Abstract
In contrast to Yersinia pestis LcrV, the recombinant V10 (rV10) variant (lacking residues 271 to 300) does not suppress the release of proinflammatory cytokines by immune cells. Immunization with rV10 generates robust antibody responses that protect mice against bubonic plague and pneumonic plague, suggesting that rV10 may serve as an improved plague vaccine.
Yersinia pestis, the causative agent of bubonic plague and pneumonic plague, is a gram-negative pathogen that infects many animal species, including humans, and is transmitted by arthropod vectors or aerosol droplets (16). Immunization with purified recombinant LcrV (rLcrV) is sufficient to generate protective immunity to both bubonic plague and pneumonic plague in mice, guinea pigs, and non-human primates (6, 7, 10, 11, 13, 30). LcrV is a multifunctional protein that is required for Y. pestis type III injection of effector proteins into immune cells (4, 22). Y. pestis lacking LcrV is avirulent in mouse models of plague disease (8, 17, 19, 20). In addition to its role in type III secretion, LcrV displays immunosuppressive properties (2, 12-14, 24, 25, 28). Brubaker and colleagues showed that LcrV injection of animals triggered release of interleukin-10 (13), a cytokine that suppresses innate immune functions (21). rLcrV also prevents the release of proinflammatory cytokines (gamma interferon and tumor necrosis factor α) in murine and human macrophages (2, 12, 13). Considering the immune modulatory properties of rLcrV, there are concerns regarding the safety of rLcrV vaccines in humans (15).
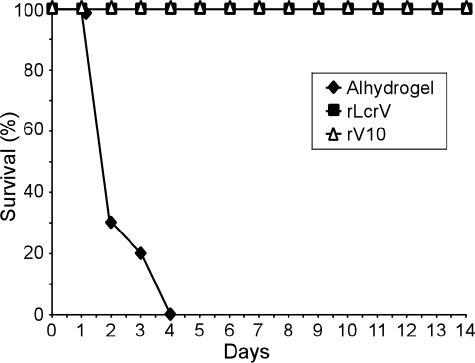
We searched for variants with reduced immune modulatory properties (15). rV10, a variant lacking amino acids 271 to 300 of LcrV, displayed a significant decrease in its ability to induce interleukin-10 and to suppress tumor necrosis factor α or gamma interferon release (15). Immunization of mice with rV10 protects against lethal plague infections caused by 1,000 mean lethal doses (MLD) of Y. pestis KIM5 (KIM D27) (15), a Δpgm (pigmentation defective), attenuated strain that causes plague infections only when inoculated into the bloodstream (3). We sought to determine whether rV10 vaccination can prevent plague disease in animal challenge studies with the fully virulent isolate Y. pestis CO92 (15). Previous work determined the 50% lethal dose of Y. pestis CO92 to be ∼1 to 2 CFU via the subcutaneous route of infection (23, 29). We measured a similar 50% lethal dose for Y. pestis CO92 in a subcutaneous infection model in BALB/c mice (data not shown). Escherichia coli BL21(DE3) carrying prLcrV or prV10 (15) was grown overnight at 37 °C in Luria-Bertani medium (Difco) with 100 μg/ml ampicillin. Bacteria were diluted in fresh medium and grown to an optical density at 600 nm of 0.8 to 1.0. T7 polymerase expression was induced with 1 mM isopropyl-β-d-thiogalactopyranoside, and bacterial growth was continued for 3 hours at 37°C. Cells were harvested by centrifugation at 10,000 × g for 10 min. Bacterial sediment was suspended in 20 ml of Tris-HCl (pH 7.5)-150 mM NaCl (column buffer) containing 100 μM phenylmethylsulfonyl fluoride, and cells were disrupted by two passages through a French pressure cell