Abstract
Formation of a membrane-associated replication complex, composed of viral proteins, replicating RNA, and altered cellular membranes, is a characteristic feature of plus-strand RNA viruses. Here, we demonstrate the presence of a specific membrane alteration, designated the membranous web, that contains hepatitis C virus (HCV) nonstructural proteins, as well as viral plus-strand RNA, in Huh-7 cells harboring autonomously replicating subgenomic HCV RNAs. Metabolic labeling with 5-bromouridine 5′-triphosphate in the presence of actinomycin D revealed that the membranous web is the site of viral RNA synthesis and therefore represents the replication complex of HCV.
Formation of a membrane-associated replication complex composed of viral proteins, replicating RNA, and altered cellular membranes is a hallmark of all of the plus-strand RNA viruses investigated thus far (4, 11, 23, 27, 29, 32). This strategy may offer multiple potential advantages, including (i) compartmentalization and local concentration of viral products, (ii) physical support and organization of the RNA replication complex (20), (iii) tethering of the viral RNA during unwinding, (iv) provision of lipid constituents important for replication (1, 34), and (v) protection of the viral RNA from double-stranded RNA-mediated host defenses or RNA interference.
Hepatitis C virus (HCV) contains a plus-strand RNA genome of approximately 9,600 nucleotides that encodes a polyprotein precursor of about 3,000 amino acid residues (17, 21). We have recently shown that expression of the HCV polyprotein in tetracycline-regulated human osteosarcoma cell lines induces distinct membrane alterations. A prominent alteration, designated the membranous web, was found to contain all of the viral structural and nonstructural proteins and therefore was proposed to represent the HCV replication complex (9). The membranous web could be induced by NS4B alone and was very similar to the “sponge-like inclusions” previously observed by electron microscopy (EM) in the livers of HCV-infected chimpanzees (24).
The recent development of replicon systems for HCV has allowed, for the first time, the study of efficient and genuine HCV RNA replication in Huh-7 human hepatoma cells in vitro (6, 13, 19, 25). The aims of the present study were to locate the site of HCV RNA replication and to identify the HCV replication complex in Huh-7 cells harboring a prototype subgenomic HCV replicon.
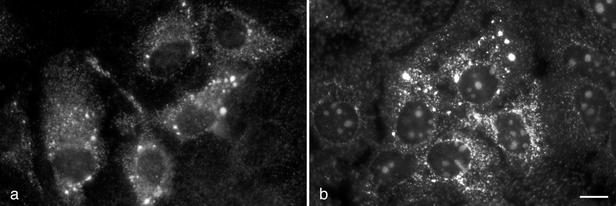
First, Huh-7-derived clone 9-13, harboring a bicistronic subgenomic replicon (19), was investigated by immunofluorescence microscopy (IF) to determine the subcellular distribution of the viral nonstructural proteins. Fixation, permeabilization, and immunostaining of cells were performed as previously described (10). As shown in Fig. 1a, NS3 was found in the cytoplasm as brightly fluorescing dots and in a reticular staining pattern. Very similar staining patterns have consistently been observed with monoclonal antibodies (MAbs) directed against NS4A, NS4B, NS5A, and NS5B (data not shown). By double-labeling IF, HCV nonstructural proteins were found to colocalize in the dot-like structures (data not shown). This is consistent with our observations for tetracycline-regulated cell lines expressing the entire HCV polyprotein (22) and suggests the formation of a multiprotein complex.
FIG. 1.
Localization of HCV nonstructural proteins and plus-strand RNA in Huh-7 cells harboring a subgenomic replicon. (a) Huh-7-derived 9-13 replicon cells were examined by IF with HCV NS3-specific MAb 1B6 (33). NS3 was located in dot-like structures and in a reticular staining pattern. (b) HCV plus-strand RNA was detected by FISH with a directly FITC-labeled riboprobe of negative polarity. HCV plus-strand RNA accumulated in dot-like structures. Bar, 10 μm. Naive Huh-7 cells stained in parallel showed no specific staining.
