Abstract
One of the candidate proteins for a mucosal vaccine antigen against Streptococcus pneumoniae is PsaA (pneumococcal surface antigen A). Vaccines targeting mucosal immunity may raise concerns as to possible alterations in the normal microbiota, especially in the case of PsaA, which was shown to have homologs with elevated sequence identity in other viridans group streptococci. In this work, we demonstrate that intranasal immunization with a cholera toxin B subunit-PsaA fusion protein is able to protect mice against colonization with S. pneumoniae but does not significantly alter the natural oral or nasopharyngeal microbiota of mice.
Immunoprophylaxis would be the most efficient way to prevent infections caused by Streptococcus pneumoniae. The most immunogenic component of pneumococci is the capsular polysaccharide, which defines the serotypes. The currently available vaccines are thus composed of polysaccharide from the most prevalent serotypes, conjugated or not with a protein carrier, providing serotype-specific protection. Recent efforts to develop new vaccines against pneumococci have focused on proteins that may be conserved throughout the different serotypes. One of the candidates is PsaA (pneumococcal surface antigen A), a 37-kDa protein initially considered to be an adhesin by homology with adhesins of other Streptococcus species (31). It was later demonstrated that the psaA gene is part of an operon responsible for metal (mainly manganese and zinc) transport (9, 27). The analysis of the crystal structure of PsaA has revealed a Zn2+ binding site and characterized it as a metal binding membrane transport protein (20). Mutation of psaA has been reported to cause deficiencies in growth, virulence, adherence, and the oxidative stress response (5, 9, 23, 35). Although it has been shown that PsaA is probably not accessible on the surface of the pneumococcus (14, 17), natural induction of anti-PsaA immunoglobulin G (IgG) and IgA through S. pneumoniae colonization or infection has been described in several countries (3, 13, 15, 19, 29, 32, 39). It has been shown that PsaA has a significant role in protection against pneumococcal carriage, and it has been proposed for use as a component of a combined mucosal protein vaccine including PspA (7). More recently, we have described an increase in both systemic and mucosal antibodies in saliva and nasal and bronchial wash samples after intranasal immunization of mice with a cholera toxin B subunit (CTB)-PsaA fusion protein (1).
PsaA has been shown to be highly conserved by restriction fragment length analysis of PCR-amplified psaA of the 23 vaccine serotypes (30) and by analysis of the reactivity of monoclonal antibodies with samples from 90 serotypes (8). More recently, a PCR-based identification method based on psaA was shown to amplify the gene from the 90 tested serotypes (26). The facts that some monoclonal antibodies raised against PsaA show immunoreactivity with homologous proteins in several viridans group streptococcal species commonly isolated from human clinical specimens (8, 16) and that the psaA gene shows elevated sequence identity with Streptococcus mitis, Streptococcus oralis, and Streptococcus anginosus homologs (16) have raised concerns about possible alterations in the normal microbiota caused by immunization with PsaA. In this work, we demonstrate that intranasal immunization with CTB-PsaA fusion protein does not significantly alter the natural oral or nasopharyngeal microbiota in mice but is able to protect mice against colonization with S. pneumoniae.
Induction of antibodies by intranasal immunization.
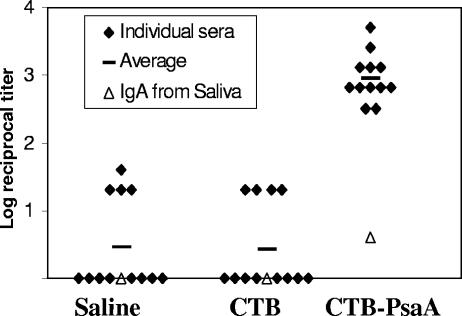
In order to test the immunogenic potential of the CTB-PsaA fusion protein, female C57BL/6 mice (Instituto Butantan, 5 to 7 weeks old,12 animals per group) were immunized intranasally twice a week for 3 consecutive weeks with saline, CTB (1.6 μg), or CTB-PsaA (5 μg). The amount of CTB in 5 μg of CTB-PsaA is the equivalent molar amount present in 1.6 μg of CTB. Ten microliters was inoculated into each nostril of anesthetized animals. Recombinant CTB and CTB-PsaA were obtained according to previously described methods (1, 2). After 21 days, serum and saliva samples were analyzed in terms of IgG and IgA anti-PsaA production, respectively, through ELISA. Titers were considered the last dilution of serum that registered an A492 of 0.1. As shown in Fig. 1, only animals immunized with the fusion protein were able to produce high titers of serum IgG and detectable levels of anti-PsaA IgA in saliva. Interestingly, immunization of C57BL/6 mice with PsaA alone or along with CTB did not result in detectable levels of either anti-PsaA IgG or IgA (data not shown), which is in accordance with our results previously obtained with BALB/c mice (1). The role of antibodies in protection against colonization is controversial. Several groups have recently shown that colonization can be prevented in the absence of antibodies (22, 25, 36), while CD4+ T cells seem to be required for protection (22, 36). Since we have only analyzed the induction of antibodies, we have used it as a parameter of induction of an immune response against PsaA.
