Abstract
Helicobacter infections are present in approximately 50% of humans, causing severe illnesses such as gastritis and malignancies. Dendritic cells (DC) are critical antigen-presenting cells which link innate and adaptive immune responses. The mechanism of dendritic cell regulation in Helicobacter-induced gastritis is poorly understood. These studies characterized DC isolated from the lamina propria of Helicobacter-infected mice and analyzed innate and adaptive immune responses elicited by Helicobacter antigen (Ag)-pulsed DC. The presence of DC was elevated in the gastric lamina propria infiltrate of infected mice in comparison with controls. After treatment with Helicobacter felis Ag, DC were polarized to secrete interleukin-6 as the dominant cytokine. In the presence of DC and Helicobacter Ag, responder allogeneic T cells in culture exhibited limited cell division. We suggest that the response of DC and T cells to Helicobacter Ag is critical to the chronic persistence of Helicobacter-induced gastritis.
The gram-negative bacterium Helicobacter pylori colonizes the mucus layer covering the gastric mucosa of an infected host and induces histologic gastritis as the bacterium multiplies and persists (9, 53, 55, 66). This bacterium infects more than 50% of the world's population. Over the course of a lifetime, in the absence of adequate eradication therapy, the chronic infection may be the gateway for chronic gastritis, gastric and duodenal ulcers, and several forms of gastric lymphoma (10, 18, 20, 26). The gastritis is characterized by neutrophilic as well as mononuclear cell inflammation and subsequently high levels of proinflammatory cytokines such as interleukin-6 (IL-6), among others, in the gastric mucosa (15, 30, 71). As at other sites of inflammation in the body, a mixed population of leukocytes and neutrophils are present in the gastric mucosa in this disease, as they are drawn by the attraction of cytokines, chemokines, and selectins. The term “active-chronic gastritis” has historically been used to describe the continued predominance of polymorphonuclear leukocytes in the inflammatory response, which also contains significant numbers of lymphocytes.
Dendritic cells (DC) are professional antigen-presenting cells (APCs) (3, 34, 43, 52) sparsely found in most tissues of the body, including the gastrointestinal (GI) mucosa (6). DC have been identified in the lamina propria of mice with chronic Helicobacter infections (54). These cells have devised mechanisms to come into direct contact with bacteria, including opening tight junctions or acquiring bacteria from other cells (60). Helicobacter cells most likely bind to the surface of DC through Toll-like receptors (TLRs) (25, 35, 63) (even though the specific TLRs utilized in this process have not yet been identified), as well as through DC-specific ICAM-3-grabbing nonintegrin (DC SIGN; CD209), a molecule found on human gastric DC in stomach sections (1, 5). There is also the potential for this bacterium or its components to interact with DC at the cytoplasmic level through the nucleotide-binding oligomerization domain 1 (Nod1; CARD 4) and Nod2 (CARD 15) pathogen recognition molecules which respond to different motifs in cell wall peptidoglycan (68). The response of antigen-presenting cells to Helicobacter organisms is poorly understood. They sense invading pathogens, capture these organisms, and become activated. DC process and present microbial particles on major histocompatibility complex (MHC) class II molecules to T cells, which elicit an immune response (31). The response of DC to a specific organism depends on the signals integrated in response to the microbial pathogen and to the cells in the environment. Interaction of Helicobacter antigens with DC surface molecules can alter the way in which these cells interact with other cell types or can induce changes in intracellular signaling pathways within the DC leading to cytokine polarization.
In our effort to address DC cytokine regulation with Helicobacter, and the ability of Helicobacter antigen (Ag)-treated DC to interact with T cells, we conducted in vivo and in vitro investigations using cells obtained from the gastric lamina propria of Helicobacter felis-infected mice and with DC exposed to a preparation from this organism, H. felis antigen. Here, we are the first authors to report the isolation of lamina propria mononuclear cells (LPMC) from the gastric mucosa of mice for phenotypic characterization of dendritic cells. After chronic H. felis infection with 107 CFU, moderate numbers of LPMC were recovered from the gastric lamina propria, compared with approximately 10-fold fewer from uninfected mice.
Because of the limitations posed by in vivo studies, to investigate the ability of Helicobacter to regulate DC innate and adaptive immune responses, we employed bone marrow-derived DC to study the potential of H. felis Ag to induce cytokines in DC cultures and to regulate T-cell proliferation induced by H. felis Ag-treated DC. We demonstrate that mouse bone marrow-derived myeloid DC respond to H. felis Ag by secreting predominately IL-6 in culture. Stimulation of T cells with DC in the presence of H. felis Ag reduced the proliferation of T cells in culture. We suggest that the increased presence of DC in the gastric mucosa of H. felis-infected mice may contribute to gastric inflammation in Helicobacter infections.
MATERIALS AND METHODS
Animals.
Adult C57BL/6J (H-2b) or BALB/c (H-2d) mice (8 to 10 weeks old) were purchased from the Jackson Laboratories (Bar Harbor, ME). Mice were maintained in a specific-pathogen-free environment in the Case Western Reserve University School of Medicine (Cleveland, OH) under conditions required by institutional guidelines.
Experimental infection of mice with Helicobacter felis and sacrifice of mice.
Mice were infected with H. felis strain CS1. H. felis was grown on Columbia agar (Difco) with 7% horse blood in bell jars under microaerobic conditions using the CampyPak Plus envelope system (5% O2, 10% CO2, and 85% N2) at 37°C for 96 h. Medium was supplemented with trimethoprim (20 μg/ml; Sigma), vancomycin (6 μg/ml), amphotericin B (2.5 μg/ml), and polymyxin B (0.125 μg/ml). Colonies were harvested from plates in 1 ml brucella broth using a sterile bacterial spreader and transferred to 50 ml brain heart infusion medium (Difco) containing 10% fetal bovine serum (FBS; Life Technologies) in 200-ml Erlenmeyer flasks. Cultures were placed in bell jars on a rotary shaker at 125 rpm at 37°C and were maintained under microaerobic conditions as described above. Organisms were determined to be sufficiently viable if >90% bacteria were motile on microscopic examination at ×400. The concentration of H. felis organisms was determined by optical density at 450 nm using a previously established growth curve for Helicobacter pylori organisms (7). C57BL/6J mice were infected with 1 × 107 CFU in 0.5 ml nutrient broth by gastric intubation, using a device consisting of flexible tubing attached to an 18-gauge needle.
Animals were sacrificed later than 2 months after infection with H. felis for isolation of LPMC. Mice were killed by CO2 asphyxiation. Thin strips of gastric tissue biopsy material were removed from the greater curvature of the stomach, including both the antrum and the cardia, and the samples were placed in Stuart's urease test broth (62). H. felis colonization was indicated by a change in broth color from orange to red, as urease was produced over a 24-h period. Since the high motility of H. felis often results in confluent growth on nutrient agar, a CFU determination could not be performed (45). A second strip of tissue was removed from the greater curvature of the stomach, including both the antral and fundic glandular mucosa, pinned flat on polystyrene floats, fixed in 10% buffered formalin, paraffin embedded, and mounted on slides as 5-μm sections. Sections were silver stained by the Steiner method (Histology Consultation Services, Everson, WA) for direct visualization of bacteria.
Isolation of gastric lamina propria mononuclear cells.
