Abstract
Nedd4 family ubiquitin protein ligases (E3s) specifically associate with latent membrane protein 2A (LMP2A) of Epstein-Barr virus. Our previous studies analyzing LMP2A function in vitro have suggested that Nedd4 family E3s regulate LMP2A function. To determine the role of Nedd4 family E3s in LMP2A B-cell signaling, LMP2A transgenic (LMP2A+) mice were crossed with mice with the Itch-deficient (Itch−/−) background. Itchy, a mouse homologue of human AIP4, is a Nedd4 family E3 and is also the most abundant Nedd4 family E3 found in LMP2A affinity precipitates from B cells. There were significantly fewer B-cell receptor-positive B cells in spleen and bone marrow B cells in LMP2A+ Itch−/− mice than in LMP2A+ mice. In addition, LMP2A+ Itch−/− bone marrow B cells formed larger colonies in cultures treated with interleukin-7 (IL-7) than control bone marrow B cells did. Finally, there was a dramatic increase in tyrosine phosphorylation of LMP2A and Syk in IL-7-cultured LMP2A+ Itch−/− B cells. These results indicate that Nedd4 family E3s, in particular Itchy, downmodulate LMP2A activity in B-cell signaling.
Epstein-Barr virus (EBV) infects primary human B lymphocytes in culture, creating immortalized lymphoblastoid cell lines (LCLs) (for reviews, see references 14 and 15). In EBV-immortalized LCLs, the EBV genome expresses a restricted set of nine viral proteins, two small viral RNAs, and the BamHI A rightward transcripts (for reviews, see references 14 and 15). Six of these latently expressed proteins are Epstein-Barr nuclear antigens (EBNA1, EBNA2, EBNA3A, EBNA3B, EBNA3C, and EBNA-LP), and three are latent membrane proteins (latent membrane protein 1 [LMP1], LMP2A, and LMP2B). EBV can be detected in a subset of nonproliferating memory B cells from EBV-infected healthy individuals (for a review, see reference 25). Studies from these latently infected B cells indicate that LMP2A may be the most readily detected latent mRNA of any of the EBV proteins associated with EBV latent infections (for a review, see reference 25). In addition, LMP2A expression is detected in many of the proliferative disorders associated with EBV infection (for reviews, see references 14, 15, and 25).
LMP2A is a 497-amino-acid type II membrane protein with 12 transmembrane domains (16, 22). The cytoplasmic amino-terminal tail of LMP2A contains phosphorylated tyrosine residues and proline-rich regions that are critical for the ability of LMP2A to interact with a variety of cellular proteins containing specific interactive modular domains, such as SH2 and WW domains. The Src family Lyn protein tyrosine kinase (PTK) binds to tyrosine 112. The Syk PTK binds to the LMP2A immunoreceptor tyrosine-based activation motif (ITAM) at tyrosine 74 and 85 via SH2-phosphotyrosine interactions (7, 8). LMP2A blocks B-cell receptor (BCR)-mediated signal transduction in EBV-immortalized LCLs. These observations have led to a model in which LMP2A maintains viral latency by blocking normal cellular signaling. The association of LMP2A with Src family and Syk PTKs is essential for this activity.
Transgenic mice expressing LMP2A downstream of the immunoglobulin (Ig) μ-heavy-chain enhancer and promoter (Eμ) have highlighted the ability of LMP2A to impart developmental and survival signals to developing B cells despite blocking BCR signal transduction in vitro (1, 2). Normally, B cells lacking a cognate BCR have been shown to rapidly undergo apoptosis (10). In LMP2A transgenic mice, however, LMP2A causes a developmental alteration where the requirement for BCR expression is bypassed, resulting in BCR-negative B cells (1, 2). This ability of LMP2A to drive B-cell development and survival in the absence of normal BCR signals is most dramatically observed when LMP2A transgenic mice are bred with mice with the recombinase-activating gene 1-deficient (RAG-1−/−) background. In this background, LMP2A drives B-cell development, resulting in B cells that are capable of exiting the bone marrow and persisting in the periphery (1, 2). These results suggest that LMP2A may promote B-cell survival or provide a specific signal that allows EBV to gain access to a particular B-cell compartment. Previously, we used our LMP2A transgenic mice to determine the importance of specific cellular proteins for LMP2A function (4, 17, 18). In these studies, a variety of proteins involved in BCR signal transduction that are required for the LMP2A-mediated B-cell developmental and survival signals have been identified. In the present study, the interaction of the Nedd4 family ubiquitin protein ligases (Nedd4 family E3s) in negatively regulating LMP2A function in our LMP2A transgenic model of in vivo EBV infection was investigated.
