Abstract
Staphylococcus epidermidis is an important cause of nosocomial infections. Virulence is attributable to elaboration of biofilms on medical surfaces that protect the organisms from immune system clearance. Even though leukocytes can penetrate biofilms, they fail to phagocytose and kill bacteria. The properties that make biofilm bacteria resistant to the immune system are not well characterized. In order to better understand the mechanisms of resistance of bacteria in biofilms to the immune system, we evaluated antibody penetration throughout the biofilm and antibody-mediated phagocytic killing of planktonic versus biofilm cells of S. epidermidis by using a rabbit antibody to poly-N-acetylglucosamine (PNAG). These antibodies are opsonic and protect against infection with planktonic cells of PNAG-positive Staphylococcus aureus and S. epidermidis. Antibody to PNAG readily penetrated the biofilm and bound to the same areas in the biofilm as did wheat germ agglutinin, a lectin known to bind to components of staphylococcal biofilms. However, biofilm cells were more resistant to opsonic killing than their planktonic counterparts in spite of producing more PNAG per cell than planktonic cells. Biofilm extracts inhibited opsonic killing mediated by antibody to PNAG, suggesting that the PNAG antigen within the biofilm matrix prevents antibody binding close to the bacterial cell surface, which is needed for efficient opsonic killing. Increased resistance of biofilm cells to opsonic killing mediated by an otherwise protective antibody was due not to a biofilm-specific phenotype but rather to high levels of antigen within the biofilm that prevented bacterial opsonization by the antibody.
Staphylococcus epidermidis and other coagulase-negative staphylococci are among the most frequent causes of nosocomial infections (29, 41) and are often associated with the use of medical devices (38). A critical virulence determinant in such infections is the ability to form biofilms on synthetic materials (3). The biofilm matrix of S. epidermidis is composed mainly of a large exopolysaccharide, poly-N-acetylglucosamine (PNAG) (31), which is involved in intercellular adhesion and is sometimes referred to as polysaccharide intercellular adhesin (21). Opsonic antibody to PNAG mediates protection against systemic infection by both S. epidermidis (16, 35) (where it was called capsular polysaccharide/adhesin) and Staphylococcus aureus (23). However, the ability of antibody to this antigen to interact with staphylococcal cells within a biofilm has not been investigated. As staphylococcal infections, particularly those associated with indwelling medical devices, are not only difficult to treat and eradicate but also associated with high rates of relapse infection (4, 9), it is possible that even if the host mounts an immune response to protective staphylococcal antigens like PNAG, as has recently been shown to occur in S. aureus-infected cystic fibrosis patients (14), these antibodies may not effectively kill bacterial cells within a biofilm.
PNAG is synthesized by proteins encoded by the icaADCB locus (10, 24) and has several described functions: it acts as an intercellular adhesin promoting cell-to-cell aggregation (7, 10), and it is responsible for biofilm maturation (38). PNAG also plays a crucial role in the protection of planktonic S. aureus and S. epidermidis cells from antibody-independent phagocytosis (17, 34, 37). However, despite these findings, the role of PNAG in the resistance of bacterial cells embedded within a biofilm to host opsonic killing mechanisms, particularly in the presence of a potentially opsonic and protective antibody, has not been reported.
When bacteria assume the biofilm phenotype, they display several properties that differ from those expressed during planktonic growth (1, 40), including enhanced resistance to antimicrobials (11) and differential gene expression (30). Biofilms also protect the resident bacteria from attack by phagocytes and complement (8, 36). A previous study (13) has shown that opsonic antibodies to Pseudomonas aeruginosa alginate can mediate killing of the alginate-overexpressing mucoid phenotype of this organism when grown as a biofilm, while another study using nonmucoid P. aeruginosa strain PAO1 biofilms showed that neutrophils could phagocytose planktonic bacteria released from the biofilm but that this made them less active against the bacterial cells within the biofilm (13). Leid et al. have shown that human leukocytes can easily penetrate S. aureus biofilms but fail to phagocytose the bacteria (19).
In order to determine the effect of the biofilm growth on the activity of an opsonic, protective antibody to S. epidermidis, we evaluated the ability of a rabbit immunoglobulin raised against PNAG to penetrate into S. epidermidis biofilms and to mediate opsonic killing. Antiserum with specificity for this antigen was chosen, as previous work has shown that normal sera contain antibodies to other S. epidermidis antigens but that these normal sera fail to mediate opsonic killing or protective immunity to S. epidermidis strains that express PNAG (16, 35). PNAG-producing S. epidermidis strains constitute a large majority of clinical isolates (27, 43). Also, during experimental infection in rabbits, S. epidermidis provokes immune responses to multiple cell wall antigens, but again, these fail to mediate in vitro opsonic killing or reduce levels of infections in tissues once elicited following infection (33, 34). Importantly, antibody-mediated opsonic killing specific to the PNAG antigen is the only well-defined antigen-antibody system which has demonstrated protection against S. epidermidis infection in animal infections and thus represents a host-microbe interaction that can be analyzed to explore mechanisms of resistance of biofilm cells to mediators of immunity.
