Abstract
Escherichia coli is the most common cause of community-acquired urinary tract infection (UTI). During murine cystitis, uropathogenic E. coli (UPEC) utilizes type 1 pili to bind and invade superficial bladder epithelial cells. UPEC then replicates within to form intracellular bacterial communities (IBCs), a process whose genetic determinants are as yet undefined. In this study, we investigated the role of SurA in the UPEC pathogenic cascade. SurA is a periplasmic prolyl isomerase/chaperone that facilitates outer membrane protein biogenesis and pilus assembly in E. coli. Invasion into bladder epithelial cells was disproportionately reduced when surA was genetically disrupted in the UPEC strain UTI89, demonstrating that binding alone is not sufficient for invasion. In a murine cystitis model, UTI89 surA::kan was unable to persist in the urinary tract. Complementation of UTI89 surA::kan with a plasmid (pDH15) containing surA under the control of an arabinose-inducible promoter restored in vivo binding and invasion events. However, the absence of arabinose within the mouse bladder resulted in depletion of SurA after invasion of the bacteria into the superficial epithelial cells. Under these conditions, invasion by UTI89/pDH15 surA::kan was normal, but in contrast to UTI89, UTI89/pDH15 surA::kan formed intracellular collections that contained fewer bacteria, were loosely organized, and lacked the normal transition to a densely packed, coccoid morphology. Our data argue that SurA is required within bladder epithelial cells for UPEC to undergo the morphological changes that underlie IBC maturation and completion of the UTI pathogenic cascade.
Urinary tract infections (UTI) represent a significant cause of morbidity and are most frequently caused by uropathogenic Escherichia coli (UPEC) (10). The ability of UPEC to establish infection in the urinary tract is most closely linked to the production of adhesive pili that interact with receptors on urinary epithelial cells. P pili are produced by pyelonephritic strains of UPEC, bind to globoseries glycolipids present on the kidney epithelium, and are critical for the establishment of pyelonephritis (27). Production of type 1 pili is required for UPEC to establish cystitis (11, 14, 29). These fibers bind to mannosylated uroplakin molecules on the surfaces of superficial facet cells of the bladder epithelium, mediating bacterial entry (21-23, 32). Once inside facet cells, UPEC multiplies and forms morphologically distinct organized colonies called intracellular bacterial communities (IBCs), which provide a safe haven from effectors of host immunity. Initially, IBCs are loose collections of rod-shaped bacteria that soon mature into coccoid organisms with a distinct, tightly packed architecture (16). In response to unknown signals, bacteria at the IBC periphery either adopt a filamentous phenotype or detach from the community, flux from facet cells, and reestablish infection in naive epithelial cells, eventually forming a quiescent reservoir undetected by host immune surveillance (24). Type 1 pili are required for the initial binding and invasion events; however, little is known about the molecular basis of IBC maturation subsequent to invasion.
The peptidyl-prolyl isomerases (PPIases) include at least three related families of proteins in eukaryotes and prokaryotes that catalyze the cis-trans conversion of peptidyl proline bonds (30). E. coli K-12 encodes at least four periplasmic enzymes in these families: the cyclophilin PpiA (20), the FK506-binding protein-like isomerase FkpA (13), and two parvulin domain-containing isomerases, SurA and PpiD (7, 8, 19). Growth of E. coli K-12 under laboratory conditions was not affected when all four PPIase genes were inactivated (17). SurA possesses periplasmic chaperone activity that localizes not to its two parvulin-like PPIase domains but to its N-terminal substrate-binding domain (3, 4, 12, 31). The peptide substrates of SurA are not precisely known, but it has been previously shown that mutation in surA in E. coli K-12 results in reduced amounts of OmpA and LamB in the outer membrane (19). In addition, maturation of the type 1 pilus usher FimD is impaired in the surA mutant of E. coli K-12 carrying a plasmid containing the fim operon. FimD is also unstable when expressed from its native chromosomal location in the surA mutant of UPEC strain UTI89 (17).
Recently, it was shown that uropathogenic strains of E. coli are able to suppress bladder epithelial cytokine responses in vitro (15), a property that may facilitate early IBC formation. This effect was possibly related to lipopolysaccharide (LPS) structure, as mutations within the LPS biosynthetic operons rfa and rfb (resulting in lack of O antigen) led to loss of suppression. In addition, inactivation of surA led to abrogation of this anti-inflammatory effect, while its LPS reactivity with O typing antiserum was intact, suggesting that SurA participates in the maturation of an additional surface-expressed or secreted factor responsible for UPEC suppression of cytokine production by epithelial cells.
Here, we investigated the steps in IBC maturation that were supported by SurA. Inactivation of surA in UPEC resulted in deficient binding and invasion of bladder epithelial cells, with a disproportionate effect on invasion. In addition, SurA was shown to be required for intracellular growth and IBC maturation during murine cystitis.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The UPEC strain UTI89 was obtained from a patient with cystitis (24), and construction of the surA mutant was previously described (18). Plasmid pAER1 (kind gift from T. Silhavy) contains the surA coding sequence under the control of the arabinose-inducible ParaBAD promoter (26). Plasmid pDH15 was constructed by MluI excision of the kanamycin resistance gene from pcomGFP (6) followed by ligation into this site of a 2.9-kb araC-ParaBAD-surA fragment from pAER1 flanked by AscI sites. pDH15 retains GFPmut3 under the control of the Ptac promoter. For all assays, bacteria were grown overnight in standing Luria broth culture with appropriate antibiotics at 37°C. Induction of surA from pDH15 was achieved by adding l-arabinose at concentrations up to 0.2%.
