Abstract
We have studied homologous (HoM) and cross-reacting (CR) immunoglobulin A (IgA) antibody responses to colonization factors (CFs) in Bangladeshi children with diarrhea due to enterotoxigenic E. coli (ETEC) strains of the CF antigen I (CFA/I) group (CFA/I, n = 25; coli surface antigen 4 [CS4], n = 8; CS14, n = 11) and the CS5 group (CS5, n = 15; CS7, n = 8), respectively. The responses to the HoM, CR, and heterologous (HeT) CF antigens in each group of patient were studied and compared to that seen in healthy children (n = 20). In the CFA/I group (CFA/I and CS14), patients responded with antibody-secreting cell (ASC) responses to HoM CFs (geometric mean, 156 to 329 ASCs/106 peripheral blood mononuclear cells [PBMCs]) and to CR CFs (≈15 to 38 ASCs/106 PBMCs) but least of all to the HeT CS5 antigen (2 to 4 ASCs/106 PBMCs). For the CS5 group of patients with ETEC (CS5 and CS7), likewise, responses to HoM CFs (230 to 372 ASCs/106 PBMCs) and CR CFs (27 to 676 ASCs/106 PBMCs) were seen, along with lower responses to the HeT CFA/I antigen (9 to 38 ASCs/106 PBMCs). Both groups of patients responded with CF-specific IgA antibodies to HoM and CR antigens in plasma but responded less to the HeT CFs. The responses in patients were seen very soon after the onset of diarrhea and peaked around 1 week after onset. Vaccinees who had received two doses of the oral, killed whole-cell ETEC vaccine (CF-BS-ETEC) responded with plasma IgA antibodies to CFA/I, a component of the vaccine, but also to the CR CS14 antigen, which was not included in the vaccine, showing that antibody responses can be stimulated by a CFA/I-containing ETEC vaccine to a CR-reacting antigen in individuals in countries where ETEC is endemic.
Enterotoxigenic Escherichia coli (ETEC) is a common cause of acute watery diarrhea in children in countries of the developing world as well as travelers to these regions (3). ETEC strains are noninvasive enteropathogens that colonize the small intestine by means of protein appendages termed colonization factors (CFs) (5, 7) and cause watery diarrhea by the production of heat-stable toxin (ST) and/or heat-labile enterotoxin (LT) (6, 20). Studies using animals and human volunteers have demonstrated that colonization factors are protective antigens that provide protection against infection with ETEC strains expressing homologous CFs (11, 12, 21-23).
Although it has been claimed that the different CFs are separate antigens, cross-reacting epitopes shared by some of the CFs have been demonstrated. Mouse monoclonal antibodies as well as human immune sera from CF antigen I (CFA/I)-infected patients have been found to cross-react immunologically with other CFs such as CS1, CS2, CS4, CS17, and CS14 (13, 18, 19).
Genetic and phylogenetic studies have classified some groups of related fimbrial or fibrillar CFs, the most prominent being the CFA/I group (including CFA/I, CS4, CS14, CS17, and CS19) and the CS5 group (including CS5 and CS7) (8, 15). Recently, a classification of CFA/I as a class 5 adhesive fimbrial type has been made, whereby the subclass 5a group includes more closely genetically related antigens, CFA/I, CS4, and CS14 (2).
To date, over 22 different CFs have been characterized (15), and the multivalent nature of these ETEC virulence factors has made vaccine development based on CFs problematic. However, in this large group of CFs, the ones most often related to pathogenesis in humans are relatively few and predominantly include the CFA/I group followed by the CS5 group of fimbriae (15, 17). Thus, if natural infection or immunization with these antigens also leads to responses to the related cross-reacting antigenic epitopes, it might be possible to have protection against a large mixture of CFs by immunization with the CFs. To test if this might occur after natural ETEC infections, we tested Bangladeshi patients infected with ETEC strains expressing fimbriae of the CFA/I and CS5 groups. The responses in children who had received the oral, killed whole-cell ETEC vaccine expressing CFA/I and CS5 antigens were also tested.
MATERIALS AND METHODS
Bacteriological analysis of fecal samples.
Stool specimens from patients suffering from watery diarrhea were cultured on MacConkey agar and CFA agar with and without bile salts (24). Bacterial colonies on CFA agar were assayed for the presence of CFs by dot blot assays using monoclonal antibodies specific for defined colonization factors (18). Production of LT and ST of Escherichia coli colonies isolated on MacConkey agar was determined by monosialoganglioside GM1-enzyme-linked immunosorbent assay (ELISA) (25, 26). The stool samples were also cultured for other enteric pathogens, e.g., Vibrio cholerae O1/O139; Salmonella, Shigella, and Campylobacter spp.; and rotavirus (27). Stool samples were tested for ETEC but not for other diarrheagenic E. coli types such as enteropathogenic E. coli or enteroaggregative E. coli. Only patients positive for ETEC but negative for V. cholerae O1/O139 and other enteric pathogens tested here were included in this study. Stool samples from study subjects were also tested by direct microscopy to detect cyst and vegetative forms of parasites and ova of helminths. Stools from healthy children were similarly screened, and those children were recruited as controls in the study if their samples were found to be negative for enteric pathogens.