at 14,000 lb/in2. The lysate was subjected to ultracentrifugation at 40,000 × g for 30 min, and the soluble fraction was applied to a nickel nitrilotriacetic acid column (1-ml bed volume) preequilibrated with 10 ml of column buffer. The column was washed with 10 ml of the same buffer, followed by a second (10 ml of column buffer with 10% glycerol) and a third (10 ml of column buffer with 10% glycerol and 20 mM imidazole) washing. Bound protein was eluted in 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, and 10% glycerol containing 250 mM imidazole. Purified proteins were subjected to three sequential Triton X-114 (Sigma) phase separations to remove endotoxins. Purified proteins were applied to a G-25 (Amersham) gel filtration column to remove residual Triton X-114 and then retrieved by phosphate-buffered saline elution. Lipopolysaccharide contamination of purified proteins was assayed with Limulus amebocyte lysate (QCL-1000; Cambrex, New Jersey) and determined to be less than 1 ng/100 μg of purified protein. Protein concentrations were determined by the bicinchoninic acid assay (Pierce Technology, Rockford, IL). Proteins were aliquoted at 1 mg/ml and stored at −80 °C for further use. Purified recombinant rLcrV and rV10 vaccine antigens were emulsified with Alhydrogel. Groups of 10 BALB/c mice were immunized with adjuvant alone or with 50 μg of rLcrV or rV10 on day 0, followed by a booster with an equal dose on day 21. Blood from 5 mice in each immunization set was taken on days 0, 14, 28, and 42 after primary immunization to measure the generation of specific antibodies. On day 43, mice were challenged with 100,000 MLD of Y. pestis CO92 via subcutaneous injection. rLcrV- or rV10-immunized mice were protected against lethal challenge, whereas mice receiving adjuvant alone succumbed to disease within 4 days after infection with an average time-to-death of 2.5 days (Fig. 1 and Table 1). These data demonstrate that, similar to rLcrV, rV10 immunization of mice provides robust protection against bubonic plague infections.
FIG. 1.
Vaccination of mice with rV10 provides protection against bubonic plague. BALB/c mice were immunized intramuscularly with adjuvant alone (Alhydrogel), rLcrV, or rV10 in a two-dose regimen (50 μg of purified, endotoxin-free antigen injected on day 0 and 21). On day 43 postimmunization, mice were challenge with 100,000 MLD of Y. pestis CO92 by subcutaneous injection, and survival was monitored.
TABLE 1.
Vaccine protection elicited by rV10 and rLcrV immunization against intranasal challenge with Y. pestis CO92
| Inoculum (CFU)a | No. of surviving animals/total no. of animals in cohort (avg time to death ± SD [days])b
|
||
|---|---|---|---|
| Alhydrogel | rLcrV | rV10 | |
| 4.5 × 105 | 0/9 (2.1) | 9/9 | 9/9 |
| 6.7 × 106 | NT | 8/9 (3.0) | 9/9 |
| 5.6 × 107 | NT | 7/9 (3.0) | 9/9 |
| 4.0 × 108 | NT | 2/9 (2.4 ± 1.2) | 3/9 (4.2 ± 2.5) |
Anesthetized BALB/c mice were infected by intranasal inoculation and observed for 14 days for the development of plague.
Statistical significance of differences in average time to death at 4.0 × 108 CFU infectious doses was interrogated with a paired Student's t test, and the P value was recorded (P < 0.0531). NT, not tested.