Gastric tissue was removed from mice, and collagenase digestion was performed (22, 67) to obtain lamina propria mononuclear cells by methods customized for this organ. In preparation, the remaining glandular portion of the stomach was removed from each mouse, cleaned with a cell scraper, washed in Hanks balanced salt solution (HBSS), and minced with scissors. Stomach tissue was pooled within each group (infected or uninfected) of mice. Epithelial cells were removed over a period of three incubations in a 37°C water bath. Tissue was first incubated in 20 ml HBSS medium containing 5% FBS (Gibco BRL, Life Technologies, Grand Island, NY), EDTA (0.05 M), penicillin-streptomycin-amphotericin B (Fungizone; 1.2% [Cambrex Bioscience, Walkersville, MD]), HEPES buffer (0.6 mM), and 15 μg/ml dithiothreitol for 15 min, with intermittent removal of tissue and vigorous pipetting. Samples were filtered in 100-μm strainers. This incubation process was repeated in HBSS medium as described above and then in RPMI medium (Gibco-BRL) supplemented with 10% FBS, penicillin-streptomycin (each at 100 units/ml), and HEPES buffer. Tissue was digested with a solution of 20 ml RPMI medium containing supplements plus collagenase (1.75 mg/ml; Boehringer Mannheim, Germany) and DNase (0.01 mg/ml; Boehringer Mannheim) for 50 min with intermittent pipetting. Recovered LPMC were serially filtered three times, using strainer sizes of 100, 40, and 40 μm, respectively.
Determination of dendritic cell percentages in gastric LPMC.
Cell surface antigen expression of LPMC was determined by dual monoclonal antibody (MAb) immunostaining (22). Briefly, after blocking nonspecific staining of cells by preincubation with Fc receptor for immunoglobulin G (IgG) III/II antibody (CD16/CD32; PharMingen), LPMC were dual labeled with CD11c-phycoerythrin (HL3), and CD40 (3/23), CD80 (B7-1; 16-10A1), CD86 (B7-2; GL1), CD11b (M1/70), or MHC class II I-Ab (AF6-120.1), all of which were fluorescein isothiocyanate (FITC) labeled (PharMingen). The appropriate species-specific IgG isotype controls were included in each experiment. At least 10,000 events were acquired on a FACScan fluorescence-activated cell sorter (Becton Dickinson), and analysis was performed using Cell Quest software.
Generation of bone marrow-derived dendritic cells.
Myeloid dendritic cells were generated from C57BL/6J (B6) mouse bone marrow cells by conventional means and as described elsewhere (21, 36). Briefly, after lysis of red blood cells, leukocytes were cultured in 24-well plates, at 2 × 106 cells/ml, in RPMI culture medium, containing RPMI buffer, supplemented with 10% FBS, 100 U/ml penicillin/100 U/ml streptomycin, nonessential amino acids (0.1 mM; Gibco-BRL), sodium pyruvate (1 mM;, Gibco-BRL), and HEPES buffer, in the presence of 7 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D Systems, Minneapolis, Minn). Cultures were placed in a 5% CO2-humidified incubator at 37°C, fed as described previously (21), and harvested after 5 days for stimulation with H. felis antigen.
Preparation of H. felis antigen.
H. felis antigen was prepared as described elsewhere (8). Briefly, H. felis was harvested from approximately 50 plates in sterile phosphate-buffered saline (PBS), and pelleted by centrifugation at 4,000 × g for 20 min. The pellet was resuspended in 2 ml PBS. Bacteria were lysed using a probe sonicator at a power setting of 5 and a duty cycle of 5% on the pulse setting by four 1-min blasts on ice. Bacteria were again centrifuged as earlier to remove whole cells, and the lysate was filtered through a 0.22-μm-pore filter. Protein concentration was determined by a method reported earlier (48).
Exposure of purified DC to H. felis antigen.
DC were enriched using CD11c Microbeads and MACS columns (miniMACS; Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions, routinely yielding cells of approximately 95% purity. H. felis antigen was diluted to a stock concentration of 1 mg/ml and added to 1 ml purified DC (1 × 106/ml) in RPMI culture medium (described above), in 24-well plates, to obtain a final concentration of 2 or 10 μg/ml H. felis Ag. Cultures were incubated at 37°C and harvested after 24 h for phenotypic analysis of cells or after 72 h for analysis of cytokines released in supernatants.
Quantitation of cytokines in culture.
Stimulated DC supernatants were centrifuged for the removal of dead cells and stored at −70°C. Cytokines released in supernatants were measured by enzyme-linked immunosorbent assay (ELISA) for bioactive IL-12 p70, IL-6, IL-10, and gamma interferon (IFN-γ), using Duoset ELISA kits (R&D Systems, Minneapolis, MN).
Labeling of CD4+ T cells with CFSE.
Spleens were harvested from mice, and single-cell preparations of leukocytes were obtained. T cells were purified using the CD4+ MACS system (Miltenyi Biotec, Auburn, CA) and adjusted to a concentration of 7.5 × 106/ml. Cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (51, 64) as described by the authors (22). Cells (2 × 106/ml) were cultured in 96-well flat-bottomed plates at 37°C in a 5% CO2-humidified incubator for periods and conditions as indicated in the figure legends, to allow incorporation of CFSE dye in dividing cells. On harvest, cells were washed twice in HBSS-1% FBS buffer, and MAb was stained with CD4-peridinin chlorophyll protein (PerCP) (L3T4; RM4-5). Greater than 25,000 events were collected in the live gate on a FACScan for analysis by Cell Quest software.
Statistical analysis.
The means and standard deviations of data are represented in figures as illustrated. Significant differences in cytokine production were determined by Student's t test, using the limit of P < 0.05 as significant.
RESULTS
The gastric mucosa of H. felis-infected mice has an enriched population of dendritic cells.
To underscore the importance of dendritic cells in gastric lamina propria inflammation of Helicobacter infections, mice were infected with 107 CFU live H. felis organisms for a period of over 2 months, after which we isolated LPMC from the gastric lamina propria for characterization of CD11c-positive dendritic cells. Stomachs were harvested from groups of age-matched Helicobacter-infected and uninfected mice, and LPMC were recovered by a collagenase digestion procedure. No inflammation was observed in the stomachs of uninfected B6 mice. However, gross morphology of harvested stomachs from mice infected with H. felis showed an obvious increase in size and paleness of tissue in comparison with uninfected stomachs. The presence of Helicobacter in gastric tissue after infections has been routinely confirmed by histological studies in earlier reports (7, 44). In addition, strips of tissue were placed in urease broth from both uninfected and infected mice. Control uninfected mouse tissue did not produce a change in color of the broth, whereas there was a definite change in color from yellow to pink over the course of 24 h in the broths containing tissue from Helicobacter-infected mice, indicating the presence of the organism.
