent Ehrlichia muris. Intradermal (i.d.) infection with IOE established mild, self-limited disease associated with minimal hepatic apoptosis, and all mice survived past 30 days. Intraperitoneal (i.p.) infection with IOE resulted in acute, severe toxic shock-like syndrome and severe multifocal hepatic apoptosis and necrosis, and all mice succumbed to disease. Compared to i.p. infection with IOE, intradermally infected mice had a 100- to 1,000-fold lower bacterial load in the spleen with limited dissemination. Compared to mice infected intraperitoneally with IOE, i.d. infection stimulated a stronger protective type-1 cell-mediated response on day 7 of infection, characterized by increased percentages of both CD4+ and CD8+ splenic T cells, generation of a greater number of IOE-specific, gamma interferon-producing CD4+ Th1 cells, and higher levels of tumor necrosis factor (TNF-α) in the spleen but lower concentrations of serum TNF-α and interleukin-10. These data suggest that under the conditions of natural route of challenge (i.e., i.d. inoculation), the immune response has the capacity to confer complete protection against monocytotropic ehrlichiosis, which is associated with a strong cell-mediated type-1 response and decreased systemic production of pro- and anti-inflammatory cytokines.
Human monocytotropic ehrlichiosis (HME) is an emerging infection in humans, and is the most prevalent life-threatening tick-borne disease in North America (20). Ehrlichia chaffeensis, the etiologic agent of HME, is an obligately intracellular bacterium (30) which causes a range of manifestations, from mild to severe, in immunocompetent patients. HME can be fatal, particularly in immunocompromised (22, 31) or elderly individuals (19).
Several animal models have been developed to examine the factors that determine host resistance or susceptibility to ehrlichiosis. E. chaffeensis does not cause disease in immunocompetent mice. Although E. chaffeensis infection in SCID mice provided some clues to the immune response to Ehrlichia (i.e., the importance of T cells, gamma interferon [IFN-γ], and antibodies in clearing infection) (4, 9, 35), the pathology does not mimic HME. Two different strains of Ehrlichia, Ehrlichia muris (21) and the unnamed strain isolated from Ixodes ovatus ticks (IOE) (27), have provided better models that mimic the pathology and clinical manifestations of HME. However, the antigenic and genetic differences between E. muris and IOE could be confounding factors in the interpretation of the results when comparing these models (26, 32). Models using the same organism provide a more advantageous situation to study differences in the mild and severe forms of ehrlichioses.
Few experimental models of ehrlichiosis have attempted to reproduce the biology of natural transmission, which would include consideration of (i) the dose (individuals can be inoculated by the ticks with variable quantities of ehrlichiae), (ii) inoculum medium (ehrlichiae are coinoculated with tick saliva that contains antihemostatic and immunomodulatory molecules that facilitate tick blood feeding), (iii) site of inoculation (infection is initiated in the skin, in which dermal and epidermal compartments contain unique cells such as keratinocytes, dendritic epidermal T cells, and Langerhans cells that enable the host to deal with the hostile external environment to which it is constantly exposed), and (iv) pathology (which mimics mild and severe monocytotropic ehrlichiosis in immunocompetent patients). Individually, these variables can have substantial effects on the outcome of infection and the host immune response against different pathogens (2, 5, 6, 16, 26, 32, 36). The fact that several of these variables can act synergistically or antagonistically in nature adds to the complexity of the disease seen in humans.
To the best of our knowledge, monocytotropic ehrlichial infection after intradermal (i.d.) inoculation, the natural route of transmission, has not been described. However, Shibata et al. did inoculate IOE, formerly referred to as an HF strain, subcutaneously and found that this route increased survival in wild-type BALB/c mice (26). The goal of this study was to establish a method by which the same ehrlichial strain could be inoculated into mice and used to compare two forms of ehrlichiosis seen in humans, namely, disease that is mild and self-limited and disease that is fatal. An additional goal was to use the natural (i.e., i.d.) route of E. chaffeensis infection for developing the mild disease, which would best represent the way humans are inoculated with the pathogen by ticks. We were able to achieve these goals by inoculating the same dose of IOE either intradermally or intraperitoneally in immunocompetent C57BL/6 mice. We then used this method to evaluate differences in the disease pathology, systemic bacterial burden, and immune responses, initiated by the two routes of inoculation. IOE inoculated intradermally resulted in mild disease characterized by minimal liver pathology, lower systemic bacterial burden, a greater number of CD4+ and CD8+ lymphocytes, and a more robust Th1 immune response compared with the same dose of IOE inoculated intraperitoneally.
MATERIALS AND METHODS
Bacterial stocks.
For most experiments, a highly virulent ehrlichial species, IOE, originally isolated from Ixodes ovatus ticks in Japan, was used (12, 26). Mildly virulent E. muris was used in the enzyme-linked immunospot (ELISPOT) studies (see Fig. 5) and the survival experiments as an example of a well-characterized animal model for mild disease and for comparison with i.d. inoculation of IOE (13). Both ehrlichial strains were kindly provided by Yasuko Rikihisa (Ohio State University). The IOE isolate was defined as HF565 in the original study (8). The stock aliquots of IOE used in all the experiments were prepared by intraperitoneal (i.p.) inoculation of C57BL/6 mice with approximately 1 × 107 organisms, and then the IOE-infected spleens were harvested when the mice appeared to be terminally ill (usually on day 7 postinfection). Each spleen was homogenized and resuspended in either 5 ml of Leibovitz medium (for animal inoculations) or fetal bovine serum with 10% dimethyl sulfoxide (for storage in liquid nitrogen). This technique reproducibly yielded aliquots containing approximately 1 × 107 IOE copies per ml as determined by real-time PCR (see below). E. muris was propagated in a P388D1 macrophage cell line (ATCC) as previously described (13). Experimental inoculation with E. muris was performed using aliquots of spleen homogenate prepared from C57BL/6 mice inoculated with the frozen stock of cell culture-associated E. muris.
FIG. 5.