In vitro HA, binding, and invasion.
Hemagglutination (HA) assays were performed as described previously (19). Cultured 5637 human bladder epithelial cells (ATCC HTB-9) were obtained from the American Type Culture Collection (Manassas, VA) and grown in RPMI 1640 supplemented with 10% fetal bovine serum (Sigma-Aldrich Co., St. Louis, MO) at 37°C in a humidified atmosphere of 95% air and 5% CO2. Forty-eight hours prior to assay, cells were detached with 0.05% trypsin-0.02% EDTA, centrifuged, resuspended in fresh medium, and allocated to wells of sterile 24-well tissue culture plates. On the day of assay, confluent monolayers were washed once with sterile phosphate-buffered saline (PBS), and fresh medium was applied prior to infection with 107 CFU/ml of the strains indicated in Table 1 and Fig. 1. Quantitative determination of bound and invaded bacteria was performed as previously described (21).
TABLE 1.
Hemagglutination titers
| Strain | HA titer
|
|
|---|---|---|
| No mannose | +2% mannose | |
| UTI89 | 1:512 | 0 |
| UTI89 surA::kan | 1:4a | 0 |
| UTI89/pDH15 surA::kan | 1:256-1:512 | 0 |
Significantly different from wild-type UTI89 (P < 0.001 by paired t test).
FIG. 1.
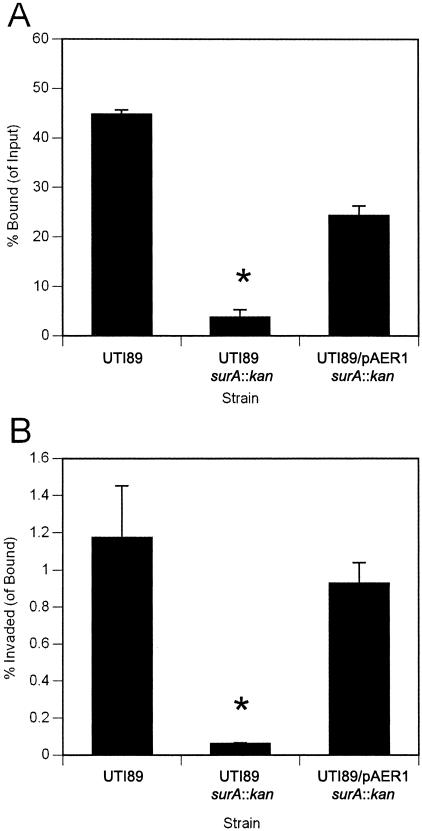
Mutation in surA confers a disproportionate effect on bacterial invasion in vitro. Monolayers of cultured 5637 bladder epithelial cells were infected with wild-type UTI89, UTI89 surA::kan, or UTI89 surA::kan complemented with plasmid pAER1, and bacteria bound (as a proportion of input bacteria) and invaded (as a proportion of bound bacteria) were quantified. The surA mutant demonstrates reduced binding (A) and an additive defect in invasion capacity (B) (*, P < 0.005 versus wild type). Experiments were repeated at least three times with similar results.
Murine cystitis and confocal microscopy.
The murine cystitis model has been described in detail (23). Briefly, 8-week-old female C3H/HeN mice fed arabinose-free chow were anesthetized and transurethrally inoculated with 107 CFU of the UPEC strains listed in Table 1. At the time points indicated in Fig. 4, 5, and 6, mice were sacrificed and bladders were removed in sterile fashion. For assessment of bacterial titers, groups of five mice were infected with each strain. The bladders were homogenized in PBS with 0.1% Triton X-100 and serial dilutions plated onto Luria-Bertani (LB) agar; the lower limit of detection is assessed to be 10 CFU/bladder. For lacZ staining (25), groups of three or four mice were infected with the UPEC strains; bladders were stretched, fixed for 30 min in 10% neutral buffered formalin (NBF), washed with lacZ wash buffer (PBS with 0.01 M MgCl2, 0.01% sodium deoxycholate, and 0.02% Igepal [{octylphenoxy}polyethoxyethanol] CA-640), incubated in lacZ stain (lacZ wash buffer with 1 mg/ml X-Gal [5-bromo-4-chloro-3-indolylphosphate], 5 mM potassium ferrocyanide, and 5 mM potassium ferricyanide) for 16 h, washed three times in PBS, and photographed by light microscopy in whole mount for enumeration of spots representing IBCs. Bladders were then further fixed in 10% NBF overnight and embedded in paraffin. Sections were deparaffinized and stained with hematoxylin and eosin for light microscopy. For confocal microscopy, freshly harvested bladders from experimental groups of three or four mice were stretched under PBS, fixed with 3% paraformaldehyde for 1 h, stained briefly with SYTO 61 red fluorescent nucleic acid stain (Molecular Probes, Eugene, OR), washed five times with PBS, mounted on glass slides under ProLong antifade reagent (Molecular Probes), and then viewed using the LSM510 Meta laser scanning confocal microscope (Carl Zeiss Inc., Thornwood, NY). Experiments were repeated at least three times.