Recent studies have shown that two proline-rich PY motifs within the LMP2A amino terminus associate with Nedd4 family E3s, including AIP4, WWP2, Nedd4, and Nedd4-2 (11, 26). This interaction causes the ubiquitination and rapid degradation of LMP2A and LMP2A-associated proteins, such as the Lyn PTK (12, 26). LCLs containing mutations in both PY motifs of LMP2A demonstrated hyperphosphorylation of tyrosines in LMP2A and LMP2A-associated Lyn and Syk, suggesting that LMP2A-mediated signaling is regulated by the ubiquitin-proteasome system (13). Itchy is the murine homologue of human AIP4. Mice with a mutation in the itchy locus (Itch−/−) develop a unique spectrum of immunological diseases (20), suggesting that Itchy plays an important role in immune development. Indeed, Itch−/− T cells show an activated phenotype and enhanced proliferation (6). Itch deficiency affects T-cell development and drives T cells to differentiate into T helper type 2 (TH2) cells (6).
In this study, LMP2A transgenic mice were crossed with mice with the Itch−/− background to investigate whether LMP2A developmental and survival signals are regulated by Itchy and the ubiquitin-proteasome system. Flow cytometry of bone marrow cells and splenocytes, in vitro growth of bone marrow B cells, and analysis of phosphorylation in LMP2A and LMP2A+ Itch−/− B cells indicates that Itchy negatively regulates LMP2A function in B lymphocytes.
Generation of LMP2A+ Itch−/− mice.
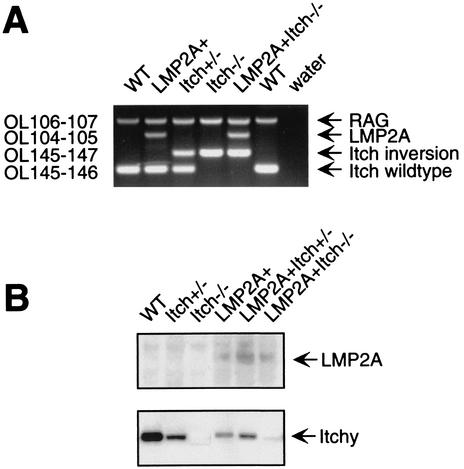
Itch−/− animals bearing a paracentric inversion in the a18H locus (20) were mated to animals of LMP2A transgenic mouse line E (LMP2A+). LMP2A+ and Itch+/− mice and their offspring were specifically bred to generate Itch+/+ (wild-type [WT]), Itch+/−, Itch−/−, LMP2A+, LMP2A+ Itch+/−, and LMP2A+ Itch−/− mice. Genotypes were determined by PCR screening for LMP2A, Itch, and recombinase-activating gene (RAG) sequences as previously described (2) (Fig. 1A). To determine the Itch−/− genotype, primers that amplify sequence across the distal breakpoint in the Itch sequence were used. Absence of Itchy expression was determined in bone marrow and splenic B cells from Itch−/− and LMP2A+ Itch−/− mice by immunoblot analysis (Fig. 1B).
FIG. 1.