In the present study, we found that the biofilm did not pose an overall diffusion barrier to the antibody, but when we compared the opsonophagocytic killing of planktonic and biofilm cells of four S. epidermidis strains, there was a distinct difference between the susceptibilities of planktonic and biofilm-grown cells to phagocytic killing. We found greatly enhanced PNAG production by the biofilm cells, suggesting that the excess antigen inhibited the antibody-mediated phagocytosis of the biofilm bacteria by preventing the deposition of quantities of antibody on the bacterial cell surface sufficient to mediate high levels of phagocytic killing.
MATERIALS AND METHODS
Growth conditions.
All S. epidermidis strains were grown at 37°C on tryptic soy agar plates. Liquid cultures were grown overnight in tryptic soy broth (TSB) supplemented with an additional 1% glucose (TSBG) at 37°C and with shaking at 200 rpm.
Bacterial strains and analysis of PNAG production.
In this study, nine distinct S. epidermidis strains isolated from infective endocarditis, dialysis-associated peritonitis, and blood were used (3), as were two control strains kindly provided by Dietrich Mack, Sansea, Wales, United Kingdom: S. epidermidis 9142, a strongly biofilm-producing strain, and S. epidermidis 9142-M10, which has a transposon in the icaADCB locus and does not form a biofilm (21). All strains were first characterized in terms of the presence of the ica operon by extracting genomic DNA and performing a PCR for the presence of the icaC gene with the following primers: 5′-ATAGTGAATCACTTATCACCGC-3′ and 5′-GAGAATCTAAGATAATTGGGTGC-3′. PNAG production and detection by immunoblotting were performed essentially as described by Cramton et al. (6). Biofilms were grown in 96-well polystyrene plates in TSBG and quantified as described by Heilmann et al. (10). We then selected the strains that were ica-positive strong PNAG producers that formed thick biofilms to use in the opsonophagocytic assays.
Opsonophagocytosis of planktonic and biofilm bacteria by serum raised to PNAG.
To prepare bacteria for evaluation of susceptibility to opsonic killing, biofilms were formed in six-well polystyrene plates (Sarsted, Germany) by growing cells at 37°C in TSBG for 24 h with a constant rocking motion. Biofilms were fragmented by scraping cells from the plastic surface and sonicating twice for 5 s at 20 W (VC600; Sonics, Newtown, CT) to homogenize the suspension. Planktonic bacteria were grown overnight in TSBG at 37°C with shaking and were sonicated as described above. Both suspensions were then diluted in TSBG to a concentration of approximately 2 × 108 cells/ml as determined by the optical density at 640 nm. The opsonophagocytic assay was performed as described by Maira-Litrán et al. (22) using a rabbit polyclonal serum raised against deacetylated PNAG conjugated to diphtheria toxoid (23) with a final bacterial cell concentration of approximately 1 × 107 cells/ml. Briefly, the opsonophagocytic assay mixture contained 100 μl of leukocytes (at a concentration of 2 × 107 cells/ml), 100 μl of a bacterial suspension at 1 × 107 cells/ml, 100 μl of a 1:15 dilution of infant rabbit serum as a complement source, and 100 μl of the antibody diluted 1:10. All components were suspended or diluted in RPMI medium with 15% heat-inactivated fetal bovine serum. Control tubes consisted of assay mixtures wherein one of the essential components (phagocytes, complement, or antibody) was individually replaced with 100 μl of RPMI-fetal bovine serum. The reaction mixture was incubated on a rotor rack at 37°C for 90 min. The tubes were vortexed for 15 s, and samples were diluted in TSB with 0.05% Tween to prevent bacterial aggregation and adherence to the walls of the dilution vessel and plated onto tryptic soy agar plates. The percentage of killing was calculated by determining the ratio of the CFU surviving in the tubes with bacteria, leukocytes, complement, and antibody to the CFU surviving in the control tubes. Each condition was evaluated in triplicate, and each assay was repeated two to three times.
CSLM analysis.
Biofilms were prepared for confocal scanning laser microscopy (CSLM) analysis as previously described (28), with some modifications. Briefly, biofilms formed on six-well polystyrene plates as described above were washed twice with 0.9% NaCl. The biofilms were incubated for 2 h at room temperature with affinity-purified rabbit immunoglobulin G (IgG) antibody (14) specific to S. aureus PNAG (23) and washed three times for 5 min each with phosphate-buffered saline (PBS). The secondary antibody used was an Alexa 488-conjugated goat anti-rabbit IgG (Molecular Probes) at 2 mg/liter; biofilms were incubated with it for 1 h at room temperature in the dark and then washed three times for 5 min each with PBS. Some of the biofilms were also incubated with wheat germ agglutinin (WGA) conjugated to Texas Red (Molecular Probes) at 10 mg/liter for 20 min at room temperature in the dark. After staining, the biofilms were gently rinsed with PBS.
