Abstract
To test the hypothesis that the Staphylococcus aureus enterotoxin gene cluster (egc) can generate new enterotoxin genes by recombination, we analyzed the egc locus in a broad panel of 666 clinical isolates of S. aureus. egc was present in 63% of isolates, confirming its high prevalence. The archetypal organization of the egc locus, consisting of five enterotoxin genes plus two pseudogenes, was found in 409 of 421 egc-positive strains. The egc locus was incomplete in a few strains and occasionally harbored an insertion sequence and transposase genes. These strains may represent evolutionary intermediates of the egc locus. One strain with an atypical egc locus produced two new enterotoxins, designated SElV and SElU2, generated by (i) recombination between selm and sei, producing selv, and (ii) a limited deletion in the ϕent1-ϕent2 pseudogenes, producing selu2. Recombinant SElV and SElU2 had superantigen activity, as they specifically activated the T-cell families Vβ 6, Vβ 18, and Vβ 21 (SElV) and Vβ 13.2 and Vβ 14 (SElU2). Immunoscope analysis showed a Gaussian CDR3 size distribution of T-cell receptor Vβ chain junctional transcripts of expanded Vβ subsets in toxin-stimulated cultures, reflecting a high level of polyclonality. These data show that egc is indeed capable of generating new superantigen genes through recombination.
Staphylococcus aureus produces a large variety of exotoxins, including staphylococcal enterotoxins A to E (SEA to SEE), SEG to SER, and SEU; staphylococcal enterotoxin-like toxins (SEls); and toxic shock syndrome (TSS) toxin-1 (5, 23). These toxins are responsible for specific acute clinical syndromes such as TSS (due to both TSS toxin-1 SEs and SEls), food poisoning (due to SEs), and staphylococcal scarlet fever (considered a mild form of TSS) (10, 26, 34).
All these toxins share certain structural and biological properties, suggesting that they derive from a common ancestor (16, 21). They exhibit superantigen activity, stimulating polyclonal T-cell proliferation through coligation between major histocompatibility complex class II molecules on antigen-presenting cells (APC) and the variable portion of the T-cell antigen receptor β chain or α chain (TCR Vβ and TCR Vα, respectively), with no need for prior APC processing (4, 13, 21, 22, 37, 39). The pattern of Vβ/Vα activation is specific to each superantigen (4, 12). T-cell/APC activation by these toxins leads to the release of various cytokines/lymphokines and interferon, enhances endotoxic shock, and causes T- and B-cell immunosuppression, all of which may undermine the immune response against bacterial infection (5, 10, 25).
All the genes encoding these toxins are harbored by mobile elements, including bacteriophages, pathogenicity islands, genomic islands, and plasmids (10, 20, 28, 36). Only the enterotoxin gene cluster (egc) is organized as an operon, consisting of two enterotoxin genes (seg and sei), three enterotoxin-like genes with proven superantigenic activity but not emetic properties (selo, selm and seln), and two pseudogenes (ϕent1 and -2). This organization suggests that egc arose through gene duplication and variation from an ancestral gene and that gene recombination created variant toxins with different biological activities (7, 12). SEs and SEls can be divided into three phylogroups, each of which contains one or more egc-encoded toxins, suggesting that all SEs and SEls potentially derive from the egc locus. Several allelic variants of egc toxin in clinical, animal, and food isolates of S. aureus have been described. At least four SEG variants and three SEI variants have been described, and most bear key amino acids involved in TCR and major histocompatibility complex interactions (3, 6, 12, 15, 27). Letertre et al. have described a new SE-like toxin, designated SEU, that apparently arises from fusion between ϕent1 and ϕent2, which itself results from a 15-nucleotide (nt) deletion in ϕent1 (15). Note that SEU should be designated “SElU,” as its emetic properties have not yet been demonstrated.
The aim of the present study was to further examine the possibility that egc can generate new enterotoxin genes by recombination and that the new enterotoxins thereby have superantigen activity. For this purpose we analyzed the egc locus in a broad collection of clinical isolates for signs of evolutionary intermediates and new egc toxins. The activities of the new recombinant enterotoxins were investigated.
MATERIALS AND METHODS
Bacteria and culture.
A panel of 666 S. aureus clinical isolates were selected from the collection of the French National Reference Center for Staphylococci (Lyon, France) between 2001 and 2003. They had been isolated from patients with suppurative infections (arthritis, skin infection, pneumonia, or infective endocarditis), acute toxemia (TSS, staphylococcal scalded skin syndrome, or staphylococcal scarlet fever), or asymptomatic nasal carriage. All isolates were identified as S. aureus by their ability to coagulate citrated rabbit plasma (Pasteurex-Staph-Plus; Sanofi Diagnostics Pasteur GmbH, Freiburg, Germany) and their catalase and DNase activities (Toluidine; Bio-Rad, Marnes-la-Coquette, France). S. aureus strain A900322 was used as the egc+ reference strain (sea−, seb−, sec−, sed−, see−, seg+, seh−, sei+, selm+, seln+, selo+, ϕent1+, ϕent2+, selp+, lukD/E+) (12). S. aureus strain RN4220 was used as a negative control for SE genes.
DNA extraction and purification.
All strains were grown in brain heart infusion at 37°C overnight, and DNA was extracted with the standard phenol-chloroform procedure (35).
PCR detection of egc toxin genes.
Sequences specific for egc toxin genes (selo, selm, sei, seln, and seg) were detected by PCR as previously described (12). Amplification of gyrA was used to confirm the quality of each DNA extract and the absence of PCR inhibitors. The PCR products were resolved by electrophoresis through 0.8% agar gel (Sigma, St. Louis, Missouri). The location of egc in the chromosome was determined by PCR with one primer (seloY) located in egc and another primer (hemY) located 2.3 kb upstream of the egc locus (11).
Cloning and sequencing of egc variants.
The Clontech Genome Walker kit (Ozyme; Montigny-Le-Bretonneux, France) was used to identify the flanking regions in selected egc variants, following the supplier's instructions, with enzymes and specific primers for each strain and target gene (Table 1).
TABLE 1.