ELISPOT analysis of Th1 and Th2 cytokine responses in the spleen following lethal i.p. IOE infection, nonlethal i.d. IOE infection, or nonlethal E. muris infection on day 7 postinfection. Splenocytes were harvested from control and infected mice, and immune splenocytes were stimulated in vitro with the relevant ehrlichial antigen. The concentration of Ehrlichia-specific cytokine-producing cells was determined by subtracting the number of spots in the wells containing no antigen from those stimulated with antigen. The intradermally infected animals had a significantly larger concentration of Ehrlichia-specific IFN-γ-producing type-1 cells, which is almost equal in magnitude to the type-1 response produced upon E. muris infection, and this was significantly higher than that observed in the intraperitoneally infected animals (P < 0.05). Data are expressed as means ± standard errors, and three mice per group were used in the analysis.
Experimental design and inoculation of mice.
Age-matched (8 to 10 weeks old) and sex-matched (female) C57BL/6J mice were used for all experiments (Jackson Laboratories, Bar Harbor, Maine). Mice were housed in the animal research center at the University of Texas Medical Branch in accordance with the institutional guidelines for animal welfare. In our preliminary studies, India ink was injected intradermally and the localization of the dye was examined by light microscopy to confirm the i.d. localization of the inoculum. In all experiments, mice were inoculated intradermally or intraperitoneally with 300 μl of IOE containing the same concentration of Ehrlichia. IOE has not been propagated in cell culture, which makes determining the exact dose of IOE an issue. We first completed a large-scale dose-response study to determine the effect of different ehrlichial concentrations on survival in both groups of (intradermally or intraperitoneally inoculated) mice. A total of 72 mice (6 mice per dose per group) were inoculated with 10-fold serial dilutions (102 to 107 IOE genome copies) of ehrlichiae to determine the dose that would result in 100% survival of the intradermally inoculated mice and 100% fatality in the intraperitoneally inoculated mice. We determined that a concentration of 5 × 104 IOE copies was optimal, and this dose was used in all the experiments in this study. For E. muris infections, mice were inoculated intraperitoneally with a high dose of E. muris (∼4 × 108 bacterial genomes). We inoculated three mice per group per time point and repeated each experiment at least three times. Mice were monitored daily for signs of illness, including the presence of distress, loss of appetite, rough hair coat, and decreased activity.
Ehrlichial load determination in E. muris and IOE inocula by quantitative real-time PCR.
Quantitative real-time PCR was used to determine the IOE or E. muris copy numbers in the frozen stock and fresh inocula. Portions of the ehrlichial dsb and human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) genes were cloned into plasmids using the TOPO TA cloning kit (Invitrogen, Carlsbad, CA). We then determined the sizes of the plasmids (including the inserts) in kilodaltons, the concentration of double-stranded DNA in each sample calculated with a NanoDrop ND-1000 spectrophotometer (Wilmington, DE), the number of cells acquired from each spleen, and the assumption of two copies of each gene per cell to determine our exact copy number per μl. These copy numbers were then used for our standards in the real-time assays (see below). We then normalized the copy numbers of IOE by dividing by the copy numbers of GAPDH.
Ehrlichial load determination in collected organs by quantitative real-time PCR.
To determine the relative differences in the ehrlichial burden between the different groups of mice, approximately 10 mg of liver, lung, spleen, and kidney (each) were collected at different time points postinfection and homogenized. DNA was extracted using the DNeasy tissue kit (QIAGEN, Valencia, CA) with an elution volume of 100 μl deionized water, and ehrlichial burdens were determined using an iCycler IQ multicolor real-time detection system (Bio-Rad, Hercules, CA). The following primers and probes targeting both IOE and E. muris dsb (which encodes a thiodisulfide oxidoreductase gene) and host GAPDH (29) genes were used and included: EM/IOE dsb F, 5′ CAG GAT GGT AAA GTA CGT GTG A 3′; EM/IOE dsb R, 5′ TAG CTA AYG CTG CCT GGA CA 3′; EM/IOE probe (5′FAM/3′BHQ1), 5′ AGG GAT TTC CCT ATA CTC GGT GAG GC 3′; GAPDH F, 5′ CAA CTA CAT GGT CTA CAT GTT C 3′; GAPDH R, 5′ TCG CTC CTG GAA GAT G 3′; and GAPDH probe (5′CY5/3′BHQ2), 5′ CGG CAC AGT CAA GGC CGA GAA TGG GAA GC 3′. The comparative cycle threshold method (3) was used for comparison of the ehrlichial burdens in the harvested organs because (i) we could not determine the number of cells present in each sample and (ii) our intent was to evaluate the relative differences between the intradermally and intraperitoneally inoculated animals.
Histology.
Formalin-fixed, paraffin-embedded samples from livers, lungs, and spleens were sectioned and stained with hematoxylin and eosin (H&E). Histologic sections were evaluated qualitatively, and liver lesions were assessed by four parameters, including hepatocyte damage, frequency of lesions, size of inflammatory lesions, and extent of perivascular inflammation.
TUNEL assays.
If apoptosis without focal necrosis was observed in the H&E-stained tissues by light microscopy, the paraffin-embedded tissues from these samples were examined by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining. Ten percent neutral-buffered formaldehyde-fixed, paraffin-embedded tissue sections (5 μm) were deparaffinized and rehydrated by passage through xylene and graded ethanol solutions. Slides were treated with Protease XXIV (BioGenex, San Ramon, CA) and then postfixed with 4% paraformaldehyde. The terminal dT (TdT)-FragEL DNA fragmentation detection kit (Calbiochem, San Diego, CA) was used to detect the DNA breaks in apoptotic nuclei according to the manufacturer's instructions. Positive controls were pretreated with DNase I, and negative controls had TdT omitted during labeling. Slides were counterstained with Mayer's modified hematoxylin (Poly Scientific, Bay Shore, NY) before mounting and were viewed under an Olympus BX51 microscope, and the images were recorded by an RT Slider digital camera (Diagnostic Instruments Inc., Sterling Heights, MI).
Preparation of host cell-free E. muris and IOE antigens.
For ex vivo IOE antigen stimulation used in the ELISPOT assay and splenocyte cultures (see below), purified host cell-free Ehrlichia were obtained from the spleens and livers of IOE-infected mice on day 7 postinfection as described previously (13). The E. muris antigens were obtained from E. muris infected P388D cells as described previously (13). For both assays, we used ∼10 μg per ml of either E. muris or IOE antigen as determined by a bicinchoninic protein assay reagent kit (Pierce, Rockford, IL).
ELISPOT assays for antigen-specific, cytokine-producing T cells.