To determine the distribution of viral plus-strand RNA, fluorescence in situ hybridization (FISH) was performed. A fluorescein isothiocyanate (FITC)-labeled riboprobe of negative polarity was prepared by in vitro transcription from HindIII-restricted plasmid pFKrepneo/R2884G (18) using T7 RNA polymerase (Roche Molecular Biochemicals, Mannheim, Germany). Labeling, alkaline hydrolysis, and purification of the 6.6-kb riboprobe were performed as previously described (10). HCV-specific plus-strand RNA was detectable as bright dots distributed over the cytoplasm, with some accumulation in the perinuclear region (Fig. 1b). The distribution and size of the dots strongly resembled those of the dots identified by IF (compare Fig. 1a and b). As commonly observed with riboprobes, there was some nonspecific labeling of the nucleoli of 9-13 replicon cells (Fig. 1b) and naive Huh-7 cells (data not shown). However, no label was found in the cytoplasm of naive Huh-7 cells, proving the specificity of the FISH reaction. In conclusion, both HCV nonstructural proteins and RNA localized to distinct, dot-like structures that are newly formed in Huh-7 cells harboring autonomously replicating HCV RNAs.
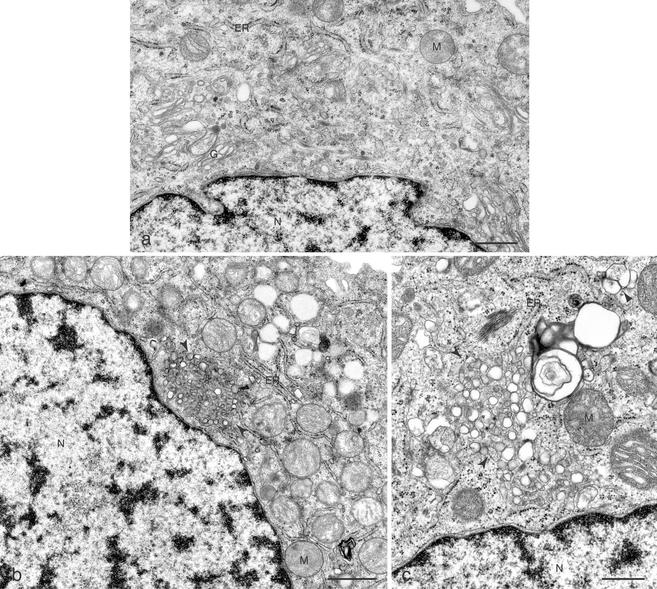
To identify the ultrastructural equivalent of the dot-like structures harboring viral proteins and RNA, we investigated naive and replicon-harboring Huh-7 cells by EM. For EM, cells were fixed in 2.5% glutaraldehyde and 2% OsO4 and embedded in Poly/Bed 812 (Polysciences, Warrington, Pa.) in accordance with standard protocols. As shown in Fig. 2a, no membrane alterations were found in naive Huh-7 cells. In contrast, a membranous web composed of small vesicles embedded in a membrane matrix was found in 9-13 cells (Fig. 2b and c). This structure was similar to the membranous web previously identified in U-2 OS human osteosarcoma-derived cell lines inducibly expressing the HCV polyprotein (9). As in these cell lines, the membranous web was often found closely associated with the rough endoplasmic reticulum (ER). On the basis of this observation, together with earlier studies demonstrating the colocalization of individually expressed HCV proteins with membranes of the ER (7, 12, 28, 33) and recent data indicating that HCV RNA replication takes place in a compartment that sustains endoglycosidase H-sensitive glycosylation (14), we speculate that the membranous web is derived from membranes of the ER.
FIG. 2.