FIG. 4.
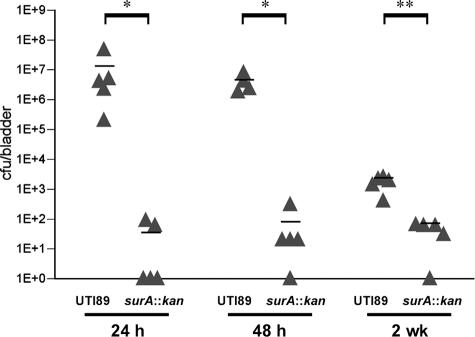
The UPEC surA mutant is deficient in establishing murine cystitis. Mice were infected with the wild type or UTI89 surA::kan and the bladders harvested for titers at the indicated time points. Wild-type UTI89 persists at 106 CFU/bladder during the first 48 h of infection, while UTI89 surA::kan titers fall off and are nearly undetectable at 2 weeks after infection. Titers of UTI89 surA::kan are significantly lower than those of wild-type UTI89 at all time points (*, P < 0.0001; **, P < 0.001). Horizontal bars indicate mean titers. Experiments were repeated at least three times.
FIG. 5.
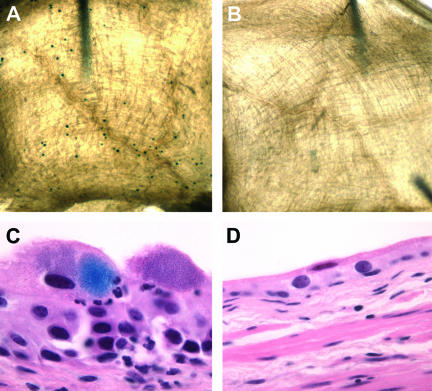
The UPEC surA mutant fails to establish IBCs. C3H/HeN mice were inoculated with wild-type UTI89, UTI89 surA::kan, or UTI89/pDH15 surA::kan; bladders were harvested at 6 h after infection and exposed to an X-Gal-containing substrate (see Materials and Methods). In whole mount, wild-type IBCs are visible as dark blue spots on the bladder surface (A), while UTI89 surA::kan (B) infection yields no such staining. By light microscopy, UTI89-infected bladders contain IBCs; panel C includes an example in which deparaffinization incompletely removed the lacZ stain from an IBC. Bladders infected with UTI89 surA::kan (D) or UTI89/pDH15 surA::kan (not shown) contain no developed IBCs. Experiments were repeated at least three times.
FIG. 6.
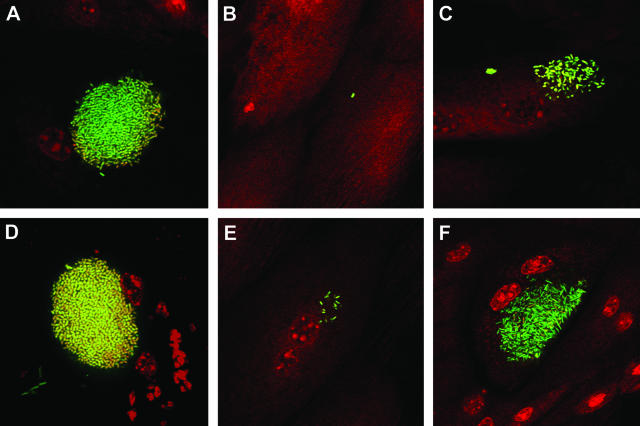
SurA is required for IBC maturation. UTI89/pcomGFP, UTI89/pcomGFP surA::kan, and UTI89/pDH15 surA::kan were used to inoculate C3H/HeN female mice, and the bladders were viewed in whole mount by confocal microscopy at 6 h (top row) and 16 h (bottom row). UTI89 forms early IBCs at 6 h (A) and matures into tightly packed coccoid IBCs by 16 h (D), while the UTI89 surA mutant fails to establish intracellular growth (B). At 16 h, a single collection of ∼20 intracellular bacteria was seen in one of >20 bladders infected with UTI89 surA::kan across several replicated experiments (E). Complementation of type 1 piliation in vitro and binding and invasion in vivo via the plasmid pDH15 permit invasion and modest intracellular replication by 6 h (C), but UTI89/pDH15 surA::kan IBCs do not mature properly by 16 h (F), demonstrating lower density, looser organization, and persistent rod-shaped bacterial morphology compared to wild-type IBCs.
Fluorescence and EM.
For immunofluorescence, bacteria were washed in PBS and incubated for 1 h with mouse antibody raised to the FimH adhesin domain (anti-FimHA) (MedImmune Inc., Gaithersburg, MD). Cells were washed several times in PBS and incubated with AlexaFluor 488-conjugated anti-mouse immunoglobulin G (IgG; Molecular Probes). After additional PBS washes, cells were applied to a glass slide and viewed using the Axioskop 2 fluorescence microscope (Carl Zeiss Inc.). Negative-stain electron microscopy (EM) was performed as described previously (17). For immunogold EM, washed bacteria were adsorbed to Formvar/carbon-coated grids, incubated sequentially with primary anti-FimHA antibody and secondary AlexaFluor 488-conjugated anti-rabbit IgG, and washed twice with distilled water before staining with 1% aqueous uranyl acetate. Samples were viewed on a 1200EX transmission electron microscope (JEOL USA, Peabody, MA).