Confocal scanning laser microscopy analysis was performed with an LSM 510 Meta (Zeiss, Germany) attached to an Axioplan II microscope (Zeiss, Germany), as previously described (18) with some modifications. Biofilms formed by S. epidermidis strain 9142 were observed using a 63× water immersion objective (Achroplan 63×/0.95W) with a multiple-track channel analysis. The first-channel excitation wavelength was set at 633 nm, with output power of 70%, and bacterial cells were detected as refracted light. A second channel, with an excitation wavelength of 488 nm and output power of 10%, was used to detect where the antibodies had bound. A third channel, with an excitation wavelength of 543 nm and output power of 70%, was used to detect where WGA had bound. The excitation beam splitter used was HFT UV/488/543/633. The filters used to detect the refracted light by the biofilm cells were NFT 635 VIS and BP 500-550 for the antibodies and LP650 for the WGA. The beam splitter used was NFT 545.
Relative quantification of PNAG in planktonic and biofilm cells and supernatants.
For relative evaluation of the concentration of PNAG in biofilms and in planktonic cells, we selected biofilms and planktonic cells of S. epidermidis strain 9142. Biofilms were scraped and sonicated as described above, and then bacterial cells from both planktonic and biofilm cultures were adjusted to a concentration of 109 cells/ml. PNAG was extracted using EDTA and heat and detected by immunoblotting as described by Cramton et al. (6). For detection of PNAG in the supernatants, an aliquot of growth medium from biofilm or planktonic cells equivalent to the volume in which 109 CFU were present was centrifuged at 10,500 × g for 10 min and filter sterilized, and the contents in the entire aliquot were immobilized on a membrane and then probed for PNAG by immunoblotting (6).
Inhibition of phagocytosis by the biofilm matrix and purified PNAG.
Inhibition of phagocytosis was evaluated as described by Maira-Litrán et al. (23), with some modifications. To obtain soluble components of the biofilm matrix formed after 24 h of growth, a biofilm culture of S. epidermidis strain 9142 was released from the solid matrix by sonication in TSB, diluted in TSB to a concentration of approximately 1 × 109 cells/ml, and centrifuged at 10,500 × g for 10 min. The supernatant was boiled in EDTA for 5 min to facilitate solubilization of the PNAG (6) and then allowed to cool down to room temperature. For each milliliter of supernatant, 20 μl of proteinase K (20 mg/ml; QIAGEN) was added, and the supernatant was then incubated for 60 min at 60°C. The proteinase K was heat inactivated for 30 min at 80°C. We also used a solution of 100 mg/liter of purified PNAG that was kindly provided by T. Maira-Litrán, Boston, MA (22), as a positive control. As a negative control, a culture of S. epidermidis 9142-M10 grown for 24 h was diluted to approximately 1 × 109 cells/ml and treated with EDTA to extract surface polysaccharides, as described previously (2). A rabbit polyclonal antiserum raised against the deacetylated PNAG conjugated to diphtheria toxoid (23) was diluted 1:5, and the antiserum was incubated for 90 min at 4°C with an equal volume of either the biofilm matrix extract, a solution of 100 mg/liter of PNAG, the cell surface extract of 9142-M10, or TSB. Subsequently, the antiserum was centrifuged and filtered, and the absorbed serum was used in an opsonophagocytic assay as described above.
Statistical analysis.
Analysis of the statistical significance between pairs of data for which the comparisons of interest were specified prior to looking at the results were conducted with a t test. Analysis of variance (ANOVA) was used for determining differences among multiple groups.
RESULTS
Characterization of S. epidermidis isolates.
Table 1 summarizes the relative levels of PNAG in and amounts of biofilm formed by, as well as the presence of the icaC gene in, the S. epidermidis strains used in this study. The positive control, S. epidermidis 9142, was icaC positive, exhibited high levels of PNAG production, and formed a thick biofilm. The negative control, S. epidermidis 9142-M10, was not able to form biofilm or to produce PNAG, as expected (21). Most of the clinical isolates of S. epidermidis had the icaC gene (six out of nine). However, only three clinical isolates were strong PNAG producers, and these were also the three strains that formed thick biofilms. Accordingly, these three clinical isolates (IE186, IE214, and M187) and also the positive control strain 9142 were selected to perform the phagocytic assays.
TABLE 1.
Characterization of the S. epidermidis strains used in this study
| Strain | ica locusa,b | PNAG blotb | Biofilm formationb |
|---|---|---|---|
| S. epidermidis 9142 | + | + | + |
| S. epidermidis 9142-M10 | − | − | − |
| S. epidermidis IE75 | − | − | − |
| S. epidermidis IE186 | + | + | + |
| S. epidermidis IE214 | + | + | + |
| S. epidermidis M129 | − | − | − |
| S. epidermidis M187 | + | + | + |
| S. epidermidis FJ6 | − | − | − |
| S. epidermidis JI6 | + | − | − |
| S. epidermidis LE7 | + | − | − |
| S. epidermidis PE9 | + | − | − |
Detected with primers for the icaC gene within the icaADCB locus.