Primers used for detection of egc genes and for SElU2 and SElV cloning and sequencing
| Primer | Location within egca | Oligonucleotide sequence (5′→3′)b |
|---|---|---|
| primer5′ | 1-19 | GTC CCG TTA GGA GTC ATA C |
| seo1 | 481-506 | AGT TTG TGT AAG AAG TCA AGT GTA GA |
| seo2 | 630-660 | ATC TTT AAA TTC AGC AGA TAT TCC ATC TAA C |
| seo3 | 427-449 | GCA TTG TTT ACA CTA CAT ATT GC |
| seo4 | 244-270 | CTG TTT GTT CAA TAG TAA GTA GGA TTG |
| seo5 | 557-581 | GTT GAT ACA ATT GAT TTT ACT GTC G |
| invseo2 | 630-660 | GTT AGA TGG AAT ATC TGC TGA ATT TAA AGA T |
| invsem1 | 1785-1811 | GTT CTC CAT TAA CCC AAA GAT TAA TAG |
| sem1 | 1785-1811 | CTA TTA ATC TTT GGG TTA ATG GAG AAC |
| sem2 | 2085-2110 | TTC AGT TTC GAC AGT TTT GTT GTC AT |
| invsei1 | 2260-2281 | CCT ACA CCA ATA TCA CCT TGA G |
| sei1 | 2260-2281 | CTC AAG GTG ATA TTG GTG TAG G |
| sei2 | 2886-2915 | GTT ACT ATC TAC ATA TGA TAT TTCGAC ATC |
| invsei2 | 2886-2915 | GAT GTC GAA ATA TCA TAT GTA GAT AGT AAC |
| yent1 | 3352-3375 | ACG TAG ATT TGT TTG GGA CAA ACT |
| yent2 | 3502-3529 | GTG CTG TTA TGT TTT TCT TAT TAG TAG G |
| invsen1 | 3969-3988 | GAC TCG TCT AAT TGC CAC GT |
| sen1 | 3969-3988 | ACG TGG CAA TTA GAC GAG TC |
| sen2 | 4415-4444 | GAT TGA TCT TGA TGA TTA TGA GAA TGA AAG |
| invsen2 | 4415-4444 | CTT TCA TTC TCA TAA TCA TCA AGA TCA ATC |
| invseg1 | 4979-5003 | CAT TAC ATT ACC CAT AGT TCC CTT A |
| seg1 | 4979-5003 | TAA GGG AAC TAT GGG TAA TGT AAT G |
| seg2 | 5514-5541 | GAA CAA AAG GTA CTA GTT CTT TTT TAG G |
| invseg2 | 5514-5541 | CCT AAA AAA GAA CTA GTA CCT TTT GTT C |
| primer3′ | 6163-6189 | CTT TAA CTT CAT AAA TTA GCA GTA GTC |
| pQE-Fc | CCC GAA AAG TGC CAC CTG | |
| PQE-Rc | GTT CTG AGG TCA TTA CTG G | |
| R-SElU2-1 | CA GGATCC ATG TTA AAT GGC AAT CCT AAA C CA | |
| R-SElU2-2 | GC CTGCAG TTA TTT TTT GGT TAA ATG AAC TTC TAC ATT AAT AGA TTT A | |
| R-SElV 1 | GCA GGATCC GAT GTC GGA GTT TTG AAT CTT AGG | |
| R-SElV 2 | TAA CTGCAG TTA GTT ACT ATC TAC ATA TGA TAT TTC GAC ATC |
Location based on the S. aureus egc sequence (GenBank accession number AFP 285760).
Restriction sites for BamHI and PstI are underlined.
Primer used for sequencing the inserted gene in the pQE-30 vector.
Production and purification of recombinant enterotoxins.
Primers were designed following the identification of suitable hybridization sites in selv and selu2 of strain A900624. DNA was extracted and used as a template for PCR amplification and recombinant protein production.
The 5′ primer was placed in the coding region of selm for selv (R-SElV 1) and of ϕent1 for selu2 (R-SElU2 1), omitting the region predicted to encode the signal peptide (http://www.cbs.dtu.dk/services/SignalP/). The 3′ primer (PstI restriction site) was chosen to overlap the stop codon of seln for selv (R-SElV 2) and of ϕent2 for selu2 (R-SElU2 2) (Table 1; see Fig. 2). PCR products were codigested with appropriate restriction enzymes (Promega, Madison, Wisconsin), purified with the High Pure PCR product purification kit (Boehringer Mannheim, Meylan, France), and ligated (T4 DNA ligase; Boehringer Mannheim, Meylan, France) in the pQE-30 expression vector (QIAGEN) digested with the same restriction enzymes. The resulting plasmid was transformed into Escherichia coli strain M15. Open reading frame (ORF) integrity was verified by sequencing the junction between pQE-30 and the insert. E. coli was grown overnight in Luria-Bertani (LB) broth with appropriate antibiotics (ampicillin, 100 μg/ml; kanamycin, 25 μg/ml). Overnight cultures were transferred (1/10) into 1 liter of LB broth containing antibiotics and were incubated until the optical density at 540 nm reached 0.5 to 0.6. Protein expression was induced by adding 1 mM isopropylthio-β-d-galactoside. After 4 hours, bacterial cells were harvested by centrifugation at 13,000 rpm for 20 min at +4°C. The cell pellets were resuspended in lysis buffer and kept at −20°C overnight. Cell debris were removed by centrifugation at 14,000 rpm for 15 min at +4°C. The recombinant protein (His6 tag) was purified from cell lysates on affinity chromatography columns according to the supplier's instructions (New England Biolabs). The protein content was determined by the Bradford method (12) as modified by Bio-Rad Laboratories (Richmond, California), using bovine serum albumin (Sigma, Steinheim, Germany) as a standard. Protein purity was verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
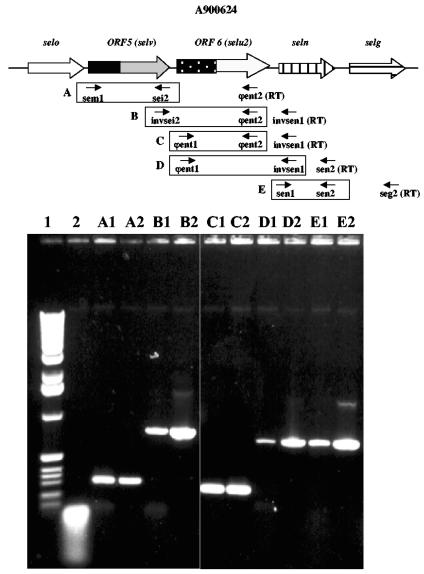
FIG. 2.
Genetic organization of the strain A900624 egc locus in comparison with the archetypal egc locus of reference strain A900322. Black arrows indicate the locations of primers used for the SElV (R-SElV 1 and R-SElV 2) and SElU2 (R-SElU2 1 and R-SElU2 2) recombinant strategy.
T-cell activation assay.
Specific activation of T cells by SElV and SElU2 toxins was measured as previously described (13). Briefly, 50 μl of fresh blood from healthy donors was incubated with 50 μl of RPMI medium, either alone (negative control) or supplemented with each toxin (from 1 μg/ml to 10 ng/ml) or with 10 μg/ml phytohemagglutinin (PHA) (positive control) for 18 h at 37°C in humidified 5% CO2-air. The cell suspension was then treated with ammonium chloride lysis buffer and centrifuged to eliminate erythrocytes. The remaining peripheral blood mononuclear cells (PBMC) were incubated for 20 min at 4°C with anti-CD3, anti-CD4, and anti-CD69 monoclonal antibodies. Activated cells were detected by CD69 staining. The cells were treated with lysis buffer, resuspended in 300 μl of sterile phosphate-buffered saline (Gibco, Invitrogen, Paisley, United Kingdom), and analyzed with a flow cytometer (FACScan; Becton Dickson, Mountain View, CA). Data were analyzed with LYSIS II software.