Membrane alterations in Huh-7 cells harboring a subgenomic replicon. (a) A naive Huh-7 cell displays unaltered organelles. Bar, 1 μm. (b) Low-power overview showing the membranous web (arrows) in a 9-13 replicon cell. Bar, 1 μm. (c) Higher magnification of the membranous web (arrows). A second membrane alteration that closely resembles the contiguous vesicles found in HCV-inducible cell lines and that did not harbor HCV proteins (9) was less abundant in replicon cells (arrowhead). Bar, 500 nm. G, Golgi apparatus; M, mitochondria; N, nucleus.
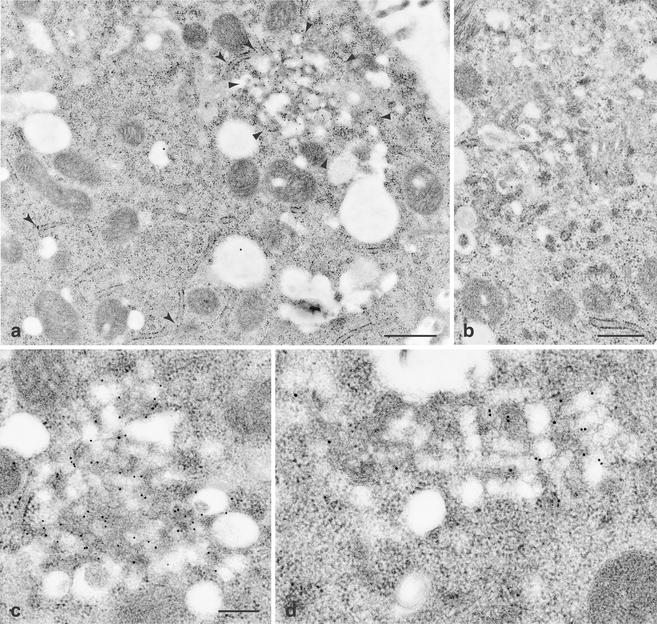
Immuno-EM was performed to investigate the localization of HCV nonstructural proteins. For immuno-EM, cells were fixed and embedded in LRGold (London Resin Co., London, United Kingdom) at −20°C as previously described (3). Sections were incubated with MAbs, followed by incubation with gold-conjugated secondary antibodies (Amersham Pharmacia Biotech, Piscataway, N.J.) as previously described (2). As shown at low magnification in Fig. 3a, NS5A-specific label strongly accumulated on a membranous structure. In addition, some label was found to be associated with the ER. Simultaneously processed naive Huh-7 cells did not show label accumulation above the background (Fig. 3b). Thus, distribution of NS5A at the ultrastructural level corresponds very well to the strong dot-like and weak reticular staining pattern observed by IF. Higher magnification allowed us to unequivocally define the structure extensively labeled by MAbs against NS5A and NS3 as the membranous web (Fig. 3c and d). Identical findings were obtained with MAbs against NS4A, NS4B, and NS5B (data not shown). Thus, all HCV nonstructural proteins are associated with the membranous web in Huh-7 cells harboring subgenomic HCV replicons. This result is in perfect agreement with our previous observations using tetracycline-regulated cell lines (9).
FIG. 3.
Immunogold detection of HCV nonstructural proteins on the membranous web in replicon cells. (a) 9-13 replicon cells were labeled with NS5A-specific MAb 11H (7; kindly provided by Jan Albert Hellings, Organon Teknika B.V., Boxtel, The Netherlands). Gold particles accumulated on a membranous structure (surrounded by arrowheads). In addition, some label was found on the ER (arrows). Bar, 500 nm. (b) Identically treated naive Huh-7 cells were virtually devoid of gold particles. Bar, 500 nm. (c and d) At higher magnification, 9-13 replicon cells labeled with NS5A-specific MAb 11H (c) and NS3-specific MAb 1B6 (d). Both antigens are found almost exclusively on the membranous web. Bar, 200 nm.