A + indicates that the icaC gene was present, a strong PNAG immunoblot signal was produced, or a biofilm was produced. A − indicates that the icaC gene locus was absent, the PNAG immunoblot signal produced was weak or absent, or no biofilm was produced.
Antibody penetration into the biofilm.
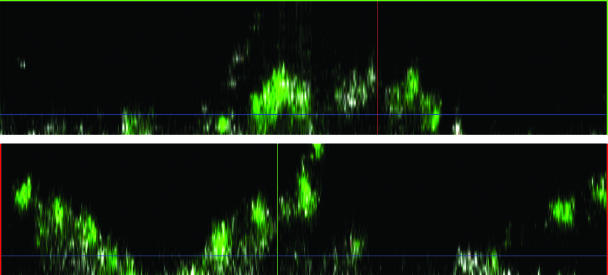
To determine whether the three-dimensional structure of the biofilm acted as a physical barrier to the penetration of antibody, we allowed PNAG-specific rabbit antibodies to bind to biofilms formed by S. epidermidis strain 9142, which were then observed by CSLM. This confocal microscopic analysis of the mixture revealed that the antibodies were able to penetrate throughout the biofilm, as visualized in the x and y cross sections of the biofilm (Fig. 1), demonstrating that S. epidermidis biofilms do not pose a permeability barrier to antibodies. Prior work measuring the diffusion of small molecules through PNAG-based biofilms of S. aureus also showed that there was easy penetration of these molecules throughout the biofilm (12). S. epidermidis biofilms reached thicknesses of approximately 100 μm (2).
FIG. 1.
Analysis of the ability of rabbit IgG antibodies to PNAG to penetrate into a biofilm formed by S. epidermidis strain 9142; the x axis (upper panel) and y axis (lower panel) cross sections of the biofilm are shown. Rabbit antibody to PNAG was visualized using goat anti-rabbit IgG conjugated to Alexa 488.
Localization of the antibody in the biofilm matrix.
Wheat germ agglutinin has been shown to bind to the biofilm matrix of S. epidermidis (28), possibly due to its ability to recognize the N-acetylglucosamine component of the PNAG antigen, although it may recognize other components, such as peptidoglycan and teichoic acid, which also contain glucosamine and form part of the biofilm matrix. We sought to determine if the antibody to PNAG bound to the same areas of the biofilm as did WGA. Figure 2 is a top view of a biofilm, representing the results obtained using three distinct confocal microscope channels: Fig. 2A shows the binding of the antibody to PNAG, Fig. 2B shows the biofilm matrix visualized as a result of the lectin binding, and Fig. 2C shows the three-dimensional structure of the biofilm. Figure 2D shows the overlap of the signals from all of the channels. It could be seen that a fair amount of the antibody signal (green) overlapped with the lectin signal (red) as indicated by the yellow color that appears in Fig. 2D, suggesting that a large proportion of the antibody is sequestered within the biofilm matrix rather than closely associated with the bacterial cells. Although it appears from this top view in Fig. 2 that the smaller lectin molecule (36 kDa) might have penetrated the biofilm somewhat better than the 150-kDa antibody molecules, the x and y cross sections shown in Fig. 1 show that the antibody can readily penetrate throughout the biofilm in both the vertical and horizontal planes.
FIG. 2.
Analysis of binding of either rabbit IgG to PNAG or WGA to components of the biofilm formed by S. epidermidis strain 9142. The following Z sections are shown: panel A, binding of rabbit IgG to PNAG visualized by a goat antibody to rabbit IgG conjugated to Alexa 488; panel B, WGA directly conjugated to Texas Red; panel C, overall biofilm structure as visualized by the refraction of far red light; panel D, colocalization of binding of antibody to PNAG and WGA. In panel D, the antibody signal is shown in green and the lectin signal is shown in red, with the overlap causing the yellow color.
Opsonophagocytosis of planktonic and biofilm cells.
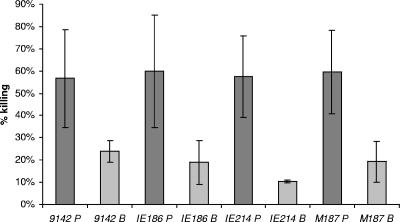
Figure 3 illustrates the antibody-mediated phagocytic killing of suspensions of planktonic and disrupted biofilm cells of S. epidermidis. It is clear that planktonic cells were more easily killed in the phagocytic assay than were the biofilm cells. The average killing of the planktonic cell suspensions was ∼60%, while in biofilm cells, even as fragments in suspension, the average percentage of phagocytic killing dropped to less than 20% (P < 0.05, paired t tests for comparisons between each pair of biofilm and planktonic cells). The levels of killing of planktonic cells of S. epidermidis were similar to previous reports for opsonic killing of S. aureus and S. epidermidis planktonic cells using antibody to PNAG (22, 23). When tested by ANOVA for homogeneity of the killing activity within groups, there were no significant differences in the killing of the four strains as planktonic cells and no significant differences in the killing of the four strains in the biofilm state.