Ex vivo gentamicin protection assay.
Eight-week-old C3H/HeN female mice (in experimental groups of four to six mice) were inoculated with ∼107 CFU of the strains listed in Table 1 as described above. One hour after infection, mice were sacrificed and bladders were removed in sterile fashion. Bladders were splayed and washed three times in 500 ml sterile PBS each time; these collected washes represent luminal bacteria. Bladders were then incubated for 90 min at 37°C with 100 μg/ml gentamicin to kill adherent extracellular bacteria. After this incubation, bladders were washed three times in 1 ml fresh sterile PBS. Bladders were homogenized in 1 ml PBS with 0.01% Triton X-100 (this lysate, representing invaded bacteria, was termed the intracellular fraction). Undiluted and 100-fold-diluted samples of luminal and intracellular fractions were plated to LB agar and incubated overnight at 37°C for colony enumeration.
RESULTS
Binding and invasion are deficient in surA mutant UPEC.
The ability of UPEC to bind and invade human cultured bladder epithelial cell monolayers is conferred by type 1 pili and, more specifically, by the type 1 tip adhesin FimH (21). Our recent work demonstrated that inactivation of surA in E. coli K-12 or in UPEC resulted in significantly decreased levels of the type 1 pilus usher FimD in the outer membrane (17). We therefore evaluated the in vitro type 1 pilus-dependent functions of the UPEC surA mutant. UTI89 surA::kan demonstrated reduced HA of guinea pig erythrocytes (Table 1); the residual HA was inhibited by the addition of 2% methyl-α-d-mannopyranoside. Correspondingly, the surA mutant exhibited a 10-fold decrease in binding to cultured 5637 bladder epithelial cells. Surprisingly, when internalized bacteria were counted relative to bound bacteria, we found a disproportionate effect on invasion in the surA mutant (Fig. 1). In UTI89 infection, there was one invasion event per 100 bound cells, while infection with UTI89 surA::kan resulted in one invasion event per 1,000 bound cells. These defects in binding and invasion were complemented with an episomal copy of surA under the control of the ParaBAD promoter.
Distribution of FimH adhesin in UTI89 surA::kan.
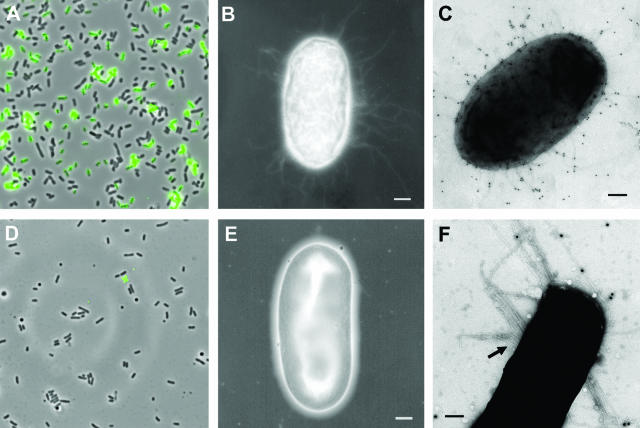
The decreased invasion efficiency of the surA mutant could result from reduced levels of functional pili and/or reflect a requirement for additional SurA-dependent proteins for invasion. In order to evaluate the presence of FimH, bacteria were studied by immunofluorescence microscopy using a mouse antibody raised to the FimH adhesin domain (FimHA). After overnight static growth, a substantial proportion of wild-type bacteria were labeled with anti-FimHA, while labeling of surA mutant bacteria was not detected using this approach (Fig. 2A and D). Next, the density of pili on the bacterial surface was evaluated by negative-stain EM. The majority of wild-type UTI89 bacteria were piliated (Fig. 2B), and immunolabeling with anti-FimHA antibody confirmed that these fibers were type 1 pili (Fig. 2C). By negative-stain EM, the majority of surA mutant bacteria were bald or sparsely piliated (Fig. 2E). In addition, approximately 10% of the bacteria produced distinct, bundled-looking fibers that did not react with the anti-FimHA antiserum (Fig. 2F). The nature of these fibers is under investigation. It is presumed that the sparse residual pili of surA mutant bacteria are responsible for the minimal HA and in vitro binding activity of UTI89 surA::kan. Decreased pilus density may contribute to the additive invasion defect in UTI89 surA::kan; however, the requirement for alternative, SurA-dependent factors that enhance invasion cannot be excluded.
FIG. 2.
Microscopy of surA mutant UPEC. Immunofluorescence of an overnight static culture of UTI89 demonstrates type 1 piliation (A), while UTI89 surA::kan is not labeled with anti-FimHA antibody (D). Negative-stain EM reveals that, in contrast to wild-type UTI89 (B), the majority of UTI89 surA::kan bacteria are nonpiliated (E). Immunogold EM with anti-FimHA antibody identifies type 1 pili on the surface of UTI89 (C) but does not consistently label the thicker, bundled fibers (arrow) expressed by a minority of surA mutant bacteria (F). Scale bars, 200 nm.