LMP2A and Itchy expression in LMP2A+ Itch−/− mice. (A) Genomic tail DNA was prepared as previously described (2). OL104 and OL105 amplify LMP2A-specific sequence (424 bp). OL145 and OL146 amplify germ line sequence from the Itch gene (194 bp). OL145 and OL147 amplify sequence across the distal breakpoint in the Itch sequence (294 bp) (20). OL106 and OL107 amplify control sequence (560 bp) from RAG as a positive control. The sequences of primers are as follows: OL104, AAC ATG GAG GAT TGA GGA CCC ACC; OL105, CGT GTG GCT TAC CTG CTG CCA ATG; OL106, TAC CCT GAG CTT CAG TTC TGC ACC; OL107, TGA CTG TGG GAA CTG CTG AAC TTT; OL145, TCT ATG CTC TGT TGT CTC CCA TGC; and OL146, ATC GTC TAC TCA CCC CAC ATA AGG; OL147 AAG AAG CAG CAG AGA CAA CGA GTG. (B) Immunoblot analysis of LMP2A and Itchy expression in spleen cells from Itch+/+ (WT), Itch+/−, Itch−/−, LMP2A+, LMP2A+ Itch+/−, and LMP2A+ Itch−/− mice were performed with rat anti-LMP2A (14B7) and rabbit anti-AIP4/Itchy antibodies as described previously (13). The positions of LMP2A and Itchy proteins are indicated by arrows.
Itch deficiency augments B-cell developmental alteration in LMP2A+ mice.
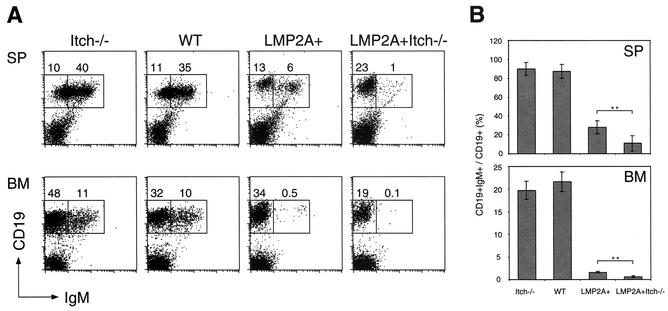
Murine B-cell development occurs in the bone marrow through ordered stages that can be delineated by cell surface markers and the status of Ig heavy-chain (HC) and light-chain (LC) gene rearrangements (10). To examine the effects of Itch deficiency on the alteration in B-cell development induced by LMP2A, flow cytometry analysis of CD19 (a pan-B-cell marker) and surface IgM (equivalent to the BCR) was performed on spleen and bone marrow cells collected from WT, Itch−/−, LMP2A+, and LMP2A+ Itch−/− animals. In WT mice, CD19+ IgM+ cells exit the bone marrow and colonize peripheral immune organs, such as the spleen, as was previously observed in WT animals (Fig. 2A). LMP2A+ animals exhibited a dramatic decrease of CD19+ IgM+ cells in the spleen, as previously characterized (Fig. 2A). Itch−/− animals showed normal B-cell development in the spleen defined by BCR expression compared to WT animals (Fig. 2A). In contrast, splenic CD19+ IgM+ cells were reduced in LMP2A+ Itch−/− animals compared to LMP2A+ animals (Fig. 2A). These results are summarized in Fig. 2B, are statistically significant, and were confirmed in multiple litters. Actual numbers of total splenic B cells were similar in LMP2A+ (1.6 × 107 ± 0.3 × 107 [n = 4]) and LMP2A+ Itch−/− (1.3 × 107 ± 0.4 × 107 [n = 4]) mice, which rules out the possibility of Itch−/− influencing splenic cell number. As a further verification of the similarity of differentiation status of the LMP2A+ and LMP2A+ Itch−/− mice, we found that both did not express IgD showing a similar immature-like differentiation stage (data not shown). To further confirm these results, bone marrow B cells from LMP2A+ Itch−/− animals were compared to those from LMP2A+ animals (Fig. 2B). Compatible with the results observed in splenic B cells, there was a slight but statistically significant reduction of CD19+ IgM+ cells in bone marrow B cells from LMP2A+ Itch−/− mice compared to LMP2A+ mice, indicating that the LMP2A phenotype was more severe in the absence of Itchy.
FIG. 2.
Decreased CD19+ IgM+ B cells in LMP2A+ Itch−/− mice. (A) Spleen (SP) and bone marrow (BM) cells prepared from mice were immunostained with phycoerythrin-conjugated CD19 and fluorescein isothiocyanate-conjugated IgM antibodies for flow cytometry as previously described (2). Inset boxes represent CD19+ IgM− (left) and CD19+ IgM+ (right) cells. The percentages of these cells within the regions are indicated. (B) Average percentages of CD19+ IgM+ cells to CD19+ cells were determined in several SP or BM samples from Itch−/− (n = 7 or 5), WT (n = 6 or 5), LMP2A+ (n = 7 or 4), and LMP2A+ Itch−/− mice (n = 7 or 4), respectively. Data were analyzed by Student's t test. Values that were statistically significantly differently from each other (P < 0.001) are indicated by two asterisks.