Splenocytes and purified T-cell subsets were assessed via ELISPOT assay for cytokine production, as described previously (23). Briefly, 96-well nitrocellulose plates (Millipore, Bedford, MA) were coated at 4°C overnight with monoclonal antibodies (1.25 μg/ml and 100 μl/well) that are specific for murine IFN-γ or interleukin-4 (IL-4) (BD Pharmingen, San Diego, CA). Twofold dilutions of spleen cells were added to wells starting at 106 to 2 × 105 cells/well in the presence of an additional 1 × 106 spleen cells from naive, unimmunized mice. The plates were developed as described previously (13, 23). The spots were counted under a dissecting microscope. In all experiments, antigen-specific spots were determined by subtraction of the background spots (spots detected in the wells containing no antigen) from spots detected in the antigen-containing wells.
Splenocyte cultures and cytokine detection.
For measurement of cytokine production in infected mice, spleens were harvested on day 7 postinfection, and single-cell suspensions were prepared. Splenocytes were cultured in six-well plates at a total cell concentration of 1 × 107 cells per well in a final volume of 5 ml of complete medium (RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 1% HEPES buffer [238.3 μg/ml], and 1% penicillin-streptomycin [100 μg/ml]) in the presence or absence of IOE antigens (∼10 μg/ml). To ensure optimal antigen presentation, 2.5 × 106 naïve syngeneic splenocytes were added to the immune splenocytes obtained from IOE-infected mice. Cultures were incubated at 37°C in an atmosphere containing 5% CO2. Supernatant fluids were harvested after 48 h and assayed for the presence of IFN-γ, tumor necrosis factor (TNF-α), and IL-10 by using Quantikine enzyme-linked immunosorbent assay (ELISA) kits (R&D, Minneapolis, MN), following the manufacturer's recommendations.
Serum cytokines.
At different time points after infection, serum samples were collected from infected mice, and the levels of IFN-γ, TNF-α, and IL-10 were determined by Quantikine ELISA.
Flow cytometry.
Splenocytes were harvested, counted, and resuspended in fluorescence-activated cell sorter staining buffer (Dulbecco's phosphate-buffered saline without Mg2+ or Ca2+ containing 1% heat-inactivated fetal calf serum and 0.09% sodium azide, filtered (0.2 μm-pore membrane; pH adjusted to 7.4 to 7.6), and aliquoted into a 96-well V-bottom plate (Costar, Corning, NY) at a concentration of 106 cells per well. All labeling was carried out on ice. Fc receptors were blocked (clone 2.4G2) for 15 min. The cells were then washed and pelleted. Fluorescein isothiocyanate-conjugated CD4 (clone GK1.5)- and phycoerythrin-conjugated CD8a (clone 53-6.7)-specific monoclonal antibodies with corresponding isotype controls were used. All antibodies were from BD Pharmingen (San Diego, CA). Lymphocyte populations were gated based on forward and side-scatter parameters, 20,000 events were collected using a BD FACSCalibur (BD Immunocytometry Systems, San Jose, CA) flow cytometer with CellQuest software (Immunocytometry Systems), and data were analyzed using FlowJo (Tree Star Inc., Ashland, OR) flow-cytometric analysis software.
Statistical analysis.
We used Student's t test for comparison between two individual groups (group 1 was made up of mice infected intraperitoneally with IOE and group 2 was made up of mice infected intradermally with IOE), and only differences with a P value of less than 0.05 were considered statistically significant. In the ELISPOT experiment, similar comparisons were made between two groups of mice (between mice infected intradermally with IOE and mice infected intraperitoneally with E. muris and between mice infected intraperitoneally with IOE and mice infected intraperitoneally with E. muris). Each experiment was repeated at least three times, and statistical comparisons between the i.d. and i.p. routes of inoculation were made for each independent experiment.
RESULTS
Effect of IOE dose on disease progression and mortality in immunocompetent C57BL/6 mice.
Our previous study showed a correlation between the dose of highly virulent Ehrlichia (IOE) and the duration of disease until death (13). To determine the dose of Ehrlichia that causes 100% survival or fatality following i.d. or i.p. inoculation, respectively, mice were inoculated with serial 10-fold dilutions over a range of doses from 102 to 107 bacterial genomes, as determined by quantitative real-time PCR. The percent survival and mean survival times (MST) in the groups of mice inoculated with various doses are shown in Table 1. i.p. inoculation of mice with high doses of IOE (104 to107 bacterial genomes) caused 100% mortality on days 7 to 10 postinfection. i.d. inoculation with 107 bacterial genomes also caused 100% mortality on day 9 postinfection (Table 1). Thirty-three percent and 67% of infected mice survived infection after i.d. inoculation with 106 and 105 organisms, respectively. However, all mice infected intradermally with 104 genome copies of IOE survived past day 30 postinfection. Moribund mice in all groups (i.e., intradermal or intraperitoneal) inoculated with doses of IOE showed clinical signs of wasting, hunched posture, ruffled fur, and finally hypothermia, suggesting that toxic shock-like syndrome was the cause of death as described previously (13). We also observed differences in mortality and MST based on the age of the mice, where mice less than 6 weeks old were more susceptible to IOE than mice with an age range of 7 to 10 weeks (data not shown). In the remaining experiments described in this study, we used 8- to 10-week-old mice inoculated with an IOE inoculum containing 5 × 104 bacterial genomes and refer to this as high-dose IOE. The survival curves for mice given this dose are shown in Fig. 1.
TABLE 1.
Outcome and survival times of infection of mice with different doses of IOE inoculated intraperitoneally and intradermally
| IOE dose (copies) | MST (days)
|
No. (%) surviving on day 30 postinfection | |
|---|---|---|---|
| Mean | SD | ||
| Intraperitoneal | |||
| 107 | 7 | 0 | 0 (0) |
| 106 | 7 | 0 | 0 (0) |
| 105 | 8 | 0 | 0 (0) |
| 104 | 10 | 0.63 | 1 (17) |
| 103 | 11 | 2.63 | 3 (50) |
| 102 | 12.5 | 2.5 | 4 (67) |
| Intradermal | |||
| 107 | 9 | 0 | 0 (0) |
| 106 | 9.5 | 0.5 | 2 (33) |
| 105 | 12.5 | 3.5 | 4 (67) |
| 104 | 30 | 0 | 6 (100) |
| 103 | 30 | 0 | 6 (100) |
| 102 | 30 | 0 | 6 (100) |
FIG. 1.