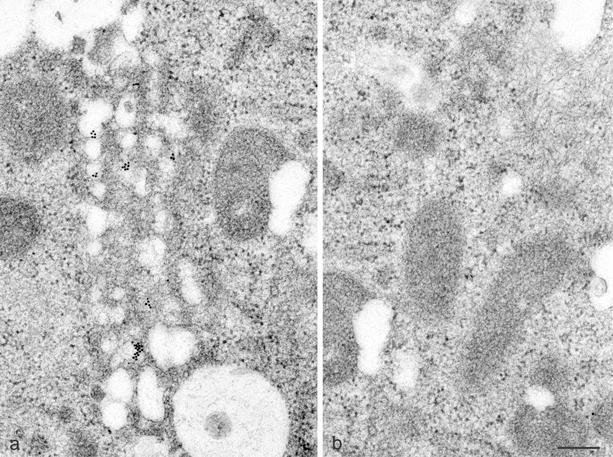
The replicon system also enabled us to localize the genuinely replicated viral RNA by EM-in situ hybridization. A digoxigenin-labeled HCV-specific riboprobe of negative polarity was prepared that was analogous to the probe used for FISH (see above). After hybridization, RNA was detected with a gold-conjugated anti-digoxigenin antibody (Aurion, Wageningen, The Netherlands). The results shown in Fig. 4a reveal that the bulk of the HCV-specific plus-strand RNA was associated with the membranous web. An adjacent area of the same cell showed only background labeling (Fig. 4b). In conclusion, the membranous web contains, besides all of the HCV nonstructural proteins, the autonomously replicated viral RNA.
FIG. 4.
EM-in situ hybridization of Huh-7 cells harboring a subgenomic HCV replicon. (a) HCV plus-strand RNA in 9-13 replicon cells, detected with a digoxigenin-labeled riboprobe of negative polarity and a gold-conjugated anti-digoxigenin antibody, is associated with the membranous web. (b) An adjacent area of the same cell shows no label accumulation above the background. Bar, 200 nm.
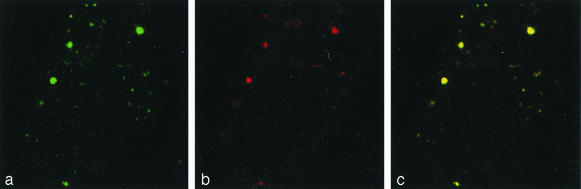
The observation that all of the viral nonstructural proteins localized to the membranous web allowed us to select arbitrarily one of the viral nonstructural proteins, i.e., NS5A, as a marker for the membranous web in subsequent IF studies. The observation that viral proteins and plus-strand RNA localized to the membranous web validated this membrane alteration as a candidate HCV replication complex. To conclusively identify the replication complex, we determined the site of viral RNA-dependent RNA synthesis by metabolic labeling with 5-bromouridine 5′-triphosphate as previously described (11). By double-labeling confocal laser scanning microscopy, we determined the localization of newly synthesized viral RNA in relation to NS5A as a marker of the membranous web. Digital optical sections were taken with a confocal laser scanning microscope (model TCS4D; Leica Lasertechnik, Heidelberg, Germany). NS5A was located with the polyclonal antiserum WU144 (kindly provided by Charles M. Rice, The Rockefeller University, New York, N.Y.) and an FITC-conjugated secondary antibody (green in Fig. 5a), and bromouridine-labeled newly synthesized viral RNA was detected with a MAb against bromodeoxyuridine (Bio Cell Consulting, Reinach, Switzerland), followed by a Texas Red-conjugated secondary antibody (red in Fig. 5b). Both signals clearly colocalized to the same cytoplasmic dot-like structures (yellow in Fig. 5c). The finding that NS5A, a marker of the membranous web, colocalizes with newly synthesized viral RNA demonstrates that the membranous web represents the site of viral RNA synthesis and, therefore, the HCV replication complex.
FIG. 5.