FIG. 3.
Opsonophagocytic killing of different S. epidermidis stains grown either in the planktonic (P) or biofilm (B) mode. Killing was determined using a 1:10 dilution of rabbit antibody raised to deacetylated PNAG conjugated to diphtheria toxoid, and the percent killing was calculated using the mean CFU in duplicate determinations from three different control tubes lacking one of the assay components as the denominator. Killing in the presence of normal rabbit serum was always <5%. There were no significant differences in killing achieved when comparing all four strains grown as planktonic cells and no significant differences in killing when comparing all four strains grown as biofilm cells (P > 0.1; ANOVA). There were significant differences in killing for each strain when comparing planktonic versus biofilm cells at P < 0.05 (unpaired samples t test).
Relative quantification of PNAG in planktonic and biofilm cells.
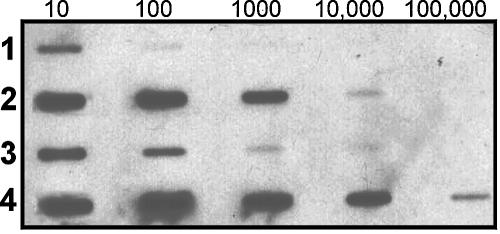
To compare the amounts of PNAG that were secreted or cell surface associated in biofilm and planktonic cells, cell culture supernatants or suspensions of disrupted biofilms and planktonic cells of S. epidermidis strain 9142 with 109 cells/ml were extracted with EDTA and heat and were analyzed. Figure 4 presents the relative quantification of PNAG in these samples as determined by immunoblotting. In both the biofilm and planktonic cells, the PNAG was largely cell surface associated (Fig. 4, lanes 2 and 4), with approximately 100-fold more PNAG on the surface than in the culture supernatant. PNAG was also more abundant on biofilm cells (∼10-fold more) than planktonic cells, and this difference was even more pronounced for the secreted polysaccharide (>10-fold more PNAG in supernatants from biofilm cells than in supernatants from planktonic cells).
FIG. 4.
Expression of PNAG in culture supernatants and cell surface extracts of S. epidermidis strain 9142. Dilutions of each sample are indicated on the top of the figure. Row 1, supernatant from planktonic cells; row 2, EDTA extract of planktonic cells; row 3, supernatant of biofilm cells; row 4, EDTA cell extract of biofilm cells.
Inhibition of phagocytosis by PNAG from the biofilm matrix.
To determine if biofilms could produce sufficient PNAG to inhibit phagocytosis, the biofilm matrix formed by S. epidermidis strain 9142 was extracted and used in an opsonic killing inhibition assay. As a positive control, purified PNAG (50 mg/liter) was also used in the inhibition assay, and as a negative control, a comparably prepared cell surface extract of strain 9142-M10 was used. Figure 5 demonstrates that addition of either undiluted or 1:10 diluted S. epidermidis 9142 biofilm matrix components to antibodies to PNAG markedly decreased the opsonic killing of planktonic cells. While noninhibited antibodies (simply diluted in fresh medium) killed ∼60% of the planktonic bacteria (comparable to results shown in Fig. 3), after incubation of the antibodies to PNAG with either undiluted or 1:10 diluted biofilm matrix there was a ∼75% inhibition of killing of an equivalent suspension of planktonic bacteria. PNAG at 50 mg/liter inhibited ∼90% of the killing, whereas the cell surface extract of strain 9142-M10 did not inhibit the opsonic killing. These results suggest that there is a sufficient amount of the PNAG component in the biofilm matrix that can bind to the opsonic antibodies to subsequently inhibit the killing of otherwise susceptible bacteria.
FIG. 5.
Inhibition of opsonic killing of planktonic cells of S. epidermidis strain 9142. A rabbit antiserum to deacetylated PNAG (diluted 1:10) was adsorbed with either TSB, strain 9142 biofilm matrix (undiluted or diluted 1:10), strain 9142-M10 cell surface extract, or purified PNAG before use in the opsonophagocytic assay. The reduction in percent killing by both the biofilm matrix (undiluted and at 1:10 dilutions) and 50 mg/liter PNAG was significant at P < 0.05 (t test) when compared with the TSB-inhibited sample.
DISCUSSION
In the last few years, several studies reporting the increased resistance of biofilms to killing by components of the immune system were published (13, 15, 25, 42). However, it is still not clear if this resistance is associated with bacterial phenotypic alterations triggered by the sessile mode of life. A better understanding of mechanisms whereby cells in biofilms resist killing by the immune system is needed, as suggested by Leid et al. in their recent study on polymorphonuclear leukocyte penetration into P. aeruginosa biofilms (20).