FIG. 1.
Induction of anti-PsaA IgG and IgA after intranasal immunization with CTB-PsaA. Anti-PsaA IgG titers of individual serum samples and IgA titers of pooled saliva collected 3 weeks after the last immunization are shown.
Protection against intranasal challenge with S. pneumoniae.
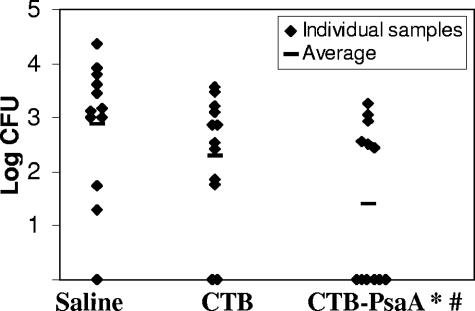
Immunized animals were challenged intranasally with S. pneumoniae, and nasopharyngeal colonization was analyzed 21 days after the last immunization. Anesthetized animals were inoculated intranasally with 10 μl of a suspension containing 5 × 106 CFU of S. pneumoniae strain 0603 (serotype 6B) (21). After 5 days, animals were sacrificed and nasal washes were performed as previously described (38). Serial dilutions of the samples were plated on blood agar containing 4 μg ml−1 gentamicin. The total number of CFU in each sample was estimated while considering the volume recovered. For representation in the graphic and statistical analysis, results were expressed as log10 values and recovery of 0 CFU was considered 1 CFU. Only mice that had been immunized with CTB-PsaA showed a statistically significant decrease in terms of the number of CFU (P < 0.01, Mann-Whitney U test) recovered from the nasopharynx, as well as in terms of the number of colonized mice (P < 0.05, Fisher exact test) (Fig. 2). However, we could not detect a correlation in individual mice between the level of IgG and protection against colonization. Immunization with PsaA either alone or along with CTB did not confer protection against challenge (data not shown). For this reason, further investigation was restricted to mice that received the CTB-PsaA fusion protein or the controls, saline and CTB, intranasally. These results are in contrast with data published by other groups showing significant antibody induction in both serum and saliva and protection through immunization with PsaA by using CTB as adjuvant (7). It is important to point out that there are important differences in the antigens used in these studies. Immunization in the work of Briles and collaborators (7) was performed with PsaA containing a signal sequence from outer membrane surface protein OspA from Borrelia burgdorferi, which renders the protein lipidated and immunogenic even in the absence of adjuvants. Furthermore, while our experiments were performed with recombinant CTB purified from Escherichia coli (and treated for endotoxin removal), CTB used in the previous work was purified from Vibrio cholerae, resulting in some contamination with intact cholera toxin.
FIG. 2.
Intranasal challenge with S. pneumoniae. Numbers of CFU recovered from nasal washes after intranasal challenge with pneumococcal strain 0603 are shown. *, values statistically different from control mice immunized with saline (Mann-Whitney U test). #, number of colonized mice statistically different from the number of control mice immunized with saline (Fisher exact test).
Analysis of the microbiota of immunized mice.