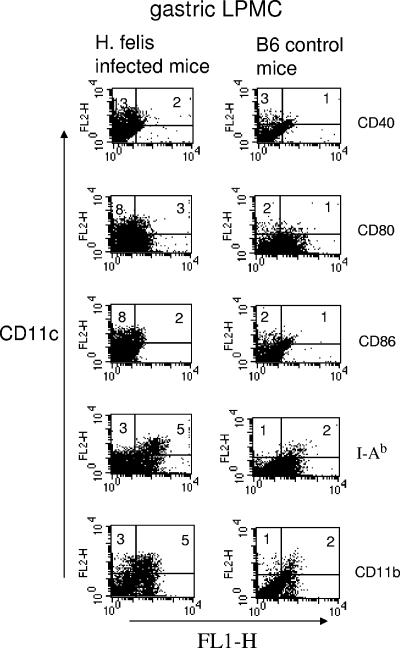
LPMC were isolated from groups of Helicobacter-infected and uninfected mice. The yield of LPMC from stomachs was low in comparison to the known cellularity of other mouse organs such as the spleen and colon. In these studies, stomachs from H. felis-infected B6 mice were pooled in groups of two to three in three separate experiments and LPMC were obtained by collagenase digestion of gastric tissue. The average recovery of LPMC per infected mouse was 2.28 × 106. LPMC numbers recovered from Helicobacter-infected mice were more than 10-fold greater than those from uninfected mice. In the latter case, we pooled stomachs from five uninfected control mice in separate experiments and recovered approximately 0.15 × 106 LPMC per mouse. The increased recovery of cells isolated from the gastric lamina propria of H. felis-infected mice correlates with higher percentages of CD11c (a characteristic DC marker)-positive stained cells in LPMC. CD11c-positive cells were mostly positive for the myeloid DC marker CD11b and for MHC class II I-Ab and expressed low to moderate levels of costimulatory molecules CD40, CD80, and CD86. The levels of CD11c staining observed in uninfected mouse LPMC were negligible (Fig. 1). Increased numbers of DC in the gastric lamina propria of H. felis-infected mice may contribute to the mechanism of persistence of Helicobacter in a chronic infection.
FIG. 1.
Phenotypes of CD11c-positive cells in the gastric mucosa. Groups of H. felis-infected and control uninfected mice (three and five mice, respectively) were sacrificed. Stomachs were removed and pooled, and LPMC were extracted by collagenase digestion. Cells were MAb dual stained for DC-specific marker CD11c-phycoerythrin and CD40-, CD80-, CD86-, CD11b-, or MHC class II I-Ab-FITC. Addition of cell percentages in the upper quadrant reflects the total percentage of CD11c-positive cells. The upper right quadrant shows the percentage of cells positive for both markers. Expression of CD11c-positive cells in H. felis-infected mice is representative of three separate experiments.
Interleukin-6 is the predominant cytokine product of H. felis antigen-pulsed dendritic cells.
Inoculation of mice with H. felis organisms resulted in a robust infection and visual inflammation of the gastric mucosa and a corresponding increase of DC in LPMC. Little is known about the significance of DC in chronic Helicobacter gastritis or the response of these cells to the organism. DC at sites of infection and inflammation have an innate capacity to alter cytokines in the immediate environment. Even though Helicobacter-infected mice have significantly more DC in the stomach than uninfected mice, it is difficult to recover vast numbers of these cells from the gastric lamina propria due to the characteristic low-cellular nature of the stomach with regard to leukocytes. Therefore, in order to determine how H. felis could alter DC, we conducted our subsequent studies using DC generated from bone marrow cells of B6 mice and investigated the ability of H. felis Ag prepared from live organisms by sonication, to polarize these cells towards the release of certain cytokines. Bone marrow-derived DC were generated in the presence of GM-CSF over the course of 5 days. To determine the cytokine response elicited by DC in response to H. felis Ag, we examined the production of IFN-γ and IL-12, since Th1 cytokines are reported to be involved in the course of Helicobacter infection (17, 61). In addition, we studied the ability of DC to release IL-6 and IL-10 in DC on stimulation with H. felis Ag. IL-6 is associated with models of chronic inflammation in the gastrointestinal tract and in H. pylori stimulation of human monocyte-derived DC (15, 39, 40, 70), whereas IL-10 is a downregulatory cytokine in the gut (2, 42).
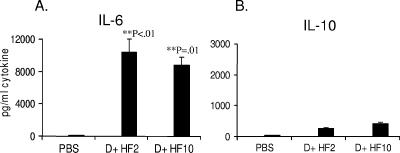
IL-6 was not released in cultures of resting DC. Stimulation of DC with H. felis Ag in culture for a period of 3 days resulted in significantly high levels of IL-6 in DC cultures. Both high (10 μg/ml) and low (2 μg/ml) doses of H. felis Ag were potent stimulators of IL-6 in DC cultures (Fig. 2A). H. felis Ag stimulated the release of low levels of IL-10 from DC (Fig. 2B), while levels of IFN-γ and IL-12 were undetectable. The levels of detection for these cytokines were 16, 31, 31, and 39 pg/ml for IL-6, IL-10, IFN-γ, and IL-12 p70, respectively.
FIG. 2.
H. felis antigen stimulates IL-6 in dendritic cell cultures. C57BL/6J mouse bone marrow-derived DC were generated in the presence of GM-CSF over 5 days. Cells were harvested and DC were purified over CD11c MACS columns. DC were treated with H. felis Ag at 2 or 10 μg/ml (D + HF2 and D + HF10, respectively) in 24-well plates and incubated for 3 days of culture. Supernatants were collected, frozen, and assayed for cytokines by ELISA. Error bars show standard deviations over duplicate wells. P values are for significant increases in cytokine production above PBS-treated control DC. Panel A shows IL-6 release, and panel B shows IL-10 release. Results are representative of three independent experiments.
H. felis antigen regulates the expression of dendritic cell surface molecules.
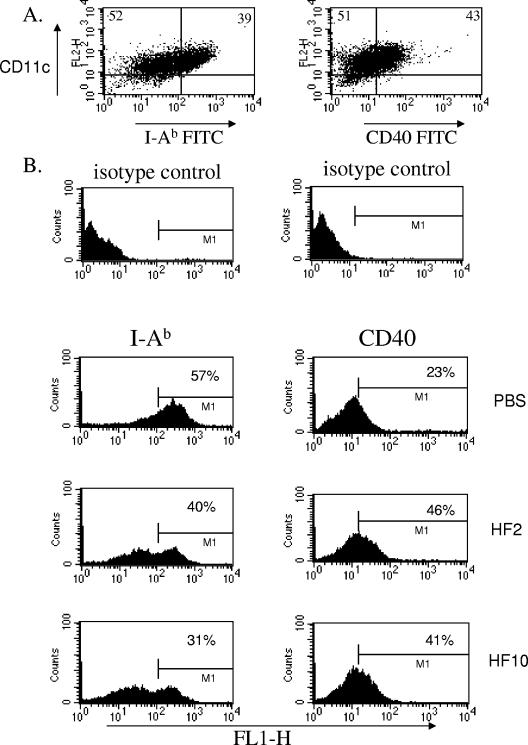
The expression of MHC class II is a function of the ability of DC to interact with and influence T-cell responses. MHC class II is critical in the process of antigen presentation of molecules to T cells and in the induction of T-cell proliferation. To investigate how H. felis Ag can regulate DC surface molecules involved in T-cell responses and cytokine production, we monitored the phenotypic response of DC MHC class II to H. felis Ag, as well as the ability of this bacterial preparation to regulate expression of costimulatory molecules CD40, CD80, and CD86 on the surface of DC. On exposure of bone marrow-derived DC to H. felis Ag for overnight culture and subsequent MAb staining, CD80 and CD86 expression on the surface of DC were not significantly altered (not shown). The expression of CD40 was increased, while MHC class II I-Ab was consistently reduced after exposure of DC to H. felis Ag for 24 h (Fig. 3A and B). Further analysis of dot plots (not shown) revealed that over three separate experiments, total CD40 expression on DC increased 10, 10, and 16% (12% on average) in H. felis Ag-treated (10 μg/ml) compared with control medium-treated DC. For this parameter, MHC class II expression was downregulated 29, 31, and 41% (34% decrease on average) with H. felis Ag treatment of DC. Similar trends in phenotypic changes were observed whether DC were exposed to 2 or 10 μg/ml H. felis Ag (Fig. 3A and B).