UTI89 surA::kan is deficient in establishing murine cystitis.
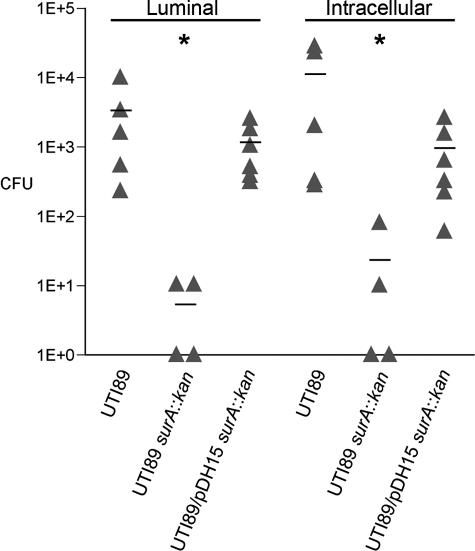
Although type 1 pili are required for binding and invasion of bladder epithelial cells in vivo (18), a role for type 1 pili or any other bacterial protein during IBC maturation (i.e., following invasion) has not been established. In addition, other substrates for SurA may be important in intracellular development. To examine the in vivo requirements for SurA during uropathogenesis, the course of murine cystitis with UTI89 surA::kan was studied. Female C3H/HeN mice were transurethrally inoculated with ∼107 CFU of wild-type UTI89, UTI89 surA::kan, or UTI89 surA::kan complemented with a plasmid carrying surA under the control of the arabinose promoter (pDH15). A gentamicin protection assay was used to verify that pDH15 complemented the binding and invasion defect of UTI89 surA::kan in vivo. Bladders were removed at 1 hour and washed in 1 ml PBS to collect the luminal bacteria. Significant numbers of luminal bacteria were observed in UTI89 and UTI89/pDH15 surA::kan infections, while the surA mutant had been largely eliminated (Fig. 3, luminal). Intracellular bacteria were enumerated following gentamicin treatment and subsequent bladder homogenization. UTI89 and UTI89/pDH15 surA::kan invaded at nearly the same levels, but UTI89 surA::kan was invasion deficient (Fig. 3, intracellular).
FIG. 3.
In vivo invasion is intact in the UTI89 surA mutant complemented with pDH15 in an ex vivo gentamicin protection assay. C3H/HeN female mice were transurethrally inoculated with wild-type UTI89, UTI89 surA::kan, or UTI89/pDH15 surA::kan, and bladders were harvested after 1 h and processed as described in Materials and Methods. Luminal bacteria and invaded bacteria (intracellular) were quantified by serial dilution and plating. UTI89 surA::kan persists poorly in the lumen over 1 h of infection and invades poorly compared to wild-type UTI89 (*, P < 0.005); complementation with pDH15 restores early luminal colonization and invasion to wild-type levels. Experiments were repeated at least three times with similar results.
Because murine tissues lack l-arabinose, the complemented strain UTI89/pDH15 surA::kan was ideally suited for investigation of the requirement for SurA substrates in vivo at time points reflecting intracellular growth, IBC maturation, and bacterial persistence. Bladders were harvested and homogenized in order to enumerate the bacterial load. Wild-type UTI89 colony counts averaged 106 CFU/bladder over the first 48 h of infection and persisted at 2 weeks after infection (Fig. 4). Bladder colony counts of UTI89 surA::kan were significantly lower over the first 48 h, an interval marked by intracellular growth in wild-type infection (16). The surA mutant was nearly eliminated by 2 weeks, further suggesting that substrates for SurA are required for intracellular growth and progression through IBC maturation and establishment of a chronic reservoir. Provision of surA in trans on plasmid pDH15 failed to restore wild-type bladder colony counts at time points >48 h (data not shown). The inability of pDH15 to complement colonization was presumably related either to reduction in plasmid copy number or to in vivo depletion of SurA due to the absence of arabinose in the animal chow and low levels of arabinose uptake by rodent cells (9). Recovered bacteria remained resistant to ampicillin, indicating the plasmid was retained (data not shown).
To determine whether the few recoverable intracellular surA mutant bacteria represented multiple invasion events not proceeding through the IBC cascade or limited IBC formation from a very few invasion events, bladders were visualized microscopically, taking advantage of the observation that UTI89 is lacZ+. To assess the number of IBCs, female C3H/HeN mice were infected transurethrally with UTI89, UTI89 surA::kan, or UTI89/pDH15 surA::kan and mice were sacrificed 6 h after infection. Bladders were harvested aseptically, fixed in 10% neutral buffered formalin, and stained with an X-Gal-containing substrate. Infection with wild-type UTI89 yielded a range of 20 to 210 IBCs per bladder (Fig. 5A), while no IBCs were detected in bladders infected with UTI89 surA::kan (Fig. 5B). Similarly, no IBCs were detected using this method when surA was provided in trans on plasmid pDH15 (data not shown). Histologic examination of the same bladders confirmed the whole-mount lacZ staining results, demonstrating the presence of IBCs after wild-type infection (Fig. 5C), while neither the UTI89 surA::kan-infected (Fig. 5D) nor UTI89/pDH15 surA::kan-infected (not shown) bladders demonstrated IBCs.