Detection of nascent HCV RNA in Huh-7 cells harboring a subgenomic replicon. 9-13 replicon cells were metabolically labeled with 5-bromouridine 5′-triphosphate in the presence of actinomycin D and then subjected to double-labeling confocal laser scanning microscopy. (a) Detection of NS5A with the polyclonal antiserum WU144 and an FITC-conjugated secondary antibody. (b) Detection of newly synthesized, bromouridine-labeled viral RNA with a MAb against bromodeoxyuridine and a Texas Red-conjugated secondary antibody. (c) The overlay demonstrates colocalization (yellow) of NS5A and nascent viral RNA. Each image covers an area of 37 by 37 μm. Negative controls included identically treated naive Huh-7 cells.
The similarity of the membranous web identified in the replicon cells to that previously found in tetracycline-regulated cell lines indicates that expression of HCV proteins is sufficient to induce formation of the membranous web that accommodates active RNA replication. This is consistent with results obtained with other plus-strand RNA viruses where single proteins expressed alone or in the context of the entire open reading frame induced membrane alterations very similar to membrane changes found during natural infection (10, 30, 31). In the replicon cells, the membranous web was found less frequently than the inducible cell lines. Also, additional membrane alterations found in the tetracycline-regulated cell lines, notably, loose vesicles and contiguous vesicles, were much less abundant in the replicon cells. This is likely due to the overall lower protein expression levels in the replicon cells and may more closely reflect the natural situation in HCV-infected human and chimpanzee hepatocytes (15, 16). In this context, it is important to note that intracellular organelles, particularly the ER and the Golgi complex, remained intact in 9-13 cells. Thus, characteristic membrane changes described in other virus systems as cytopathic effects (5, 8) were not observed. This is consistent with unaltered growth characteristics of these cells compared to those of naive Huh-7 cells (26).
In our initial ultrastructural studies of Huh-7 cells carrying a persistently replicating HCV replicon, we did not detect the membranous web (26). The most likely reasons for this are differences in the specimen preparation methods that influence the recognizability of this structure in EM. Moreover, the previous identification of the membranous web in inducible cell lines that express high levels of the HCV polyprotein facilitated the finding of this structure in cells with a replicon where expression levels of HCV proteins are lower and formation of the membranous web is less frequent. Accordingly, we have very recently also identified the membranous web in Huh-7 cells harboring genomic length HCV replicons (25) (R.G. et al., unpublished observations).
In conclusion, ultrastructural analyses, combined with metabolic labeling of nascent viral RNA, have allowed us to identify, for the first time, the HCV replication complex in Huh-7 cells harboring autonomously replicating subgenomic HCV RNAs. Future studies will be aimed at isolating and further characterizing this complex and at defining the viral and cellular processes involved in formation of the membranous web. Elucidation of these processes will, in all likelihood, allow the definition of novel targets for antiviral intervention against hepatitis C.
Acknowledgments
We gratefully acknowledge Lukas Landmann, Institute of Anatomy, University of Basel, Basel, Switzerland, for access to the confocal laser scanning microscope and Jan Albert Hellings and Charles M. Rice for antibodies.
This work was supported by grant Mo 799/1-3 and SFB 490, Teilprojekt A2 from the Deutsche Forschungsgemeinschaft, grant 31-055397.98 from the Swiss National Science Foundation, grants QLK2-CT1999-00356 and QLK2-CT2002-01329 from the European Commission, grant 01 KI 9951 from the Bundesministerium für Bildung und Forschung, the Lucie Bolte Foundation, and the Jubiläumsstiftung der Schweizerischen Rentenanstalt/Swiss Life.