Robust proliferation of LMP2A+ Itch−/− bone marrow B cells in IL-7-treated culture compared to LMP2A+ cells.
To examine the effect of Itchy on the previously described ability of LMP2A to promote B-cell growth in cultures treated with interleukin-7 (IL-7), in vitro bone marrow cultures were grown for each genotype. The growth of B cells in IL-7-containing medium in the absence of stromal cell contact requires transition from a CD43+ pro-B stage to a CD43− pre-B stage, a process dependent upon Ig HC gene rearrangement and expression (5, 23). CD43 expression in Itch−/− bone marrow cells was normal compared to that in WT animals (data not shown). Fewer B cells were observed in the bone marrow of LMP2A+ Itch−/− mice, but the phenotype of LMP2A+ Itch−/− bone marrow B cells in regard to CD43 expression was similar to the phenotype of LMP2A+ mice (1, 2) (data not shown).
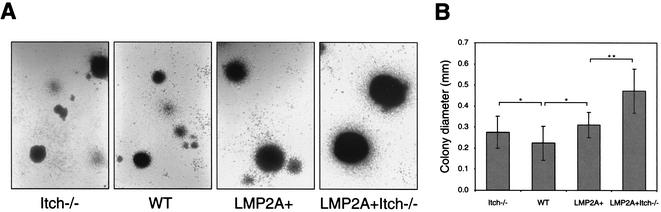
After 9 days of culture in IL-7-containing methylcellulose medium, bone marrow cells from WT, Itch−/−, LMP2A+, and LMP2A+ Itch−/− littermates formed B-cell colonies (Fig. 3A). As previously reported (17), LMP2A+ B cells formed more colonies in cultures treated with IL-7 (data not shown). In proportion to the number of bone marrow B cells, the number of colonies that grew from cells from LMP2A+ Itch−/− mice was less than the number from LMP2A+ mice and similar to the numbers from WT and Itch−/− mice (data not shown). Of particular interest was the statistically significantly larger colony size exhibited by LMP2A+ Itch−/− B cells (Fig. 3B). The colony size in LMP2A+ Itch−/− cultures was typically 1.5 times larger than colonies from LMP2A+ cultures. However, colony sizes from either Itch−/− or LMP2A+ cultures were not dramatically different from those from WT cultures. These results indicate that Itch deficiency enhances LMP2A-derived proliferation of bone marrow B cells in IL-7-treated culture.
FIG. 3.
Formation of colonies from bone marrow cells cultured in methylcellulose medium containing IL-7. A total of 106 bone marrow cells from mice were incubated in 3 ml of IL-7-containing methylcellulose medium for 9 days as previously described (1). (A) Cells in the four micrographs were photographed at the same magnification. (B) Colony diameter was measured in Itch−/− (n = 19), WT (n = 31), LMP2A+ (n = 55), and LMP2A+ Itch−/− cultures (n = 14). Data were analyzed by Student's t test. Values that were statistically significantly differently from each other are indicated (★, P < 0.05; ★★, P < 0.001).
Phosphorylation increases in LMP2A+ Itch−/− bone marrow B cells.