Flow cytometric analysis of Vβ TCR-triggered PBMC proliferation.
PBMC were isolated from heparinized venous blood of healthy donors by Ficoll density gradient sedimentation (PANCOLL; PAN Biotech GmbH, Aidenbach, Germany). The cells were washed three times in Hanks balanced salt solution (Sigma-Aldrich) and suspended in RPMI 1640 culture medium (Gibco, Invitrogen Corporation) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Invitrogen Corporation), 20 mM HEPES buffer, 2 mM l-glutamine (Sigma-Aldrich), 100 μg/ml streptomycin, and 100 μg/ml penicillin (Gibco-Invitrogen Corporation). Cells (2 × 106 to 5 × 106/ml) were cultured for 3 days with each toxin (500 ng/ml) in 24-well plates (Falcon Becton-Dickinson) and then washed in Hanks balanced salt solution and suspended in culture medium containing increasing concentrations (20 to 100 U/ml) of human interleukin-2 (Eurobio, France) for 12 to 14 days. The Vβ profile was then determined by flow cytometry using the IOTest Beta Mark (Immunotech, Marseille, France), according to the supplier's instructions. The multiparameter data files were analyzed with the Cellquest program (Becton Dickinson). Cells incubated in culture medium or stimulated with PHA (10 μg/ml) were used as negative and positive controls, respectively. For kinetic experiments, cells were cultured for 10 days and the Vβ profile was determined on days 0, 1, 3, 4, 6, and 10 by using the same procedure.
Quantitative Immunoscope analysis.
Total RNA was extracted with the GenElute Total RNA Miniprep kit (Sigma-Aldrich, St. Quentin Fallavier, France) as recommended by the manufacturer. RNA (50 μl) was reverse transcribed with oligo(dT)17 and 400 U of SuperScript II RNase H reverse transcriptase (Invitrogen, Cergy Pontoise, France). An aliquot of cDNA synthesis reaction mix was amplified with each of the 24 TCR Vβ family-specific primers, together with a TCRCβ primer and a fluorochrome-labeled nested oligonucleotide TaqMan probe for TCRC Vβ. Real-time PCR was carried out in an ABI7300 system (Applied Biosystems, Courtaboeuf, France). All quantitative PCRs took place in a total volume of 25 μl with 1× TaqMan buffer (Applied Biosystems), 25 mU of AmpliTaq, 5 mM MgCl2, 0.2 mM deoxynucleoside triphosphates, 400 nM each primer, and 200 nM probe. The thermal cycling conditions consisted of 95°C for 10 min followed by 95°C for 15 seconds and 60°C for 1 min for 40 cycles. The relative usage of each TCR Vβ segment was computed as described elsewhere (17). Two microliters of each amplification product was used as template in a runoff reaction initiated with a nested TCRCβ fluorescent primer in a total volume of 10 μl with 1× polymerase buffer (Promega, Charbonnières, France), 25 mU of Taq DNA polymerase, 3 mM MgCl2, 0.2 mM deoxynucleoside triphosphates, and 0.1 mM nested TCRCβ fluorescent primer. The fluorescent products were then separated and run on an automated 3730 DNA sequencer (Applied Biosystems) and analyzed with Immunoscope software (32) in order to profile the TCR Vβ repertoire.
Detection of bacterial RNA by RT-PCR.
Total RNA was extracted from staphylococcal cultures by using RNeasy spin columns (QIAGEN, Courtaboeuf, France). cDNA was synthesized using Ready-To-Go reverse transcriptase PCR (RT-PCR) beads (Pharmacia Biotech, Orsay, France) by incubating 0.1 μg of total RNA with the following pairs of primers: primer5′ and seo3, seo4 and seo5, seo1 and seo2, invseo2 and invsem1, sem1 and invsei1, sei1 and sei2, invsei2 and yent2, yent1 and invsen1, sen1 and sen2, invsen2 and invseg1, seg1 and seg2, and invseg2 and primer3′ (Table 1). The reaction mixtures were incubated with each primer pair described above at 42°C for 30 min for reverse transcription, followed by 30 cycles of amplification (1 min of denaturation at 94°C, 1 min of annealing at 55°C, and 1 min of extension at 72°C). The RT-PCR products were then analyzed by electrophoresis through a 1% agarose gel. RNA extracts were tested for DNA contamination by preincubating the reaction mixtures at 95°C for 10 min to inactivate reverse transcriptase prior to the RT-PCRs.
RESULTS
Detection of incomplete egc.
Among the 666 isolates tested by PCR for the presence of the egc toxin genes, 421 isolates were positive for at least one gene. The egc was complete (including selo, selm, sei, seln seg, and the two pseudogenes ϕent1 and ϕent2) in 409 isolates and was atypical in 12 isolates. Seven of these 12 atypical isolates harbored five egc toxin genes, one had four, one had three, one had two, and two had one (Table 2). In order to analyze the most common egc variations, we selected for further investigation 6 of these 12 isolates (A950227, A980341, A940440, A900624, LY19991222, and LY1991287), representing each of the above egc variations. The chromosomal location of egc in these six strains was investigated by using a PCR primer annealing to the hemY gene located 2.3 kb upstream of the egc locus and another primer located within seo. PCR was positive with all six strains and with the egc+ control strain, indicating that the egc loci were all located in the same chromosomal region. The complete sequence of each of the genes and their arrangement within the egc locus were determined, and the results were compared with those for the egc locus of the reference strains A900322 and Mu50 (1). Schematic representation of the egc loci in these variants (Fig. 1) showed that the overall organization and sequence of the archetypal egc locus were conserved. However, we observed two types of genetic events that generated variations in the egc locus: (i) two types of insertion sequence (IS) and (ii) new enterotoxin genes generated by homologous recombination between egc genes or pseudogenes.
TABLE 2.
Distribution of the egc genes in the 666 S. aureus isolates
| Category and strain |
egc gene contenta
|
Additional genesb | n (%) | |||||
|---|---|---|---|---|---|---|---|---|
| selo | selm | sei | ent1- ent2 | seln | seg | |||
| Strains with complete egc cluster | + | + | + | + | + | + | 409 (61.4) | |
| Strains with 5 egc genes or pseudogenes | 7 (1.05) | |||||||
| HT20011243 | + | + | + | + | + | − | ND | |
| HT2000480 | + | + | + | + | + | − | ND | |
| LY1991222 | + | + | + | + | + | − | TC | |
| A950227 | + | + | + | + | + | − | TC | |
| LY1990182 | + | + | + | + | + | − | ND | |
| A980341 | + | + | − | + | + | + | TC | |
| LY1991287 | + | + | + | + | − | + | TC | |
| Strain with 4 egc genes or pseudogenes, A950062 | + | − | + | + | + | − | TC | 1 (0.15) |
| Strain with 3 egc genes or pseudogenes, A900624 | + | − | − | − | + | + | selu2, selv | 1 (0.15) |
| Strain with 2 egc genes, A940440 | + | + | − | − | − | − | TC | 1 (0.15) |
| Strains with 1 egc gene | 2 (0.3) | |||||||
| HT2000461 | + | − | − | − | − | − | ND | |
| HT2000458 | + | − | − | − | − | − | ND | |
| Total strains with at least 1 egc gene | 421 (63.2) | |||||||
| Total strains with no egc gene or pseudogene | 245 (36.7) | |||||||
The egc genes are represented in their order of transcription (from left to right). seo was previously designated sel; sen was previously designated sek. The nomenclature for the other genes is unchanged (12).