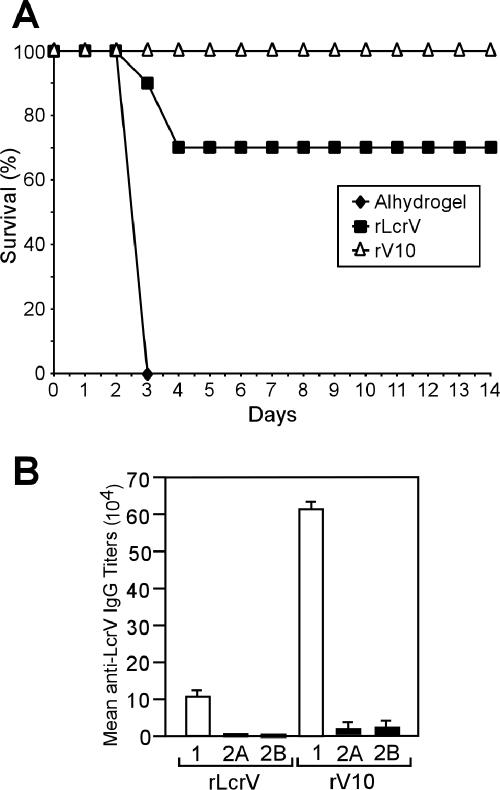
Pneumonic plague infections in mice can be precipitated via aerosol inhalation or intranasal infection. Aerosol infection of mice is technically demanding and requires high doses of Y. pestis (1). To develop an intranasal infection model of Y. pestis CO92, groups of 10 BALB/c mice were infected with bacterial suspensions delivered by the intranasal route. Actual deposition in the lungs was determined by postmortem removal of lungs 60 min after inoculation, followed by plating of tissue homogenate and colony formation. After infection with 1.9 × 105 CFU of Y. pestis CO92, greater than 80% of inoculated bacteria were found deposited in lung tissues. Animals were monitored for 14 days for signs of lethal disease or death and time-to-death was recorded. An average dose of 389 CFU (MLD) caused lethal disease in half of all experimental animals, consistent with previous observations using Y. pestis biovar Medievalis strain KIM (26). The average time to death varied, depending on dose, with high-dose animals succumbing to infection on day 2, while animals infected with lower doses developed lethal infections 4 days after inoculation. Groups of 10 BALB/c mice were immunized with rLcrV or rV10 according to the two-dose regimen described above. On day 43 after primary immunization, mice were challenged with 2,570 MLD of Y. pestis CO92 (1,000,000 CFU) by intranasal inoculation. rLcrV vaccination provided 70% protection in this experiment, whereas mice immunized with rV10 were completely protected (Fig. 2). These data suggest that rV10 vaccination is at least as efficacious against lethal pneumonic plague challenge as rLcrV immunization. Sera collected from immunized mice on day 42 after primary immunization were analyzed by enzyme-linked immunosorbent assay (ELISA) for total immunoglobulin G (IgG) specific for rLcrV or rV10. The data revealed a significant increase in anti-rLcrV IgG antibody titer in rV10-vaccinated mice compared with rLcrV-immunized animals (1.3 × 105 [± 8.2 × 103] for rV10 and 2.5 × 104 [± 0] for rLcrV; P < 0.001, determined with a Student's t test).
FIG. 2.
Vaccination of mice with rV10 provides protection against pneumonic plague. (A) BALB/c mice were immunized intramuscularly by following the standard two-dose regimen with adjuvant alone, rLcrV, or rV10. On day 43 postimmunization, mice were challenged with 1,000,000 CFU (equivalent to 2,570 MLD) of Y. pestis CO92 via intranasal instillation, and survival was monitored. (B) rLcrV-specific IgG1, IgG2A, and IgG2B antibodies were measured by ELISA in sera of five animals immunized with either rLcrV or rV10. rV10 immunization generated significantly higher titers for IgG1 (P < 0.001), IgG2A (P < 0.0181), and IgG2B (P < 0.0064). Statistical significance of differences in IgG titers was interrogated with a paired Student's t test, and P values were recorded.
To compare rV10 and rLcrV vaccine efficacy, we investigated breakthrough challenges in mice immunized with adjuvant, rLcrV, or rV10. On day 43 after immunization, animals were infected intranasally with doses ranging from 4.5 × 105 to 4.0 × 108 CFU. Adjuvant control mice succumbed to disease. Mice vaccinated with rLcrV or rV10 were fully protected at a challenge dose of 4.5 × 105 CFU. Mice immunized with rV10 and challenged with 6.7 × 106 or 5.6 × 107 CFU were also fully protected; however, mice immunized with rLcrV were only partially protected as 1/9 and 2/9 animals, respectively, succumbed to infection. At the highest dose, 4.0 × 108 CFU, rV10 offered partial protection as 6/9 mice succumbed to infection, whereas rLcrV vaccination protected only 2/9 mice. Thus, in comparison with rLcrV, rV10 vaccination offered at least equal levels of plague vaccine protection.