SurA is essential for intracellular growth and morphological transition.
UTI89/pDH15 surA::kan bacteria, though successful at initial invasion, failed to progress to mature IBCs as determined by light microscopy. Thus, to dissect the apparent need for SurA substrates during IBC maturation, we examined the morphology of IBCs by fluorescence confocal microscopy. Female C3H/HeN mice were inoculated with UTI89 or UTI89 surA::kan transformed with pcomGFP or UTI89/pDH15 surA::kan, and bladders were harvested and viewed 6 and 16 h after inoculation. When compared to wild-type UTI89, establishment of IBCs was sharply impaired in UTI89 surA::kan (Fig. 6). Consistent with previous studies (2, 16), UTI89 formed early IBCs of loose, rod-shaped bacteria at 6 h, which had progressed to larger collections of tightly packed, coccoid bacteria by 16 h (Fig. 6A and D). No bacteria were observed in most bladders infected with UTI89 surA::kan at either of these time points (not shown). However, a few isolated surface-bound surA mutant bacteria (Fig. 6B) were occasionally seen at 6 h, and in one instance a loose intracellular collection of ∼20 surA mutant bacteria was observed at 16 h (Fig. 6E). Examination of the UTI89/pDH15 surA::kan-infected bladders confirmed a failure to carry out the IBC program. At 6 h, very small loose collections of intracellular UTI89/pDH15 surA::kan bacteria were observed in a small number of superficial facet cells (Fig. 6C). At 16 h, limited intracellular bacterial growth had taken place but bacterial density and morphology were still significantly reduced compared to that seen in wild-type UTI89 infection. Specifically, intracellular collections of UTI89/pDH15 surA::kan bacteria did not assume the tightly packed, coccoid morphology typical of mature wild-type IBCs (compare Fig. 6F to D). Of note, UTI89 surA::kan demonstrated no growth defect in Luria broth culture when compared to the wild type (data not shown).
DISCUSSION
Protein folding in the periplasm of gram-negative bacteria is accomplished via a set of ATP-independent catalysts, including disulfide bond isomerases (e.g., DsbA), pilus chaperones (e.g., PapD), and PPIases. The PPIases include PpiA, PpiD, FkpA, and SurA, all of which display homology to members of immunophilin families in eukaryotes. In E. coli K-12, SurA is unique among the prolyl isomerases in that surA mutants display increased membrane permeability and sensitivity to certain antibiotics and dyes (19, 28). In addition, a surA mutation is synthetically lethal with mutations in genes encoding the chaperone Skp or the periplasmic protease DegP, suggesting that SurA functions in parallel with these chaperones in the periplasm (26). A quadruple mutant in all of the four PPIases in E. coli K-12 remains viable but has a mild growth defect and is unable to assemble pili via the chaperone/usher pathway (17). In this study, we discovered that SurA plays critical roles in the intracellular lifestyle of UTI pathogenesis.
The role of SurA in binding and invading bladder epithelial cells was investigated using a human bladder carcinoma cell line. Consistent with previous studies with E. coli K-12 (17), the chromosomal surA mutation in the clinical isolate UTI89 sharply reduced but did not abolish type 1 piliation. In addition, a small subpopulation of surA mutant bacteria produced thicker, bundled appendages that did not label consistently with anti-FimHA antibody by immunogold EM, suggesting that disruption of surA results in altered pilus regulation. UTI89 surA::kan exhibited a corresponding 10-fold reduction in ability to bind to cultured cells, but, surprisingly, when invaded bacteria were quantified as a proportion of bound bacteria, UTI89 surA::kan showed an additional 10-fold defect in invasion. Thus, FimH-mediated binding to bladder epithelial cells is necessary but may not be sufficient for bacterial invasion. These data argue that either a threshold number of type 1 pili are necessary to mediate invasion or that an alternative substrate for SurA is involved in potentiating invasion.
In a well-characterized model of murine cystitis, UTI89 surA::kan was severely attenuated and was eliminated from the urinary tract by 2 weeks postinfection, in contrast to wild-type UTI89, which forms a persistent intracellular reservoir (24). Using an in vivo gentamicin protection assay, we discovered that UTI89 surA::kan invaded the bladder epithelium 100-fold less efficiently than wild-type UTI89. This reduced level of bacterial invasion, while still detectable, did not lead to IBC formation by UTI89 surA::kan, as determined by whole-mount lacZ staining and by fluorescence microscopy. Complementation of UTI89 surA::kan with pDH15 (surA) restored the early binding and invasion events in vivo; wild-type and UTI89 surA::kan CFU recovered from infected bladders treated with gentamicin were similar at 1 hour postinfection. However, due to the absence of the arabinose inducer within the mouse bladder, surA expression was thought to be lost during the course of UTI89/pDH15 surA::kan infection, and we discovered that IBC formation by UTI89/pDH15 surA::kan was defective. In contrast to wild-type IBCs, the intracellular collections of UTI89/pDH15 surA::kan seen at 6 and 16 h contained fewer bacteria, were loosely organized, and lacked the normal transition to a densely packed, coccoid morphology. Thus, SurA in UPEC acts on substrates that are required for IBC maturation. Further, in vitro growth of UTI89 surA::kan was equivalent to wild type, suggesting that the intracellular environment uniquely requires SurA function for bacterial growth.