While it is well known that bacteria have a number of mechanisms by which they evade immune defenses, such as capsule synthesis (26, 32), surface protein modification, and molecular mimicry, it is not clear how these factors might contribute to biofilm-specific properties that confer resistance to clearance by the immune system. Furthermore, studies comparing the susceptibilities of planktonic and biofilm cells to antibodies established to be opsonic and protective against infection with planktonic cells have been limited (13). Here we found that S. epidermidis cells within the biofilm matrix were more resistant to opsonic killing mediated by antibody to PNAG than planktonic cells. However, the well-known increase in synthesis of PNAG associated with the formation of biofilms by S. epidermidis did not establish a barrier to antibody diffusion throughout the biofilm, which indicates that this potential mechanism of resistance to opsonic killing was unlikely to account for the reduced killing of cells. Rather, the increased production of PNAG within the biofilm appeared to overwhelm the antibody added and was able to inhibit killing of planktonic cells when the biofilm matrix was mixed with antibody prior to use in a phagocytosis assay. Whether this mechanism of biofilm cell resistance to phagocytosis is used for other antigenic targets is not known, but to date no other clearly characterized antigenic target of opsonic and protective antibody has been described for PNAG-producing S. epidermidis.
Phagocytic assays have been widely used to test for resistance of bacteria to components of the immune system (32, 37, 39). However, application of this technique to biofilms poses some serious technical limitations. The assay is functional only with a low number of bacterial cells (normally about 107 CFU/ml in order to maintain a balanced proportion of bacteria and leukocytes), and mature biofilms have a much higher cell density. In order to compare biofilm with planktonic bacteria phagocytosis, we disrupted the biofilm and diluted the suspension until a lower bacterial cell concentration, the same as that used for planktonic bacteria, was reached, as described previously by Meluleni et al. (25). Although it can be questioned whether disrupted biofilms are representative of cells within a native three-dimensional biofilm, fragmented biofilms do have the same physiological state as do cells within a mature biofilm (38), and this physiologic state is thought to be a possible reason that some infections are resistant to host immune effectors (5). In addition, it is not clear whether the biofilms formed under optimal in vitro conditions are true mimics of those formed on infected medical devices, which in most infections are unlikely to be as extensively covered with cells and components of the biofilm matrixes as can be achieved by growth in vitro in tissue culture wells. Thus, analyzing smaller fragments of biofilms in our assays likely has some relevance to biofilms formed in vivo.
Overall, the results presented here show that the biofilm matrix can protect bacteria from antibody-mediated phagocytosis in the presence of an antibody opsonically active against planktonic cells, probably due to the large amount of PNAG antigen present within the matrix. This situation minimizes antibody binding close to the bacterial cell surface, where it needs to be in order to promote opsonic killing. We also found much more PNAG produced per cell within the biofilm matrix, supporting the conclusion that this large amount of antigen can inhibit antibody binding to the bacterial cell surface. While PNAG is known to protect planktonic bacteria against antibody-independent phagocytosis (17, 39), it appears that even in the presence of opsonic antibody to PNAG the excess production of the target antigen within the biofilm can prevent efficient opsonic killing. It does not appear that the resistance to opsonic killing is due to poor penetration of antibody, complement, or phagocytes into the biofilm, as some residual killing was always seen; this indicates that if the proper combination of opsonins and phagocytes in sufficient concentration can be achieved within a biofilm, opsonic antibody may also have some protective effects in this setting. However, whether the concentrations of antibody needed to overcome this inhibitory effect can be feasibly achieved in vivo is not known.
Acknowledgments
We acknowledge the financial support of FCT through the project FCT POCTI/ESP/42688/2001 and also the grant SFRH/BD/8676/2002. N.C. was also supported by the Portuguese Fulbright Commission. G.B.P. was supported by NIH grant AI46706, and K.K.J. was supported by NIH grant R21AI61590.
We thank Michelle Ocana and the Center for Brain Imaging, Harvard Center for Neurodegeneration and Repair, Harvard University, Boston, MA, for use of their confocal microscope.