rLcrV vaccination generates antibodies that provide protection against plague by improving the efficiency of host polymorphonuclear cell phagocytosis of Y. pestis and by blocking bacterial type III injection of effector proteins into host cells (18, 27). Immune sera from BALB/c mice vaccinated with either rLcrV or rV10 were characterized for the immunoglobulin isotype profile by ELISA. ELISA plates were coated with 100 μl of the purified recombinant antigens at a concentration of 1 ìg/ml and incubated overnight at 4 °C. Serially diluted mouse serum (100 μl) was added to each well and assayed in triplicate after blocking. The plates were incubated with horseradish peroxidase-conjugated anti-mouse IgG diluted at 1:10,000 (100 μl per well), followed by washing, and were finally developed with 3,3′,5,5′-tetramethybenzidine solution (100 μl per well). The reactions were stopped by adding 25 μl of 2 M H2SO4, and the plates were read at the optical density at 450 nm. An IgG1 or IgG2A or IgG2B isotype-specific ELISA was conducted as described above, using horseradish peroxidase-conjugated goat-anti-mouse IgG1 or IgG2A or IgG2B (Jackson Immunoresearch, Pennsylvania) at a dilution of 1:1,000, 1:500, or 1:500, respectively. Specific IgG titers were estimated as the maximum dilution of serum generating absorbance at 450 nm of 0.1 units over background. Using this antibody titer, mean antibody titers ± standard error of the mean were derived per immunization group. Four independent experiments were performed to verify reproducibility.
Animals receiving either one of these two vaccines responded with the predominant production of IgG1-type antibodies (Fig. 2). Mice immunized with rLcrV showed little to no IgG2a or IgG2b isotype (5). rV10 immunization generated significantly increased humoral immune responses for IgG1, 2a, and 2b isotypes (Fig. 2). To characterize cellular immune responses to vaccination, the proliferation of T cells isolated from immunized mice was measured in response to incubation with homologous or heterologous antigens. The data suggest that rV10 booster immunizations generated elevated T-cell responses compared to rLcrV booster immunizations.
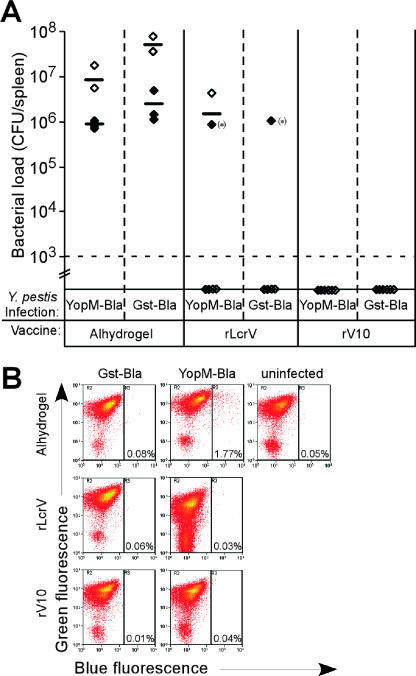
Y. pestis strain KIM5 (pMM83) was used as a reporter to analyze the effect of rV10 and rLcrV immunization on the type III injection of effector Yops into immune cells. pMM83 encodes YopM-Bla, a hybrid between the YopM effector with a C-terminal fusion to β-lactamase (9). CCF2-AM is a membrane-permeant ester with two fluorophores attached to cephalosporin that exhibit fluorescence resonance energy transfer. Excitation of coumarin (409 nm) results in green fluorescence emission from fluorescein (520 nm) in intact CCF2-AM. When the YopM-Bla is injected into host cells, β-lactamase cleaves CCF2-AM, thereby disrupting fluorescence resonance energy transfer and establishing blue fluorescence emission that can be measured by flow cytometry. Groups of seven mice were immunized with adjuvant alone, rLcrV, or rV10 by following the two-dose regimen. On day 43, mice were infected intravenously with 1,000 CFU of Y. pestis KIM5 expressing either YopM-Bla or GST-Bla, a control hybrid that cannot travel the type III pathway (9). On day 2 postinfection, mice were euthanized, and splenocytes were treated with CCF2-AM and subjected to flow cytometry to analyze the injection of YopM-Bla into immune cells (Fig. 3). Mice that received adjuvant developed high bacterial titers in the spleen, ranging from 106 to 107 CFU. Only one of the mice that had been immunized with LcrV developed colonization of the spleen, though no disease symptoms were apparent in this animal. Mice that had been immunized with rV10 harbored no detectable bacteria in the spleen. When analyzed by flow cytometry, mice immunized with adjuvant alone and then infected with Y. pestis expressing YopM-Bla harbored a significant proportion of blue cells (1.77% of splenocytes). As a control, adjuvant mice infected with glutathione transferase (GST)-Bla Yersinia harbored no blue cells. One mouse that had been vaccinated with LcrV harbored Y. pestis KIM5 (pMM83) in the spleen, and 1% of its splenocytes stained blue. No blue cells could be found in the other two rLcrV-immunized mice that did not harbor bacteria in the spleen. None of the rV10-vaccinated mice harbored blue cells or were infected with Y. pestis, suggesting that rV10 vaccination efficiently clears the infection (Fig. 3).