Purified antigens.
CFs were purified from homogenized CF-positive bacteria using standard ETEC strains (4). These included control ETEC strains H10407 (CFA/I+ O78:H− ST/LT), E11881A (CS4+ CS6+ O25:H42 ST/LT), E17018A (CS5+ O167:H5 ST), 334A (CS7+ O15:H11 ST), and E7476A (CS14+ O166:H27 ST) purified by either ammonium sulfate fractionation (4) or salt and isoelectric precipitation (10) followed by ion-exchange chromatography and further purification by isopycnic cesium chloride gradient centrifugation at 110,000 × g for 18 h (4). The purity and concentration of the preparations were determined by spectrophotometry (A280 to A260 values) and inhibition ELISA (18). In addition, sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting were carried out, which showed bands corresponding to the different CF subunits when specific monoclonal antibodies or antisera raised against whole fimbriated bacteria were used (18). The CF antigens prepared were highly pure and relatively free of other antigens and bacterial products.
Study subjects and sample collection.
Children aged 2 to 5 years with acute watery diarrhea caused by ETEC as the only enteric pathogen were included in the study from the Dhaka hospital of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) (Table 1). Venous blood (5 ml) was collected from the patients at the acute stage of the disease, i.e., on the second day of hospitalization, which was considered to be approximately 2 days after the onset of diarrhea (day 2), and then at different times after onset (day 7, day 14, and day 30). Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood by gradient centrifugation on a Ficoll-Isopaque gradient (Pharmacia, Uppsala, Sweden). Plasma collected from the top of the Ficoll gradient was stored in aliquots at −20°C until ELISA tests were performed.
TABLE 1.
Characteristics of patients with diarrhea due to ETEC strains expressing different CFs
| CF of ETEC strains | Total no. of patientsa | Median agea (yr) | Dehydration statusb (%) |
Time from onset of diarrhea to first blood drawnc (median h) | ||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | ||||
| CFA/I | 25 | 2.0 | 20 | 44 | 36 | 48 |
| CS4 + CS6 | 8 | 3.5 | 25 | 75 | 42 | |
| CS5 + CS6 | 15 | 3.5 | 14 | 36 | 50 | 54 |
| CS7 | 8 | 2.0 | 57 | 43 | 96 | |
| CS14 | 11 | 4.0 | 27 | 27 | 46 | 48 |
| Total | 67 | 3.5 | 29 | 30 | 41 | 56 |
Children 2 to 5 years of age (median age, 3.5 years) with diarrhea due to ETEC strains expressing different CFs were studied.
The dehydration status was graded as severe, moderate, or mild.
The first blood samples were drawn from patients about 24 h after arrival to the hospital, which is shown together with the average time of onset of diarrhea prior to admission at the ICDDR,B hospital.
Plasma samples collected from children (n = 27; 2 to 5 years of age; median age, 2.4 years) who had been orally immunized with two doses of the oral, killed whole-cell ETEC vaccine (CF-BS-ETEC) at an interval of 14 days were also analyzed in the study (14, 16). Samples collected prior to immunization (day 0) and 7 days after the intake of each dose of the vaccine were tested for responses to CFA/I and the CR CS14 antigen by ELISA.
Twenty healthy children of similar age as the patients and of similar socioeconomic background living in urban areas of Dhaka city but with no history of diarrhea during the previous 3 months were included as endemic control subjects. Stool samples from these subjects were screened for enteric pathogens as described above for the patients, and only those who did not harbor any of the enteric pathogens described above were recruited. Blood samples (5 ml) were collected at only one time point from the controls and processed as described above for the patients.
Informed consent was obtained from the patients and control subjects. The study was approved by the Ethical Review Committee of the ICDDR,B and Goteborg University.
Determination of antibody-secreting cells.