Survival curve of mice infected with Ixodes ovatus Ehrlichia (IOE) or E. muris. C57BL/6 mice were inoculated intradermally (i.d.) or intraperitoneally (i.p.) with 300 μl containing the same dose (∼5 × 104 bacterial genomes) of IOE or intraperitoneally with high-dose E. muris (∼4 × 108 bacterial genomes). Three mice from each group were sacrificed on days 4 and 7 postinfection, and the rest were monitored for signs of illness and survival. All of the remaining intradermally inoculated mice survived past day 30 postinfection, and all of the animals infected intraperitoneally with IOE succumbed to disease by day 10 postinfection. Results are from three independent experiments in which the intraperitoneally inoculated animals succumbed to disease between days 8 and 10 postinfection. A total of nine mice per group (from the three independent experiments) are included in the survival curve.
Intradermal inoculation of IOE resulted in mild disease.
While intraperitoneally infected animals developed severe hepatic apoptosis and multifocal necrosis on day 7 postinfection, all of the intradermally infected mice developed relatively mild hepatic cellular infiltration and minimal hepatocellular apoptosis. The major differences in the hepatic pathology in both groups were seen as early as day 4 postinfection (data not shown). The intradermally inoculated animals showed only mild, lymphohistiocytic infiltration (LHI), which was localized around blood vessels and portal triads. In contrast, the intraperitoneally inoculated animals had a dispersed inflammatory response, which was present throughout the liver within the majority of sinusoids.
Quantitative analysis of the TUNEL assays detected relatively few apoptotic cells in the livers of the intradermally inoculated animals, mainly observed within the areas of hepatic LHI. We observed apoptosis of both hepatocytes and Kupffer cells throughout the liver in the intraperitoneally inoculated mice (Fig. 2) on day 6 postinfection. Extensive apoptosis was consistently observed in the liver one day prior to the day when massive hepatic necrosis appeared.
FIG. 2.
Mild liver pathology and minimal apoptosis following intradermal inoculation with Ehrlichia compared with intraperitoneal inoculation. On day 6 after i.d. or i.p. infection with IOE, livers were harvested, fixed, and stained by H&E. TUNEL assay was performed to detect apoptotic cells. Uninfected control mice did not have lesions or apoptosis in the liver (TUNEL assay) (A). Mice infected intraperitoneally had many apoptotic hepatocytes (arrows) in H&E-stained sections (B) and those stained by TUNEL assays (C). In mice infected intradermally, apoptotic cells (arrows) were observed principally in the foci of hepatic inflammation (D).
By day 7 postinfection (Fig. 3), the LHI in the intradermally infected animals had increased. The hepatic architecture at sites without inflammation appeared relatively normal. We observed severe congestion, extensive apoptosis, and focal hepatic necrosis in the intraperitoneally inoculated animals on day 7 postinfection.
FIG. 3.
Hepatic histopathology in the intradermally (A) or intraperitoneally (B) inoculated animals on day 7 postinfection. The intradermally inoculated animals had localized lymphohistiocytic infiltrates mainly near blood vessels and portal triads (arrow), and the surrounding liver architecture appeared normal. In contrast, extensive apoptosis (arrowheads) and hepatic necrosis (arrows) were observed in the intraperitoneally inoculated animals.
Intradermal inoculation resulted in decreased systemic bacterial burden.
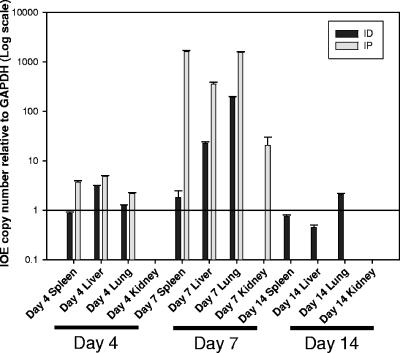
The bacterial burden in the intradermally inoculated animals was most similar to that detected in i.p. infected mice on day 4 postinfection. On day 7 postinfection, C57BL/6 mice inoculated intradermally with IOE had a significant increase in the number of ehrlichiae detected in the liver and lung (P < 0.01) compared to the levels detected on day 4 postinfection. There was no significant increase in the splenic bacterial burden from day 4 to day 7 postinfection. Intradermally infected mice that survived ehrlichial infection harbored ehrlichial DNA in their livers, spleens, and lungs on day 14 postinfection. In addition, ehrlichial DNA was not detected in the kidneys of the intradermally inoculated mice at any time point (Fig. 4).
FIG. 4.
Kinetic analysis of the bacterial burden in C57BL/6 mice after i.p. or i.d. challenge with high-dose IOE. Mice were sacrificed on days 4 and 7 postinfection and analyzed for the presence of ehrlichial DNA using real-time PCR with primers and probes targeting the Ehrlichia dsb and host cell GAPDH genes. Cycle threshold values greater than 40 were considered negative. The intradermally infected mice had significantly lower concentrations of bacteria on days 4 and 7 in livers, lungs, and spleens (P < 0.05). Day 14 data show the bacterial burdens in the intradermally inoculated mice only, as all the intraperitoneally infected mice had already succumbed to the disease. Data are expressed as means plus standard errors, and three mice per group were used in the analysis.
Inoculation of IOE intradermally resulted in a higher number of antigen-specific IFN-γ-producing T cells.
We then evaluated the differences in the antigen-specific Th1 and Th2 immune responses between the models of severe (i.p. IOE) ehrlichiosis and mild (i.d. IOE) ehrlichiosis and compared them with the mild (i.p. E. muris) ehrlichiosis model using an ELISPOT assay (Fig. 5). All groups contained negligible numbers of IOE-specific, IL-4-producing cells (Fig. 5), and we did not detect significant IL-4 production in the culture supernatant of antigen-stimulated splenocytes from any infected group by ELISA (data not shown).
We then determined if the increased IFN-γ production observed in the ELISPOT assay following i.d. or i.p. infections was due to differences in the frequency of CD4+ or CD8+ T cells in the spleen. To test this possibility, the percentages of CD4+ and CD8+ T cells in mice infected intraperitoneally or intradermally with the same dose of IOE were measured by flow cytometry on day 7 postinfection. The intradermally infected animals had significantly higher (P < 0.01) percentages of both CD4+ and CD8+ T cells (17.47% and 11.08% of total gated lymphocytes, respectively) than those detected in intraperitoneally inoculated mice (10.56% and 4.03% of total gated lymphocytes were CD4+ and CD8+ T cells, respectively) (Fig. 6).