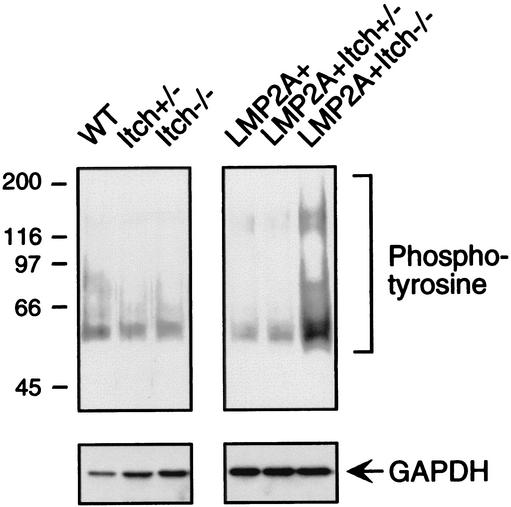
The increased proliferation of LMP2A+ Itch−/− bone marrow B cells in culture suggests that Itchy negatively regulates LMP2A-derived signals. We had previously suggested that Nedd4 family E3s, such as Itchy, downmodulates LMP2A-associated PTK activity by ubiquitin conjugation. In support of this model, we have previously shown in tissue culture cells that a LMP2A mutant protein deficient in Nedd4 family E3 association exhibits hyperphosphorylation of LMP2A-associated Lyn and Syk PTKs (13). To investigate this possibility, lysates from bone marrow cells cultured in IL-7-containing media were analyzed using antibodies directed against phosphotyrosine. As shown in Fig. 4, the results of this analysis demonstrate the dramatic hyperphosphorylation of a variety of cellular proteins compared to proteins in littermate controls. To verify that the lysates contained similar amounts of protein, immunoblot analysis was performed using antibodies directed against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Fig. 4).
FIG. 4.
Tyrosine phosphorylation in cultured bone marrow cells. After the bone marrow cells were cultured for 7 days in IL-7-containing methylcellulose medium, the cells were lysed with 1% Triton X-100, immunoprecipitated with mouse antiphosphotyrosine antibody (4G10; Upstate Biotechnology), and immunoblotted with antiphosphotyrosine peptide (RC20; Transduction Laboratory). GAPDH, a control for protein loading, was immunoblotted with mouse anti-GAPDH antibody (6C5; Novus Biologicals) with whole Triton X-100 lysates. The positions of molecular size markers (in kilodaltons) are shown to the left of the gel.
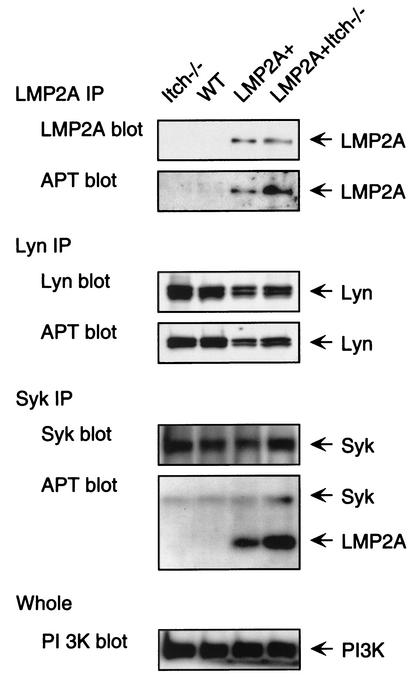
To further characterize proteins with phosphorylated tyrosines in bone marrow B cells in culture, tyrosine phosphorylation of specific proteins was examined. Data are representative of the results from three experiments. Cell lysates from bone marrow B cells cultured in IL-7-containing medium were immunoprecipitated with anti-LMP2A antibody and subsequently detected with an anti-LMP2A antibody to verify equivalent amounts of LMP2A in the immunoprecipitation or detected with antiphosphotyrosine antibody to determine the level of LMP2A phosphorylation (Fig. 5A). Similar amounts of LMP2A were present in the LMP2A immunoprecipitation from LMP2A+ and LMP2A+ Itch−/− cells. Interestingly, LMP2A in LMP2A+ Itch−/− mice exhibited an increase of phosphorylation compared to LMP2A+ mice, indicating that the Itch mutation increased phosphorylation of LMP2A. Next, phosphorylation of Lyn and Syk was determined by a procedure similar to the one described above to detect LMP2A phosphorylation. In LMP2A+ mice, a modest decrease in Lyn expression and phosphorylation was observed, while this decrease was not observed in LMP2A+ Itch−/− mice (Fig. 5). This is similar to results when Lyn phosphorylation is examined in LMP2A-expressing human B cells in vitro (9, 19). In contrast to the Lyn results, a modest yet reproducible increase in Syk phosphorylation was observed in LMP2A+ Itch−/− cells compared to LMP2A+ cells (Fig. 5, Syk IP, APT blot). LMP2A phosphorylation was increased in Syk immunoprecipitates (Fig. 5, Syk IP, APT blot), a result comparable with our previous observations in tissue culture cells expressing a LMP2A mutant unable to associate with Nedd4 family E3s.