The Ficoll-separated PBMCs were assayed for numbers of CF-specific antibody-secreting cells (ASCs) by a two-color enzyme-linked immunospot technique (16) using samples obtained from patients at study day 2 and day 7 only. Numbers of cells secreting antibodies of the immunoglobulin A (IgA) isotype were determined. Individual wells of nitrocellulose-bottomed 96-well plates (Millititer HA; Millipore Corp., Bedford, MA) were coated with 0.1 ml of purified CFs (10 μg/ml) and included CFA/I, CS4, CS5, CS7, and CS14. Patients were assayed for ASC responses against the homologous CF of the infecting strain (HoM) and against a “cross-reacting” CF preparation (CR) and a heterologous antigen (HeT). In this study, CFs cross-reacting with CFA/I included CS4 and CS14, and the heterologous CF was CS5. For CS5-infected children, immune responses against CS5 (HoM), CS7 (CR), and the heterologous CFA/I were tested. For CS7-infected children, responses to CS7 (HoM), CS5 (CR), and CFA/I (HeT) were studied. IgA ASC responses in the healthy children were tested in duplicates to the CF antigens. Values are expressed as geometric means (GM) for HoM, CR, or HeT antigens and ranges (±standard errors of the means [SEM]). A CF-specific ASC level of ≥5 ASCs/106 PBMCs was used to signify a response in a patient (the cutoff was based on CF-specific ASC levels in healthy controls) (CFA/I = 2 ASCs/106 PBMCs; CS14 = 1 ASC/106 PBMCs; CS4 = 1 ASC/106 PBMCs; CS5 = 5 ASCs/106 PBMCs; CS7 = 2 ASCs/106 PBMCs). Results are expressed as a range of GM values in different study groups or as individual GM values for a certain CF in a study group.
Antibody responses in plasma.
Plasma samples obtained from study subjects were tested by ELISA using previously described methods using the purified antigens described above at a 1-μg/ml concentration (100 μl/well) (1, 16). Samples collected at study days 2, 7, 14, and 30 were tested. Titer calculations were carried out using the computer-based program MULTI (DataTree Inc., Watthams, MA). The values are expressed as GMs and ranges (±SEM). For analyses using plasma, the titer at a study day that was greater or equal to the GM ± 2 SEM of that seen in healthy controls was considered a response (titers for CFA/I:CS4:CS5:CS7:CS14, 62:101:87:86:45, respectively).
Analyses.
The CF-specific ASC and plasma antibodies were compared between the acute and convalescent stages of infection in the patients or to that seen in the healthy controls. The increase in the magnitude of antibody levels (ASCs or titers in plasma) between different study days in the patients or in comparison to the healthy controls was considered a response in a group, and comparisons were carried out using the Wilcoxon signed-rank test or the Mann-Whitney U test as necessary. The Kruskal-Wallis one-way analysis of variance was used to compare the ASC and ELISA measurements seen at one time point in the healthy controls with that seen at different study days for patients. For the vaccinees, responder frequency (percent) indicates numbers responding with a ≥2-fold increase in titer in plasma after vaccination compared to that seen prior to immunization. A P value of ≤0.05 was considered a significant difference. Statistical analyses were carried out using the SigmaStat computer program (SPSS, San Rafael, CA). Paired samples were compared using the Wilcoxon signed-rank test, nonpaired samples were compared by the Mann-Whitney U test, and comparisons between multiple groups were performed with the Kruskal-Wallis test.
P values of ≤0.05 were considered to be statistically significant. Geometric means and ranges (±SEM) of CF-specific ASCs/106 PBMCs and antibody titers were determined.
RESULTS
Clinical history of patients and phenotype of ETEC strains.
The patients with ETEC (age, 2 to 5 years; median age, 3.5 years) enrolled in this study had a history of diarrhea ranging between 2 h to 120 h prior to arrival at the treatment center (median duration, 24 h) (Table 1). Thus, the day of arrival was assumed to be day 1 after onset of diarrhea in this study. About 34% of the patients came with severe dehydration, 61% came with mild to moderate dehydration, and 4.5% showed no signs of dehydration. Of the patients, 88% vomited from a minimum of 1 time to a maximum of 20 times per day upon hospitalization. Upon arrival at the hospital, patients were hospitalized for 1 to 5 days (median duration, 2 days). In about 92% of the cases, patients received antibiotics as soon as they were hospitalized. About 66% were given erythromycin, 22% were given doxycycline, 1.5% were given amoxicillin, 1.5% were given metronidazole, and 0.75% were given tetracycline.
The first venous blood sample was collected on the second day after admission, after confirmation of the CF of the ETEC strain (day 2, acute stage of infection), and approximately 5 to 6 days later (day 7). In addition, samples from the convalescent stage were collected around 14 and 30 days after the onset of disease whenever possible (Table 1).
The patients enrolled in the study included those that were positive for CFA/I (n = 25), CS4 and CS6 (n = 8), CS5 and CS6 (n = 15), CS7 (n = 8), and CS14 (n = 11). No other bacterial enteropathogen was cocultured from the stool samples of the patients participating in the study. However, stool microscopy revealed the presence of Giardia lamblia in 4 of the 20 patients (vegetative cells in three patients and cysts in one patient).
CF-specific antibody-secreting cell response in peripheral blood of study subjects.