FIG. 3.
H. felis antigen regulates MHC class II expression on DC. Bone marrow-derived DC cultured from B6 mice were harvested and purified on CD11c MACS columns to obtain the DC population. Cells were treated with medium or H. felis Ag at 2 or 10 μg/ml (D + HF2 and D + HF10, respectively) for 24 h of culture. (A) DC were treated with H. felis Ag (2 μg/ml), harvested, and MAb stained with CD11c-phycoerythrin and I-Ab-FITC or CD40-FITC. Panel B shows changes in phenotypic expression of CD11c-positive cells on exposure to different doses of H. felis Ag. Isotype control background percentages were subtracted. Results are representative of three separate experiments.
Helicobacter antigen stimulates low synergistic release of interleukin-6 in DC-T-cell cultures.
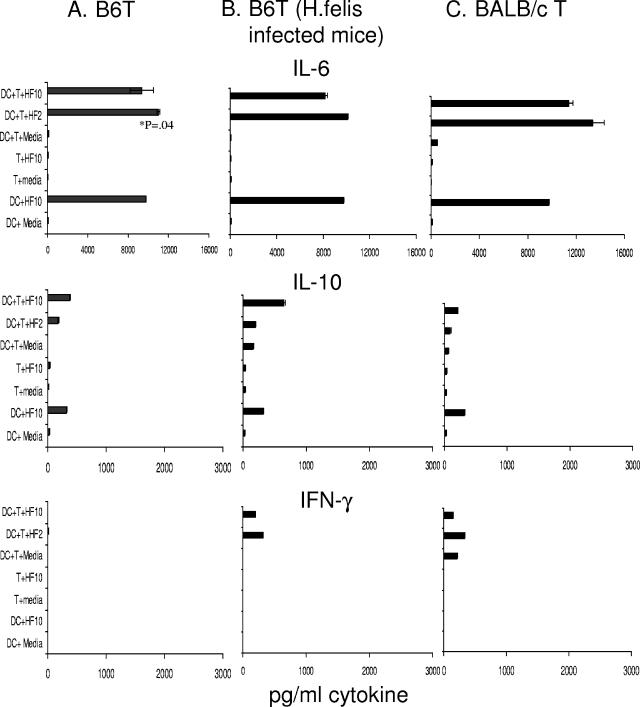
In the host, dendritic cell-T-cell interaction is capable of inducing adaptive immune responses in T cells. The amplitude of this response may be regulated by the presence of microorganisms interacting with DC and subsequent changes in DC. In our system, we investigated the ability of H. felis Ag to modulate cytokine production in B6 DC cocultured with T cells for a period of 4 days. Purified CD4+ T cells were obtained from spleens of uninfected B6 or BALB/c mice or from H. felis-infected B6 mice to address the possibility that T cells from infected mice may elicit a different immune response from those of control mice.
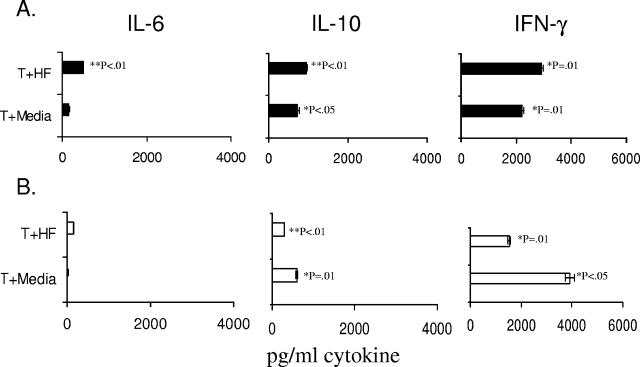
We observed that B6 mice DC incubated with H. felis Ag stimulated the release of IL-6. When BALB/c T cells were incubated with DC in the presence of H. felis Ag, there was an increase in IL-6 levels in cultures in comparison with cultures without H. felis Ag. This increase was not statistically significant. DC cocultured with B6T cells from naïve mice or H. felis-infected mice released similar levels of IL-6 in the presence of H. felis Ag in three of three experiments. As seen in Fig. 4, this increase in IL-6 release was only marginally significant (P = 0.04) in comparison with H. felis Ag stimulation of DC alone in culture and only occurred in one group. Thus, in DC-T-cell cocultures, the greater response observed is due to the stimulation of DC by H. felis Ag (Fig. 2 and 4) rather than to the action of H. felis Ag on T cells. In comparison with high levels of IL-6 release in cocultures, levels of interleukin-10 detected were low. Low levels of IFN-γ were detected from H. felis Ag-treated DC cultured with or without the addition of T cells (Fig. 4A, B, and C). IL-12 p70 levels were below the limits of detection in all cultures (not shown). H. felis Ag did not stimulate significant levels of any cytokine examined in cultures of T cells alone (Fig. 4A, B, and C).
FIG. 4.
IL-6 release in DC-T-cell cultures induced by H. felis Ag treatment. Purified B6 DC (1 × 106/ml) were added to purified CD4+ T cells (2 × 106/ml) in 96-well flat-bottomed plates. H. felis Ag (10-μl volume) was added at 2 and 10 μg/ml (HF2 and HF10, respectively), and cultures were incubated for 4 days. Supernatants were harvested and assayed for cytokines by ELISA. Results are shown for experiments using T cells from unmanipulated B6 control mice (A), H. felis-infected B6 mice (B), and unmanipulated BALB/c mice (C). Means and standard deviations over duplicate assay wells are indicated. Results are representative of two to three separate experiments.
Because there was not potent augmentation of cytokine release on exposure of DC-T-cell cultures to Helicobacter Ag, we conducted experiments to determine whether T cells used in these studies were capable of eliciting cytokine responses in the presence of H. felis Ag modulation. Thus, we incubated C57BL/6 or BALB/c T cells in the absence of DC in 24-well plates coated with anti-CD3 antibody. H. felis Ag and soluble anti-CD28 were added to these cultures, which were then incubated for 4 days. As described above (Fig. 4), T cells cultured in medium alone released minimal levels of cytokines. Both BALB/c and B6 mouse T cells cultured in the presence of H. felis Ag and additional stimulation (anti-CD3/CD28) exhibited robust cytokine responses. The predominant cytokine released was IFN-γ, with moderate levels of IL-10 and minimal levels of IL-6 (Fig. 5A and B).
FIG. 5.