FIG. 5.
Tyrosine phosphorylation of LMP2A, Lyn, and Syk in bone marrow cells cultured in IL-7-containing methylcellulose medium. Triton X-100 lysates from methylcellulose-cultured bone marrow B cells were subjected to three different treatments: (i) immunoprecipitation (IP) with rat anti-LMP2A antibody and immunoblotting with rat anti-LMP2A or antiphosphotyrosine peptide (APT), (ii) immunoprecipitation with rabbit anti-Lyn antibody (SC-15; Santa Cruz) and immunoblotting with rabbit anti-Lyn or antiphosphotyrosine peptide, or (iii) immunoprecipitation with rabbit anti-Syk (C-20; Santa Cruz) and immunoblotting with rabbit anti-Syk antibody or antiphosphotyrosine peptide. As a protein content control, the Triton X-100 lysates were immunoblotted with rabbit anti-phosphatidylinositol 3-kinase (PI3K) p85 subunit (Upstate Biotechnology).
The interaction of LMP2A with cellular proteins, particularly Lyn and Syk PTKs, has been well characterized using in vitro model systems. However, it has not been possible to analyze the LMP2A-mediated B-cell growth and survival signals without the use of LMP2A transgenic mice. We have used LMP2A transgenic mice to characterize the dependence of this LMP2A-specific signal on many cellular proteins involved in normal BCR signal transduction. By and large, with some modest exceptions, LMP2A functions in many ways as a constitutively active homologue of the BCR.
The present model of LMP2A function in transgenic mice can be refined using the data described here. LMP2A is first recruited to the lipid raft fraction within cellular membranes (3). This allows LMP2A to become phosphorylated and associate with the Lyn PTK, since Lyn is constitutively localized to lipid rafts (9). Following this phosphorylation, LMP2A binds the Syk PTK via interaction of the Syk SH2 domains with the LMP2A ITAM (8). Subsequently, other proteins important for BCR signal transduction (such as Btk, SLP-65/BLNK, and Akt) are activated, providing a BCR-like signal that allows B cells to develop and survive in LMP2A transgenic mice in the absence of normal BCR signals.
We hypothesize that after this signaling event, the Nedd4 family ubiquitin ligases (particularly Itchy) bind to LMP2A, resulting in downmodulation of the LMP2A signal. This is based upon the enhanced tyrosine phosphorylation observed in LMP2A+ Itch−/− mice compared to LMP2A+ mice. LMP2A is also able to provide a stronger growth signal in the absence of Itchy, as suggested by increased IL-7-dependent proliferation of LMP2A+ Itch−/− bone marrow cells and reduction of CD19+ IgM+ cells in the spleen and bone marrow of LMP2A+ Itch−/− mice. However, a stronger LMP2A signal may also mediate apoptosis, as may be interpreted by the reduction in bone marrow B cells in the LMP2A+ Itch−/− mice from that in WT mice. In BCR signaling, strong signals result in apoptosis of immature B cells, while the same signals mediate cell growth in mature B cells. Most of the immature B cells exiting to the periphery are destined to die by apoptosis because they will not receive the antigen-driven BCR activation that is required for the positive selection of mature B cells. The LMP2A signal functions in development, providing the required signal to allow Ig expression to be bypassed, and in the periphery where it ensures B-cell survival. Thus, as we have shown in this study, alterations in the strength of the LMP2A signal may result in an increase in apoptosis in B cells as they develop in the bone marrow (similar to the negative selection of B cells) and, as would be expected, increases the severity of the LMP2A phenotype in the periphery, as monitored by Ig-negative B cells. In our previous studies using EBV-transformed LCLs containing a specific mutation in LMP2A unable to bind Nedd4 E3s, we demonstrated enhanced phosphorylation of LMP2A and LMP2A-associated Lyn and Syk PTKs (13). However, the functional consequences of this observation were not determined. In this study, we now show that the interaction of LMP2A with Nedd4 E3s is vital in regulating the relative strength of LMP2A signal. Providing an appropriate BCR-like signal will be important for LMP2A function in latently infected B cells.