FIG. 6.
Ex vivo analysis of CD4+ and CD8+ T-cell percentages in harvested splenocytes from the intradermally and intraperitoneally infected mice on day 7 postinfection. Harvested splenocytes were incubated concurrently with fluorescein isothiocyanate-conjugated anti-CD4 and phycoerythrin-conjugated anti-CD8 antibodies. Lymphocytes were gated based on size and granularity, and 20,000 events were collected. The intradermally inoculated animals had significantly (P < 0.01) higher percentages of both CD4+ and CD8+ T cells in the spleen compared to the intraperitoneally infected mice. Results are representative of all three mice per group, and similar results were obtained from three independent experiments.
Intradermal inoculation of IOE did not cause toxic shock-like syndrome.
Next, we compared the differences in cytokine concentrations produced both locally and systemically in the intradermally and intraperitoneally inoculated animals by analysis of antigen-stimulated splenocyte supernatants and sera, respectively. Since our previous study suggested that overproduction of TNF-α is associated with toxic shock-like syndrome following i.p. infection with high-dose IOE, we focused on the proinflammatory cytokines IFN-γ and TNF-α and the anti-inflammatory cytokine IL-10.
On day 7 postinfection, spleens of the animals inoculated intradermally with IOE contained cells capable of producing significantly higher concentrations of IFN-γ when stimulated by IOE antigen than those of the intraperitoneally infected animals (P < 0.01) (Fig. 7, top panels). However, the intraperitoneally infected mice had significantly higher serum levels of IFN-γ than the intradermally infected mice (P < 0.05) (Fig. 7, top panels). The relative concentrations of TNF-α in sera and antigen-stimulated splenocyte cultures from mice infected intradermally or intraperitoneally with IOE were similar to those of IFN-γ in these animals. The intradermally inoculated mice had significantly higher TNF-α production in the spleen than the i.p. inoculated mice (P < 0.05), while the intraperitoneally inoculated mice had higher levels of TNF-α in the serum (P < 0.01), which also continued to increase as the animals approached death (Fig. 7, middle panels). The IL-10 concentrations produced by antigen-stimulated splenocytes from the mice infected with IOE intradermally were not significantly different than those of the intraperitoneally infected mice (P > 0.05) (Fig. 7, bottom panels). In contrast, i.d. inoculation was associated with significantly lower serum levels of IL-10 than those detected in mice infected intraperitoneally with IOE (P < 0.05) (Fig. 7, bottom panels).
FIG. 7.
Comparison of cytokine concentrations in antigen-stimulated splenocyte cultures and sera from the intradermally and intraperitoneally infected mice on day 7 postinfection. Animals were sacrificed on day 7 postinfection, and spleens and sera were collected. Splenocyte cultures (left panels) were stimulated ex vivo with IOE antigen in the presence of syngeneic naïve splenocytes, and supernatant was collected after 48 h. IFN-γ (P < 0.01) and TNF-α (P < 0.05) concentrations in splenocyte culture supernatants 48 h post-IOE antigen stimulation were significantly higher in the intradermally inoculated animals (top and middle panels, respectively). In contrast, the systemic serum levels of IFN-γ (P < 0.05) and TNF-α (P < 0.05) were significantly higher in the intraperitoneally infected animals (top and middle panels, respectively). The concentration of IL-10 (P < 0.01) was significantly higher in the serum of intraperitoneally infected animals (bottom panels), but the difference in IL-10 produced by the cultured splenocytes was not significant between the two groups. Mice infected intradermally with IOE had significantly lower levels of all three cytokines in the serum (right panels) than the intraperitoneally inoculated mice (all panels). The syngeneic splenocytes (APCs only) did not produce significant concentrations of cytokines after stimulation with IOE antigen. Data are expressed as means plus standard errors, three mice per group were used in the analysis, and similar results were obtained from three independent experiments.
DISCUSSION
E. chaffeensis infection in humans can be a mild, asymptomatic infection or may be fatal (31). In an attempt to mimic the biology of natural transmission, we have established a model of mild monocytotropic ehrlichiosis by using i.d. inoculation of C57BL/6 mice with the highly virulent IOE, which is closely related to E. chaffeensis genetically and antigenically (26). In our previous study, we characterized the immunological correlates of resistance or susceptibility to severe ehrlichiosis following i.p. infection with two ehrlichial species in which differences in the bacterial genetic and antigenic profiles were confounding factors in the interpretation of the results. In this study, we examined the mechanisms of protection against Ehrlichia by using a single ehrlichial strain with a more natural route of infection. We established a dose of IOE that resulted in 100% survival of the mice when inoculated intradermally and was 100% fatal when given intraperitoneally. The outcome of infection and immune responses of mice infected intradermally were compared to those of mice infected intraperitoneally with the same doses of IOE. In some experiments, we compared mice infected intraperitoneally or intradermally with IOE with wild-type mice infected intraperitoneally with a high dose of mildly virulent E. muris because it represents a well-characterized animal model of mild ehrlichiosis associated with induction of protective antiehrlichial cell-mediated immunity.
Because there was a significant increase in ehrlichial burden in livers and lungs between days 4 and 7, it is apparent that IOE disseminates and replicates in the intradermally inoculated mice. However, bacterial replication and dissemination to different nonlymphoid organs was lower in the intradermally inoculated mice. This was supported by our observations that intradermally inoculated mice harbor a lower number of bacteria in the liver, lung, and spleen than intraperitoneally infected mice, and ehrlichial DNA was undetectable in the kidneys of the intradermally inoculated mice (Fig. 4). The effect of i.d. inoculation with IOE on the immune response and outcome of infection was dose dependent (Table 1). We predict that the intradermally inoculated mice succumb to the disease when many bacteria escape the draining lymph node and enter the systemic circulation, triggering the pathogenic immune response.