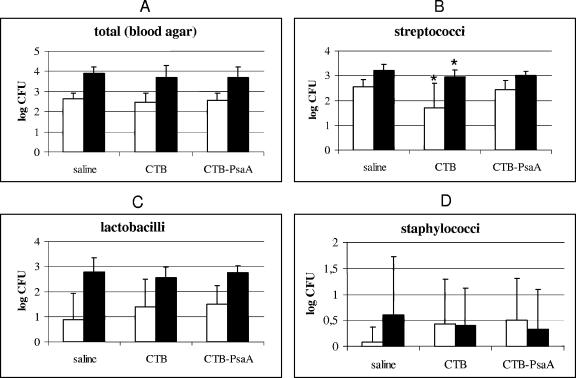
In order to evaluate the possible impact of the immune response elicited against PsaA on the nasopharyngeal and oral microbiota of mice, we compared the total numbers of CFU in nasal wash and saliva samples from mice inoculated with saline, CTB, or CTB-PsaA. Appropriate sample dilutions were plated on blood agar (5% sheep blood), mitis salivarius agar (Difco), Rogosa agar (Difco), and mannitol salt agar (Difco). The plates were incubated in a candle jar at 37°C for 24, 48, or 72 h. The microorganisms were counted according to the colony morphology on blood agar. Colonies resembling viridans group streptococci were counted on mitis salivarius agar, seeded in thioglycolate medium (Difco), and identified by microscopic observation, Gram staining, biochemical tests (4, 10, 34), and in API 20 Strep (BioMérieux) as well. Identification of staphylococci was performed by Gram staining, colony morphology, mannitol fermentation, catalase production, and coagulase production (18). Lactobacilli were presumptively grouped into four taxa by Gram stain morphology, catalase production, raffinose fermentation, and vancomycin susceptibility (28). For statistical analysis, distribution of data on CFU was analyzed through the Kolmogorov-Smirnov test. Experiments with normal distribution of data were analyzed by Student's t test, whereas those not normally distributed were analyzed by the Mann-Whitney U test (P ≤ 0.05). As shown in Fig. 3A, no differences were detected between the groups in total counts of bacteria recovered from either nasal washes or saliva and grown on blood agar plates 3 weeks after the last immunization. When we evaluated streptococci (Fig. 3B), lactobacilli (Fig. 3C), and staphylococci (Fig. 3D) individually, we could not detect any statistically significant differences in bacterial loads in animals from the CTB-PsaA group in relation to the control group. Interestingly, a statistically lower load of streptococci (but not of lactobacilli or staphylococci) was observed in the control group of mice immunized with the adjuvant CTB alone, in both nasal wash and saliva samples. Since we have obtained similar results when analyzing nasal wash and saliva samples, these results further indicate that the impact of nasal immunization on nasopharyngeal samples and that on oral samples are comparable. In order to address whether the effect of immunization with the CTB adjuvant on streptococci was transitory, we next analyzed streptococci in the saliva of animals inoculated with saline, CTB, or CTB-PsaA 8 weeks after the last immunization. In accordance with the results obtained with nasal wash and saliva samples 3 weeks after the last immunization, no alteration was detected in mice immunized with CTB-PsaA. As for immunization with CTB, the reduction in the number of streptococci detected in both nasopharyngeal and oral samples at 3 weeks continued to be detected at 8 weeks after the last immunization (data not shown). One hypothesis for the unexpected finding that CTB affects the microbiota and an equivalent amount of CTB in CTB-PsaA does not is that the structures of the two proteins are quite different; although the fusion protein also assembled into a pentamer, each monomer of CTB has a molecule of PsaA fused to it. Although CTB-PsaA is capable of binding the GM1 receptor in vitro (1), it is possible that its binding capacity in vivo varies considerably from that of the CTB pentamer.
FIG. 3.
Composition of the nasopharyngeal and oral microbiota. CFU of total bacteria (A), streptococci (B), lactobacilli (C), and staphylococci (D) recovered 3 weeks after the last immunization from nasal wash (empty bars) and saliva (solid bars) samples are shown. *, value statistically different from that of control mice inoculated with saline (Student's t test).
Identification of streptococci, lactobacilli, and staphylococci.
The microorganisms obtained from nasal wash and saliva samples were identified by biochemical tests, and the isolated species are presented in Table 1. The streptococcal species recovered were Streptococcus orisratti, Streptococcus ratti, Streptococcus criceti (mutans group), Streptococcus mitis, Streptococcus oralis (mitis group), and Streptococcus vestibularis (salivarius group). In terms of the alterations in streptococcal species in the CTB group, the numbers of animals colonized by S. criceti in nasal washes and S. oralis and S. vestibularis in saliva were found to be significantly reduced; however, S. ratti was found to be increased (results not shown). Similar alterations in the colonization of the animals with the individual species were also observed in the CTB-PsaA group, but the total amount was not altered since the reduction in S. vestibularis was compensated for by an increase in S. ratti. In this case, S. oralis was not changed (results not shown). Coagulase-negative staphylococci were isolated in all nasal washes and saliva groups. Three weeks after the last immunization, Staphylococcus aureus was identified in the saliva of all mouse groups. According to the group-based lactobacillus classification, it was possible to identify three groups in the nasal washes and saliva: Lactobacillus murinus, Lactobacillus reuteri, and Lactobacillus casei. On the whole, the microbiota was very similar in terms of the species of streptococci, staphylococci, and lactobacilli identified in the nasal wash and saliva samples from the saline and CTB control groups and the group immunized with CTB-PsaA. The variation in species isolated at 3 and 8 weeks after the last immunization in the case of animals inoculated only with saline shows that some of the differences detected between groups might be due to transitory temporal alterations in the microbiota of the animals.