In addition, we demonstrated that at least four members of Nedd4 family E3s associate with LMP2A in vitro (11). On the basis of the results of this study and our previous observation that AIP4, the human homologue of Itchy, is the most abundant protein precipitated with bacterially expressed LMP2A, we feel that there is little redundancy in the regulation of LMP2A by other Nedd-4 family E3s. To verify this conclusion, we are currently constructing transgenic mice expressing LMP2A containing a mutation of the LMP2A PY motifs that are required for LMP2A binding to the Nedd4 family E3s.
One of the most remarkable aspects of this study is the result indicating that Itchy plays a role in negatively regulating LMP2A activity. Our previous studies have shown that LMP2A largely mimics an activated BCR and uses a similar group of B-cell proteins to provide this signal. Interestingly, in this study, we have shown that LMP2A may have at least one activity that is quite different from a normal BCR. Recent work has suggested that ubiquitin ligases may be important in regulating both B- and T-cell receptor signal transduction, but these studies have suggested a role for Cbl in receptor regulation (24). Studies analyzing T cells from the Itch mice suggest a role of Itchy in regulating T-cell function, but it was shown that Itchy regulated the degradation of JunB, a transcription factor important in TH2 differentiation (6). These data indicate that Itchy is not important for regulating membrane proximal events during T-cell signal transduction. At this point, there are no available data regarding a possible role of Itchy in regulating B-cell signal transduction. It is too early to determine whether the results presented indicate an undiscovered aspect of BCR signal transduction or if the interaction of LMP2A with Itchy indicates a bifurcation of the LMP2A-mediated pathway of B-cell activation compared to the normal signal transduction pathways activated by the BCR. Results of recent gene expression analysis (21) with LMP2A transgenic mice support the latter supposition. In these experiments (21), major differences in the expression of global transcription factors were observed in LMP2A transgenic B cells compared to WT mice, indicating potential differences in the LMP2A signal compared to a BCR signal. Further studies, using both in vitro and in vivo methodologies, will elucidate the function of this intriguing viral protein.
Acknowledgments
We thank Bill Perry and Nancy Jenkins for providing Itch mice.
R.L. is supported by Public Health Service grants CA62234, CA73507, and CA93444 from the National Cancer Institute and grant DE13127 from the National Institute of Dental and Craniofacial Research. R.L. is a Stohlman Scholar of the Leukemia and Lymphoma Society of America. M.I. is a special fellow of the Leukemia and Lymphoma Society of America.
REFERENCES
- 1.Caldwell, R. G., R. C. Brown, and R. Longnecker. 2000. Epstein-Barr virus LMP2A-induced B-cell survival in two unique classes of EmuLMP2A transgenic mice. J. Virol. 74:1101-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caldwell, R. G., J. B. Wilson, S. J. Anderson, and R. Longnecker. 1998. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity 9:405-411. [DOI] [PubMed] [Google Scholar]
- 3.Dykstra, M. L., R. Longnecker, and S. K. Pierce. 2001. Epstein-Barr virus co-opts lipid rafts to block the signaling and antigen transport functions of the BCR. Immunity 14:57-67. [DOI] [PubMed] [Google Scholar]
- 4.Engels, N., M. Merchant, R. Pappu, A. C. Chan, R. Longnecker, and J. Wienands. 2001. Epstein-Barr virus latent membrane protein 2A (LMP2A) employs the SLP-65 signaling module. J. Exp. Med. 194:255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Era, T., M. Ogawa, S. Nishikawa, M. Okamoto, T. Honjo, K. Akagi, J. Miyazaki, and K. Yamamura. 1991. Differentiation of growth signal requirement of B lymphocyte precursor is directed by expression of immunoglobulin. EMBO J. 10:337-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang, D., C. Elly, B. Gao, N. Fang, Y. Altman, C. Joazeiro, T. Hunter, N. Copeland, N. Jenkins, and Y. C. Liu. 2002. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat. Immunol. 3:281-287. [DOI] [PubMed] [Google Scholar]