ND, not determined; TC, transposase cassette.
FIG. 1.
Organization of the egc locus in reference and atypical strains. A900322 (GenBank accession number AFP285760) is the egc reference strain. A900624 harbors two new enterotoxin genes, selu2 and selv. LY19991287, A950227, LY19991222, A980341, and A940440 have an insertion sequence (Rve-like 1-transposase 8 or Rve-like 2-IstB-like). These sequences are shown schematically below the genes into which they are inserted.
Insertion sequences.
The first IS was detected in four strains (Fig. 1) and in three different genes (sei, seln, and seg). It was composed of 0.757 to 1.222 kb of DNA containing two very short terminal inverted repeats and two additional open reading frames (ORF 1 and ORF 2, of 282 and 807 bp, respectively). ORF 1 encoded 93 amino acids (aa) showing 96% sequence identity (91/93 aa) to transposase 8 (Tnase 8) (from the methicillin-resistant S. aureus [MRSA] 252 genome) and is referred to below as transposase 8-like. ORF 2 encoded a 268-aa peptide that had 95% sequence identity (257/268 aa) with MRSA 252 transposase, 96% (182/188 aa) with the truncated transposase from strain Mu50, and 88% (198/224 aa) with the truncated transposase from strain MW2. This protein belonged to the Rve family, which corresponds to the catalytic domains of numerous transposases and is referred to below as Rve-like protein. This cassette (Tnase 8-like/Rve-like) was in the same orientation as the egc operon in strains A950227, LY991222, and A940440 and in the opposite orientation to that in strain LY991287 (Fig. 1). The intergenic sequence of 41 nucleotides between the Tnase 8-like and Rve-like genes was strictly identical in the four strains, while the stretch between the Tnase 8-like gene and the next egc gene varied in sequence and length (11 to 110 nt), as did the stretch between the Rve-like gene and the next egc gene (16 to 17 nt).
A putative −10 and −35 promoter sequence (GATTTT-N3-TATTGT-N27-ATG) was found upstream of the Tnase 8-like start codon in three of the four strains. In addition, the Tnase 8-like and Rve-like genes had appropriate Shine-Dalgarno sequences. In three strains the direct repeats corresponding to the insertion site of the Tnase 8-like/Rve-like cassette were ATTT, AAGG, and CATGAT. These nucleotide motifs were detected 102, 19, and 5 times, respectively, in the egc sequence of the prototype strain (GenBank accession number AFP 285760).
The second type of IS was detected only in strain A980341 (Fig. 1). It was composed of a 2.122-kb DNA sequence containing two terminal inverted repeats of 8 bp and 14 bp and two ORFs (ORF 3 and ORF 4) of 1,197 and 768 bp, respectively. These ORFs encoded 398 aa and 255 aa, respectively. Comparison of the deduced amino acid sequences of these ORFs with translated sequences from GenBank showed that the putative translation product of ORF 3 had 55% identity (220/393 aa) to the putative integrase core domain (Rve) of Enterococcus faecium and to the transposase conserved domain and 54% identity (213/393 aa) to the transposase-like protein of Lactobacillus collinoides. This protein was designated Rve-like 2. The peptide sequence encoded by ORF 4 was homologous to the putative IstB-like ATP-binding protein and the DNA replication protein conserved domain and had 52% identity (127/242) to a transposition helper protein of E. faecium. It was designated IstB-like protein. A putative −10 and −35 promoter sequence (TATTTT-N5-ATGACA-N26-ATG) was found upstream of the Rve-like 2 start codon. In addition, Rve-like 2 and IstB-like had an appropriate Shine-Dalgarno sequence. The direct repeat sequence CTTCA, corresponding to the insertion site of the Rve-like 2/IstB-like cassette, was detected only once in the egc sequence of the prototype strain.
Two new enterotoxin genes generated by egc gene recombination and/or deletion.
Two additional ORFs (ORF 5 and ORF 6) of 720 and 771 bp, respectively, both related to staphylococcal enterotoxins, were found in strain A900624 (Fig. 2). ORF 5 had 99% identity (424/428) to the 5′ end of the selm gene (nt 1 to 419) and almost 100% identity (300/301) to the 3′ end of the sei gene (nt 452 to 730) of the reference strain. Thus, ORF 5 appears to result from a recombination event between selm and sei. Interestingly, a 23-nucleotide sequence (TAGTAACAGCTCAAGAAATTGAT; nt 418 to 441) overlapping the junction region was shared by the selm and sei genes.
Analysis of the deduced amino acid sequence of ORF 5 showed the same features, with 97.2% identity (139/143) of the first 143 aa with the N-terminal part of SElM and 100% identity of aa 144 to 239 with the C-terminal part of SEI. Globally, however, ORF 5 shared only 87.5% amino acid identity with the SEM and less with other SEs/SEls. Based on the recently described nomenclature for staphylococcal superantigens (18), ORF 5 was designated “selv” and the corresponding protein “SE-like V” (SElV).
ORF 6 appears to result from another type of molecular reorganization. Analysis of the DNA sequence showed that the first part of this ORF (nt 1 to 402) had 99.3% identity (399/402) to the ϕent1 pseudogene of strain Mu50 and that the second part (nt 376 to 771) had 100% identity to the ϕent2 pseudogene of the same strain. As with ORF 5, the junction region of ORF 6 corresponded to a 25-nt conserved sequence between the 3′ end of ϕent1 and the 5′ end of ϕent2 (Fig. 2). ORF 6 contained a single adenine deletion (A 365) very close to the end of ϕent1, which abolished the ochre stop codon present in the end of ϕent1. This generated a 256-aa translation product corresponding to fusion of ϕent1 and ϕent2. Analysis of the deduced amino acid sequence showed strong similarity with SElU (94% identity) and moderate similarity with SEC1 and SEC3 (53% identity). The gene was designated selu2 and its product SElU2. The putative Shine-Dalgarno sequences of selu2 and selv are, respectively, TGGAGT and GGAGAA. To verify that ORFs 5 and 6 correspond to transcribed genes in strain A900624, RT-PCR analyses using primers specific for these two genes were performed. In both cases a positive RT-PCR signal was obtained and the signal intensity was comparable to that of sen (Fig. 3).
FIG. 3.