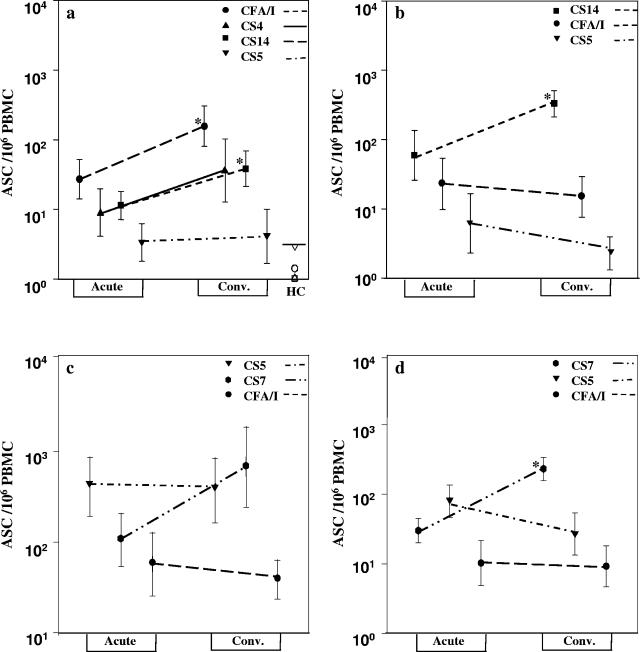
The IgA ASC responses in the blood to the HoM, CR, and HeT CF antigens were analyzed in the majority of the patients. These analyses showed that most patients responded with ASCs specific to the HoM CF very soon, i.e., on day 2 after onset of infection (GM = 27 to 407 ASCs/106 PBMCs) (Fig. 1). In most cases, this response further increased around 7 days after onset of diarrhea (GM = 156 to 329 ASCs/106 PBMCs; P = 0.05 to 0.009). The CF-specific IgA ASC response to the different CF antigens tested at one time point in the healthy controls was negligible and significantly lower than those seen in the patients at either day 2 or day 7 after onset of diarrhea (P≤ 0.001 in comparison to all antigens). The IgA ASC responses to the HoM CF antigen at the acute and convalescent stages were significantly higher than those seen with corresponding CF antigens in healthy control subjects.
FIG. 1.
IgA ASC response to HoM, CR, and HeT CFs in patients infected with enterotoxigenic Escherichia coli. The IgA antibody-secreting cell responses in patients with CFA/I (a [n = 25])-, CS14 (b [n = 11])-, CS5 (c [n = 15])-, and CS7 (d [n = 8])-expressing ETEC to HoM, CR, and HeT CF antigens are shown. The responses to CFA/I (•), CS4 (▴), CS5 (▾), CS7 ( ), and CS14 (▪) after onset of diarrhea (Acute) (day 2) and at early convalescence stage (Conv.) (day 7) are shown. Asterisks indicate statistically significant differences (*, P < 0.05 to 0.01; **, P < 0.01 to 0.001) between the acute and convalescent phases. The symbols indicate geometric means, and vertical lines indicate SEM. The responses in the 20 healthy subjects are also shown in panel a (GM of responses are depicted as open symbols for CFA/I [○], CS4 [▵], CS5 [▿], CS7 [○], and CS14 [□]). The cutoff value for a response to CF antigens is also shown in panel a (≥5 ASCs/106 PBMCs [—]) for healthy control (HC).
Patients infected with CFA/I ETEC responded well to the homologous fimbrial antigen but also with ASC responses to the cross-reacting CS4 and CS14 antigens; low responses to the heterologous CS5 antigen were seen (Fig. 1a). The magnitude of the response to the HoM antigen was about fivefold higher for CFA/I than those seen with CS4 and CS14 antigens. Similarly, CS14 ETEC-infected patients responded well to HoM antigen, although less-pronounced (Fig. 1b) responses to the CR CFA/I antigen were seen; poor responses to CS5 were seen.
The CS5 group of patients showed a trend similar to that of the CFA/I group, with responses to both HoM CS5 (Fig. 1c) or CS7 (Fig. 1d) antigen as well as the CR CF types. These patients responded poorly to HeT CFA/I.
When the anti-CF responses to these antigens in all the patients were grouped together, the response to the HoM CF type appeared to be highest (Fig. 1) in comparison to the CR (P≤ 0.001) or the HeT (P≤ 0.001) antigens at convalescence. The ASC responses followed a pattern of response to HoM CF (GM = 257 ASCs/106 PBMCs), followed by the response to the CR CF (GM = 52 ASCs/106 mononuclear cells), which was followed by the response to the HeT antigen, which was usually poor (GM = 7.0 ASCs/106 PBMCs). The CF-specific response in the healthy individuals was usually lower than that seen in the patients when they were tested against the HeT antigen.
Since the numbers of CF-specific ASCs in the patients before ETEC infection could not be determined for obvious reasons, the magnitude of the ASC responses against CFs in patients was calculated by comparing patient ASC levels with the levels of ASCs to the same CF determined in the blood of healthy volunteers. By such comparisons, it was found that the mean level of CFA/I-specific IgA ASCs in patients infected with a CFA/I-expressing ETEC strain was 15-fold higher at the acute stage in patient specimens than in the samples collected from the healthy individuals. When the IgA ASC response levels to CS5 in blood of the healthy volunteers were compared to the responses in patients infected with a CS5-expressing ETEC strain, a 78-fold increase was seen at the early stage of infection. Corresponding values for CS7 and CS14 differences between patients and healthy controls were 11-fold and 40-fold, respectively.