Stimulation of cytokine release in T cells on exposure to H. felis Ag. Purified CD4+ T cells (1 × 106) were cultured in 24-well plates coated with anti-CD3 Ab (1 μg/ml), and soluble anti-CD28 (2 μg/ml) was added to wells. Cultures were set up with and without H. felis Ag (10 μg/ml) and incubated in a 37°C, 5% CO2-humidified incubator for 4 days. Supernatants were harvested for cytokine analysis by ELISA. The standard deviations and means over duplicate wells are shown for experiments using C57BL6/J (A) or BALB/c (B) mouse T cells. Significance was determined against unstimulated T cells in medium (<50 pg/ml cytokine in each case). P values were determined for cytokine levels of >300 pg/ml.
Helicobacter antigen limits T-cell division in DC-T-cell cocultures.
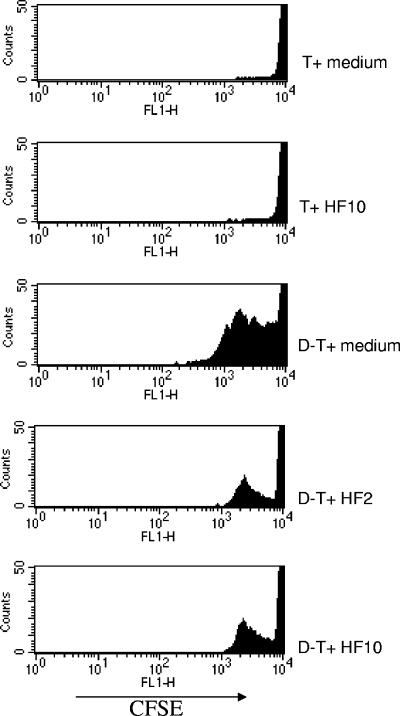
In inflammatory conditions of the gut, dendritic cells and T cells are in close proximity (47). In H. felis-infected mice, in addition to the population of cells recruited to the gastric mucosa, DC can also potentially induce cell division and proliferation in T cells. To test the hypothesis that H. felis Ag-treated DC may regulate the level of T-cell division, naïve CD4+ T cells were purified from BALB/c mouse spleens, labeled with CFSE, added to B6 DC (ratio of 1 DC to 2 T cells) in the presence of H. felis Ag, and cultured for 4 days. Cells were harvested and then labeled with CD4-PerCP, and CFSE dye inclusion was determined by flow cytometry. As shown by the amplitude and numbers of cell division peaks, there was robust T-cell division when DC were cultured with T cells in the presence of medium, in comparison with that observed for T cells alone. DC-T-cell division resulted in five division peaks in T cells. When H. felis Ag (2 or 10 μg/ml) was added to cocultures with BALB/c T cells and B6 DC, there was then significantly less division in T cells, as shown by the reduction in division peaks. T cells alone did not proliferate in response to H. felis Ag (Fig. 6).
FIG. 6.
H. felis Ag inhibits T-cell division in DC-T-cell cultures. Purified B6 DC (1 × 106/ml) and CFSE-labeled BALB/c T cells (2 × 106/ml) were incubated in 96-well flat-bottomed plates in multiple wells, in the presence and absence of H. felis Ag (2 and 10 μg/ml [HF2 and HF10, respectively]). At day 4 after the start of culture, wells were harvested and pooled. Cells were MAb stained with CD4-PerCP. CFSE dye incorporation in T cells was assessed by flow cytometry (FL1 channel). Dead cells were gated out, and >25,000 events were collected to analyze differences in cell division peaks. Results are representative of two independent experiments.
DISCUSSION
The present study demonstrates that gastritis observed in H. felis-infected mice has a distinct and significant portion of dendritic cells in the lamina propria infiltrate. We observed a paucity of dendritic cells in the stomachs of naïve control C57BL6/J mice (and BALB/c mice; M. Drakes, personal observations, 2006), whereas approximately 10% of the lamina propria cell population recovered from the gastric tissue of H. felis-infected mice stained positive for DC markers. A similar elevation in dendritic cells was recently reported in the lamina propria of the intestine of IBD mice by the authors (22) and is consistent with the notion that in the gastrointestinal tract the increased presence of DC drives inflammation and dysregulation of immune responses. H. felis was specifically chosen for these studies since many mouse-adapted H. pylori strains induce either mild disease or require lengthy colonization periods to develop significant levels of gastritis. The limited gastritis achieved by H. pylori infection would make it impractical to isolate DC from the gastric mucosa since, unlike the colon, few leukocytes are present in the absence of disease.
This is the first study to characterize DC in isolated lamina propria mononuclear cells of gastric tissue. We employed a modification of our multistep isolation procedure originally developed to recover LPMC from the large bowel of mice with colitis (22, 24). Isolation of cells from the lamina propria of the gastrointestinal mucosa is difficult in that the epithelial cell monolayer must be removed from the tissue and then cells must be released from the extracellular matrix through collagenase digestion. Even when cell release is highly efficient, large numbers of contaminating epithelial cells and monocytes are typically present. The present study utilized a novel procedure applicable to the gastric lamina propria, allowing for suitable numbers of cells to be retrieved for phenotypic characterization of DC through MAb staining and flow cytometry, providing for a more quantitative analysis than by immunohistochemistry (54).
Gastric lamina propria DC of Helicobacter-infected mice were of the myeloid subclass, expressing the characteristic CD11b, as well as moderate levels of costimulatory molecules, the latter of which are essential for interaction with effector T cells in the gastrointestinal tract. Although enough cells can be isolated for phenotypic characterization, the numbers of dendritic cells present in the gastric mucosa of these mice make it difficult to perform the ex vivo investigations required to evaluate dendritic cell function. With the knowledge that DC process and present bacterial Ag to T cells during the course of infections, we evaluated the effect of H. felis Ag on DC function using DC propagated from mouse bone marrow cells in the presence of GM-CSF conditioning medium. Bone marrow-derived and peripheral blood dendritic cells have been used almost exclusively to examine dendritic cell function for Helicobacter infections (32, 33, 41, 59). The present study goes beyond the scope of previous reports by investigating not only the innate immune response of DC to Helicobacter Ag in light of cytokine polarization, but by also examining several aspects of the adaptive immune response that is elicited by T cells on stimulation with DC and H. felis Ag. Our system was designed to model immunological events in the gastric lamina propria in an “active-chronic” Helicobacter infection, with the components of infiltrated DC and other cells, in the presence of bacterial antigens.
Infection of mice with H. felis, an original cat isolate (44), has been widely used as a model for the study of H. pylori pathogenesis and immunity. H. felis readily infects mice and induces the type of chronic hyperinflammation observed in gastric biopsies of H. pylori-infected patients. Although clinical isolates of H. pylori have since been adapted to mice, experimental H. felis infections continue to be more appropriate for several different aspects of Helicobacter research. First, H. felis infection causes a pronounced gastritis to develop within weeks of infection, facilitating the study of Helicobacter-associated immunopathogenesis (44). This is in contrast to most mouse-adapted strains of H. pylori which require longer periods to induce gastric inflammation and yet yield a much milder form of the disease. The dynamics of H. felis-associated gastritis in murine models allow for studies regarding vaccine-induced protective immunity (14, 16, 27, 46, 56), the role of T regulatory cells in persistent infection (4, 37), and the contribution of innate factors in immunopathogenesis (37). Second, the advanced pathology associated with H. felis infections includes prominent lymphoid aggregates and severe progressive gastritis which advances to metaplasia, dysplasia, and cancer. Thus, the H. felis model is an effective tool to study the effects of Helicobacter infection and eradication on gastric mucosa-associated lymphoid tissue lymphoma and gastric adenocarcinoma (13, 38).