FIG. 3.
Effect of rLcrV and rV10 immunization on plague pathogenesis. (A) Y. pestis strain KIM5 carrying plasmid pMM83 (for expression and type III injection of YopM-Bla) or pMM91 (for expression—but not type III secretion—of GST-Bla) were used for intravenous infection of mice that had been immunized with adjuvant alone (Alhydrogel), rLcrV, or rV10. Bacterial load in the spleen of infected animals was quantified by colony formation of tissue homogenate. The dashed line indicates the limit of detection. (B) Yersinia type III injection of splenic phagocytes measured with CCF2-AM staining (blue fluorescence) and flow cytometry of cells isolated from representative animals from panel A immunized with Alhydrogel (control), rLcrV, or rV10.
In sum, using bubonic plague and pneumonic plague models, we observed high levels of protection afforded by rV10 immunization in mice that correlated with the development of specific humoral and cellular immune responses that are at least equivalent to those raised with rLcrV. As rV10 displays reduced immune modulatory properties, this antigen may serve as a safe and effective subunit vaccine for humans. To test this prediction, rV10 vaccine efficacy and safety will need to be examined in non-human primates with aerosol challenge of Y. pestis CO92 as a measure for protection against pneumonic plague.
Acknowledgments
The authors acknowledge membership within and support from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (NIH Award 1-U54-AI-057153).
Editor: V. J. DiRita
REFERENCES
- 1.Anderson, G. W., Jr., S. E. C. Leary, E. D. Williamson, R. C. Titball, S. C. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect. Immun. 64:4580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brubaker, R. R. 2003. Interleukin-10 and the inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 71:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brubaker, R. R. 1969. Mutation rate to nonpigmentation in Pasteurella pestis. J. Bacteriol. 98:1404-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones, S. M., F. Day, A. J. Stagg, and E. D. Williamson. 2001. Protection conferred by a fully recombinant sub-unit vaccine against Yersinia pestis in male and female mice of four inbred strains. Vaccine 19:358-366. [DOI] [PubMed] [Google Scholar]
- 6.Jones, S. M., K. F. Griffin, I. Hodgson, and E. D. Williamson. 2003. Protective efficacy of a fully recombinant plague vaccine in the guinea pig. Vaccine 21:3912-3918. [DOI] [PubMed] [Google Scholar]
- 7.Leary, S. E. C., E. D. Williamson, K. F. Griffin, P. Russell, S. M. Eley, and R. W. Titball. 1995. Active immunization with recombinant V antigen from Yersinia pestis protects mice against plague. Infect. Immun. 63:2854-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee, V. T., C. Tam, and O. Schneewind. 2000. LcrV, a substrate for Yersinia enterocolitica type III secretion, is required for toxin targeting into the cytosol of HeLa cells. J. Biol. Chem. 275:36869-36875. [DOI] [PubMed] [Google Scholar]
- 9.Marketon, M. M., R. W. DePaolo, K. L. DeBord, B. Jabri, and O. Schneewind. 2005. Plague bacteria target immune cells during infection. Science 309:1739-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motin, V. L., R. Nakajima, G. B. Smirvov, and R. R. Brubaker. 1994. Passive immunity to yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect. Immun. 62:4192-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motin, V. M., Y. A. Nedialkov, and R. R. Brubaker. 1996. V antigen-polyhistidine fusion peptide: binding to LcrH and active immunity against plague. Infect. Immun. 64:4313-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakajima, R., and R. R. Brubaker. 1993. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 61:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakajima, R., V. L. Motin, and R. R. Brubaker. 1995. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect. Immun. 