- 7.Fruehling, S., S. K. Lee, R. Herrold, B. Frech, G. Laux, E. Kremmer, F. A. Grasser, and R. Longnecker. 1996. Identification of latent membrane protein 2A (LMP2A) domains essential for the LMP2A dominant-negative effect on B-lymphocyte surface immunoglobulin signal transduction. J. Virol. 70:6216-6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fruehling, S., and R. Longnecker. 1997. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology 235:241-251. [DOI] [PubMed] [Google Scholar]
- 9.Fruehling, S., R. Swart, K. M. Dolwick, E. Kremmer, and R. Longnecker. 1998. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency. J. Virol. 72:7796-7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardy, R. R. 2000. B-cell commitment, development and selection. Curr. Opin. Immunol. 12:346-353. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda, M., A. Ikeda, L. C. Longan, and R. Longnecker. 2000. The Epstein-Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology 268:178-191. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda, M., A. Ikeda, and R. Longnecker. 2002. Lysine-independent ubiquitination of Epstein-Barr virus LMP2A. Virology 300:153-159. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda, M., A. Ikeda, and R. Longnecker. 2001. PY motifs of Epstein-Barr virus LMP2A regulate protein stability and phosphorylation of LMP2A-associated proteins. J. Virol. 75:5711-5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kieff, E. 1996. Epstein-Barr virus and its replication, p. 1109-1162. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fundamental virology. Lippincoft-Raven, New York, N.Y.
- 15.Longnecker, R. 1998. Molecular biology of Epstein-Barr virus, p. 133-172. In D. J. McCance (ed.), Human tumor viruses. American Society for Microbiology, Washington, D.C.
- 16.Longnecker, R., and E. Kieff. 1990. A second Epstein-Barr virus membrane protein (LMP2) is expressed in latent infection and colocalizes with LMP1. J. Virol. 64:2319-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merchant, M., R. G. Caldwell, and R. Longnecker. 2000. The LMP2A ITAM is essential for providing B cells with development and survival signals in vivo. J. Virol. 74:9115-9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merchant, M., and R. Longnecker. 2001. LMP2A survival and developmental signals are transmitted through Btk-dependent and Btk-independent pathways. Virology 291:46-54. [DOI] [PubMed] [Google Scholar]
- 19.Miller, C. L., A. L. Burkhardt, J. H. Lee, B. Stealey, R. Longnecker, J. B. Bolen, and E. Kieff. 1995. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity 2:155-166. [DOI] [PubMed] [Google Scholar]
- 20.Perry, W. L., C. M. Hustad, D. A. Swing, T. N. O'Sullivan, N. A. Jenkins, and N. G. Copeland. 1998. The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18H mice. Nat. Genet. 18:143-146. [DOI] [PubMed] [Google Scholar]
- 21.Portis, T., and R. Longnecker. 2003. Epstein-Barr virus LMP2A interferes with global transcription factor regulation when expressed during B-lymphocyte development. J. Virol. 77:105-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sample, J., D. Liebowitz, and E. Kieff. 1989. Two related Epstein-Barr virus membrane proteins are encoded by separate genes. J. Virol. 63:933-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spanopoulou, E., C. A. Roman, L. M. Corcoran, M. S. Schlissel, D. P. Silver, D. Nemazee, M. C. Nussenzweig, S. A. Shinton, R. R. Hardy, and D. Baltimore. 1994. Functional immunoglobulin transgenes guide ordered B-cell differentiation in Rag-1-deficient mice. Genes Dev. 8:1030-1042. [DOI] [PubMed] [Google Scholar]
- 24.Thien, C. B., and W. Y. Langdon. 2001. Cbl: many adaptations to regulate protein tyrosine kinases. Nat. Rev. Mol. Cell. Biol. 2:294-307. [DOI] [PubMed] [Google Scholar]
- 25.Thorley-Lawson, D. A. 2001. Epstein-Barr virus: exploiting the immune system. Nat. Rev. Immunol. 1:75-82. [DOI] [PubMed] [Google Scholar]
- 26.Winberg, G., L. Matskova, F. Chen, P. Plant, D. Rotin, G. Gish, R. Ingham, I. Ernberg, and T. Pawson. 2000. Latent membrane protein 2A of Epstein-Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases. Mol. Cell. Biol. 20:8526-8535. [DOI] [PMC free article] [PubMed] [Google Scholar]