Plasma IgA antibody response to CF antigens in patients.
Overall, the titers of the antibody response to the HoM CF antigen in plasma were already elevated at the acute stage but increased further by day 7 after onset of diarrhea, and the responses had decreased around 3 weeks later. The IgA antibody levels at the acute stage and at the different time periods were, however, always elevated in comparison to the levels seen in healthy controls (P < 0.001) (Tables 2 and 3). The CFA/I ETEC-infected patients also responded with IgA antibodies to the CR CS14 and CS4 antigens, which were significantly elevated at all time points in comparison to the healthy controls (Table 2). A similar response to the HoM or CR antigen, that is, to either the CS14 or the CS4 antigen, was seen in the patients. The response to the HeT CS5 antigen was not significantly increased either in comparison to the controls or during the different stages of disease. The CS14 ETEC patients showed a more pronounced response to HoM antigen than to the CR CFs CFA/I and CS14 and also responded to the CR CFA/I. The CS5 group of patients (those infected with CS5- or CS7-positive ETEC) responded to HoM CS5 or CS7 antigen as well as to the CR antigen CS7 or CS5. A poor response to the HeT CFA/I antigen was seen.
TABLE 2.
IgA antibody response to homologous, cross-reacting, and heterologous antigens of patients infected with CFA/I- and CS14-expressing ETEC strains
| CF of infecting ETEC strain | CF tested for HoM, CR, and HeT antigens | Parameter | Response to homologous CF |
Response to cross-reacting CFs |
Response to heterologous CF |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 2 | Day 7 | Day 14 | Day 30 | CS14 |
CS4 |
CS5 |
||||||||||||
| Day 2 | Day 7 | Day 14 | Day 30 | Day 2 | Day 7 | Day 14 | Day 30 | Day 2 | Day 7 | Day 14 | Day 30 | |||||||
| CFA/I | CFA/I (HoM); CS14, CS4 (CR); CS5 (HeT) | GM | 342 | 1,884 | 2,838 | 849 | 256 | 574 | 726 | 470 | 350 | 622 | 1,047 | 701 | 107 | 102 | 166 | 142 |
| Range | 293-488 | 1,083-3,273 | 1,759-4,581 | 548-1,315 | 219-300 | 414-794 | 516-1,020 | 397-556 | 263-464 | 439-881 | 737-1,485 | 545-90 | 76-148 | 73-143 | 110-252 | 100-203 | ||
| P valuea | ||||||||||||||||||
| (control) | 0.003 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | NS | 0.015 | 0.003 | 0.003 | NS | NS | NS | NS | ||
| P valueb | ||||||||||||||||||
| (days) | 0.006 | <0.001 | NS | NS | 0.033 | NS | NS | 0.044 | NS | NS | NS | NS | ||||||
| CFA/I |
CS4 |
CS5 |
||||||||||||||||
| CS14 | CS14 (HoM); CFA/I, CS4 (CR); CS5 (HeT) | GM | 421 | 1,318 | 601 | 519 | 390 | 430 | 490 | 269 | 251 | 466 | 130 | 188 | 26 | 35 | 37 | 83 |
| Range | 331-534 | 996-1,798 | 337-1,074 | 347-774 | 250-607 | 248-743 | 282-851 | 194-372 | 183-341 | 361-601 | 97-174 | 129-274 | 16-42 | 21-55 | 26-50 | 53-127 | ||
| P value | ||||||||||||||||||
| (control) | 0.002 | <0.001 | 0.014 | 0.008 | 0.015 | 0.038 | 0.028 | 0.045 | NS | 0.045 | NS | NS | 0.024 | 0.005 | 0.006 | 0.001 | ||
| P value | ||||||||||||||||||
| (days) | 0.010 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||||
Antibody titers at acute (day 2) and at convalescence (day 7, day 14, and day 30) stages after onset of diarrhea in patients were compared with the levels in healthy controls.
Titers at different time periods at convalescence (day 7, day 14, and day 30) were compared with the titer at the acute phase (day 2) of diarrhea in the patients.
TABLE 3.