Analysis of selv and selu2 transcripts by RT-PCR. cDNA was prepared from S. aureus A900624 total RNA using the RT primers shown (RT), followed by PCR using primer pairs A to E (boxed). Lanes A to E, results obtained using the corresponding primer pairs. Lane 1, 1-kb molecular size marker; lane 2, RT-PCR negative control (heat inactivation of reverse transcriptase); lanes A1 to E1, RT-PCR from extract of A900624; lanes A2 to E2, PCR positive control (A900624 DNA as template).
Superantigen activities of recombinant SElV and SElU2.
Purified recombinant SElV and SElU2 expressed in E. coli were first studied for their ability to induce T-cell activation in the CD69 assay (19). When incubated overnight with PBMC, both toxins induced concentration-dependent CD69 expression on CD3+ T cells, with the proportions of CD3+ CD69+ cells ranging from 1.5% to 11.8% (not shown) (PHA [48.6%] and RPMI medium [0.5%] were used as positive and negative controls, respectively).
The toxins were then examined for their ability to induce TCR Vβ-selective expansion of T cells in 12-day PBMC culture. As shown in Table 3, SElU2 induced selective expansion of Vβ 14 and Vβ 13.2 T-cell subsets, while SElV induced Vβ 18 and Vβ 21.3 expansion. However, the extent of Vβ 18 expansion varied from one donor to another.
TABLE 3.
FACS and RT-PCR analysis of PBMC from three donors cultured for 12 days in the presence of SE/U2 or SE/V
| Sample | TCR Vβa | % of total CD3+ cells in cultureb for donor:
|
|||||
|---|---|---|---|---|---|---|---|
| A
|
B
|
C
|
|||||
| FACS | RT-PCR | FACS | RT-PCR | FACS | RT-PCR | ||
| Day 0 | 13 | 4.52 | 4.5 | 1.33 | 8.8 | 1.35 | 3.9 |
| 14 | 5 | 1.1 | 1.6 | 0.2 | 0.71 | 0.2 | |
| 18 | 3.1 | 4.6 | 1.6 | 4.9 | 1.3 | 3.6 | |
| 21 | 3.22 | 9.4 | 3.34 | 1.6 | 3.28 | 7.6 | |
| PHAc | 13 | 3.12 | 10.1 | 1.03 | 4.1 | 1.13 | 11.3 |
| 14 | 4.68 | 1.1 | 3.57 | 1.2 | 2.7 | 0.6 | |
| 18 | 0.42 | 6.9 | 0.5 | 9.2 | 0.64 | 4.6 | |
| 21 | 2.22 | 8.6 | 1.4 | 9.8 | 1.84 | 8.1 | |
| SelU2 | 13 | 16.2 | 4.7 | 7.46 | 11.6 | 3.9 | 8.9 |
| 14 | 54.28 | 56.4 | 76.37 | 68.3 | 71.39 | 48.8 | |
| 18 | 0.46 | 0.9 | 0.11 | 0.9 | 0.03 | 0.8 | |
| 21 | 1.28 | 2 | 2,13 | 0.2 | 1.47 | 1 | |
| SelV | 13 | 1.96 | 7 | 1.76 | 9 | 0.67 | 4.3 |
| 14 | 3.9 | 0.1 | 1.23 | 0.6 | 1.9 | 0.3 | |
| 18 | 5.04 | 14.8 | 1.81 | 14.8 | 1.3 | 28.3 | |
| 21 | 5.76 | 30.5 | 7.22 | 37.8 | 5.4 | 40.8 | |
13, Vβ 13.2 in FACS assay and Vβ 13a in RT-PCR quantification assay; 21, Vβ 21.3 in FACS assay and Vβ 21f in RT-PCR quantification assay.
Values in boldface indicate specific expansions.
Four-day PHA stimulation.
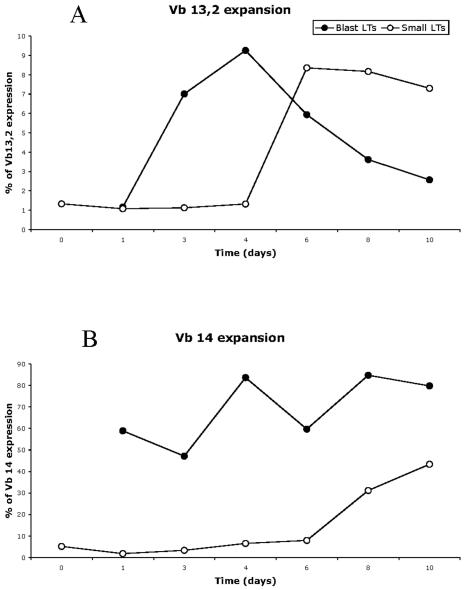
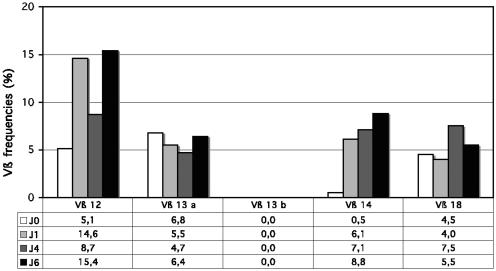
To document the TCR Vβ composition of superantigen-stimulated T cells and the repertoire diversity of the expanded TCR Vβ subsets, the size distribution of PCR-amplified TCR Vβ chain junctional products was studied by using the Immunoscope method (14-16). This method enables the quantification of each of the TCR Vβ usage frequencies within total T cells and the visualization of the TCR third complementary region (CDR3) length distribution for each Vβ-Jβ rearrangement. Therefore, it gives a representation of the diversity of the TCR repertoire. Polyclonal populations show a typical Gaussian distribution of the CDR3 lengths, whereas oligoclonal populations are characterized by the presence of prominent peaks. TCR Vβ quantitative Immunoscope analysis, shown in Fig. 4, confirmed that SElV triggered the expansion of the Vβ 18 and Vβ 21 T-cell subsets. In addition, it showed the expansion of Vβ 6b cells, a subset which is not covered by the IOTest Beta Mark method. As shown in Table 3, TCR Vβ quantitative Immunoscope analysis showed, for all the donors, a constant and significant expansion of Vβ 18-expressing T cells following stimulation with SElV. This was confirmed by fluorescence-activated cell sorter (FACS) analysis for donor A but hardly for donors B and C. Based on the clear-cut RT-PCR data, we believe that this apparent discrepancy is linked to the poor reactivity of the Vβ 18-specific antibody included in the IOTest. Regarding SElU2, Immunoscope analysis confirmed the expansion of Vβ 14 cells but not of Vβ 13 cells. To investigate this apparent discrepancy, we performed early kinetic studies (from day 1 to day 10 of stimulation with SEIU2), in which FACS analyses were carried out on both small lymphocytes and blasts (gated according to forward scatter/side scatter criteria). As shown in Fig. 5, SEIU2 triggered the expansion of Vβ 13.2 cells, as Vβ 13.2 blasts were detected from day 3 to day 10, with a peak at day 4, while Vβ 13.2 small lymphocytes were detected later (day 6 to at least day 10). These kinetic studies also confirmed that Vβ 14 cells were triggered by SEIU2, as BLAST expansion was detected after as little as 1 day of stimulation, while small-lymphocyte expansion was detected only on day 8. It is noteworthy that both the kinetics and the proportions of Vβ 14 and Vβ 13 expansion triggered by SEIU2 were markedly different, with the proportion of the Vβ 13 BLAST cells being almost nine times smaller than the proportion of the Vβ 14 cells (9% versus 80% in this experiment). Similar kinetic experiments were performed for TCR Vβ quantitative Immunoscope analysis (Fig. 6). Vβ 14 expansion was detected from day 1 (6.1%, versus 0.5% at day 0) and was still high on day 6 (8.8%). In contrast, no specific Vβ 13a or Vβ 13b expansion was detected.