TABLE 1.
Identification of streptococci, staphylococci, and lactobacilli in nasopharyngeal and oral microbiota of immunized mice
| Microorganisms | Species detected in:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nasal wash (3 wk)
|
Saliva (3 wk)
|
Saliva (8 wk)
|
|||||||
| Saline | CTB | CTB-PsaA | Saline | CTB | CTB-PsaA | Saline | CTB | CTB-PsaA | |
| Streptococci | S. ratti, S. orisratti, S. mitis, S. criceti | S. ratti, S. orisratti, S. mitis | S. ratti, S. orisratti, S. mitis, S. oralis | S. ratti, S. orisratti, S. oralis, S. vestibularis | S. ratti, S. orisratti | S. ratti, S. orisratti, S. oralis | S. ratti, S. orisratti, S. oralis, S. mitis, S. vestibularis | S. ratti, S. orisratti, S. oralis | S. ratti, S. orisratti, S. oralis, S. vestibularis |
| Staphylococci | CNS,aS. aureus | CNS | CNS | CNS, S. aureus | CNS, S. aureus | CNS, S. aureus | CNS | CNS | CNS, S. aureus |
| Lactobacilli (group) | L. murinus, L. reuteri, L. casei | L. murinus, L. reuteri | L. murinus, L. reuteri | L. murinus, L. reuteri, L. casei | L. reuteri | L. murinus, L. reuteri | L. murinus, L. reuteri | L. murinus, L. reuteri, L. casei | L. murinus, L. reuteri, L. casei |
CNS, coagulase-negative staphylococci.
Immunoreactivity with anti-PsaA antiserum in isolated streptococci.
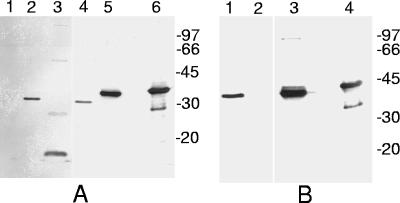
Since our purpose was to address the impact of the immune responses against PsaA on the natural microbiota of mice, because of the concern about the presence of homologous proteins in indigenous streptococci, it is essential to evaluate whether the streptococcal species isolated from mice have proteins that cross-react with anti-PsaA antibodies. Western blot analysis of all of the streptococcal species isolated (Fig. 4) showed that S. oralis and S. mitis, both from the mitis group, and S. orisratti have bands reactive with anti-PsaA antibodies. S. vestibularis (salivarius group) also has reactive bands, but with a molecular mass very different from those of S. pneumoniae St 491/00 (serotype 6B), the S. mitis reference strain (kindly provided by M. C. C. Brandileone, Instituto Adolfo Lutz, Brazil), and recombinant PsaA (rPsaA) controls. S. ratti and S. criceti, both from the mutans group, did not show any reactivity with anti-PsaA antiserum. Thus, there are streptococci in the natural microbiota of mice with proteins that cross-react with anti-PsaA antibodies. Most importantly, immunization with CTB-PsaA did not alter the total amounts of streptococci nor did it eliminate any streptococcal species showing reaction with anti-PsaA antiserum (S. oralis, S. mitis, and S. orisratti). The alterations observed in the number of animals with the different species of Streptococcus showed no correlation with the presence or absence of PsaA (results not shown).
FIG. 4.
Immunoblot analysis of streptococci with anti-PsaA antiserum. Soluble cell extracts of streptococci were subjected to Western blotting with anti-PsaA antiserum. (A) Lanes: 1, S. ratti; 2, S. orisratti; 3, S. vestibularis; 4, S. oralis; 5, S. mitis reference strain; 6, rPsaA. (B) Lanes: 1, S. mitis; 2, S. criceti; 3, S. pneumoniae 491/00; 4, rPsaA. Molecular mass markers in kilodaltons are indicated on the right.