Our animal model of mild ehrlichiosis after i.d. inoculation with highly virulent IOE demonstrated a strong association between the severity of hepatic necrosis and apoptosis and the development of fatal toxic shock-like syndrome. On days 4 and 7 postinfection, we observed mild hepatic apoptosis in the intradermally infected animals which was not associated with necrosis. In contrast, the presence of extensive liver apoptosis and necrosis following lethal i.p. infection was associated with a fatal outcome. Our previous studies have suggested that severe and fatal murine ehrlichiosis in immunocompetent hosts is caused by immune mediated pathology (13, 14). This conclusion was also supported by two findings in the current study. First, animals infected with a lethal dose of IOE via the i.p. route developed extensive hepatic apoptosis and necrosis in the absence of an overwhelming ehrlichial burden. Second, although ehrlichial organisms were detected mainly in cells lining the blood vessels and hepatic sinusoids, including Kupffer cells, endothelial cells, and monocytes, extensive apoptosis and necrosis were observed in hepatocytes mainly in the midzone of the liver (compare Fig. 2C with D and 3B). These data are consistent with previous studies in mice and humans showing a lack of correlation between the cells that are infected and those that undergo cell death (7, 17, 27). Therefore, our present data further support the hypothesis that immune mediated pathology is a potential mechanism accounting for the development of severe and fatal ehrlichiosis.
Several pathogenic and immune factors could mediate hepatic injury and tissue damage at different time points postinfection (11). Apoptotic or necrotic cell death following infections with several intracellular pathogens is mediated by cytotoxic CD8+ T cells via different cytotoxic pathways, including lysis of infected target cells by perforin/granulysin or cytotoxic killing of other host cells via death receptors (TNFR and FasL) (1, 28). Our previous study suggested a potential role of Ehrlichia-specific CD8+ T cells in the pathogenesis of fatal ehrlichiosis. TNF-α-producing CD8+ T cells were detected at higher frequencies in the spleens of intraperitoneally infected mice, and their expansion was strongly correlated with marked liver apoptosis and necrosis (13). We have demonstrated recently that TNF receptors p55 and p75 mediate, to some extent, hepatic injury in mice infected intraperitoneally with high-dose IOE (14). TNFR p55/p75 double-knockout mice infected with a lethal dose of IOE had prolonged survival, which was associated with a substantial decrease in the degree of hepatic injury. However, neutralization of TNF-α did not influence survival. These data suggested that there are additional mechanisms that account for the observed apoptosis and necrosis of host cells. Activation of macrophages and Kupffer cells can cause tissue damage via the release of toxic mediators such as nitric oxide and other reactive oxygen species (15, 24). This hypothesis has been suggested by previous studies with animal models of sepsis caused by infection with other gram-negative bacteria. We consistently observed inflammatory cells in close proximity to the apoptotic hepatocytes and Kupffer cells in the intraperitoneally inoculated mice (Fig. 2B and C). In contrast, with the intradermally inoculated animals, we observed mild hepatocyte injury and fewer apoptotic cells (Fig. 2D) that were present only in the regions of LHI which contained not only Kupffer cells but also a mixed inflammatory infiltrate.
We hypothesize that survival of IOE-infected mice following i.d. inoculation could be due to two interrelated factors. The first factor is an initial regional containment of the bacteria, and the second factor is that i.d. inoculation may promote accelerated priming of Ehrlichia-specific T cells secondary to optimal and effective antigen presentation and T-cell activation by professional antigen presenting cells, leading to the generation of a strong protective type-1 response. Strikingly, IOE inoculated intradermally resulted in a higher number of IFN-γ-producing cells (per 106 spleen cells) than i.p. IOE infection (Fig. 5). The concentration of IFN-γ-producing cells in the animals inoculated intradermally with IOE was similar to that of the E. muris-inoculated animals, with both being significantly higher than that induced by fatal i.p. infection with IOE. Thus, i.d. inoculation with IOE shifted the adaptive immune response against Ehrlichia in the direction of the protective Th1 phenotype in immunocompetent C57BL/6 mice. Whether the presence of a particular cytokine is beneficial or detrimental to the host's protective immune response depends on several factors, such as the time during infection when it is produced, the concentration of the cytokine, and whether it is present at high levels systemically in the serum or locally in the foci of infection (18). In this study, local production of proinflammatory cytokines, such as TNF-α and IFN-γ, in the spleen was observed following both i.p. and i.d. inoculation (Fig. 5 and 7, top and middle panels). However, greater production of IFN-γ and TNF-α by antigen-stimulated splenocytes was observed in the intradermally inoculated mice than in those inoculated intraperitoneally and was associated with a 1,000-fold-lower quantity of Ehrlichia organisms in the spleen of the intradermally infected animals. Furthermore, compared to intraperitoneally infected mice that succumbed to infection, survival of intradermally infected mice was associated with very low concentrations of TNF-α or IL-10 in the serum, which could explain why they did not develop toxic shock-like syndrome. The presence of high concentrations of IL-10 in the spleens of the intradermally infected animals suggests that local IL-10 production plays a role in protection. These data are consistent with the observations in the other animal model of mild ehrlichiosis caused by mildly virulent E. muris (13).
Apoptotic cells are a strong stimulus for production of pro- and anti-inflammatory cytokines, such as TNF-α and IL-10, respectively (33). If apoptotic cells are not cleared in a timely manner, they may undergo secondary necrosis, which can lead to persistent pro- or anti-inflammatory conditions. Our data show a strong correlation between the presence of apoptosis/necrosis and the presence of very high levels of serum TNF-α and IL-10 in intraperitoneally infected mice (Fig. 2, 3, and 7). However, unlike the case with lipopolysaccharide (LPS)-containing gram-negative bacteria which stimulate TNF-α and IL-10 production by macrophages upon ligation of toll-like receptors, the high systemic concentrations of TNF-α and IL-10 are produced by T cells in fatal ehrlichiosis induced by i.p. infection with IOE that lacks LPS (13, 14). Recent studies have reported that although the apoptosis that occurs as a part of normal homeostasis is an anti-inflammatory or immune-silencing process, when it occurs during infection, it is more commonly a proinflammatory event. This effect occurs because apoptotic vesicles may contain adjuvants, such as pattern-associated molecular patterns, that enhance the inflammatory response (34).