IgA antibody responses to homologous, cross-reacting, and heterologous CFs in CS5- and CS7-infected patients
| CF of infecting ETEC strain | CF tested for HoM, CR, and HeT antigens | Parameter | Response to homologous CF |
Response to cross-reacting CF CS7 |
Response to heterologous CF CFA/I |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 2 | Day 7 | Day 14 | Day 30 | Day 2 | Day 7 | Day 14 | Day 30 | Day 2 | Day 7 | Day 14 | Day 30 | |||
| CS5 | CS5 (HoM); CS7 (CR); CFA/I (HeT) | GM | 199 | 723 | 879 | 412 | 166 | 1,138 | 724 | 313 | 122 | 122 | 122 | 127 |
| Range | 142-279 | 375-1,393 | 512-1,510 | 290-586 | 115-239 | 535-2,421 | 471-1,114 | 203-482 | 71-208 | 71-208 | 87-173 | 99-162 | ||
| P valuea (control) | 0.05 | 0.001 | 0.001 | 0.05 | NS | 0.006 | 0.001 | 0.030 | NS | NS | NS | NS | ||
| P valueb (days) | NS | 0.036 | NS | 0.045 | 0.05 | NS | NS | NS | NS | |||||
| CS5 |
CFA/I |
|||||||||||||
| CS7 | CS7 (HoM); CS5 (CR); CFA/I (HeT) | GM | 423 | 2,716 | 1,596 | 294 | 214 | 453 | 456 | 168 | 71 | 115 | 123 | 95 |
| Range | 272-656 | 1,309-5,636 | 1,086-2,344 | 152-569 | 137-333 | 256-802 | 238-837 | 79-356 | 27-122 | 70-129 | 85-147 | 58-139 | ||
| P value (control) | 0.021 | 0.003 | <0.001 | NS | NS | 0.01 | 0.01 | NS | NS | NS | NS | NS | ||
| P value (days) | 0.035 | 0.035 | NS | NS | NS | NS | NS | NS | NS | |||||
Antibody titers at acute (day 2) and at convalescence (day 7, day 14, and day 30) stages after onset of diarrhea in patients were compared with the levels in healthy controls.
Titers at different time periods at convalescence (day 7, day 14, and day 30) were compared with the titer at the acute phase (day 2) of diarrhea in the patients.
For the 25 CFA/I ETEC-infected patients, we attempted to determine if the age, disease severity, and time of first sampling of blood after onset of diarrhea had any effect on the CF-specific ASC or plasma antibody responses. No association between age (those 3 years of age [n = 15] compared to those older [n = 10]) and duration of diarrhea at first sampling (2 days or longer) was found. However, patients with more severe illness had a lower ASC response at the acute stage than those who had moderate to mild illness (P = 0.037). This effect was not seen at the day 7 sampling (P value was not significant [NS]). No difference in the antibody response in plasma was seen. This analysis could not be carried out in other groups of patients with ETEC-related diarrhea due to the lower numbers of patients. However, the CS5-infected children who had a longer duration of illness prior to hospitalization had a higher magnitude of specific ASCs than the other groups at the acute stage, suggesting that the longer duration of acute illness resulted in a more robust response (Fig. 1c). However, differences in responses between acute and early convalescent stages were not seen if samples, such as those from CFA/I patients, were collected earlier than samples from those who had a longer duration of illness before coming to the treatment center, e.g., those with CS5 ETEC-induced infection (P value was NS).
Immune responses in vaccinees.
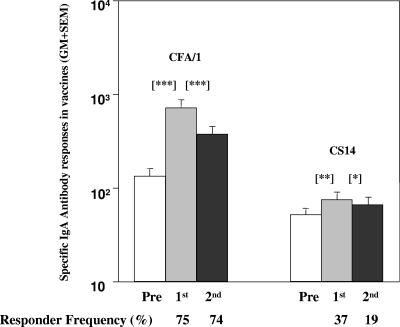
Plasma samples collected from 27 vaccinees who had received 2 doses of the ETEC-CF-BS vaccine during previous studies were tested against CFA/I and CS14 (Fig. 2). Over 70% of the children responded to the CFA/I antigen, which is a component of the ETEC vaccine. Although about 40% of vaccinees responded to CR CS14 (which is not a component of the vaccine), the magnitude and responder frequency were lower than those seen for the CFA/I antigen. The magnitude of the response in the vaccinees 7 days after receiving 1 and/or 2 doses of the vaccine was lower than the magnitude of the response to CS14 seen in the CFA/I-infected patients at day 7 or day 14 after onset of infection (P≤ 0.001).
FIG. 2.
CFA/I- and CS14-specific IgA antibody responses in the plasma of ETEC vaccine recipients. Data for the IgA antibody response in plasma in children after receiving 2 doses of the CF-BS-ETEC vaccine to CFA/I and to a cross-reacting CS14 antigen are shown. Responses are shown prior to immunization (Pre) and 7 days after intake of the first (1st) and the second vaccine dose given at an interval of 14 days (2nd). Bars are geometric means, and the lines are SEM. The responder frequency (percent) indicates the number responding per total number studied. Asterisks indicate statistically significant differences in comparisons of the preimmunization titer (Pre) to that seen 7 days after the first or second vaccine dose (*, P < 0.05; **, P = 0.001; ***, P < 0.001).