The strategy of eradication of colonization is in contrast with targeting only invasive infection and raises concerns about its effect on the natural balance between pneumococci and cocolonizing species (6). The nasopharyngeal microbiota seems to be very dynamic, and replacement of colonization with vaccine serotypes by nonvaccine serotypes has been described following immunization with the pneumococcal conjugate vaccine (12, 37). An augmentation of recurrent acute otitis media caused by S. aureus has also been described following administration of the conjugate vaccine (37). As for interactions with nonpathogenic bacteria, in vitro inhibition of S. pneumoniae by alpha-hemolytic streptococci isolated from children (33) and a decline in carriage of resident viridans group streptococci during active infection in children with otitis media caused by S. pneumoniae, Haemophilus influenza, and Moraxella catarrhalis (11) have already been described. To our knowledge, the effect of vaccination against pneumococcal colonization on the resident nonpathogenic microbiota has not been addressed yet. Here we show that the induction of an immune response against PsaA can be protective against colonization and has a negligible impact on the natural nasopharyngeal and oral microbiota of mice. Total bacterial counts, streptococci, staphylococci, and lactobacilli were not affected either quantitatively or qualitatively. However, we could detect a consistent reduction of streptococcal CFU after nasal immunization with the adjuvant CTB alone. CTB is one of the most important mucosal adjuvants tested currently and, when administered intranasally with an influenza vaccine, was shown to induce protection against an influenza virus challenge through the activation of nonspecific innate immunity (24). Such activation of innate immunity could explain the effect of CTB on streptococci in the oral microbiota. We have analyzed the oral microbiota up to 8 weeks after the last immunization, and we could still detect a reduction of streptococci in saliva after immunization with CTB alone. This effect will be further investigated, but we expect it to be transitory and not deleterious. On the whole, our results show that induction of an immune response against PsaA can lead to reduced colonization by pneumococci and that it should have a negligible effect on the natural microbiota.
Acknowledgments
This work was supported by CNPq, FAPESP, and Fundação Butantan. A. L. S. S. de Andrade was supported by grant 308043/2004-9, and L. C. C. Leite was supported by grant 51133/1997-6.
We thank M. C. C. Brandileone (Instituto Adolfo Lutz, São Paulo) for providing the S. mitis reference strain.
Editor: J. N. Weiser
REFERENCES
- 1.Areas, A. P., M. L. Oliveira, E. N. Miyaji, L. C. Leite, K. A. Aires, W. O. Dias, and P. L. Ho. 2004. Expression and characterization of cholera toxin B-pneumococcal surface adhesin A fusion protein in Escherichia coli: ability of CTB-PsaA to induce humoral immune response in mice. Biochem. Biophys. Res. Commun. 321:192-196. [DOI] [PubMed] [Google Scholar]
- 2.Areas, A. P., M. L. Oliveira, C. R. Ramos, M. E. Sbrogio-Almeida, I. Raw, and P. L. Ho. 2002. Synthesis of cholera toxin B subunit gene: cloning and expression of a functional 6XHis-tagged protein in Escherichia coli. Protein Expr. Purif. 25:481-487. [DOI] [PubMed] [Google Scholar]
- 3.Baril, L., D. E. Briles, P. Crozier, J. King, M. Punar, S. K. Hollingshead, and J. B. McCormick. 2004. Characterization of antibodies to PspA and PsaA in adults over 50 years of age with invasive pneumococcal disease. Vaccine 23:789-793. [DOI] [PubMed] [Google Scholar]
- 4.Beighton, D., J. M. Hardie, and R. A. Whiley. 1991. A scheme for the identification of viridans streptococci. J. Med. Microbiol. 35:367-372. [DOI] [PubMed] [Google Scholar]
- 5.Berry, A. M., and J. C. Paton. 1996. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 64:5255-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogaert, D., R. De Groot, and P. W. Hermans. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144-154. [DOI] [PubMed] [Google Scholar]