REFERENCES
- 1.Ahola, T., A. Lampio, P. Auvinen, and L. Kääriäinen. 1999. Semliki Forest virus mRNA capping enzyme requires association with anionic membrane phospholipids for activity. EMBO J. 18:3164-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bienz, K., and D. Egger. 1995. Immunocytochemistry and in situ hybridization in the electron microscope: combined application in the study of virus-infected cells. Histochem. Cell Biol. 103:325-338. [DOI] [PubMed] [Google Scholar]
- 3.Bienz, K., D. Egger, and L. Pasamontes. 1987. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology 160:220-226. [DOI] [PubMed] [Google Scholar]
- 4.Bienz, K., D. Egger, T. Pfister, and M. Troxler. 1992. Structural and functional characterization of the poliovirus replication complex. J. Virol. 66:2740-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bienz, K., D. Egger, Y. Rasser, and W. Bossart. 1980. Kinetics and location of poliovirus macromolecular synthesis in correlation to virus-induced cytopathology. Virology 100:390-399. [DOI] [PubMed] [Google Scholar]
- 6.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1974. [DOI] [PubMed] [Google Scholar]
- 7.Brass, V., E. Bieck, R. Montserret, B. Wölk, J. A. Hellings, H. E. Blum, F. Penin, and D. Moradpour. 2002. An amino-terminal amphipathic alpha-helix mediates membrane association of the hepatitis C virus nonstructural protein 5A. J. Biol. Chem. 277:8130-8139. [DOI] [PubMed] [Google Scholar]
- 8.Egger, D., R. Gosert, and K. Bienz. 2002. Role of cellular structures in viral RNA replication, p. 247-253. In B. Semler and E. Wimmer (ed.), Molecular biology of picornaviruses. ASM Press, Washington, D.C.
- 9.Egger, D., B. Wölk, R. Gosert, L. Bianchi, H. E. Blum, D. Moradpour, and K. Bienz. 2002. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J. Virol. 76:5974-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gosert, R., D. Egger, and K. Bienz. 2000. A cytopathic and a cell culture adapted hepatitis A virus strain differ in cell killing but not in intracellular membrane rearrangements. Virology 266:157-169. [DOI] [PubMed] [Google Scholar]
- 11.Gosert, R., A. Kanjanahaluethai, D. Egger, K. Bienz, and S. C. Baker. 2002. RNA replication of mouse hepatitis virus takes place at double-membrane vesicles. J. Virol. 76:3697-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hügle, T., F. Fehrmann, E. Bieck, M. Kohara, H.-G. Kräusslich, C. M. Rice, H. E. Blum, and D. Moradpour. 2001. The hepatitis C virus nonstructural protein 4B is an integral endoplasmic reticulum membrane protein. Virology 284:70-81. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda, M., M. Yi, K. Li, and S. M. Lemon. 2002. Selectable subgenomic and genome-length dicistronic RNAs derived from an infectious molecular clone of the HCV-N strain of hepatitis C virus replicate efficiently in cultured Huh7 cells. J. Virol. 76:2997-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivashkina, N., B. Wölk, V. Lohmann, R. Bartenschlager, H. E. Blum, F. Penin, and D. Moradpour. 2002. The hepatitis C virus RNA-dependent RNA polymerase membrane insertion sequence is a transmembrane segment. J. Virol. 76:13088-13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krawczynski, K., M. J. Beach, D. W. Bradley, G. Kuo, A. M. Di Bisceglie, M. Houghton, G. R. Reyes, J. P. Kim, Q.-L. Choo, and M. J. Alter. 1992. Hepatitis C virus antigen in hepatocytes: immunomorphologic detection and identification. Gastroenterology 103:622-629. [DOI] [PubMed] [Google Scholar]
- 16.Lau, J. Y. N., K. Krawczynski, F. Negro, and R. P. González-Peralta. 1996. In situ detection of hepatitis C virus—a critical appraisal. J. Hepatol. 24(Suppl. 2):43-51. [PubMed] [Google Scholar]
- 17.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1041. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, Pa.