FIG. 4.
TCR Vβ repertoire analysis of superantigen-stimulated PBMC by the Immunoscope approach. (A) Quantitative Vβ repertoire determined by real-time PCR analysis on day 0 (D0) and day 14 (D14) following stimulation with SElV or SElU2. The x axis indicates Vβ families and the y axis their relative frequency of usage. Selective Vβ expansion was considered to occur when the D14/D0 Vβ frequency ratio was >3. (B) Immunoscope profiles of the fluorescent Vβ-Cβ runoff products obtained with 14-day-stimulated mononuclear cells. The x axis indicates the CDR3 length. Only profiles corresponding to Vβ expansion are shown.
FIG. 5.
Flow cytometric kinetic analysis of T cell stimulation by SElU2. Time courses of Vβ 13.2 (A) and Vβ 14 (B) expression in blast cells (closed circles) and small cells (open circles), identified with forward scatter/side scatter criteria, after stimulation of PBMC with 500 ng/ml SElU2 are shown. Vβ expansion was determined by flow cytometry. Data are percentages of CD3 T cells expressing the corresponding Vβ chains.
FIG. 6.
Immunoscope analysis of the time course of T-cell stimulation by SElU2. The quantitative Vβ repertoire determined with real-time PCR analysis on days 0, 1, 4, and 6 following stimulation with SElU2 is shown. The x axis indicates Vβ families and the y axis their relative frequency of usage.
Immunoscope analysis of the CDR3 size distribution of TCR Vβ chain junctional transcripts within expanded Vβ subsets showed Gaussian profiles in all toxin-stimulated cultures, reflecting a high level of polyclonality (Fig. 4). This was further confirmed by sequence analysis of TCR Vβ junctional transcripts derived from some expanded TCR Vβ subsets (not shown). Taken together, these TCR Vβ repertoire studies confirmed the superantigenic nature of the two new toxins described here.
DISCUSSION
The aim of this study was to test our hypothesis that the S. aureus egc locus is a “superantigen nursery.” We obtained two major lines of evidence in support of this hypothesis: (i) some strains harbored an incomplete egc and possibly correspond to evolutionary intermediates. and (ii) we identified two new enterotoxins that apparently arise from egc gene recombination events.
Analysis of the egc locus in a panel of 666 strains confirmed that egc is highly prevalent (63%) in S. aureus strains from nasal colonization, suppurative infections, and acute toxemia (2, 8, 12, 24, 29). As shown in other studies, the classical organization of the egc locus, i.e., the five enterotoxin genes plus two pseudogenes, was most frequent (409 of 421 egc-positive strains) (Table 2), suggesting that this organization is currently the most successful. However, the egc locus of a few strains contained the egc promoter and the first gene (selo) but lacked one or more of the downstream genes of the operon, suggesting that these strains might correspond to evolutionary intermediates (Table 2). Unlike Omoe et al., we found no strains with a nonarchetypal egc operon that lacked the selo gene (29).
How might this tandem duplication and recombination occur? In five strains, two different insertion sequences were always inserted in the middle of an egc gene (Fig. 1). These ISs were typical, being composed of two ORFs encoding a transposase and a transposase helper protein, flanked by short terminal inverted repeat sequences. The transposases observed in our strains are related to Tnase 8-Rve or to IstB-Rve. Tnase 8 is a member of the IS3 family, which consists of various E. coli insertion elements and other bacterial transposases. Tnase 8 is required for efficient DNA transposition and could be involved in DNA replication, recombination, and repair. Proteins of the Rve family are described as integrase core domains, in that they have a catalytic integrase domain function. Insertion sequences have been found in the MRSA 252 genome (9) and in the staphylococcal cassette chromosome SCCmec type IVc of S. aureus strain MR 108 (11), in which their putative function is to facilitate mutations by transpositional mutagenesis. Thus, the five strains carrying an IS might represent evolutionary intermediates of the contemporary egc operon. In the same way, these insertion sequences might be involved in egc toxin gene export. Indeed, during transposition, they could integrate within an adjacent gene and transfer an egc toxin gene to a region outside the egc locus; this is the case for seg2, which is encoded by phage φ SA3 in strains MW2 and MSSA 476 (1).
A similar model of genetic variation involving tandem duplication and recombination, associated with the IS/transposase cassette, has been proposed for the DR13 locus. DR13 possesses various combinations of genes encoding staphylococcal exotoxin-like proteins (set). As in egc, all the set genes are similar to one another, suggesting that they were generated by successive duplication and variation of an ancestral gene. By sequence analysis of the DR13 region, Fitzgerald et al. showed that the DR13 locus could contain from 5 to 11 set genes (7). Interestingly, those authors identified a transposase gene downstream of the DR13 locus. Genetic analyses confirmed that all the set genes had a common ancestor and that multiple events of gene acquisition, duplication, recombination, and/or loss had led to the diversification of the DR13 locus.