Finally, we postulate that the significant amount of cell death observed in the animal model of fatal ehrlichiosis after i.p. infection with IOE could involve not only infected target cells but also antigen-specific T cells. This hypothesis is supported by our current data showing expansion of CD4+ and CD8+ T cells on day 7 postinfection, which was associated with substantial production of IFN-γ in the spleens of intradermally inoculated mice (Fig. 5 and 7, top panels). In contrast, i.p. inoculation with IOE resulted in a significant reduction in the percentages of CD4+ and CD8+ T cells in the spleen (Fig. 6). The lower percentages of CD4+ and CD8+ T cells in the spleen of intraperitoneally infected mice compared to intradermally infected animals could be due to increased migration of T cells to the site of infection (i.e., peripheral nonlymphoid organs) following i.p. inoculation, or due to apoptosis of activated Ehrlichia-specific T cells. Currently, there is no direct evidence that supports one hypothesis versus the other. Our previous study showed an early expansion of splenic T cells followed by a significant decline in T-cell number, particularly IFN-γ-producing Ehrlichia-specific CD4+ Th1 cells, two days before death of C57BL/6 mice infected with a low dose of IOE (13). In support of this conclusion, laboratory data from severe human and canine ehrlichioses reveal marked lymphopenia (7, 10, 25). Nevertheless, future studies should directly examine whether lymphopenia observed in murine, canine, and human ehrlichioses is due to T-cell migration or apoptosis.
In conclusion, we predict that initial bacterial containment, priming of the immune response, and induction of host cell apoptosis are the critical factors determining resistance or susceptibility to ehrlichiosis. In the model of mild disease caused by i.d. ehrlichial infection, very little apoptosis was observed. The presence of extensive apoptosis and necrosis of both infected and uninfected host cells, the presence of inflammatory cells near apoptotic bodies, and the marked decrease in CD4+ and CD8+ T cells following lethal i.p. infection with IOE support the concept that fatal ehrlichiosis is due to immune-mediated phenomena and not direct bacterial effects. Utilizing the same ehrlichial species to examine immunological correlates of resistance and susceptibility to severe ehrlichiosis has provided additional evidence about the pathogenesis of fatal ehrlichiosis, while avoiding limitations of interpreting the data that compare infection caused by two different ehrlichial species that differ in virulence (i.e., E. muris and IOE). Moreover, the special characteristics of this bacterium, including its lack of LPS and its ability to cause toxic shock-like syndrome, will provide understanding of novel mechanisms of host immunity in future studies.
Acknowledgments
We thank Sherrill Hebert and Melissa Yancey for their excellent secretarial assistance and Jere McBride for his guidance with our real-time PCR assays. We also express our gratitude to Nagaraja Thirumalapura, Mengyi Ye, Emily Crossley, Colette Keng, Donald Bouyer, and Patricia Crocquet-Valdes for their support and assistance during this project.
This work was supported in part by the CDC Fellowship Training in Vector-Borne Infectious Diseases (grant T01/CCT622892) and a grant from the National Institute of Allergy and Infectious Disease (AI31431).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abougergi, M. S., S. J. Gidner, D. K. Spady, B. C. Miller, and D. L. Thiele. 2005. Fas and TNFR1, but not cytolytic granule-dependent mechanisms, mediate clearance of murine liver adenoviral infection. Hepatology 41:97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, S., M. Colmenares, L. Soong, K. Goldsmith-Pestana, L. Munstermann, R. Molina, and D. Mahon-Pratt. 2003. Intradermal infection model for pathogenesis and vaccine studies of murine visceral leishmaniasis. Infect. Immun. 71:401-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belperio, J. A., M. P. Keane, M. D. Burdick, J. P. Lynch III, Y. Y. Xue, K. Li, D. J. Ross, and R. M. Strieter. 2002. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J. Immunol. 169:1037-1049. [DOI] [PubMed] [Google Scholar]
- 4.Bitsaktsis, C., J. Huntington, and G. Winslow. 2004. Production of IFN-gamma by CD4 T cells is essential for resolving Ehrlichia infection. J. Immunol. 172:6894-6901. [DOI] [PubMed] [Google Scholar]
- 5.Brossard, M., and S. K. Wikel. 2004. Tick immunobiology. Parasitology 129(Suppl.):S161-S176. [DOI] [PubMed] [Google Scholar]
- 6.Chen, W., H. Shen, A. Webb, R. KuoLee, and J. W. Conlan. 2003. Tularemia in BALB/c and C57BL/6 mice vaccinated with Francisella tularensis LVS and challenged intradermally, or by aerosol with virulent isolates of the pathogen: protection varies depending on pathogen virulence, route of exposure, and host genetic background. Vaccine 21:3690-3700. [DOI] [PubMed] [Google Scholar]
- 7.Dumler, J. S., J. E. Dawson, and D. H. Walker. 1993. Human ehrlichiosis: hematopathology and immunohistologic detection of Ehrlichia chaffeensis. Hum. Pathol. 24:391-396. [DOI] [PubMed] [Google Scholar]
- 8.Fujita, H., and Y. Watanabe. 1994. Ehrlichial organisms isolated from Ixodes ovatus ticks and field rodents in Japan. Annu. Rep. Ohara Hosp. 37:13-17. [Google Scholar]
- 9.Ganta, R. R., C. Cheng, M. J. Wilkerson, and S. K. Chapes. 2004. Delayed clearance of Ehrlichia chaffeensis infection in CD4+ T-cell knockout mice. Infect. Immun. 72:159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrus, S., P. H. Kass, E. Klement, and T. Waner. 1997. Canine monocytic ehrlichiosis: a retrospective study of 100 cases, and an epidemiological investigation of prognostic indicators for the disease. Vet. Rec. 141:360-363. [DOI] [PubMed] [Google Scholar]