- 7.Briles, D. E., E. Ades, J. C. Paton, J. S. Sampson, G. M. Carlone, R. C. Huebner, A. Virolainen, E. Swiatlo, and S. K. Hollingshead. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crook, J., J. A. Tharpe, S. E. Johnson, D. B. Williams, A. R. Stinson, R. R. Facklam, E. W. Ades, G. M. Carlone, and J. S. Sampson. 1998. Immunoreactivity of five monoclonal antibodies against the 37-kilodalton common cell wall protein (PsaA) of Streptococcus pneumoniae. Clin. Diagn. Lab. Immunol. 5:205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dintilhac, A., G. Alloing, C. Granadel, and J. P. Claverys. 1997. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol. Microbiol. 25:727-739. [DOI] [PubMed] [Google Scholar]
- 10.Facklam, R. 2002. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin. Microbiol. Rev. 15:613-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faden, H., J. Stanievich, L. Brodsky, J. Bernstein, and P. L. Ogra. 1990. Changes in nasopharyngeal flora during otitis media of childhood. Pediatr. Infect. Dis. J. 9:623-626. [PubMed] [Google Scholar]
- 12.Ghaffar, F., T. Barton, J. Lozano, L. S. Muniz, P. Hicks, V. Gan, N. Ahmad, and G. H. McCracken, Jr. 2004. Effect of the 7-valent pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae in the first 2 years of life. Clin. Infect. Dis. 39:930-938. [DOI] [PubMed] [Google Scholar]
- 13.Goldblatt, D., M. Hussain, N. Andrews, L. Ashton, C. Virta, A. Melegaro, R. Pebody, R. George, A. Soininen, J. Edmunds, N. Gay, H. Kayhty, and E. Miller. 2005. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J. Infect. Dis. 192:387-393. [DOI] [PubMed] [Google Scholar]
- 14.Gor, D. O., X. Ding, D. E. Briles, M. R. Jacobs, and N. S. Greenspan. 2005. Relationship between surface accessibility for PpmA, PsaA, and PspA and antibody-mediated immunity to systemic infection by Streptococcus pneumoniae. Infect. Immun. 73:1304-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmlund, E., B. Quiambao, J. Ollgren, H. Nohynek, and H. Kayhty. 2005. Development of natural antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A and pneumolysin in Filipino pregnant women and their infants in relation to pneumococcal carriage. Vaccine 24:57-65. [DOI] [PubMed] [Google Scholar]
- 16.Jado, I., A. Fenoll, J. Casal, and A. Perez. 2001. Identification of the psaA gene, coding for pneumococcal surface adhesin A, in viridans group streptococci other than Streptococcus pneumoniae. Clin. Diagn. Lab. Immunol. 8:895-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston, J. W., L. E. Myers, M. M. Ochs, W. H. Benjamin, Jr., D. E. Briles, and S. K. Hollingshead. 2004. Lipoprotein PsaA in virulence of Streptococcus pneumoniae: surface accessibility and role in protection from superoxide. Infect. Immun. 72:5858-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kloos, W. E., and T. L. Bannerman. 1999. Staphylococcus and Micrococcus, p. 264-282. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 19.Laine, C., T. Mwangi, C. M. Thompson, J. Obiero, M. Lipsitch, and J. A. Scott. 2004. Age-specific immunoglobulin G (IgG) and IgA to pneumococcal protein antigens in a population in coastal Kenya. Infect. Immun. 72:3331-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrence, M. C., P. A. Pilling, V. C. Epa, A. M. Berry, A. D. Ogunniyi, and J. C. Paton. 1998. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure 6:1553-1561. [DOI] [PubMed] [Google Scholar]
- 21.Malley, R., S. C. Morse, L. C. Leite, A. P. Areas, P. L. Ho, F. S. Kubrusly, I. C. Almeida, and P. Anderson. 2004. Multiserotype protection of mice against pneumococcal colonization of the nasopharynx and middle ear by killed nonencapsulated cells given intranasally with a nontoxic adjuvant. Infect. Immun. 72:4290-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malley, R., K. Trzcinski, A. Srivastava, C. M. Thompson, P. W. Anderson, and M. Lipsitch. 2005. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. USA 102:4848-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marra, A., S. Lawson, J. S. Asundi, D. Brigham, and A. E. Hromockyj. 2002. In vivo characterization of the psa genes from Streptococcus pneumoniae in multiple models of infection. Microbiology 148:1483-1491. [DOI] [PubMed] [Google Scholar]