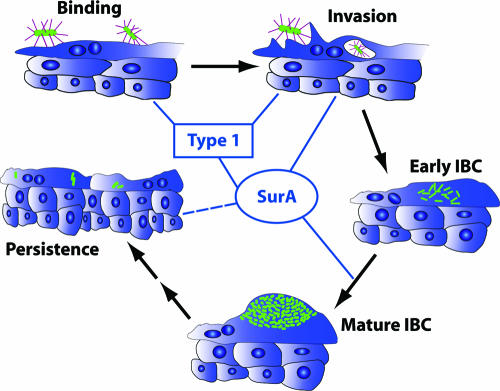
We propose that the conserved periplasmic chaperone SurA supports UPEC virulence and the IBC cascade in multiple ways (Fig. 7). SurA activity clearly underlies type 1 pilus assembly (17) and, in turn, the ability of UPEC to bind to and invade bladder epithelial cells, both in vitro and in vivo. In this study, we have demonstrated that the intracellular phenotype of SurA depletion includes deficient growth, persistent bacillary morphology, and failure of persistence, suggesting that IBC maturation is critical in UTI pathogenesis and requires substrates of SurA. Subsequent to bacterial invasion, it is unclear what role, if any, type 1 pili would play in these processes. The adverse effect of surA disruption on intracellular growth and IBC maturation may result from effects on interbacterial sensing mechanisms, nutrient acquisition pathways, or other surface factors, including the products of a multitude of chaperone-usher pathways found in UPEC (5). Identification of other SurA substrates in UPEC therefore may lead to an understanding of the key proteins involved in IBC maturation and bacterial persistence. For instance, our prior in vitro data suggest that mutation in surA leads to a loss of the anticytokine effect of UPEC on bladder epithelial cells (15). Thus, increased susceptibility to host immune factors may contribute directly to the pathogenic defect in the UTI89 surA mutant, a hypothesis that is currently under further study.
FIG. 7.
Model depicting the contributions of SurA to steps in the UPEC pathogenic cascade. SurA supports binding and invasion of bladder epithelial cells through type 1 pilus assembly. In addition, other SurA substrates may potentiate invasion. SurA activity is required for intracellular growth of UPEC and for maturation of IBCs. Finally, suppression of bladder epithelial cytokines in vitro also requires SurA (15), which may impact bacterial persistence (dashed line).
Our observations point to E. coli SurA as a critical mediator of uropathogenesis via pleiotropic effects on bacterial systems required for establishment of intracellular bacterial communities during murine cystitis. SurA is highly conserved among other gram-negative pathogens (1) and thus may represent an ideal target for inhibition by novel, nonlethal anti-infective compounds. Ongoing studies of the spectrum of SurA substrates and their interactions with this distinctive chaperone at a molecular level promise to further this aim.
Acknowledgments
This work was supported by National Institutes of Health grants F32-DK10168 (S.S.J.); P50-DK64540, R01-DK51406, and R01-AI29549 (S.J.H.); and K08-DK067894 (D.A.H.).
We thank W. Beatty for microscopy, P. Seed for technical advice, and T. Silhavy for helpful discussions.
Editor: A. D. O'Brien
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed] [Google Scholar]
- 3.Behrens, S., R. Maier, H. de Cock, F. X. Schmid, and C. A. Gross. 2001. The SurA periplasmic PPIase lacking its parvulin domains functions in vivo and has chaperone activity. EMBO J. 20:285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bitto, E., and D. B. McKay. 2003. The periplasmic molecular chaperone protein SurA binds a peptide motif that is characteristic of integral outer membrane proteins. J. Biol. Chem. 278:49316-49322. [DOI] [PubMed] [Google Scholar]
- 5.Chen, S. L., C. S. Hung, J. Xu, C. S. Reigstad, V. Magrini, A. Sabo, D. Blasiar, T. Bieri, R. R. Meyer, P. Ozersky, J. R. Armstrong, R. S. Fulton, J. P. Latreille, J. Spieth, T. M. Hooton, E. R. Mardis, S. J. Hultgren, and J. I. Gordon. 2006. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc. Natl. Acad. Sci. USA 103:5977-5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. [DOI] [PubMed] [Google Scholar]
- 7.Dartigalongue, C., and S. Raina. 1998. A new heat-shock gene, ppiD, encodes a peptidyl-prolyl isomerase required for folding of outer membrane proteins in Escherichia coli. EMBO J. 17:3968-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duguay, A. R., and T. J. Silhavy. 2004. Quality control in the bacterial periplasm. Biochim. Biophys. Acta 1694:121-134. [DOI] [PubMed] [Google Scholar]
- 9.Foley, J. E., S. W. Cushman, and L. B. Salans. 1978. Glucose transport in isolated rat adipocytes with measurements of l-arabinose uptake. Am. J. Physiol. 234:E112-E119. [DOI] [PubMed] [Google Scholar]
- 10.Foxman, B., and P. Brown. 2003. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect. Dis. Clin. N. Am. 17:227-241. [DOI] [PubMed] [Google Scholar]
- 11.Gunther, I. N., J. A. Snyder, V. Lockatell, I. Blomfield, D. E. Johnson, and H. L. Mobley. 2002. Assessment of virulence of uropathogenic Escherichia coli type 1 fimbrial mutants in which the invertible element is phase-locked on or off. Infect. Immun. 70:3344-3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hennecke, G., J. Nolte, R. Volkmer-Engert, J. Schneider-Mergener, and S. Behrens. 2005. The periplasmic chaperone SurA exploits two features characteristic of integral outer membrane proteins for selective substrate recognition. J. Biol. Chem. 280:23540-23548. [DOI] [PubMed] [Google Scholar]