DISCUSSION
This study shows that patients infected with CFA/I and CS5 groups responded to homologous as well as to cross-reacting CF antigens with IgA antibody-secreting cells. This is the first report in which we have been able to document that patients mount ASC responses to the CF antigens of the infecting strain as well as to the cross-reacting CF antigen. That this is not only a bystander response or a nonspecific response was shown by the low reactivity to the heterologous CF antigen. Patients also responded with antibodies in plasma to the HoM and CR CF antigens but responded less to the Het antigen. We also tested the antibody response in plasma from vaccinees who had received an ETEC vaccine expressing CFA/I and CS1-CS5, which is the major CF to which antibody responses are generated (14, 16). Our finding that children also responded, albeit with a lower frequency, to CS14 antigen also suggests that primed children in developing countries may respond not only to homologous but also to cross-reacting CFs. We were not able to test ASC responses to the different types of antigens after vaccination since ASCs were not available to us at the time when these studies were carried out (14, 16). In future studies, however, when testing of ETEC vaccines in children in areas of endemicity is carried out, we will need to test the ASC responses to the different HoM and CR CF antigens expressed in such vaccines and better evaluate if it will be possible to immunize against a limited number of antigens and generate responses against many more antigens. However, these studies will also need to show if the responses generated to the HeT CFs are also protective. The healthy children living in the same socioeconomic background and setting as the patients showed significantly lower CF-specific ASC and plasma antibody responses than the children with ETEC diarrhea. However, based on the high prevalence of ETEC infections in Bangladesh (15), it is very likely that these children have been exposed previously. The levels of antibodies in these children are higher than that seen in response to enteric antigens in children (9) or adults in developed countries (1).
The CF-specific IgA immune responses to the HoM and CR CF antigens in the two groups of ETEC patients are very encouraging. However, we will also need to test the IgG antibody responses to these antigens to determine if a similar or different response compared to that seen with the IgA isotype is obtained. This may be important since in Egyptian children with ETEC infections, the CFA/I IgG responses correlated better than the IgA response towards protection (17).
Studies should also be undertaken to test the antibody responses to the minor subunit, which has been shown to be the adhesin of the CFA/I group of fimbrial antigens (2). New interest in a better understanding of the components of CFA/I, which shares genetic and biochemical features with eight other fimbrial antigens including CS4 and CS14 (subclass 5a of the class 5 fimbriae), has also been generated. The minor subunits of class 5a fimbriae were found to be immunologically and biologically cross-reactive in hemagglutination inhibition and Caco-2 adherence assays (2). These minor subunits need to be tested to determine the magnitude of the response to the minor subunits compared to the major colonization factor subunit. These need to be tested in patients with ETEC diarrhea and in vaccine recipients. Such studies will help to elucidate which factors are the most important and need to be included in an effective ETEC vaccine.
Acknowledgments
This research was supported by the Swedish Agency for Research and Economic Cooperation (Sida-SAREC; grant no. 2001-3970), the Swedish Medical Research Council, and the International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B). The ICDDR,B is supported by countries and agencies that share its concern for the health problems of developing countries.
Gudrun Wilkund is gratefully acknowledged for purifying the different CFs used in the study.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Ahren, C., M. Jertborn, and A. M. Svennerholm. 1998. Intestinal immune responses to an inactivated oral enterotoxigenic Escherichia coli vaccine and associated immunoglobulin A responses in blood. Infect. Immun. 66:3311-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anantha, R. P., A. L. McVeigh, L. H. Lee, M. K. Agnew, F. J. Cassels, D. A. Scott, T. S. Whittam, and S. J. Savarino. 2004. Evolutionary and functional relationships of colonization factor antigen I and other class 5 adhesive fimbriae of enterotoxigenic Escherichia coli. Infect. Immun. 72:7190-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black, R. E. 1993. Epidemiology of diarrhoeal disease: implications for control by vaccines. Vaccine 11:100-106. [DOI] [PubMed] [Google Scholar]