A major result of our study is the identification, in one strain, of two new enterotoxins encoded by the egc locus and generated by (i) recombination between selm and sei, producing selv, and (ii) a limited deletion in the ϕent1-ϕent2 pseudogenes, producing selu2. We reconstructed the selv gene in silico by concatenation of the first 442 nucleotides of selm with nucleotides 451 to 730 of sei. Interestingly, we identified a 23-nucleotide sequence present in both the selm and sei genes, representing a potential template for recombination. This indicates that recombination between two toxin genes may lead to the emergence of new toxins in S. aureus. RT-PCR analysis showed that these two new toxin genes are transcribed at a level similar to that of other egc genes such as sen (Fig. 3). The biological activity of SElV is consistent with that of a superantigen, specifically triggering T-cell subsets of the Vβ 6, Vβ 18, and Vβ 21 families. This pattern of activation is different from that reported for SElM (6, 8, 9, 18 and 21.3) and SEI (1, 5.1, 5.3, and 23), in keeping with reports that slight amino acid sequence differences between members of the pyrogenic family can result in markedly altered biological properties (38). The selu2 gene likely results from a simple deletion at the 3′ end of the ϕent1 gene. The deletion abolishes the stop codon of ϕent1 and restores an open reading frame extending from the beginning of ϕent1 to the end of ϕent2. Letertre et al. described a similar mechanism generating the selu gene (15). However, selu and selu2 differ slightly in their nucleotide sequences, notably by the presence of an additional 15-nucleotide sequence at position 206 of the selu gene. As these 15 nucleotides are present in the ϕent1 gene of the archetypal egc locus of strain A900322 (12) but are missing from the ϕent1 gene of strain MU50 (14), selu would appear to derive from ϕent1-ϕent2 in strain A900322, while selu2 would derive from ϕent1-ϕent2 in strain MU50. The superantigenic activity of SElU2 was demonstrated by specific activation of Vβ 13.2 and Vβ 14 T cell subsets. In contrast to SElV, for which both flow cytometric analysis and the TCR Vβ quantitative Immunoscope approach showed Vβ 6, 18, and 21 T-cell expansion (with the exception of Vβ 6, which is not covered by the IOtest BetaMark), no SEIU2-dependent triggering of Vβ 13 T cells was detected by the Immunoscope method. This could have been due to the broad specificity of the primer used to detect the Vβ 13a family (it amplifies Vβ 13.1, 13.2, 13.3, 13.4, 13.6, 13.7, 13.8, and 13.9), whereas the antibody used for flow cytometry detected only the Vβ 13.2 subset. Therefore, unless a Vβ 13.2-specific primer is used, the mild Vβ 13.2 T-cell expansion (fewer than 10% of CD3 T cells) cannot be detected by RT-PCR among total Vβ 13 cells. Selective toxin stimulation of a Vβ subset within a Vβ family has previously been reported with recombinant SElP, which stimulates Vβ 5.1 and no other Vβ 5 subset (Vβ 5.2 or 5.3) (30), and with recombinant SElR, which selectively stimulates Vβ 13.2 cells within the Vβ 13 family (31). In addition to the selective expansion of TCR Vβ subsets observed with the different toxins, Immunoscope analysis revealed that the CDR3 size distribution of TCR β-chain junctional transcripts within expanded Vβ subsets was Gaussian, reflecting a high level of polyclonality (Fig. 4).
The widespread distribution of the archetypal egc organization in strains from various genetic backgrounds (12, 33) and its high prevalence in S. aureus strains of various clinical origins suggest that this organization confers an optimal selective advantage. The single strain that was shown here to produce two new superantigens, SElV (Vβ 6, 18, and 21.3) and SElU2 (Vβ 13.2 and 14) but that lacked SElM (Vβ 6, 8, 9, 18, and 21.3) and SEI (Vβ 1, 5.1, 5.2, and 5.3), stimulated different and, in theory, fewer Vβ subsets than archetypal egc strains; it is thus tempting to speculate that this strain might be less efficient at stimulating polyclonal T-cell proliferation. This egc variant might be counterselective, possibly explaining its rarity. Conversely, it is possible that ongoing variations within the egc locus, illustrated by strains carrying the insertion sequence, may produce new superantigen variants that could extend the stimulated Vβ subset and thereby supersede strains possessing the classical egc locus.
Acknowledgments
We thank Evelyne Jouvin-Marche and Marc Bonneville for their scientific advice. We thank Annick Lim, Cedric Badiou, Nicole Viollant, Christine Courtier, Christine Cardon, and Florence Couzon for their technical advice and David Young for editorial guidance.
D.Y.T. was supported by a Ph.D. grant from the French Ministry of Research.
Editor: J. T. Barbieri
REFERENCES
- 1.Baba, T., F. Takeuchi, M. Kuroda, H. Yuzawa, K. Aoki, A. Oguchi, Y. Nagai, N. Iwama, K. Asano, T. Naimi, H. Kuroda, L. Cui, K. Yamamoto, and K. Hiramatsu. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359:1819-1827. [DOI] [PubMed] [Google Scholar]
- 2.Becker, K., A. W. Friedrich, G. Lubritz, M. Weilert, G. Peters, and C. Von Eiff. 2003. Prevalence of genes encoding pyrogenic toxin superantigens and exfoliative toxins among strains of Staphylococcus aureus isolated from blood and nasal specimens. J. Clin. Microbiol. 41:1434-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaiotta, G., D. Ercolini, C. Pennacchia, V. Fusco, A. Casaburi, O. Pepe, and F. Villani. 2004. PCR detection of staphylococcal enterotoxin genes in Staphylococcus spp. strains isolated from meat and dairy products. Evidence for new variants of seG and seI in S. aureus AB-8802. J. Appl. Microbiol. 97:719-730. [DOI] [PubMed] [Google Scholar]
- 4.Choi, Y. W., B. Kotzin, L. Herron, J. Callahan, P. Marrack, and J. Kappler. 1989. Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc. Natl. Acad. Sci. USA 86:8941-8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinges, M. M., P. M. Orwin, and P. M. Schlievert. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez, M. M., M. C. De Marzi, P. Berguer, D. Burzyn, R. J. Langley, I. Piazzon, R. A. Mariuzza, and E. L. Malchiodi. 2006. Binding of natural variants of staphylococcal superantigens SEG and SEI to TCR and MHC class II molecule. Mol. Immunol. 43:927-938. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald, J. R., S. R. Monday, T. J. Foster, G. A. Bohach, P. J. Hartigan, W. J. Meaney, and C. J. Smyth. 2001. Characterization of a putative pathogenicity island from bovine Staphylococcus aureus encoding multiple superantigens. J. Bacteriol. 183:63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fueyo, J. M., M. C. Mendoza, M. A. Alvarez, and M. C. Martin. 2005. Relationships between toxin gene content and genetic background in nasal carried isolates of Staphylococcus aureus from Asturias, Spain. FEMS Microbiol. Lett. 243:447-454. [DOI] [PubMed] [Google Scholar]