63:3021-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nedialkov, Y. A., V. L. Motin, and R. R. Brubaker. 1997. Resistance to lipopolysaccharide mediated by the Yersinia pestis V antigen-polyhistidine fusion peptide: amplification of interleukin-10. Infect. Immun. 65:1196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Overheim, K. A., R. W. Depaolo, K. L. Debord, E. M. Morrin, D. M. Anderson, N. M. Green, R. R. Brubaker, B. Jabri, and O. Schneewind. 2005. LcrV plague vaccine with altered immunomodulatory properties. Infect. Immun. 73:5152-5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 10:35-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perry, R. D., P. A. Harmon, W. S. Bowmer, and S. C. Straley. 1986. A low-Ca2+ response operon encodes the V antigen of Yersinia pestis. Infect. Immun. 54:428-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philipovskiy, A. V., C. Cowan, C. R. Wulff-Strobel, S. H. Burnett, E. J. Kerschen, D. A. Cohen, A. M. Kaplan, and S. C. Straley. 2005. Antibody against V antigen prevents Yop-dependent growth of Yersinia pestis. Infect. Immun. 73:1532-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price, S. B., C. Cowan, R. D. Perry, and S. C. Straley. 1991. The Yersinia pestis V antigen is a regulatory protein necessary for Ca2+-dependent growth and maximal expression of low-Ca2+ response virulence genes. J. Bacteriol. 173:2649-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price, S. B., K. Y. Leung, S. S. Barve, and S. C. Straley. 1989. Molecular analysis of lcrGVH, the V antigen operon of Yersinia pestis. J. Bacteriol. 171:5646-5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redpath, S., P. Ghazal, and N. R. Gascoigne. 2001. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol. 9:86-92. [DOI] [PubMed] [Google Scholar]
- 22.Rosqvist, R., K.-E. Magnusson, and H. Wolf-Watz. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russel, P., S. M. Eley, S. E. Hibbs, R. J. Manchee, A. J. Stagg, and R. W. Titball. 1995. A comparison of plague vaccine, USP and EV76 vaccine induced protection against Yersinia pestis in a murine model. Vaccine 13:1551-1556. [DOI] [PubMed] [Google Scholar]
- 24.Sing, A., D. Reithmeier-Rost, K. Granfors, J. Hill, A. Roggenkamp, and J. Heesemann. 2005. A hypervariable N-terminal region of Yersinia LcrV determines Toll-like receptor 2-mediated IL-10 induction and mouse virulence. Proc. Natl. Acad. Sci. USA 102:16049-16054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sing, A., D. Rost, N. Tvardovaskaia, A. Roggenkamp, A. Wiedemann, C. Kirschning, J. M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196:1017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, S., D. Heilman, F. Liu, T. Giehl, S. Joshi, X. Huang, T. H. Chou, J. Goguen, and S. Lu. 2004. A DNA vaccine producing LcrV antigen in oligomers is effective in protecting mice from lethal mucosal challenge of plague. Vaccine 22:3348-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weeks, S., J. Hill, A. Friedlander, and S. Welkos. 2002. Anti-V antigen antibody protects macrophages from Yersinia pestis-induced cell death and promotes phagocytosis. Microb. Pathog. 32:227-237. [DOI] [PubMed] [Google Scholar]
- 28.Welkos, S., A. Friedlander, D. McDowel, J. Weeks, and S. Tobery. 1998. V antigen of Yersinia pestis inhibits neutrophil chemotaxis. Microb. Pathog. 24:185-196. [DOI] [PubMed] [Google Scholar]
- 29.Welkos, S. L., A. M. Friedlander, and K. J. Davis. 1997. Studies on the role of plasminogen activator in systemic infection by virulent Yersinia pestis strain C092. Microb. Pathog. 23:211-223. [DOI] [PubMed] [Google Scholar]
- 30.Williamson, E. D., H. C. Flick-Smith, C. LeButt, C. A. Rowland, S. M. Jones, E. L. Waters, R. J. Gwyther, J. Miller, P. J. Packer, and M. Irving. 2005. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect. Immun. 73:3598-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]