In our studies, we determined cytokine levels in supernatants harvested over a period of 3 to 4 days as indicated, to better correlate with the optimum time of day 4 at which we harvested assays for monitoring of T-cell division. Stimulation of dendritic cells with a sonicated preparation of H. felis organisms (H. felis Ag) induced significant levels of IL-6 and some IL-10. This is consistent with the findings of Kranzer and coworkers, who noted abundant IL-6 and IL-10 production in cells stimulated with live H. pylori in two separate studies (40, 41). Polarization of DC to produce IL-6 in the presence of H. felis Ag is of direct interest to the process of gastric inflammation. IL-6 is becoming recognized as a marker of activated DC and of dysregulation in the GI tract. It is a proinflammatory cytokine produced by intestinal DC of IBD mice in the absence of exogenous stimulation of these cells (22). It has been identified in a range of inflammatory diseases in the GI tract and at other sites in the body, including the Helicobacter-infected mucosa (15, 39, 70).
We also detected low levels of IFN-γ on stimulation of DC with H. felis Ag, but no IL-12. Although several published studies have reported the induction of DC IL-12 ranging from 103 to 105 pg/ml (32, 33, 41), in general, those laboratories have used live H. pylori as a stimulus for their dendritic cells. In keeping with our findings, Voland and colleagues reported only 50 pg/ml IL-12 when using the purified H. pylori antigens HpaA and Omp18 (69), a level which may not be of biological significance in our system. Additionally, stimulation assays by Kranzer and coworkers reported differential cytokine responses when stimulating DC with live H. pylori organisms and H. pylori sonicate. These workers showed that stimulation with live H. pylori generated abundant IL-12, whereas H. pylori sonicate failed to stimulate any detectable IL-12 (41), as observed in our study. In separate studies, others have reported that only intact bacteria will induce significant levels of IL-12 in DC (19).
Our results showed that treatment of DC with H. felis Ag resulted in upregulation of costimulatory molecule CD40. This indicates that H. felis sonicate is capable of activating DC. We also showed that DC exposed to H. felis Ag in vitro displayed reduced levels of surface MHC class II I-Ab. The observation of reduced MHC class II expression is in contrast to other studies in which HLA DR was shown to be elevated in expression after stimulation with live H. pylori (33, 41). We consider the possibility that in our studies, H. felis Ag may have caused a transient increase in DC maturation followed by cell death of the more mature DC early in culture. However, examination of our ex vivo T-cell allostimulation studies seems to argue against this latter possibility. In these studies, we observed that H. felis sonicate-pulsed DC inhibited T-cell division. There was significantly reduced allogeneic T-cell division when T cells were cultured with DC in the presence of H. felis Ag as compared to in culture medium. DC expressing low MHC class II induce little T-cell proliferation (23, 49), and hence in our studies reduced T-cell division may be as a consequence of H. felis Ag-induced MHC class II downregulation. T-cell division in response to treatment of Helicobacter proteins has been previously studied, but not in the context of concomitant DC stimulation, as we have done here. In other studies, specific proteins from Helicobacter including vacuolating cytotoxin A have been reported to inhibit T-cell proliferation to subsequent antigen challenge (28, 29, 65). Our studies are of prime importance, because these investigations have shed light on the ability of T cells to elicit an immune response to DC stimulation, under the regulation of Helicobacter Ag, a system of great relevance to the events occurring in the gastric lamina propria in infection. The observed inhibition of T-cell proliferation does not appear to be due to the death of T cells, since in preliminary investigations H. felis Ag did not induce apoptosis in T cells (M. Drakes, personal observations). Additionally, T cells stimulated with anti-CD3 (plate bound)/CD28 (soluble) in the presence of H. felis Ag released high levels of IFN-γ in cultures, indicating that T cells are functional in the presence of H. felis Ag. Thus, other mechanisms of H. felis Ag-induced T-cell inhibition may be responsible; for example, Helicobacter Ag may be inducing cell cycle arrest or the suppression of nuclear factor of activated T cells (29, 65).
In a series of novel investigations, we have characterized the phenotypes of DC from the gastric lamina propria of H. felis-infected mice. We suggest a prime role for these classical antigen-presenting cells as initiators of immune dysregulation in the gastric lamina propria. IL-6, the dominant cytokine released on H. felis Ag stimulation of DC, may be a crucial cytokine to drive the inflammatory responses and create an appropriate environment for the continued recruitment of cells to the site of inflammation. On the other hand, the chronicity of the infection may be perpetuated and maintained by the ability of Helicobacter Ag-primed DC to limit division of T cells at the site of inflammation and by the potential of DC-T-cell interaction to release low but critical levels of IL-10 or to induce T regulatory cells. In fact, increasing evidence is being reported with respect to immunosuppressive activity of H. pylori (12, 28) and the presence of downregulatory IL-10-producing T cells in the gastric mucosa of infected mice and humans (11, 37), and it is likely that local dendritic cells play a role in limiting the T-cell response to Helicobacter infections. Although infection is associated with gastritis, the absence of either IL-10-producing T cells or CD25+ regulatory T cells results in more severe gastritis and a reduction or clearance of bacteria from the gastric mucosa (50, 57, 58). We suggest that in a Helicobacter infection, the balance of events occurring between the recruitment of cells to the gastric lamina propria combined with limited T-cell expansion and other downregulatory factors such as the presence of IL-10, may be a sufficient condition to render a state of “active-chronic” inflammation in the stomach.
Acknowledgments
This study was supported by National Institutes of Health grants DK-65859, AI-055710, DK-46461, and DK-046461-1151. Fluorescence-activated cell sorting was performed at the Flow Cytometry Core Facility of the Comprehensive Cancer Center of Case Western Reserve University, supported by grant P30 CA43703.
The authors thank Melvin Berger of the Department of Pediatrics, Case Western Reserve University, for the kind use of his analytical flow cytometer.