Editor: J. T. Barbieri
REFERENCES
- 1.Cerca, N., S. Martins, F. Cerca, K. K. Jefferson, G. B. Pier, R. Oliveira, and J. Azeredo. 2005. Comparative assessment of antibiotic susceptibility of coagulase-negative staphylococci in biofilm versus planktonic culture as assessed by bacterial enumeration or rapid XTT colorimetry. J. Antimicrob. Chemother. 56:331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerca, N., S. Martins, S. Sillankorva, K. K. Jefferson, G. B. Pier, R. Oliveira, and J. Azeredo. 2005. Effects of growth in the presence of subinhibitory concentrations of dicloxacillin on Staphylococcus epidermidis and Staphylococcus haemolyticus biofilms. Appl. Environ. Microbiol. 71:8677-8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerca, N., G. B. Pier, M. Vilanova, R. Oliveira, and J. Azeredo. 2005. Quantitative analysis of adhesion and biofilm formation on hydrophilic and hydrophobic surfaces of clinical isolates of Staphylococcus epidermidis. Res. Microbiol. 156:506-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu, V. H., D. R. Crosslin, J. Y. Friedman, S. D. Reed, C. H. Cabell, R. I. Griffiths, L. E. Masselink, K. S. Kaye, G. R. Corey, L. B. Reller, M. E. Stryjewski, K. A. Schulman, and V. G. Fowler, Jr. 2005. Staphylococcus aureus bacteremia in patients with prosthetic devices: costs and outcomes. Am. J. Med. 118:1416.e19-1416.e22. [DOI] [PubMed] [Google Scholar]
- 5.Costerton, J., P. Stewart, and P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 6.Cramton, S. E., M. Ulrich, F. Götz, and G. Döring. 2001. Anaerobic conditions induce expression of polysaccharide intercellular adhesin in Staphylococcus aureus and Staphylococcus epidermidis. Infect. Immun. 69:4079-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Götz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fowler, V. G., Jr., L. K. Kong, G. R. Corey, G. S. Gottlieb, R. S. McClelland, D. J. Sexton, D. Gesty-Palmer, and L. J. Harrell. 1999. Recurrent Staphylococcus aureus bacteremia: pulsed-field gel electrophoresis findings in 29 patients. J. Infect. Dis. 179:1157-1161. [DOI] [PubMed] [Google Scholar]
- 10.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Gotz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 11.Hogan, D., and R. Kolter. 2002. Why are bacteria refractory to antimicrobials? Curr. Opin. Microbiol. 5:472-477. [DOI] [PubMed] [Google Scholar]
- 12.Jefferson, K., D. Goldmann, and G. Pier. 2005. Use of confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 49:2467-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jesaitis, A. J., M. J. Franklin, D. Berglund, M. Sasaki, C. I. Lord, J. B. Bleazard, J. E. Duffy, H. Beyenal, and Z. Lewandowski. 2003. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J. Immunol. 171:4329-4339. [DOI] [PubMed] [Google Scholar]
- 14.Kelly-Quintos, C., A. Kropec, S. Briggs, C. L. Ordonez, D. A. Goldmann, and G. B. Pier. 2005. The role of epitope specificity in the human opsonic antibody response to staphylococcal surface polysaccharide poly N-acetyl glucosamine. J. Infect. Dis. 192:2012-2019. [DOI] [PubMed] [Google Scholar]
- 15.Klingenberg, C., E. Aarag, A. Ronnestad, J. E. Sollid, T. G. Abrahamsen, G. Kjeldsen, and T. Flaegstad. 2005. Coagulase-negative staphylococcal sepsis in neonates: association between antibiotic resistance, biofilm formation and the host inflammatory response. Pediatr. Infect. Dis. J. 24:817-822. [DOI] [PubMed] [Google Scholar]
- 16.Kojima, Y., M. Tojo, D. A. Goldmann, T. D. Tosteson, and G. B. Pier. 1990. Antibody to the capsular polysaccharide/adhesin protects rabbits against catheter-related bacteremia due to coagulase-negative staphylococci. J. Infect. Dis. 162:435-441. [DOI] [PubMed] [Google Scholar]
- 17.Kropec, A., T. Maira-Litrán, K. K. Jefferson, M. Grout, S. E. Cramton, F. Götz, D. A. Goldmann, and G. B. Pier. 2005. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect. Immun. 73:6868-6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence, J. R., G. D. W. Swerhone, G. G. Leppard, T. Araki, X. Zhang, M. M. West, and A. P. Hitchcock. 2003. Scanning transmission X-ray, laser scanning, and transmission electron microscopy mapping of the exopolymeric matrix of microbial biofilms. Appl. Environ. Microbiol. 69:5543-5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leid, J. G., M. E. Shirtliff, J. W. Costerton, and A. P. Stoodley. 2002. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect. Immun. 70:6339-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leid, J. G., C. J. Willson, M. E. Shirtliff, D. J. Hassett, M. R. Parsek, and A. K. Jeffers. 2005. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J. Immunol. 175:7512-7518. [DOI] [PubMed] [Google Scholar]