- 9.Holden, M. T., E. J. Feil, J. A. Lindsay, S. J. Peacock, N. P. Day, M. C. Enright, T. J. Foster, C. E. Moore, L. Hurst, R. Atkin, A. Barron, N. Bason, S. D. Bentley, C. Chillingworth, T. Chillingworth, C. Churcher, L. Clark, C. Corton, A. Cronin, J. Doggett, L. Dowd, T. Feltwell, Z. Hance, B. Harris, H. Hauser, S. Holroyd, K. Jagels, K. D. James, N. Lennard, A. Line, R. Mayes, S. Moule, K. Mungall, D. Ormond, M. A. Quail, E. Rabbinowitsch, K. Rutherford, M. Sanders, S. Sharp, M. Simmonds, K. Stevens, S. Whitehead, B. G. Barrell, B. G. Spratt, and J. Parkhill. 2004. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc. Natl. Acad. Sci. USA 101:9786-9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtfreter, S., and B. M. Broker. 2005. Staphylococcal superantigens: do they play a role in sepsis? Arch. Immunol. Ther. Exp. 53:13-27. [PubMed] [Google Scholar]
- 11.Ito, T., K. Okuma, X. X. Ma, H. Yuzawa, and K. Hiramatsu. 2003. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: genomic island SCC. Drug Resist. Update 6:41-52. [DOI] [PubMed] [Google Scholar]
- 12.Jarraud, S., M. A. Peyrat, A. Lim, A. Tristan, M. Bes, C. Mougel, J. Etienne, F. Vandenesch, M. Bonneville, and G. Lina. 2001. egc, a highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J. Immunol. 166:669-677. [DOI] [PubMed] [Google Scholar]
- 13.Kappler, J., B. Kotzin, L. Herron, E. W. Gelfand, R. D. Bigler, A. Boylston, S. Carrel, D. N. Posnett, Y. Choi, and P. Marrack. 1989. V beta-specific stimulation of human T cells by staphylococcal toxins. Science 244:811-813. [DOI] [PubMed] [Google Scholar]
- 14.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, H. Murakami, A. Hosoyama, Y. Mizutani-Ui, N. K. Takahashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Furuya, C. Yoshino, T. Shiba, M. Hattori, N. Ogasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 15.Letertre, C., S. Perelle, F. Dilasser, and P. Fach. 2003. Identification of a new putative enterotoxin SEU encoded by the egc cluster of Staphylococcus aureus. J. Appl. Microbiol. 95:38-43. [DOI] [PubMed] [Google Scholar]
- 16.Li, H., A. Llera, E. L. Malchiodi, and R. A. Mariuzza. 1999. The structural basis of T cell activation by superantigens. Annu. Rev. Immunol. 17:435-466. [DOI] [PubMed] [Google Scholar]
- 17.Lim, A., V. Baron, L. Ferradini, M. Bonneville, P. Kourilsky, and C. Pannetier. 2002. Combination of MHC-peptide multimer-based T cell sorting with the Immunoscope permits sensitive ex vivo quantitation and follow-up of human CD8+ T cell immune responses. J. Immunol. Methods 261:177-194. [DOI] [PubMed] [Google Scholar]
- 18.Lina, G., G. A. Bohach, S. P. Nair, K. Hiramatsu, E. Jouvin-Marche, and R. Mariuzza. 2004. Standard nomenclature for the superantigens expressed by Staphylococcus. J. Infect. Dis. 189:2334-2336. [DOI] [PubMed] [Google Scholar]
- 19.Lina, G., G. Cozon, J. Ferrandiz, T. Greenland, F. Vandenesch, and J. Etienne. 1998. Detection of staphylococcal superantigenic toxins by a CD69-specific cytofluorimetric assay measuring T-cell activation. J. Clin. Microbiol. 36:1042-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindsay, J. A., and M. T. Holden. 2004. Staphylococcus aureus: superbug, super genome? Trends Microbiol. 12:378-385. [DOI] [PubMed] [Google Scholar]
- 21.Marrack, P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248:705-711. [DOI] [PubMed] [Google Scholar]
- 22.McCormick, J. K., T. J. Tripp, A. S. Llera, E. J. Sundberg, M. M. Dinges, R. A. Mariuzza, and P. M. Schlievert. 2003. Functional analysis of the TCR binding domain of toxic shock syndrome toxin-1 predicts further diversity in MHC class II/superantigen/TCR ternary complexes. J. Immunol. 171:1385-1392. [DOI] [PubMed] [Google Scholar]
- 23.McCormick, J. K., J. M. Yarwood, and P. M. Schlievert. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77-104. [DOI] [PubMed] [Google Scholar]
- 24.Mempel, M., G. Lina, M. Hojka, C. Schnopp, H. P. Seidl, T. Schafer, J. Ring, F. Vandenesch, and D. Abeck. 2003. High prevalence of superantigens associated with the egc locus in Staphylococcus aureus isolates from patients with atopic eczema. Eur. J. Clin. Microbiol. Infect. Dis. 22:306-309. [DOI] [PubMed] [Google Scholar]
- 25.Miller, C., J. A. Ragheb, and R. H. Schwartz. 1999. Anergy and cytokine-mediated suppression as distinct superantigen-induced tolerance mechanisms in vivo. J. Exp. Med. 190:53-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller-Alouf, H., C. Carnoy, M. Simonet, and J. E. Alouf. 2001. Superantigen bacterial toxins: state of the art. Toxicon 39:1691-1701. [DOI] [PubMed] [Google Scholar]
- 27.Munson, S. H., M. T. Tremaine, M. J. Betley, and R. A. Welch. 1998. Identification and characterization of staphylococcal enterotoxin types G and I from Staphylococcus aureus. Infect. Immun. 66:3337-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novick, R. P. 2003. Mobile genetic elements and bacterial toxinoses: the superantigen-encoding pathogenicity islands of Staphylococcus aureus. Plasmid 49:93-105. [DOI] [PubMed] [Google Scholar]
- 29.Omoe, K., D. L. Hu, H. Takahashi-Omoe, A. Nakane, and K. Shinagawa. 2005. Comprehensive analysis of classical and newly described staphylococcal superantigenic toxin genes in Staphylococcus aureus isolates. FEMS Microbiol. Lett. 246:191-198. [DOI] [PubMed] [Google Scholar]
- 30.Omoe, K., K. Imanishi, D. L. Hu, H. Kato, Y. Fugane, Y. Abe, S. Hamaoka, Y. Watanabe, A. Nakane, T. Uchiyama, and K. Shinagawa. 2005. Characterization of novel staphylococcal enterotoxin-like toxin type p. Infect. Immun. 73:5540-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omoe, K., K. Imanishi, D. L. Hu, H. Kato, H. Takahashi-Omoe, A. Nakane, T. Uchiyama, and K. Shinagawa. 2004. Biological properties of staphylococcal enterotoxin-like toxin type R. Infect. Immun. 72:3664-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pannetier, C., M. Cochet, S. Darche, A. Casrouge, M. Zoller, and P. Kourilsky. 1993. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc. Natl. Acad. Sci. USA 90:4319-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peacock, S. J., C. E. Moore, A. Justice, M. Kantzanou, L. Story, K. Mackie, G. O'Neill, and N. P. Day. 2002. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect. Immun. 70:4987-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proft, T., and J. D. Fraser. 2003. Bacterial superantigens. Clin. Exp. Immunol. 133:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 36.Schmidt, H., and M. Hensel. 2004. Pathogenicity islands in bacterial pathogenesis. Clin. Microbiol. Rev. 17:14-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White, J., A. Herman, A. M. Pullen, R. Kubo, J. W. Kappler, and P. Marrack. 1989. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell 56:27-35. [DOI] [PubMed] [Google Scholar]
- 38.Williams, R. J., J. M. Ward, B. Henderson, S. Poole, B. P. O'Hara, M. Wilson, and S. P. Nair. 2000. Identification of a novel gene cluster encoding staphylococcal exotoxin-like proteins: characterization of the prototypic gene and its protein product, SET1. Infect. Immun. 68:4407-4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yagi, J., J. Baron, S. Buxser, and C. A. Janeway Jr. 1990. Bacterial proteins that mediate the association of a defined subset of T cell receptor:CD4 complexes with class II MHC. J. Immunol. 144:892-901. [PubMed] [Google Scholar]