- 18.Lohmann, V., F. Körner, A. Dobierzewska, and R. Bartenschlager. 2001. Mutations in hepatitis C virus RNAs conferring cell culture adaptation. J. Virol. 75:1437-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohmann, V., F. Körner, J.-O. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 20.Lyle, J. M., E. Bullitt, K. Bienz, and K. Kirkegaard. 2002. Visualization and functional analysis of RNA-dependent RNA polymerase lattices. Science 296:2218-2222. [DOI] [PubMed] [Google Scholar]
- 21.Moradpour, D., V. Brass, R. Gosert, B. Wölk, and H. E. Blum. 2002. Hepatitis C: molecular virology and antiviral targets. Trends Mol. Med. 8:476-482. [DOI] [PubMed] [Google Scholar]
- 22.Moradpour, D., P. Kary, C. M. Rice, and H. E. Blum. 1998. Continuous human cell lines inducibly expressing hepatitis C virus structural and nonstructural proteins. Hepatology 28:192-201. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen, K. W., Y. van der Meer, N. Roos, and E. J. Snijder. 1999. Open reading frame 1a-encoded subunits of the arterivirus replicase induce endoplasmic reticulum-derived double-membrane vesicles which carry the viral replication complex. J. Virol. 73:2016-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfeifer, U., R. Thomssen, K. Legler, U. Böttcher, W. Gerlich, E. Weinmann, and O. Klinge. 1980. Experimental non-A, non-B hepatitis: four types of cytoplasmic alteration in hepatocytes of infected chimpanzees. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 33:233-243. [DOI] [PubMed] [Google Scholar]
- 25.Pietschmann, T., V. Lohmann, A. Kaul, N. Krieger, G. Rinck, G. Rutter, D. Strand, and R. Bartenschlager. 2002. Persistent and transient replication of full-length hepatitis C virus genomes in cell culture. J. Virol. 76:4008-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietschmann, T., V. Lohmann, G. Rutter, K. Kurpanek, and R. Bartenschlager. 2001. Characterization of cell lines carrying self-replicating hepatitis C virus RNAs. J. Virol. 75:1252-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaad, M. C., P. E. Jensen, and J. C. Carrington. 1997. Formation of plant RNA virus replication complexes on membranes: role of an endoplasmic reticulum-targeted viral protein. EMBO J. 16:4049-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt-Mende, J., E. Bieck, T. Hügle, F. Penin, C. M. Rice, H. E. Blum, and D. Moradpour. 2001. Determinants for membrane association of the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 276:44052-44063. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz, M., J. Chen, M. Janda, M. Sullivan, J. den Boon, and P. Ahlquist. 2002. A positive-strand RNA virus replication complex parallels form and function of retrovirus capsids. Mol. Cell 9:505-514. [DOI] [PubMed] [Google Scholar]
- 30.Snijder, E. J., H. van Tol, N. Roos, and K. W. Pedersen. 2001. Non-structural proteins 2 and 3 interact to modify host cell membranes during the formation of the arterivirus replication complex. J. Gen. Virol. 82:985-994. [DOI] [PubMed] [Google Scholar]
- 31.Teterina, N. L., D. Egger, K. Bienz, D. M. Brown, B. L. Semler, and E. Ehrenfeld. 2001. Requirements for assembly of poliovirus replication complexes and negative-strand RNA synthesis. J. Virol. 75:3841-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westaway, E. G., A. A. Khromykh, and J. M. Mackenzie. 1999. Nascent flavivirus RNA colocalized in situ with double-stranded RNA in stable replication complexes. Virology 258:108-117. [DOI] [PubMed] [Google Scholar]
- 33.Wölk, B., D. Sansonno, H.-G. Kräusslich, F. Dammacco, C. M. Rice, H. E. Blum, and D. Moradpour. 2000. Subcellular localization, stability and trans-cleavage competence of the hepatitis C virus NS3-NS4A complex expressed in tetracycline-regulated cell lines. J. Virol. 74:2293-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu, S.-X., P. Ahlquist, and P. Kaesberg. 1992. Active complete in vitro replication of nodavirus RNA requires glycerophospholipid. Proc. Natl. Acad. Sci. USA 89:11136-11140. [DOI] [PMC free article] [PubMed] [Google Scholar]