- 13.Horne, S. M., and K. D. Young. 1995. Escherichia coli and other species of the Enterobacteriaceae encode a protein similar to the family of Mip-like FK506-binding proteins. Arch. Microbiol. 163:357-365. [DOI] [PubMed] [Google Scholar]
- 14.Hultgren, S. J., T. N. Porter, A. J. Schaeffer, and J. L. Duncan. 1985. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect. Immun. 50:370-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunstad, D. A., S. S. Justice, C. S. Hung, S. R. Lauer, and S. J. Hultgren. 2005. Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect. Immun. 73:3999-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Justice, S. S., C. Hung, J. A. Theriot, D. A. Fletcher, G. G. Anderson, M. J. Footer, and S. J. Hultgren. 2004. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. USA 101:1333-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Justice, S. S., D. A. Hunstad, J. R. Harper, A. R. Duguay, J. S. Pinkner, J. Bann, C. Frieden, T. J. Silhavy, and S. J. Hultgren. 2005. Periplasmic peptidyl prolyl cis-trans isomerases are not essential for viability, but SurA is required for pilus biogenesis in Escherichia coli. J. Bacteriol. 187:7680-7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langermann, S., S. Palaszynski, M. Barnhart, G. Auguste, J. S. Pinkner, J. Burlein, P. Barren, S. Koenig, S. Leath, C. H. Jones, and S. J. Hultgren. 1997. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 276:607-611. [DOI] [PubMed] [Google Scholar]
- 19.Lazar, S. W., and R. Kolter. 1996. SurA assists the folding of Escherichia coli outer membrane proteins. J. Bacteriol. 178:1770-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, J., and C. T. Walsh. 1990. Peptidyl-prolyl cis-trans-isomerase from Escherichia coli: a periplasmic homolog of cyclophilin that is not inhibited by cyclosporin A. Proc. Natl. Acad. Sci. USA 87:4028-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez, J. J., M. A. Mulvey, J. D. Schilling, J. S. Pinkner, and S. J. Hultgren. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19:2803-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min, G., M. Stolz, G. Zhou, F. Liang, P. Sebbel, D. Stoffler, R. Glockshuber, T. T. Sun, U. Aebi, and X. P. Kong. 2002. Localization of uroplakin Ia, the urothelial receptor for bacterial adhesin FimH, on the six inner domains of the 16 nm urothelial plaque particle. J. Mol. Biol. 317:697-706. [DOI] [PubMed] [Google Scholar]
- 23.Mulvey, M. A., Y. S. Lopez-Boado, C. L. Wilson, R. Roth, W. C. Parks, J. Heuser, and S. J. Hultgren. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494-1497. [DOI] [PubMed] [Google Scholar]
- 24.Mulvey, M. A., J. D. Schilling, and S. J. Hultgren. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69:4572-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkinson, R. A., and P. A. Norton. 1997. Expression of the mouse fibronectin gene and fibronectin-lacZ transgenes during somitogenesis. Dev. Dyn. 208:244-254. [DOI] [PubMed] [Google Scholar]
- 26.Rizzitello, A. E., J. R. Harper, and T. J. Silhavy. 2001. Genetic evidence for parallel pathways of chaperone activity in the periplasm of Escherichia coli. J. Bacteriol. 183:6794-6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts, J. A., B. I. Marklund, D. Ilver, D. Haslam, M. B. Kaack, G. Baskin, M. Louis, R. Mollby, J. Winberg, and S. Normark. 1994. The Gal(α1-4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc. Natl. Acad. Sci. USA 91:11889-11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rouviere, P. E., and C. A. Gross. 1996. SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes Dev. 10:3170-3182. [DOI] [PubMed] [Google Scholar]
- 29.Schaeffer, A. J., W. R. Schwan, S. J. Hultgren, and J. L. Duncan. 1987. Relationship of type 1 pilus expression in Escherichia coli to ascending urinary tract infections in mice. Infect. Immun. 55:373-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw, P. E. 2002. Peptidyl-prolyl isomerases: a new twist to transcription. EMBO Rep. 3:521-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb, H. M., L. W. Ruddock, R. J. Marchant, K. Jonas, and P. Klappa. 2001. Interaction of the periplasmic peptidylprolyl cis-trans isomerase SurA with model peptides. The N-terminal region of SurA is essential and sufficient for peptide binding. J. Biol. Chem. 276:45622-45627. [DOI] [PubMed] [Google Scholar]
- 32.Wu, X. R., T. T. Sun, and J. J. Medina. 1996. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins 1a and 1b: relation to urinary tract infections. Proc. Natl. Acad. Sci. USA 93:9630-9635. [DOI] [PMC free article] [PubMed] [Google Scholar]