- 4.Evans, D. G., D. J. Evans, Jr., S. Clegg, and J. A. Pauley. 1979. Purification and characterization of the CFA/I antigen of enterotoxigenic Escherichia coli. Infect. Immun. 25:738-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans, D. G., R. P. Silver, D. J. Evans, Jr., D. G. Chase, and S. L. Gorbach. 1975. Plasmid-controlled colonization factor associated with virulence in Escherichia coli enterotoxigenic for humans. Infect. Immun. 12:656-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Field, M., L. H. Graf, Jr., W. J. Laird, and P. L. Smith. 1978. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc. Natl. Acad. Sci. USA 75:2800-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaastra, W., and F. K. de Graaf. 1982. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol. Rev. 46:129-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaastra, W., and A. M. Svennerholm. 1996. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 4:444-452. [DOI] [PubMed] [Google Scholar]
- 9.Hallander, H. O., M. Paniagua, F. Espinoza, P. Askelof, E. Corrales, M. Ringman, and J. Storsaeter. 2002. Calibrated serological techniques demonstrate significant different serum response rates to an oral killed cholera vaccine between Swedish and Nicaraguan children. Vaccine 21:138-145. [DOI] [PubMed] [Google Scholar]
- 10.Klemm, P. 1985. Fimbrial adhesions of Escherichia coli. Rev. Infect. Dis. 7:321-340. [DOI] [PubMed] [Google Scholar]
- 11.Levine, M. M. 1990. Modern vaccines. Enteric infections. Lancet 335:958-961. [DOI] [PubMed] [Google Scholar]
- 12.Levine, M. M., and R. Edelman. 1990. Future vaccines against enteric pathogens. Infect. Dis. Clin. N. Am. 4:105-121. [PubMed] [Google Scholar]
- 13.McConnell, M. M., H. Chart, and B. Rowe. 1989. Antigenic homology within human enterotoxigenic Escherichia coli fimbrial colonization factor antigens: CFA/I, coli-surface-associated antigens (CS)1, CS2, CS4 and CS17. FEMS Microbiol. Lett. 52:105-108. [DOI] [PubMed] [Google Scholar]
- 14.Qadri, F., T. Ahmed, F. Ahmed, R. B. Sack, D. A. Sack, and A. M. Svennerholm. 2003. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi children 18-36 months of age. Vaccine 21:2394-2403. [DOI] [PubMed] [Google Scholar]
- 15.Qadri, F., A. M. Svennerholm, A. S. Faruque, and R. B. Sack. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18:465-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qadri, F., C. Wenneras, F. Ahmed, M. Asaduzzaman, D. Saha, M. J. Albert, R. B. Sack, and A. Svennerholm. 2000. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi adults and children. Vaccine 18:2704-2712. [DOI] [PubMed] [Google Scholar]
- 17.Rao, M. R., T. F. Wierzba, S. J. Savarino, R. Abu-Elyazeed, N. El-Ghoreb, E. R. Hall, A. Naficy, I. Abdel-Messih, R. W. Frenck, Jr., A. M. Svennerholm, and J. D. Clemens. 2005. Serologic correlates of protection against enterotoxigenic Escherichia coli diarrhea. J. Infect. Dis. 191:562-570. [DOI] [PubMed] [Google Scholar]
- 18.Rudin, A., M. M. McConnell, and A. M. Svennerholm. 1994. Monoclonal antibodies against enterotoxigenic Escherichia coli colonization factor antigen I (CFA/I) that cross-react immunologically with heterologous CFAs. Infect. Immun. 62:4339-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudin, A., G. Wiklund, C. Wenneras, and F. Qadri. 1997. Infection with colonization factor antigen I-expressing enterotoxigenic Escherichia coli boosts antibody responses against heterologous colonization factors in primed subjects. Epidemiol. Infect. 119:391-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sixma, T. K., K. H. Kalk, B. A. van Zanten, Z. Dauter, J. Kingma, B. Witholt, and W. G. Hol. 1993. Refined structure of Escherichia coli heat-labile enterotoxin, a close relative of cholera toxin. J. Mol. Biol. 230:890-918. [DOI] [PubMed] [Google Scholar]
- 21.Svennerholm, A. M., and J. Holmgren. 1995. Oral vaccines against cholera and enterotoxigenic Escherichia coli diarrhea. Adv. Exp. Med. Biol. 371B:1623-1628. [PubMed] [Google Scholar]
- 22.Svennerholm, A.-M., and J. Holmgren. 1995. Oral B-subunit whole-cell vaccines against cholera and enterotoxigenic Escherichia coli diarrhea. John Wiley & Sons, New York, N.Y. [PubMed]
- 23.Svennerholm, A.-M., Y. L. Vidal, J. Holmgren, M. M. McConnell, and B. Rowe. 1988. Role of PCF8775 antigen and its coli surface subcomponents for colonization, disease, and protective immunogenicity of enterotoxigenic Escherichia coli in rabbits. Infect. Immun. 56:523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Svennerholm, A.-M., C. Wennerås, J. Holmgren, M. M. McConnell, and B. Rowe. 1990. Roles of different coli surface antigens of colonization factor antigen II in colonization by and protective immunogenicity of enterotoxigenic Escherichia coli in rabbits. Infect. Immun. 58:341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svennerholm, A.-M., and G. Wiklund. 1983. Rapid GM1-enzyme-linked immunosorbent assay with visual reading for identification of Escherichia coli heat-labile enterotoxin. J. Clin. Microbiol. 17:596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svennerholm, A.-M., M. Wikstrom, M. Lindblad, and J. Holmgren. 1986. Monoclonal antibodies against Escherichia coli heat-stable toxin (STa) and their use in a diagnostic ST ganglioside GM1-enzyme-linked immunosorbent assay. J. Clin. Microbiol. 24:585-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. 1987. Programme for control of diarrhoeal diseases (CDD/93.3 Rev. 1), p. 9-20. In Manual for laboratory investigations of acute enteric infections. World Health Organization, Geneva, Switzerland.