- 11.Herkel, J., M. Schuchmann, G. Tiegs, and A. W. Lohse. 2005. Immune-mediated liver injury. J. Hepatol. 42:920-923. [DOI] [PubMed] [Google Scholar]
- 12.Inayoshi, M., H. Naitou, F. Kawamori, T. Masuzawa, and N. Ohashi. 2004. Characterization of Ehrlichia species from Ixodes ovatus ticks at the foot of Mt. Fuji, Japan. Microbiol. Immunol. 48:737-745. [DOI] [PubMed] [Google Scholar]
- 13.Ismail, N., L. Soong, J. W. McBride, G. Valbuena, J. P. Olano, H. M. Feng, and D. H. Walker. 2004. Overproduction of TNF-alpha by CD8+ type 1 cells and down-regulation of IFN-gamma production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J. Immunol. 172:1786-1800. [DOI] [PubMed] [Google Scholar]
- 14.Ismail, N., H. L. Stevenson, and D. H. Walker. 2006. Role of tumor necrosis factor alpha (TNF-alpha) and interleukin-10 in the pathogenesis of severe murine monocytotropic ehrlichiosis: increased resistance of TNF receptor p55- and p75-deficient mice to fatal ehrlichial infection. Infect. Immun. 74:1846-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorgensen, P. F., P. Gotzinger, T. Scholz, Y. Gundersen, T. Sautner, R. Fugger, P. Lilleaasen, and A. O. Aasen. 2001. The role of Kupffer cell inhibition in porcine endotoxemia. Shock 16:466-472. [DOI] [PubMed] [Google Scholar]
- 16.Laskay, T., A. Diefenbach, M. Rollinghoff, and W. Solbach. 1995. Early parasite containment is decisive for resistance to Leishmania major infection. Eur. J. Immunol. 25:2220-2227. [DOI] [PubMed] [Google Scholar]
- 17.Martin, M. E., K. Caspersen, and J. S. Dumler. 2001. Immunopathology and ehrlichial propagation are regulated by interferon-gamma and interleukin-10 in a murine model of human granulocytic ehrlichiosis. Am. J. Pathol. 158:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mordue, D. G., F. Monroy, M. La Regina, C. A. Dinarello, and L. D. Sibley. 2001. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J. Immunol. 167:4574-4584. [DOI] [PubMed] [Google Scholar]
- 19.Olano, J. P., E. Masters, W. Hogrefe, and D. H. Walker. 2003. Human monocytotropic ehrlichiosis, Missouri. Emerg. Infect. Dis. 9:1579-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olano, J. P., and D. H. Walker. 2002. Human ehrlichioses. Med. Clin. N. Am. 86:375-392. [DOI] [PubMed] [Google Scholar]
- 21.Olano, J. P., G. Wen, H. M. Feng, J. W. McBride, and D. H. Walker. 2004. Histologic, serologic, and molecular analysis of persistent ehrlichiosis in a murine model. Am. J. Pathol. 165:997-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paddock, C. D., D. P. Suchard, K. L. Grumbach, W. K. Hadley, R. L. Kerschmann, N. W. Abbey, J. E. Dawson, B. E. Anderson, K. G. Sims, J. S. Dumler, et al. 1993. Brief report: fatal seronegative ehrlichiosis in a patient with HIV infection. N. Engl. J. Med. 329:1164-1167. [DOI] [PubMed] [Google Scholar]
- 23.Power, C. A., C. L. Grand, N. Ismail, N. C. Peters, D. P. Yurkowski, and P. A. Bretscher. 1999. A valid ELISPOT assay for enumeration of ex vivo, antigen-specific, IFN gamma-producing T cells. J. Immunol. Methods 227:99-107. [DOI] [PubMed] [Google Scholar]
- 24.Sass, G., K. Koerber, R. Bang, H. Guehring, and G. Tiegs. 2001. Inducible nitric oxide synthase is critical for immune-mediated liver injury in mice. J. Clin. Investig. 107:439-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schutze, G. E., and R. F. Jacobs. 1997. Human monocytic ehrlichiosis in children. Pediatrics 100:E10. [DOI] [PubMed] [Google Scholar]
- 26.Shibata, S., M. Kawahara, Y. Rikihisa, H. Fujita, Y. Watanabe, C. Suto, and T. Ito. 2000. New Ehrlichia species closely related to Ehrlichia chaffeensis isolated from Ixodes ovatus ticks in Japan. J. Clin. Microbiol. 38:1331-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sotomayor, E. A., V. L. Popov, H. M. Feng, D. H. Walker, and J. P. Olano. 2001. Animal model of fatal human monocytotropic ehrlichiosis. Am. J. Pathol. 158:757-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thoma-Uszynski, S., S. Stenger, and R. L. Modlin. 2000. CTL-mediated killing of intracellular Mycobacterium tuberculosis is independent of target cell nuclear apoptosis. J. Immunol. 165:5773-5779. [DOI] [PubMed] [Google Scholar]
- 29.Valbuena, G., and D. H. Walker. 2004. Effect of blocking the CXCL9/10-CXCR3 chemokine system in the outcome of endothelial-target rickettsial infections. Am. J. Trop. Med. Hyg. 71:393-399. [PubMed] [Google Scholar]
- 30.Walker, D. H. 1998. Tick-transmitted infectious diseases in the United States. Annu. Rev. Public Health 19:237-269. [DOI] [PubMed] [Google Scholar]
- 31.Walker, D. H., and J. S. Dumler. 1997. Human monocytic and granulocytic ehrlichioses. Discovery and diagnosis of emerging tick-borne infections and the critical role of the pathologist. Arch. Pathol. Lab. Med. 121:785-791. [PubMed] [Google Scholar]
- 32.Williams, J. C., I. Kakoma, M. Ristic, and National Institute of Allergy and Infectious Diseases. 1990. Ehrlichiosis a vector-borne disease of animals and humans. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 33.Winau, F., S. H. Kaufmann, and U. E. Schaible. 2004. Apoptosis paves the detour path for CD8 T cell activation against intracellular bacteria. Cell Microbiol. 6:599-607. [DOI] [PubMed] [Google Scholar]
- 34.Winau, F., S. Weber, S. Sad, J. de Diego, S. L. Hoops, B. Breiden, K. Sandhoff, V. Brinkmann, S. H. Kaufmann, and U. E. Schaible. 2006. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity 24:105-117. [DOI] [PubMed] [Google Scholar]
- 35.Winslow, G. M., E. Yager, K. Shilo, D. N. Collins, and F. K. Chu. 1998. Infection of the laboratory mouse with the intracellular pathogen Ehrlichia chaffeensis. Infect. Immun. 66:3892-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wormser, G. P., D. McKenna, J. Carlin, R. B. Nadelman, L. F. Cavaliere, D. Holmgren, D. W. Byrne, and J. Nowakowski. 2005. Brief communication: hematogenous dissemination in early Lyme disease. Ann. Intern. Med. 142:751-755. [DOI] [PubMed] [Google Scholar]