Editor: F. C. Fang
REFERENCES
- 1.Appelmelk, B. J., I. van Die, S. J. van Vliet, C. M. Vandenbroucke-Grauls, T. B. Geijtenbeek, and Y. van Kooyk. 2003. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 170:1635-1639. [DOI] [PubMed] [Google Scholar]
- 2.Asseman, C., S. Mauze, M. W. Leach, R. L. Coffman, and F. Powrie. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190:995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed]
- 4.Berg, D. J., N. A. Lynch, R. G. Lynch, and D. M. Lauricella. 1998. Rapid development of severe hyperplastic gastritis with gastric epithelial dedifferentiation in Helicobacter felis-infected IL-10(−/−) mice. Am. J. Pathol. 152:1377-1386. [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman, M. P., A. Engering, H. H. Smits, S. J. van Vliet, A. A. van Bodegraven, H. P. Wirth, M. L. Kapsenberg, C. M. Vandenbroucke-Grauls, Y. van Kooyk, and B. J. Appelmelk. 2004. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 200:979-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilsborough, J., and J. L. Viney. 2004. Gastrointestinal dendritic cells play a role in immunity, tolerance, and disease. Gastroenterology 127:300-309. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard, T. G., S. J. Czinn, R. W. Redline, N. Sigmund, G. Harriman, and J. G. Nedrud. 1999. Antibody-independent protective mucosal immunity to gastric helicobacter infection in mice. Cell Immunol. 191:74-80. [DOI] [PubMed] [Google Scholar]
- 8.Blanchard, T. G., N. Lycke, S. J. Czinn, and J. G. Nedrud. 1998. Recombinant cholera toxin B subunit is not an effective mucosal adjuvant for oral immunization of mice against Helicobacter felis. Immunology 94:22-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaser, M. J., and J. C. Atherton. 2004. Helicobacter pylori persistence: biology and disease. J. Clin. Investig. 113:321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaser, M. J., G. I. Perez-Perez, H. Kleanthous, T. L. Cover, R. M. Peek, P. H. Chyou, G. N. Stemmermann, and A. Nomura. 1995. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 55:2111-2115. [PubMed] [Google Scholar]
- 11.Bodger, K., K. Bromelow, J. I. Wyatt, and R. V. Heatley. 2001. Interleukin 10 in Helicobacter pylori associated gastritis: immunohistochemical localisation and in vitro effects on cytokine secretion. J. Clin. Pathol. 54:285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boncristiano, M., S. R. Paccani, S. Barone, C. Ulivieri, L. Patrussi, D. Ilver, A. Amedei, M. M. D'Elios, J. L. Telford, and C. T. Baldari. 2003. The Helicobacter pylori vacuolating toxin inhibits T cell activation by two independent mechanisms. J. Exp. Med. 198:1887-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai, X., J. Carlson, C. Stoicov, H. Li, T. C. Wang, and J. Houghton. 2005. Helicobacter felis eradication restores normal architecture and inhibits gastric cancer progression in C57BL/6 mice. Gastroenterology 128:1937-1952. [DOI] [PubMed] [Google Scholar]
- 14.Corthesy-Theulaz, I., N. Porta, M. Glauser, E. Saraga, A.-C. Vaney, R. Haas, J. P. Kraehenbuhl, A. L. Blum, and P. Michetti. 1995. Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterology 109:115-121. [DOI] [PubMed] [Google Scholar]
- 15.Crabtree, J. E., T. M. Shallcross, R. V. Heatley, and J. I. Wyatt. 1991. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut 32:1473-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czinn, S. J., A. Cai, and J. G. Nedrud. 1993. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine 11:637-642. [DOI] [PubMed] [Google Scholar]
- 17.D'Elios, M. M., M. Manghetti, M. De Carli, F. Costa, C. T. Baldari, D. Burroni, J. L. Telford, S. Romagnani, and G. Del Prete. 1997. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 158:962-967. [PubMed] [Google Scholar]
- 18.Di Napoli, A., R. Petrino, M. Boero, D. Bellis, and L. Chiandussi. 1992. Quantitative assessment of histological changes in chronic gastritis after eradication of Helicobacter pylori. J. Clin. Pathol. 45:796-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon, G. L. J., P. J. Newton, B. M. Chain, D. Katz, S. R. Andersen, S. Wong, P. van der Ley, N. Klein, and R. E. Callard. 2001. Dendritic cell activation and cytokine production induced by group B Neisseria meningitidis: interleukin-12 production depends on lipopolysaccharide expression in intact bacteria. Infect. Immun. 69:4351-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1996. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 20:1161-1181. [DOI] [PubMed] [Google Scholar]
- 21.Drakes, M., T. Blanchard, and S. Czinn. 2004. Bacterial probiotic modulation of dendritic cells. Infect. Immun. 72:3299-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drakes, M. L., T. G. Blanchard, and S. J. Czinn. 2005. Colon lamina propria dendritic cells induce a proinflammatory cytokine response in lamina propria T cells in the SCID mouse model of colitis. J. Leukoc. Biol. 78:1291-1300. [DOI] [PubMed] [Google Scholar]
- 23.Drakes, M. L., L. Lu, V. M. Subbotin, and A. W. Thomson. 1997. In vivo administration of flt3 ligand markedly stimulates generation of dendritic cell progenitors from mouse liver. J. Immunol. 159:4268-4278. [PubMed] [Google Scholar]
- 24.Drakes, M. L., S. J. Czinn, and T. G. Blanchard. 2004. Isolation and purification of colon lamina propria dendritic cells from mice with colitis. Cytotechnology 46:151-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards, A. D., S. P. Manickasingham, R. Sporri, S. S. Diebold, O. Schulz, A. Sher, T. Kaisho, S. Akira, and C. Reis e Sousa. 2002. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J. Immunol. 169:3652-3660. [DOI] [PubMed] [Google Scholar]
- 26.Ferrero, R. L. 2005. Innate immune recognition of the extracellular mucosal pathogen, Helicobacter pylori. Mol. Immunol. 42:879-885. [DOI] [PubMed] [Google Scholar]
- 27.Ferrero, R. L., J.-M. Thiberge, I. Kansau, N. Wuscher, M. Huerre, and A. Labigne. 1995. The groES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc. Natl. Acad. Sci. USA 92:6499-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gebert, B., W. Fischer, E. Weiss, R. Hoffmann, and R. Haas. 2003. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science 301:1099-1102. [DOI] [PubMed] [Google Scholar]
- 29.Gerhard, M., C. Schmees, P. Voland, N. Endres, M. Sander, W. Reindl, R. Rad, M. Oelsner, T. Decker, M. Mempel, L. Hengst, and C. Prinz. 2005. A secreted low-molecular-weight protein from Helicobacter pylori induces cell-cycle arrest of T cells. Gastroenterology 128:1327-1339. [DOI] [PubMed] [Google Scholar]
- 30.Gionchetti, P., D. Vaira, M. Campieri, J. Holton, M. Menegatti, A. Belluzzi, E. Bertinelli, M. Ferretti, C. Brignola, M. Miglioli, et al. 1994. Enhanced mucosal interleukin-6 and -8 in Helicobacter pylori-positive dyspeptic patients. Am. J. Gastroenterol. 89:883-887. [PubMed] [Google Scholar]
- 31.Guermonprez, P., J. Valladeau, L. Zitvogel, C. Thery, and S. Amigorena. 2002. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20:621-667. [DOI] [PubMed] [Google Scholar]
- 32.Guiney, D. G., P. Hasegawa, and S. P. Cole. 2003. Helicobacter pylori preferentially induces interleukin 12 (IL-12) rather than IL-6 or IL-10 in human dendritic cells. Infect. Immun. 71:4163-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hafsi, N., P. Voland, S. Schwendy, R. Rad, W. Reindl, M. Gerhard, and C. Prinz. 2004. Human dendritic cells respond to Helicobacter pylori, promoting NK cell and Th1-effector responses in vitro. J. Immunol. 173:1249-1257. [DOI] [PubMed] [Google Scholar]