- 24.Matsuo, K., T. Yoshikawa, H. Asanuma, T. Iwasaki, Y. Hagiwara, Z. Chen, S. E. Kadowaki, H. Tsujimoto, T. Kurata, and S. I. Tamura. 2000. Induction of innate immunity by nasal influenza vaccine administered in combination with an adjuvant (cholera toxin). Vaccine 18:2713-2722. [DOI] [PubMed] [Google Scholar]
- 25.McCool, T. L., and J. N. Weiser. 2004. Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infect. Immun. 72:5807-5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison, K. E., D. Lake, J. Crook, G. M. Carlone, E. Ades, R. Facklam, and J. S. Sampson. 2000. Confirmation of psaA in all 90 serotypes of Streptococcus pneumoniae by PCR and potential of this assay for identification and diagnosis. J. Clin. Microbiol. 38:434-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novak, R., J. S. Braun, E. Charpentier, and E. Tuomanen. 1998. Penicillin tolerance genes of Streptococcus pneumoniae: the ABC-type manganese permease complex Psa. Mol. Microbiol. 29:1285-1296. [DOI] [PubMed] [Google Scholar]
- 28.Pena, J. A., S. Y. Li, P. H. Wilson, S. A. Thibodeau, A. J. Szary, and J. Versalovic. 2004. Genotypic and phenotypic studies of murine intestinal lactobacilli: species differences in mice with and without colitis. Appl. Environ. Microbiol. 70:558-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rapola, S., V. Jantti, R. Haikala, R. Syrjanen, G. M. Carlone, J. S. Sampson, D. E. Briles, J. C. Paton, A. K. Takala, T. M. Kilpi, and H. Kayhty. 2000. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J. Infect. Dis. 182:1146-1152. [DOI] [PubMed] [Google Scholar]
- 30.Sampson, J. S., Z. Furlow, A. M. Whitney, D. Williams, R. Facklam, and G. M. Carlone. 1997. Limited diversity of Streptococcus pneumoniae psaA among pneumococcal vaccine serotypes. Infect. Immun. 65:1967-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sampson, J. S., S. P. O'Connor, A. R. Stinson, J. A. Tharpe, and H. Russell. 1994. Cloning and nucleotide sequence analysis of psaA, the Streptococcus pneumoniae gene encoding a 37-kilodalton protein homologous to previously reported Streptococcus sp. adhesins. Infect. Immun. 62:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simell, B., M. Korkeila, H. Pursiainen, T. M. Kilpi, and H. Kayhty. 2001. Pneumococcal carriage and otitis media induce salivary antibodies to pneumococcal surface adhesin A, pneumolysin, and pneumococcal surface protein A in children. J. Infect. Dis. 183:887-896. [DOI] [PubMed] [Google Scholar]
- 33.Tano, K., C. Olofsson, E. Grahn-Hakansson, and S. E. Holm. 1999. In vitro inhibition of S. pneumoniae, nontypable H. influenzae and M. catharralis by alpha-hemolytic streptococci from healthy children. Int. J. Pediatr. Otorhinolaryngol. 47:49-56. [DOI] [PubMed] [Google Scholar]
- 34.Trudel, L., L. St-Amand, M. Bareil, P. Cardinal, and M. C. Lavoie. 1986. Bacteriology of the oral cavity of BALB/c mice. Can. J. Microbiol. 32:673-678. [DOI] [PubMed] [Google Scholar]
- 35.Tseng, H. J., A. G. McEwan, J. C. Paton, and M. P. Jennings. 2002. Virulence of Streptococcus pneumoniae: psaA mutants are hypersensitive to oxidative stress. Infect. Immun. 70:1635-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Rossum, A. M., E. S. Lysenko, and J. N. Weiser. 2005. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect. Immun. 73:7718-7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veenhoven, R., D. Bogaert, C. Uiterwaal, C. Brouwer, H. Kiezebrink, J. Bruin, E. IJzerman, P. Hermans, R. de Groot, B. Zegers, W. Kuis, G. Rijkers, A. Schilder, and E. Sanders. 2003. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: a randomised study. Lancet 361:2189-2195. [DOI] [PubMed] [Google Scholar]
- 38.Wu, H. Y., M. H. Nahm, Y. Guo, M. W. Russell, and D. E. Briles. 1997. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J. Infect. Dis. 175:839-846. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, Q., S. Choo, and A. Finn. 2002. Immune responses to novel pneumococcal proteins pneumolysin, PspA, PsaA, and CbpA in adenoidal B cells from children. Infect. Immun. 70:5363-5369. [DOI] [PMC free article] [PubMed] [Google Scholar]