- 21.Mack, D., M. Nedelmann, A. Krokotsch, A. Schwarzkopf, J. Heesemann, and R. Laufs. 1994. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect. Immun. 62:3244-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maira-Litrán, T., A. Kropec, C. Abeygunawardana, J. Joyce, G. Mark III, D. A. Goldmann, and G. B. Pier. 2002. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect. Immun. 70:4433-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maira-Litrán, T., A. Kropec, D. A. Goldmann, and G. B. Pier. 2005. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-N-acetyl-β-(1-6)-glucosamine. Infect. Immun. 73:6752-6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKenney, D., J. Hubner, E. Muller, Y. Wang, D. A. Goldmann, and G. B. Pier. 1998. The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect. Immun. 66:4711-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meluleni, G. J., M. Grout, D. J. Evans, and G. B. Pier. 1995. Mucoid Pseudomonas aeruginosa growing in a biofilm in vitro are killed by opsonic antibodies to the mucoid exopolysaccharide capsule but not by antibodies produced during chronic lung infection in cystic fibrosis patients. J. Immunol. 155:2029-2038. [PubMed] [Google Scholar]
- 26.Moran, F. J., C. Garcia, C. Perez-Giraldo, C. Hurtado, M. T. Blanco, and A. C. Gomez-Garcia. 1998. Phagocytosis and killing of slime-producing Staphylococcus epidermidis by polymorphonuclear leukocytes. Effects of sparfloxacin. Rev. Esp. Quimioter. 11:52-57. [PubMed] [Google Scholar]
- 27.Muller, E., J. Hubner, N. Gutierrez, S. Takeda, D. A. Goldmann, and G. B. Pier. 1993. Isolation and characterization of transposon mutants of Staphylococcus epidermidis deficient in capsular polysaccharide/adhesin and slime. Infect. Immun. 61:551-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neu, T., G. D. Swerhone, and J. R. Lawrence. 2001. Assessment of lectin-binding analysis for in situ detection of glycoconjugates in biofilm systems. Microbiology 147:299-313. [DOI] [PubMed] [Google Scholar]
- 29.O'Gara, J., and H. Humphreys. 2001. Staphylococcus epidermidis biofilms: importance and implications. J. Med. Microbiol. 50:582-587. [DOI] [PubMed] [Google Scholar]
- 30.Resch, A., R. Rosenstein, C. Nerz, and F. Götz. 2005. Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl. Environ. Microbiol. 71:2663-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadavskaya, I., E. Vinogradov, S. Flahaut, G. Kogan, and S. Jabbouri. 2005. Extracellular carbohydrate-containing polymers of a model biofilm-producing strain, Staphylococcus epidermidis RP62A. Infect. Immun. 73:3007-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiau, A. L., and C. L. Wu. 1998. The inhibitory effect of Staphylococcus epidermidis slime on the phagocytosis of murine peritoneal macrophages is interferon-independent. Microbiol. Immunol. 42:33-40. [DOI] [PubMed] [Google Scholar]
- 33.Shiro, H., G. Meluleni, A. Groll, E. Muller, T. Tosteson, D. Goldmann, and G. B. Pier. 1995. The pathogenic role of Staphylococcus epidermidis capsular polysaccharide/adhesin in a low-inoculum rabbit model of prosthetic valve endocarditis. Circulation 92:2715-2722. [DOI] [PubMed] [Google Scholar]
- 34.Shiro, H., E. Muller, N. Gutierrez, S. Boisot, M. Grout, T. D. Tosteson, D. Goldmann, and G. B. Pier. 1994. Transposon mutants of Staphylococcus epidermidis deficient in elaboration of capsular polysaccharide/adhesin and slime are avirulent in a rabbit model of endocarditis. J. Infect. Dis. 169:1042-1049. [DOI] [PubMed] [Google Scholar]
- 35.Takeda, S., G. B. Pier, Y. Kojima, M. Tojo, E. Muller, T. Tosteson, and D. A. Goldmann. 1991. Protection against endocarditis due to Staphylococcus epidermidis by immunization with capsular polysaccharide/adhesin. Circulation 84:2539-2546. [DOI] [PubMed] [Google Scholar]
- 36.von Eiff, C., C. Heilmann, and G. Peters. 1999. New aspects in the molecular basis of polymer-associated infections due to staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 18:843-846. [DOI] [PubMed] [Google Scholar]
- 37.Vuong, C., S. Kocianova, J. M. Voyich, Y. Yao, E. R. Fischer, F. R. DeLeo, and M. Otto. 2004. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J. Biol. Chem. 279:54881-54886. [DOI] [PubMed] [Google Scholar]
- 38.Vuong, C., and M. Otto. 2002. Staphylococcus epidermidis infections. Microbes Infect. 4:481-489. [DOI] [PubMed] [Google Scholar]
- 39.Vuong, C., J. M. Voyich, E. R. Fischer, K. R. Braughton, A. R. Whitney, F. R. DeLeo, and M. Otto. 2004. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell. Microbiol. 6:269-275. [DOI] [PubMed] [Google Scholar]
- 40.Watnick, P., and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309-317. [DOI] [PubMed] [Google Scholar]
- 42.Yasuda, H., Y. Ajiki, J. Aoyama, and T. Yokota. 1994. Interaction between human polymorphonuclear leucocytes and bacteria released from in-vitro bacterial biofilm models. J. Med. Microbiol. 41:359-367. [DOI] [PubMed] [Google Scholar]
- 43.Zieburh, W., C. Heilmann, F. Götz, P. Meyer, K. Wilms, E. Straube, and J. Hacker. 1997. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect. Immun. 65:890-896. [DOI] [PMC free article] [PubMed] [Google Scholar]