- 34.Hart, D. N. 1997. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 90:3245-3287. [PubMed] [Google Scholar]
- 35.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 36.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ismail, H. F., P. Fick, J. Zhang, R. G. Lynch, and D. J. Berg. 2003. Depletion of neutrophils in IL-10−/− mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to Helicobacter. J. Immunol. 170:3782-3789. [DOI] [PubMed] [Google Scholar]
- 38.Ismail, H. F., J. Zhang, R. G. Lynch, Y. Wang, and D. J. Berg. 2003. Role for complement in development of Helicobacter-induced gastritis in interleukin-10-deficient mice. Infect. Immun. 71:7140-7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito, H., M. Takazoe, Y. Fukuda, T. Hibi, K. Kusugami, A. Andoh, T. Matsumoto, T. Yamamura, J. Azuma, N. Nishimoto, K. Yoshizaki, T. Shimoyama, and T. Kishimoto. 2004. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology 126:947, 989-996. [DOI] [PubMed] [Google Scholar]
- 40.Kranzer, K., A. Eckhardt, M. Aigner, G. Knoll, L. Deml, C. Speth, N. Lehn, M. Rehli, and W. Schneider-Brachert. 2004. Induction of maturation and cytokine release of human dendritic cells by Helicobacter pylori. Infect. Immun. 72:4416-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kranzer, K., L. Söllner, M. Aigner, N. Lehn, L. Deml, M. Rehli, and W. Schneider-Brachert. 2005. Impact of Helicobacter pylori virulence factors and compounds on activation and maturation of human dendritic cells. Infect. Immun. 73:4180-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuhn, R., J. Lohler, D. Rennick, K. Rajewsky, and W. Muller.1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263-274. [DOI] [PubMed] [Google Scholar]
- 43.Lanzavecchia, A., and F. Sallusto. 2001. Regulation of T cell immunity by dendritic cells. Cell 106:263-266. [DOI] [PubMed] [Google Scholar]
- 44.Lee, A., J. G. Fox, G. Otto, and J. Murphy. 1990. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology 99:1315-1323. [DOI] [PubMed] [Google Scholar]
- 45.Lee, A., S. L. Hazell, J. O'Rourke, and S. Kouprach. 1988. Isolation of a spiral-shaped bacterium from the cat stomach. Infect. Immun. 56:2843-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee, C. K., R. Weltzin, W. D. J. Thomas, H. Kleanthous, T. H. Ermak, G. Soman, J. E. Hill, S. K. Ackerman, and T. P. Monath. 1995. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J. Infect. Dis. 172:161-172. [DOI] [PubMed] [Google Scholar]
- 47.Leithauser, F., T. Krajina, Z. Trobonjaca, and J. Reimann. 2002. Early events in the pathogenesis of a murine transfer colitis. Pathobiology 70:156-163. [DOI] [PubMed] [Google Scholar]
- 48.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 49.Lu, L., J. Woo, A. S. Rao, Y. Li, S. C. Watkins, S. Qian, T. E. Starzl, A. J. Demetris, and A. W. Thomson. 1994. Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen. J. Exp. Med. 179:1823-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lundgren, A., E. Suri-Payer, K. Enarsson, A.-M. Svennerholm, and B. S. Lundin. 2003. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect. Immun. 71:1755-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lyons, A. B. 2000. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J. Immunol. Methods 243:147-154. [DOI] [PubMed] [Google Scholar]
- 52.Mellman, I., and R. M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255-258. [DOI] [PubMed] [Google Scholar]
- 53.Montecucco, C., and R. Rappuoli. 2001. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat. Rev. Mol. Cell. Biol. 2:457-466. [DOI] [PubMed] [Google Scholar]
- 54.Nishi, T., K. Okazaki, K. Kawasaki, T. Fukui, H. Tamaki, M. Matsuura, M. Asada, T. Watanabe, K. Uchida, N. Watanabe, H. Nakase, M. Ohana, H. Hiai, and T. Chiba. 2003. Involvement of myeloid dendritic cells in the development of gastric secondary lymphoid follicles in Helicobacter pylori-infected neonatally thymectomized BALB/c mice. Infect. Immun. 71:2153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parsonnet, J., G. D. Friedman, N. Orentreich, and H. Vogelman. 1997. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 40:297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radcliff, F. J., M. Chen, and A. Lee. 1996. Protective immunization against Helicobacter stimulates long-term immunity. Vaccine 14:780-784. [DOI] [PubMed] [Google Scholar]
- 57.Raghavan, S., M. Fredriksson, A. M. Svennerholm, J. Holmgren, and E. Suri-Payer. 2003. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clin. Exp. Immunol. 132:393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raghavan, S., E. Suri-Payer, and J. Holmgren. 2004. Antigen-specific in vitro suppression of murine Helicobacter pylori-reactive immunopathological T cells by CD4CD25 regulatory T cells. Scand. J. Immunol. 60:82-88. [DOI] [PubMed] [Google Scholar]
- 59.Rathinavelu, S., J. Y. Kao, Y. Zavros, and J. L. Merchant. 2005. Helicobacter pylori outer membrane protein 18 (Hp1125) induces dendritic cell maturation and function. Helicobacter 10:424-432. [DOI] [PubMed] [Google Scholar]
- 60.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J. P. Kraehenbuhl, and P. Ricciardi-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2:361-367. [DOI] [PubMed] [Google Scholar]
- 61.Smythies, L. E., K. B. Waites, J. R. Lindsey, P. R. Harris, P. Ghiara, and P. D. Smith. 2000. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J. Immunol. 165:1022-1029. [DOI] [PubMed] [Google Scholar]
- 62.Stuart, C. A., E. Van Stratum, and R. Rustigan. 1945. Further studies on urease production by Proteus and related organisms. J. Bacteriol. 49:437-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su, B., P. J. M. Ceponis, S. Lebel, H. Huynh, and P. M. Sherman. 2003. Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect. Immun. 71:3496-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suchin, E. J., P. B. Langmuir, E. Palmer, M. H. Sayegh, A. D. Wells, and L. A. Turka. 2001. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J. Immunol. 166:973-981. [DOI] [PubMed] [Google Scholar]
- 65.Sundrud, M. S., V. J. Torres, D. Unutmaz, and T. L. Cover. 2004. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proc. Natl. Acad. Sci. USA 101:7727-7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valle, J., M. Kekki, P. Sipponen, T. Ihamaki, and M. Siurala. 1996. Long-term course and consequences of Helicobacter pylori gastritis. Results of a 32-year follow-up study. Scand. J. Gastroenterol. 31:546-550. [DOI] [PubMed] [Google Scholar]
- 67.Van der Heijden, P. J., and W. Stok. 1987. Improved procedure for the isolation of functionally active lymphoid cells from the murine intestine. J. Immunol. Methods 103:161-167. [DOI] [PubMed] [Google Scholar]
- 68.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Girardin, A. P. Moran, R. Athman, S. Memet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166-1174. [DOI] [PubMed] [Google Scholar]
- 69.Voland, P., N. Hafsi, M. Zeitner, S. Laforsch, H. Wagner, and C. Prinz. 2003. Antigenic properties of HpaA and Omp18, two outer membrane proteins of Helicobacter pylori. Infect. Immun. 71:3837-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamamoto, M., K. Yoshizaki, T. Kishimoto, and H. Ito. 2000. IL-6 is required for the development of Th1 cell-mediated murine colitis. J. Immunol. 164:4878-4882. [DOI] [PubMed] [Google Scholar]
- 71.Yamaoka, Y., M. Kita, T. Kodama, N. Sawai, K. Kashima, and J. Imanishi. 1995. Expression of cytokine mRNA in gastric mucosa with Helicobacter pylori infection. Scand. J. Gastroenterol. 30:1153-1159. [DOI] [PubMed] [Google Scholar]